Abstract
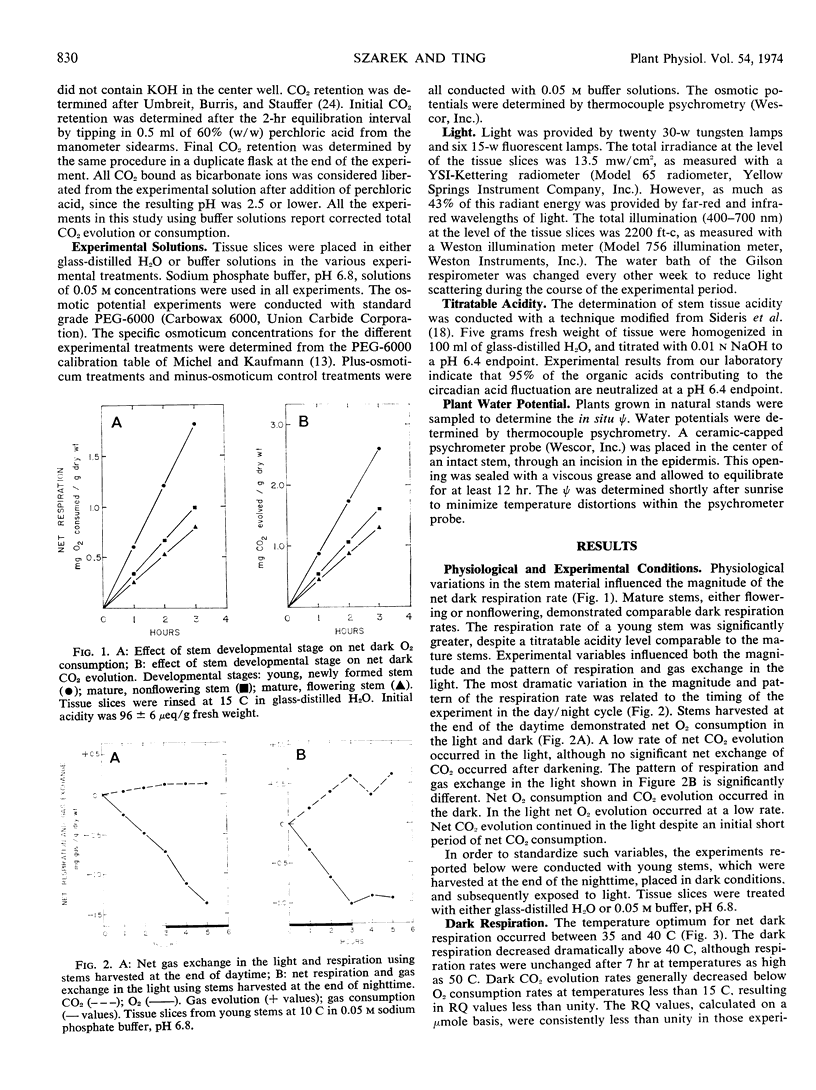
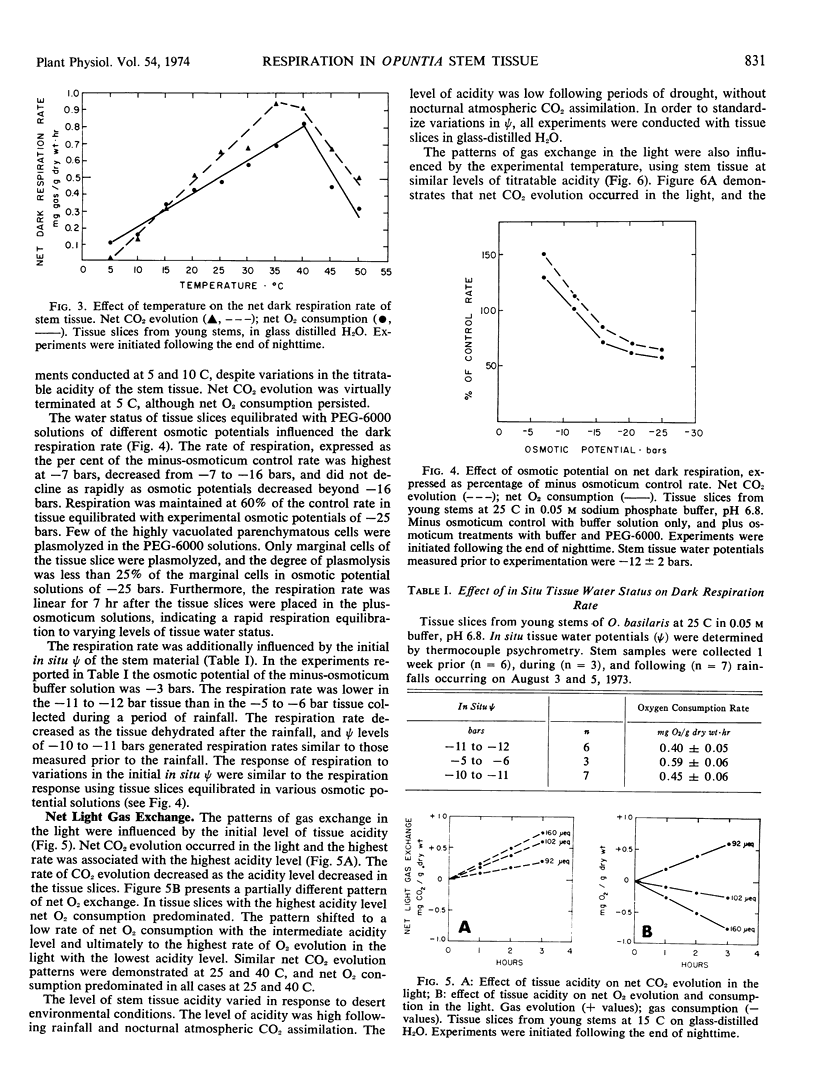
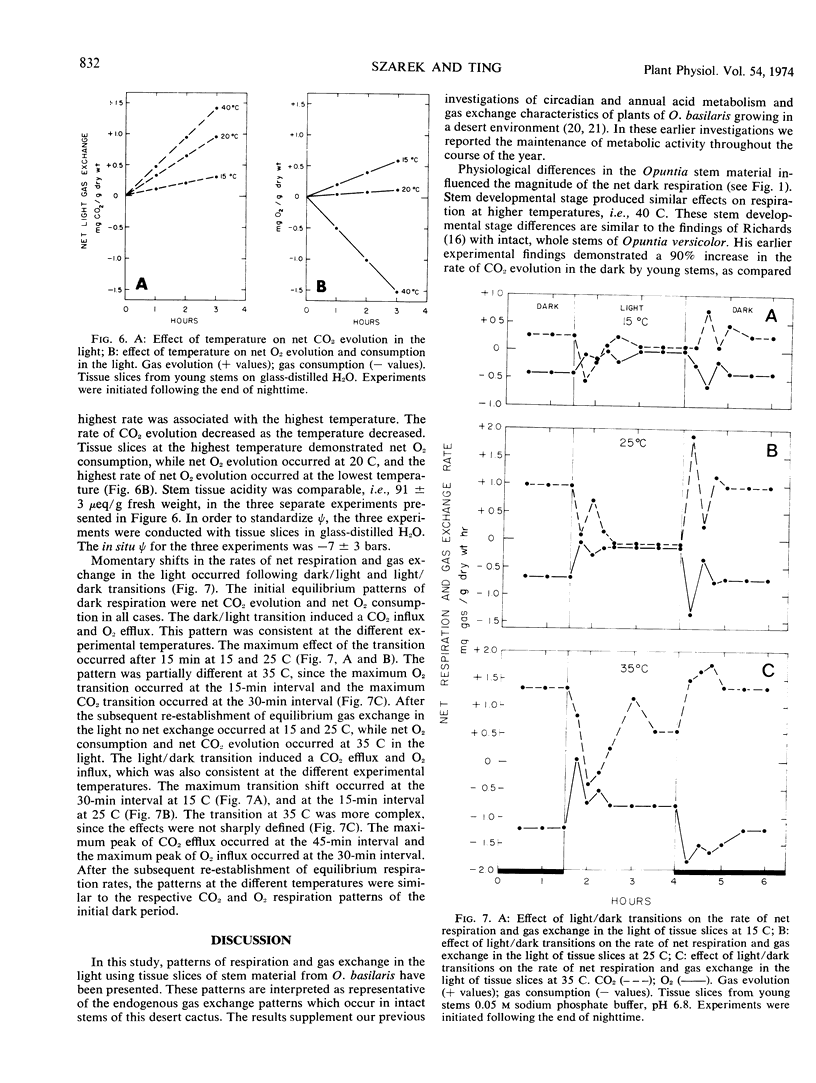
Respiration and gas exchange in the light were studied manometrically with tissue slices from stem material of Opuntia basilaris Engelm. and Bigel. Dark respiration rates were greater in young stems than in mature stems. The timing of the experiment in the day/night cycle influences the magnitude and pattern of respiration and gas exchange in the light. Net dark respiration has a temperature optimum between 35 and 40 C, and is maintained at 60% of the control rate in tissue equilibrated with experimental osmotic potentials of −25 bars. Net gas exchange in the light is regulated by the titratable acidity of the tissue and by the tissue temperature. Increased rates of net CO2 evolution and net O2 consumption occur in the light with high levels of titratable acidity and high temperatures. An efflux of CO2 and influx of O2 occur following light/dark transitions. These patterns are reversed following dark/light transitions. Similar results were demonstrated at 15, 25, and 35 C, and are interpreted as a mechanism of adaptation to desert environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell D. T., Koeppe D. E., Miller R. J. The effects of drought stress on respiration of isolated corn mitochondria. Plant Physiol. 1971 Oct;48(4):413–415. doi: 10.1104/pp.48.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon P. C. Temperature features of enzymes affecting crassulacean Acid metabolism. Plant Physiol. 1967 Jul;42(7):977–984. doi: 10.1104/pp.42.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory F. G., Spear I., Thimann K. V. The Interrelation between CO(2) Metabolism and Photoperiodism in Kalanchoë. Plant Physiol. 1954 May;29(3):220–229. doi: 10.1104/pp.29.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E. Further Comparisons between Carbowax 6000 and Mannitol as Suppressants of Cucumber Hypocotyl Elongation. Plant Physiol. 1971 Nov;48(5):513–516. doi: 10.1104/pp.48.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E., Kaufmann M. R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973 May;51(5):914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz O., Morel C. Photoperiodism and enzyme activity: towards a model for the control of circadian metabolic rhythms in the crassulacean Acid metabolism. Plant Physiol. 1974 Apr;53(4):596–602. doi: 10.1104/pp.53.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhani I., Vines H. M. Isolation of Mesophyll Cells from Sedum telephium Leaves. Plant Physiol. 1973 Jan;51(1):97–103. doi: 10.1104/pp.51.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideris C. P., Young H. Y., Chun H. H. DIURNAL CHANGES AND GROWTH RATES AS ASSOCIATED WITH ASCORBIC ACID, TITRATABLE ACIDITY, CARBOHYDRATE AND NITROGENOUS FRACTIONS IN THE LEAVES OF ANANAS COMOSUS (L.) MERR. Plant Physiol. 1948 Jan;23(1):38–69. doi: 10.1104/pp.23.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Johnson H. B., Ting I. P. Drought Adaptation in Opuntia basilaris: Significance of Recycling Carbon through Crassulacean Acid Metabolism. Plant Physiol. 1973 Dec;52(6):539–541. doi: 10.1104/pp.52.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek S. R., Ting I. P. Seasonal Patterns of Acid Metabolism and Gas Exchange in Opuntia basilaris. Plant Physiol. 1974 Jul;54(1):76–81. doi: 10.1104/pp.54.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Transhydrogenation in Root Tissue: Mediation by Carbon Dioxide. Science. 1965 Dec 24;150(3704):1727–1728. doi: 10.1126/science.150.3704.1727. [DOI] [PubMed] [Google Scholar]
- Wynn T., Brown H. Dark Release of CO(2) from Higher Plant Leaves. Plant Physiol. 1973 Sep;52(3):288–291. doi: 10.1104/pp.52.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]


