Abstract
The purpose of our study was to evaluate the use of bleeding-avoidance strategies (BAS) and risk-adjusted bleeding over time in patients ≥80 years of age undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction. We analyzed data from the CathPCI Registry from July 1, 2006 through June 30, 2009. Patients were included if they were ≥80 years old, presented with ST-segment elevation myocardial infarction, and underwent primary PCI. We evaluated trends in use of BAS (direct thrombin inhibitors, vascular closure devices, and radial access) and risk-adjusted bleeding over time. Of 10,469 patients ≥80 years old undergoing primary PCI, 1,002, (9.6%) developed a bleeding complication. Use of direct thrombin inhibitors and vascular closure devices increased over time (12.8% to 24.9% and 29.2% to 32.7%, p <0.01 and <0.05 for trends, respectively). Radial access was extremely uncommon (<1%) and did not change over the course of the study. In multivariable analyses, use of BAS was associated with lower bleeding. However, over the course of the study period, overall risk-adjusted bleeding did not decrease significantly (9.9% to 9.4%, p = 0.14 for trend). In conclusion, patients ≥80 years old undergoing primary PCI are at high risk of bleeding, and despite significant increases in use of BAS, the overall rate of bleeding complications remains high.
Patients ≥80 years of age with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) are at high risk for bleeding complications.1–6 However, the use and effectiveness of strategies to decrease bleeding are not well understood. The aim of the present study therefore was to describe the use of bleeding-avoidance strategies (BAS) and associated in-hospital bleeding rates in patients ≥80 years of age with STEMI undergoing primary PCI. Furthermore, we sought to assess whether changes in BAS over time have been associated with decreases in risk-adjusted bleeding. To accomplish these goals, we analyzed data from the National Cardiovascular Data Registry (NCDR) Catheter Percutaneous Coronary Intervention (CathPCI) Registry, which provides detailed clinical information on patients undergoing PCI at >1,000 United States hospitals. A major strength of the NCDR is that it captures patients seen in routine clinical practice, many of whom may have been excluded from randomized trials.
Methods
The NCDR CathPCI Registry is a large voluntary quality improvement program cosponsored by the American College of Cardiology and the Society for Cardiovascular Angiography and Interventions.7 The registry includes clinical and in-hospital outcome data on patients undergoing cardiac catheterization and PCI procedures including baseline clinical and sociodemographic characteristics, adjunctive therapies, complications, and mortality. Definitions for data elements within the registry are available on the NCDR Web site (http://www.ncdr.com).8
For the present study we identified patients who presented with STEMI and underwent PCI (emergency or salvage) within 6 hours of arrival. Patients with a door-to-balloon time of <15 minutes were excluded to eliminate possible coding errors.9 We restricted our analysis to PCIs performed at hospitals that participated in the CathPCI Registry continuously from July 1, 2006 through June 30, 2009 (i.e., reported ≥1 PCI in each consecutive year). Patients transferred from another acute-care hospital for PCI were excluded because details of their initial presentation and management were not captured. In addition, we considered information only from the first PCI performed during a hospital stay.
Anticoagulant strategies were divided into approaches commonly used in clinical practice: (1) unfractionated heparin (UFH) alone, (2) UFH plus glycoprotein IIb/IIIa inhibitor, (3) low-molecular weight heparin plus glycoprotein IIb/IIIa, and (4) direct thrombin inhibitor. Arterial access site was characterized as femoral, radial, or brachial. Hemostasis techniques were dichotomized into vascular closure device (suture, staple, sealant, or other) or manual compression (which included mechanical compression devices). Given the uncertainty of the devices included under “other,” we performed a sensitivity analysis for vascular closure devices that excluded this classification. Because there was no significant change in results, the designation of other was retained in the final analysis. We used the term “bleeding-avoidance strategies” to refer to the use of thrombin inhibitors, vascular closure devices, or radial access based on previous studies showing lower bleeding rates with each intervention compared to other therapies.10–13
The primary outcome was in-hospital bleeding, defined as any bleeding event (percutaneous entry site, retroperitoneal, gastrointestinal, genitourinary, or other) causing a decrease in hemoglobin >3.0 g/dl, requiring a transfusion, or prolonging hospital stay. Secondary outcomes included thrombocytopenia, transfusion of blood products, vascular complications (including access site occlusion, peripheral embolization, dissection, pseudoaneurysm, and arteriovenous fistula), and in-hospital mortality.
To characterize bleeding risk associated with primary PCI in elderly patients, we compared unadjusted outcomes observed between patients ≥80 and patients <80 years of age using the chi-square test. We focused the remainder of the analyses on patients ≥80 years old because they represented the primary subgroup of interest. In patients ≥80 years old we compared characteristics of patients who developed a bleeding event to those of patients with no bleeding using a 2-sided t test for continuous variables and chi-square test for categorical variables. We then examined the independent association of use of BAS with bleeding events. We also stratified the population by use of 0 BAS, 1 BAS, or ≥2 BAS and examined the effects of the number of BAS on the outcome of bleeding. Unadjusted and adjusted odds ratios were reported.
To account for differences in patients’ risk of bleeding, we used a previously developed NCDR bleeding risk model.14 The model adjusts for age, female gender, weight, cardiogenic shock, previous congestive heart failure, previous valvular surgery, cerebrovascular disease, peripheral vascular disease, hypertension, previous PCI, intra-aortic balloon pump, estimated glomerular filtration rate, New York Heart Association class, and PCI status of salvage/emergency. We also investigated the presence of a risk–treatment paradox (where patients at higher risk are less likely to receive beneficial strategies)15 by comparing use of BAS across strata of predicted bleeding risk (lower <6%, intermediate 6% to 12%, and higher ≥12%).
We examined trends in BAS and risk-adjusted bleeding rates from 2006 through 2009. Risk-adjusted bleeding rates were calculated using the NCDR bleeding risk model. Changes in BAS were examined using the Cochran-Amit-age trend test. Changes in risk-adjusted bleeding rates over time were evaluated using linear regression.
Results
From an initial sample of 218,935 cases with primary PCI for STEMI from July 2006 through June 2009, the following were excluded: 1,050 for not being the first PCI, 16,175 for PCI not coded as emergency or salvage, 54,511 for transfer from another hospital, 4,083 with door-to-balloon time <15 minutes or >6 hours, and 39,540 for PCI performed at hospitals that did not participate continuously in the CathPCI Registry from 2006 through 2009. This left a final sample of 103,576 procedures for analysis. Of these cases, 10.1% were performed in patients ≥80 years of age (n = 10,469). In these patients, unadjusted bleeding was nearly 2 times as common as in patients <80 years old (9.6% vs 4.9%, p <0.01). Similarly, rates of other adverse outcomes were higher in older patients including transfusion (16.0% vs 7.7%, p <0.01), thrombocytopenia (2.0% vs 1.1%, p <0.01), vascular complications (1.3% vs 0.8%, p <0.001), and crude in-hospital mortality (13.5% vs 3.9%, p <0.01).
A comparison of characteristics in patients ≥80 with and without bleeding is presented in Table 1. Patients with bleeding were more likely to be women (63.6% vs 54.7%, p <0.01), have a history of hypertension (77.6% vs 73.7%, p <0.01) and chronic lung disease (17.2% vs 13.9%, p <0.01), and to present in congestive heart failure (23.9% vs 16.0%, p <0.01) or cardiogenic shock (20.5% vs 13.6%, p <0.01). There were no significant differences between groups in rates of renal insufficiency, diabetes, or previous MI. Bleeding at remote sites (gastrointestinal, genitourinary, or other) was more common in these patients than bleeding related to arterial access (percutaneous entry site or retroperitoneal, 67.7% vs 34.9%) and increased as a proportion of total bleeding over time (61.1% in 2006 vs 78.5% in 2009).
Table 1.
Characteristics of patients ≥80 years old with bleeding versus no bleeding
| Variable | Bleeding
|
p Value | |
|---|---|---|---|
| Yes (n = 1,002) | No (n = 9,467) | ||
| Age (years), mean ± SD | 84.5 ± 3.7 | 84.7 ± 4.3 | 0.27 |
| Women | 63.6% | 54.7% | <0.01 |
| Race | 0.27 | ||
| Caucasian | 89.9% | 87.9% | |
| Black | 3.6% | 3.7% | |
| Hispanic | 1.7% | 2.7% | |
| Other* | 4.7% | 5.6% | |
| Current smoker | 6.8% | 7.2% | 0.65 |
| Chronic renal insufficiency | 8.6% | 7.0% | 0.06 |
| Diabetes mellitus | 20.7% | 19.4% | 0.34 |
| Previous myocardial infarction | 17.2% | 19.2% | 0.12 |
| Hypertension† | 77.6% | 73.7% | <0.01 |
| Dyslipidemia‡ | 53.7% | 53.7% | 0.99 |
| Previous congestive heart failure | 11.7% | 9.9% | 0.08 |
| Chronic lung disease | 17.2% | 13.9% | <0.01 |
| Cerebrovascular disease | 15.7% | 15.5% | 0.89 |
| Previous percutaneous coronary intervention | 13.3% | 18.0% | <0.01 |
| Previous coronary bypass | 6.2% | 8.6% | <0.01 |
| Clinical presentation | |||
| Congestive heart failure | 23.9% | 16.0% | <0.01 |
| Cardiogenic shock | 20.5% | 13.6% | <0.01 |
| Symptom onset >12 hours | 10.4% | 9.6% | 0.40 |
| Right heart catheterization | 7.5% | 3.7% | <0.01 |
Includes Asian, Native-American, and “other.”
Blood pressure >140/90 mm Hg or on antihypertensive therapy.
Total cholesterol >200 mg/dl or low-density lipoprotein ≤130 mg/dl or high-density lipoprotein <30 mg/dl or triglycerides >150 mg/dl or on dyslipidemia therapy.
Thrombin inhibitors were used in 18.3% of cases. Vascular closure devices were used in 31.7% of cases, and radial arterial access was uncommon (<1%). A comparison of characteristics in patients who received 0 BAS, 1 BAS, and ≥2 BAS is presented in Table 2. Baseline co-morbidities were similar in the 2 groups. Patients presenting in congestive heart failure were more common in the group receiving 0 BAS (17.6%) compared to 1 BAS or ≥2 BAS (16.1% and 13.6% respectively, p <0.01). A similar trend was seen in patients who presented in cardiogenic shock (17.3% 0 BAS, 10.8% 1 BAS, 7.5% ≥2 BAS, p <0.01) and in those who received an intra-aortic balloon pump (16.9% 0 BAS, 7.6% 1 BAS, 2.9% ≥2 BAS, p <0.01).
Table 2.
Patient characteristics stratified by number of bleeding-avoidance strategies
| Variable | Total (n = 10,469) | 0 (n = 5,849) | 1 (n = 3,942) | ≥2 (n = 678) | p Value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 84.6 ± 4.2 | 84.6 ± 4.1 | 84.7 ± 4.3 | 85.0 ± 4.2 | <0.05 |
| Women | 55.6% | 55.5% | 55.9% | 54.3% | 0.71 |
| Race | <0.01 | ||||
| Caucasian | 88.1% | 88.6% | 87.1% | 88.9% | |
| Black | 3.7% | 4.0% | 3.3% | 3.0% | |
| Hispanic | 2.6% | 2.6% | 2.6% | 2.8% | |
| Other* | 5.5% | 4.8% | 6.8% | 5.2% | |
| Body mass index (kg/m2), mean ± SD | 25.7 ± 5.0 | 25.7 ± 4.9 | 25.8 ± 5.1 | 25.5 ± 5.1 | 0.33 |
| Current smoker | 7.1% | 7.6% | 6.6% | 6.1% | 0.08 |
| Chronic renal insufficiency | 7.1% | 7.0% | 7.4% | 6.2% | 0.51 |
| Diabetes mellitus | 19.5% | 19.5% | 19.8% | 18.1% | 0.61 |
| Previous myocardial infarction | 19.0% | 19.3% | 18.9% | 16.5% | 0.21 |
| Hypertension† | 74.1% | 74.2% | 74.3% | 72.0% | 0.42 |
| Dyslipidemia‡ | 53.7% | 53.2% | 54.6% | 53.0% | 0.36 |
| Previous congestive heart failure | 10.1% | 10.4% | 9.8% | 8.9% | 0.39 |
| Chronic lung disease | 14.2% | 14.5% | 13.7% | 14.9% | 0.45 |
| Cerebrovascular disease | 15.5% | 15.6% | 15.5% | 15.0% | 0.93 |
| Previous percutaneous coronary intervention | 17.6% | 17.3% | 18.1% | 16.5% | 0.42 |
| Previous coronary bypass | 8.4% | 8.5% | 8.5% | 6.3% | 0.15 |
| Clinical presentation | |||||
| Congestive heart failure | 16.8% | 17.6% | 16.1% | 13.6% | <0.01 |
| Cardiogenic shock | 14.2% | 17.3% | 10.8% | 7.5% | <0.01 |
| Symptom onset >12 hours | 9.6% | 9.4% | 10.0% | 9.6% | 0.65 |
| Intra-aortic balloon pump | 12.5% | 16.9% | 7.6% | 2.9% | <0.01 |
| Right heart catheterization | 4.1% | 5.3% | 2.9% | 1.9% | <0.01 |
| Bleeding | 9.6% | 11.1% | 8.1% | 5.0% | <0.01 |
Includes Asian, Native-American, and “other.”
Blood pressure >140/90 mm Hg or on antihypertensive therapy.
Total cholesterol >200 mg/dl or low-density lipoprotein ≤130 mg/dl or high-density lipoprotein <30 mg/dl or triglycerides >150 mg/dl or on dyslipidemia therapy.
Patients at higher risk of bleeding were modestly more likely to receive thrombin inhibitors than lower-risk patients (18.7% vs 16.9%, p <0.01; Table 3) but less likely to receive vascular closure devices (25.8% vs 37.3%, p <0.01). The combination strategy of thrombin inhibitor plus vascular closure device was used less frequently in higher-risk compared to lower-risk patients (5.3% vs 7.0%, p <0.05; Table 3). In patients who received 1 BAS the adjusted odds ratio for in-hospital bleeding was 0.76 (95% confidence interval 0.66 to 0.88), and in patients receiving ≥2 BAS the odds ratio was 0.45 (95% confidence interval 0.32 to 0.63; Table 4).
Table 3.
Use of strategies based on National Cardiovascular Data Registry bleeding risk score in patients ≥80 years old
| Strategy | Lower Risk (<6%) (n = 2,152) | Average Risk (6–12%) (n = 5,281) | Higher Risk (≥12%) (n = 3,036) | p Value |
|---|---|---|---|---|
| Anticoagulants | <0.01 | |||
| Unfractionated heparin alone | 12.8% | 15.2% | 18.5% | |
| Unfractionated heparin + glycoprotein IIb/IIIa inhibitor | 58.6% | 56.8% | 54.2% | |
| Low-molecular-weight heparin + glycoprotein IIb/IIIa inhibitor | 3.3% | 3.1% | 2.4% | |
| Thrombin inhibitor | 16.9% | 18.6% | 18.7% | |
| Vascular closure device | 37.3% | 32.6% | 25.8% | <0.01 |
| Thrombin inhibitor + vascular closure device | 7.0% | 6.8% | 5.3% | <0.05 |
Table 4.
Bleeding according to number of bleeding-avoidance strategies*
| Outcome | 1 BAS | ≥2 BAS | p Value |
|---|---|---|---|
| Unadjusted bleeding | 0.73 (0.63–0.84) | 0.41 (0.29–0.58) | <0.01 |
| Risk-adjusted bleeding | 0.76 (0.66–0.88) | 0.45 (0.32–0.63) | <0.01 |
Values are presented as odds ratio (95% confidence interval).
Reference = 0 strategy.
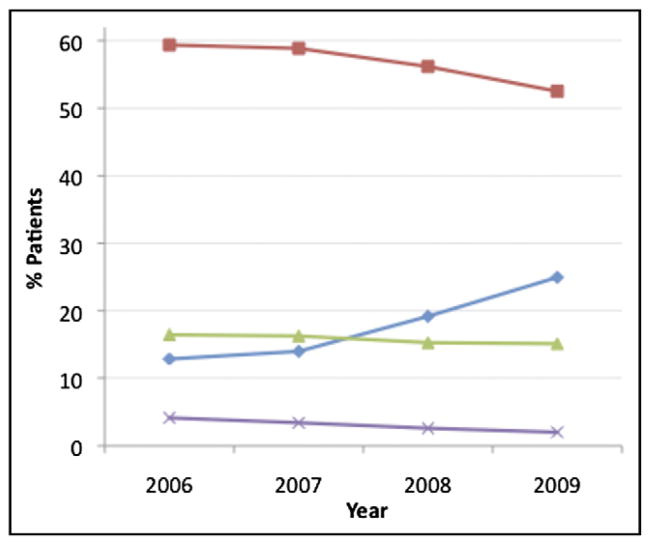
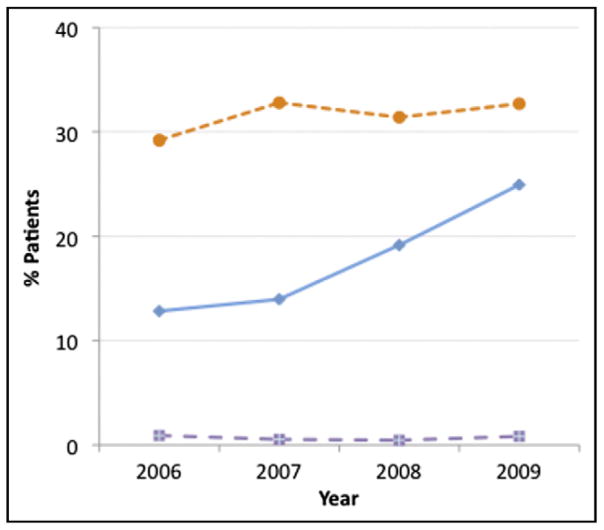
There was a steady increase in use of thrombin inhibitors and decrease in use of UFH plus glycoprotein IIb/IIIa in patients ≥80 years old over time (p <0.001 for trend; Figure 1). Of note, in patients receiving a thrombin inhibitor, many also received heparins and/or IIb/IIIa inhibitors, although this decreased modestly over time (thrombin inhibitor plus UFH 43.8% in 2006, 40.8% in 2007, 41.0% in 2008, 39.4% in 2009; thrombin inhibitor plus IIb/IIIa 53.9% in 2006, 46.3% in 2007, 42.3% in 2008, 34.8% in 2009, p <0.001 for trends). Use of vascular closure devices increased from 29.2% to 32.7% (p <0.05 for trend; Figure 2). In-hospital bleeding rate in patients ≥80 years of age was 9.6% during the entire study period. After risk adjustment using the NCDR bleeding model, there was a nonsignificant decrease in risk-adjusted bleeding over the 4 years (9.9% in 2006, 9.5% in 2007, 9.6% in 2008, 9.4% in 2009, p = 0.14 for trend). There was no significant decrease in risk-adjusted mortality over the same period (12.8% in 2006, 12.4% in 2007, 12.8% in 2008; 12.0% in 2009, p = 0.33 for trend).
Figure 1.
Trends in use of unfractionated heparin plus glycoprotein IIb/IIIa (squares), thrombin inhibitor (diamonds), unfractionated heparin alone (triangles), and low-molecular-weight heparin plus glycoprotein IIb/IIIa (x) in patients ≥80 years old, 2006 to 2009.
Figure 2.
Trends in bleeding-avoidance strategies, namely vascular closure device (circles), thrombin inhibitor (diamonds), and radial access (squares), in patents ≥80 years old, 2006 to 2009.
Discussion
In a large cohort of patients undergoing primary PCI for STEMI, those ≥80 years of age had a nearly twofold increased risk of in-hospital bleeding compared to patients <80. In multivariable analyses, BAS were associated with a lower risk of bleeding, but <1/2 of population received even 1 BAS. Furthermore, although there were modest increases in use of BAS in patients ≥80 years old over the study period, observed risk-adjusted bleeding rates and risk-adjusted mortality did not significantly decrease over time. These findings suggest the need to further refine approaches to decreasing bleeding in these high-risk patients, which may include increasing the use of existing BAS and development of novel technical and pharmacologic approaches to improve outcomes.
Major bleeding after PCI is an outcome of interest because of immediate consequences such as prolonged hospitalization and longer-term associations with early and late mortality.16,17 Numerous studies have documented that the oldest patients are at highest risk for bleeding after PCI,1,5,6 which may be due to a combination of age-associated physiologic changes18 and under-recognition of hepatic or renal impairments that alter drug metabolism.19
There are few studies that have evaluated the use of strategies to decrease bleeding events in patients ≥80 years of age. Therefore, clinicians largely have had to extrapolate from trials in younger patients. The currently best studied BAS is the direct thrombin inhibitor bivalirudin, which has been shown to decrease bleeding complications in several randomized trials.10,20 There are no studies that have specifically addressed the efficacy of bivalirudin in the oldest patients, although several observational case series and subgroup analyses have suggested that it is similarly effective in these patients.6,21
Evidence for decrease in bleeding complications with vascular closure devices is less compelling,22,23 although several investigators have considered them part of the BAS armamentarium.11,24 Previous studies have been limited by small samples and the inclusion of multiple devices with variable complication rates,22 although several retrospective analyses have suggested a clinical benefit in bleeding decrease25,26 or a trend toward benefit23 with specific devices. Data in the oldest patients are limited, although advanced age has been associated with an increasing likelihood of vascular closure device failure,27,28 which may be due in part to the calcified vessels and tortuous anatomy common in these patients.
Radial access has been associated with fewer bleeding and vascular complications than the femoral approach in several studies12,13 including the recent Radial Versus Femoral InvEstigation in ST Elevation Acute Coronary Syndrome (RIFLE-STEACS) trial, which demonstrated decreased bleeding with radial access in STEMI (7.8% vs 12.2% for femoral access, p = 0.026) (see the preliminary results presented by Enrico Romagnoli at Transcatheter Cardiovascular Therapeutics (TCT) in November 2011). Currently, radial access is underused in the United States compared to other developed countries29 and its use is especially low in older patients.12 Although the overall very low rate of radial access in our population (<1%) limits our ability to comment on the effectiveness of this strategy, available data suggest that there may be room for increased use in select older adults with STEMI.
Given the general paucity of evidence for BAS in the oldest patients outside a controlled research setting, we investigated trends in use of these strategies and risk-adjusted bleeding over time in a large registry population. Our findings suggest that the oldest patients may benefit from the use of BAS: patients who received 1 BAS were 24% less likely to develop a bleeding event and patients receiving ≥2 BAS were 55% less likely to develop an event. These findings are similar to those observed in a previous study analyzing a broader population of NCDR CathPCI Registry patients.11 However, we also found that, despite significant increases in the use of 2 of these strategies (direct thrombin inhibitors and vascular closure devices), there was no associated decrease in risk-adjusted bleeding.
There are several potential explanations for our findings. The increase in use of BAS from 2006 to 2009 may have been insufficient to result in an offset in bleeding complications. In addition, <7% of patients in our study received ≥2 BAS, and an increased application of a combined approach (e.g., bivalirudin plus vascular closure device) is worthy of further study. Thrombin inhibitors were often used with adjunctive therapies (UFH or IIb/IIIa) in our sample, and current data suggest that a thrombin inhibitor strategy alone (without adjunctive anticoagulants) is superior to other therapies in decreasing PCI-related bleeding complications.10,20 It is also possible that BAS were not employed in patients who stood to benefit the most. For example, we found that patients in the highest tertile of bleeding risk, although slightly more likely to receive direct thrombin inhibitors, were much less likely to receive vascular closure devices or the combination of bivalirudin plus vascular closure devices. This may have been secondary to procedural characteristics precluding the use of closure devices or beliefs that devices did not decrease bleeding. An additional factor to consider is that most bleeding events were not related to access site, and 2 of the 3 available BAS would not be expected to decrease the risk of nonaccess site bleeding. In addition, the apparent benefit of BAS in this and other analyses may in fact be confounded by unmeasured factors associated with the decision to use a BAS and risk of bleeding events.
Our study has several limitations that warrant consideration. Because we performed retrospective analyses of registry data, our results are associative and hypothesis generating rather than definitive. Bleeding events were reported by site, but we believe it is unlikely that reporting of bleeding events would vary by use of BAS. The CathPCI Registry also does not capture information on all co-morbidities of interest (e.g., atrial fibrillation), does not have data on whether anticoagulants were dosed properly, and does not have information on contraindications to use of specific BAS (such as tortuous anatomy that may preclude the use of vascular closure devices). We also did not have information on the temporal relation of adjunctive strategies that were used with bivalirudin (e.g., IIb/IIIa as bailout vs upstream use), although we did find that there was a modest overall decrease in use of adjunctive strategies over time with no significant change in risk-adjusted bleeding. Our models for risk-adjusted bleeding may have been affected by unmeasured confounders, although the CathPCI Registry captures a wide variety of clinical factors that have previously been shown to be strongly associated with bleeding.14
Acknowledgments
The project described was supported by Award U01HL105270 from the National Heart, Lung, and Blood Institute, Bethesda, Maryland. Dr. Dodson is supported by Training Grant T32 AG019134 in Geriatric Clinical Epidemiology from the National Institutes of Health/National Institute on Aging, Bethesda, Maryland. Dr. Chaudhry is supported by Beeson Career Development Award K23 AG030986 from the National Institutes of Health/National Institute on Aging, Bethesda, Maryland. The CathPCI Registry is an initiative of the American College of Cardiology Foundation and the Society for Cardiovascular Angiography and Interventions.
References
- 1.Avezum A, Makdisse M, Spencer F, Gore JM, Fox KA, Montalescot G, Eagle KA, White K, Mehta RH, Knobel E, Collet JP GRACE Investigators. Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2005;149:67–73. doi: 10.1016/j.ahj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Claessen BEPM, Kikkert WJ, Engstrom AE, Hoebers LPC, Damman P, Vis MM, Koch KT, Baan J, Meuwissen M, van der Schaaf JR, de Winter RJ, Tijssen JGP, Piek JJ, Henriques JPS. Primary percutaneous coronary intervention for ST elevation myocardial infarction in octogenarians: trends and outcomes. Heart. 2010;96:843–847. doi: 10.1136/hrt.2009.185678. [DOI] [PubMed] [Google Scholar]
- 3.Merchant FM, Weiner RB, Rao SR, Lawrence R, Healy JL, Pomerantsev E, Rosenfield K, Jang IK. In-hospital outcomes of emergent and elective percutaneous coronary intervention in octogenarians. Coron Artery Dis. 2009;20:118–123. doi: 10.1097/MCA.0b013e3283292ae1. [DOI] [PubMed] [Google Scholar]
- 4.Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24:1815–1823. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 5.Kinnaird T, Anderson R, Hill J, Thomas M. Bleeding during percutaneous intervention: tailoring the approach to minimise risk. Heart. 2009;95:15–19. doi: 10.1136/hrt.2007.131284. [DOI] [PubMed] [Google Scholar]
- 6.Lemesle G, De Labriolle A, Bonello L, Syed A, Collins S, Maluenda G, Torguson R, Kaneshige K, Xue Z, Suddath WO, Satler LF, Kent KM, Lindsay J, Pichard AD, Waksman R. Impact of bivalirudin on in-hospital bleeding and six-month outcomes in octogenarians undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009;74:428–435. doi: 10.1002/ccd.22007. [DOI] [PubMed] [Google Scholar]
- 7.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology–National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed January 3, 2011.];National Cardiovascular Data Registry. Available at: http://www.ncdr.com/WebNCDR/ELEMENTS.ASPX.
- 9.Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, Krumholz HM. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. Bmj. 2009;338:b1807. doi: 10.1136/bmj.b1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, White HD, Pocock SJ, Ware JH, Feit F, Colombo A, Aylward PE, Cequier AR, Darius H, Desmet W, Ebrahimi R, Hamon M, Rasmussen LH, Rupprecht HJ, Hoekstra J, Mehran R, Ohman EM, ACUITY Investigators Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 11.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS National Cardiovascular Data Registry. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. Jama. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 12.Rao SV, Ou F, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR RIVAL Trial Group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 14.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP National Cardiovascular Data Registry. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–229. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Tu JV, Juurlink DN, Alter DA, Ko DT, Austin PC, Chong A, Stukel TA, Levy D, Laupacis A. Risk-treatment mismatch in the pharmacotherapy of heart failure. Jama. 2005;294:1240–1247. doi: 10.1001/jama.294.10.1240. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, Manoukian SV, Nikolsky E, Lansky AJ, Kirtane A, White HD, Colombo A, Ware JH, Moses JW, Ohman EM. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30:1457–1466. doi: 10.1093/eurheartj/ehp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. doi: 10.1016/s0002-9149(03)00972-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang TY, Gutierrez A, Peterson ED. Percutaneous coronary intervention in the elderly. Nat Rev Cardiol. 2011;8:79–90. doi: 10.1038/nrcardio.2010.184. [DOI] [PubMed] [Google Scholar]
- 19.Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, Pollack C, Gibler WB, Ohman EM, Peterson ED for the CRUSADE Investigators. Excess dosing of antiplatelet and anti-thrombin agents in the treatment of non–ST-segment elevation acute coronary syndromes. Jama. 2005;294:3108–3116. doi: 10.1001/jama.294.24.3108. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 21.Lopes RD, Alexander KP, Manoukian SV, Bertrand ME, Feit F, White HD, Pollack CV, Jr, Hoekstra J, Gersh BJ, Stone GW, Ohman EM. Advanced age, antithrombotic strategy, and bleeding in non-ST-segment elevation acute coronary syndromes: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol. 2009;53:1021–1030. doi: 10.1016/j.jacc.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Jneid H, Derdeyn CP, Klein LW, Levine GN, Lookstein RA, White CJ, Yeghiazarians Y, Rosenfield K American Heart Association Diagnostic and Interventional Cardiac Catheterization Committee of the Council on Clinical Cardiology, Council on Cardiovascular Radiology and InterventionCouncil on Peripheral Vascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Arteriotomy closure devices for cardiovascular procedures: a scientific statement from the American Heart Association. Circulation. 2010;122:1882–1893. doi: 10.1161/CIR.0b013e3181f9b345. [DOI] [PubMed] [Google Scholar]
- 23.Koreny M, Riedmüller E, Nikfardjam M, Siostrzonek P, Müllner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. Jama. 2004;291:350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 24.Dauerman HL, Rao SV, Resnic FS, Applegate RJ. Bleeding avoidance strategies. Consensus and controversy. J Am Coll Cardiol. 2011;58:1–10. doi: 10.1016/j.jacc.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaitkus PT. A meta-analysis of percutaneous vascular closure devices after diagnostic catheterization and percutaneous coronary intervention. J Invasive Cardiol. 2004;16:243–246. [PubMed] [Google Scholar]
- 26.Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153:606–611. doi: 10.1016/j.ahj.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Bangalore S, Arora N, Resnic FS. Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ Cardiovasc Interv. 2009;2:549–556. doi: 10.1161/CIRCINTERVENTIONS.109.877407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dauerman HL, Applegate RJ, Cohen DJ. Vascular closure devices: the second decade. J Am Coll Cardiol. 2007;50:1617–1626. doi: 10.1016/j.jacc.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, Frasier D, Gulati R, Skelding K, Bertrand O, Patel T. Transradial arterial access for coronary and peripheral procedures: executive summary by the transradial committee of the SCAI. Catheter Cardiovasc Interv. 2011;78:823–829. doi: 10.1002/ccd.23052. [DOI] [PubMed] [Google Scholar]