Abstract
Establishing extracellular milieus to stimulate neuronal regeneration is a critical need in neuronal tissue engineering. Many studies have used a soluble factor (such as nerve growth factor or retinoic acid [RA]), micropatterned substrate, and electrical stimulation to induce enhanced neurogenesis in neuronal precursor cells. However, little attention has been paid to mechanical stimulation because neuronal cells are not generally recognized as being mechanically functional, a characteristic of mechanoresponsive cells such as osteoblasts, chondrocytes, and muscle cells. In this study, we performed proof-of-concept experiments to demonstrate the potential anabolic effects of mechanical stretch to enhance cellular neurogenesis. We cultured human neuroblastoma (SH-SY5Y) cells on collagen-coated membrane and applied 10% equibiaxial dynamic stretch (0.25 Hz, 120 min/d for 7 days) using a Flexcell device. Interestingly, cell stretch alone, even without a soluble neurogenic stimulatory factor (RA), produced significantly more and longer neurites than the non–RA-treated, static control. Specific neuronal differentiation and cytoskeletal markers (e.g., microtubule-associated protein 2 and neurofilament light chain) displayed compatible variations with respect to stretch stimulation.
Key words: mechanical cell stretch, neurite outgrowth, neuronal differentiation, neuronal regenerative medicine, retinoic acid
Introduction
Injuries to the nervous system and many neurological disorders are characterized by the loss of neuronal functional circuits. The damaged neuronal system seldom exhibits spontaneous recovery due to the inferior regenerative capability of neurons.1 Regeneration of injured neurons accompanies complex cellular activities, including cytoskeletal reorganization and expression of neuronal genes and proteins. The development of optimal extracellular signals that can stimulate these neuronal regenerative activities is of significant interest.
Many attempts have been made to provide extracellular cues for enhancing cellular neurogenesis, including soluble factors (such as nerve growth factor or retinoic acid [RA]),2–4 micropatterned substrate,5–8 and electrical stimulation.9,10 Static physical cell confinement within protein micropattern could induce enhanced neurogenesis. We recently showed that micropatterning of neuronal cells within narrow (5 and 10 μm) collagen lanes enhanced neurite growth relative to an unpatterned control.8 However, there has been very limited effort to reveal the role of dynamic mechanical stimulation on cell neurogenesis. This may be because neuronal cells have not been recognized as being mechanoresponsive, a characteristic found in cells such as osteoblasts, chondrocytes, and muscle cells. We demonstrated anabolic effects from mechanical stretch for enhancing cellular neurogenesis. Specifically, we showed for the first time that cell stretch alone, even without soluble neurogenic stimulatory factor, could produce significant neurite outgrowth and potentially support neuronal differentiation.
Materials and Methods
Mechanical cell stretching
SH-SY5Y human neuroblastoma cells (ATCC, CRL-2266) were maintained using growth media (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 1% penicillin-streptomycin, all from Invitrogen, Grand Island, NY). Cells were seeded at 3×104 cells/well on collagen-coated membranes (Bioflex 6-well plate, Flexcell, Hillsborough, NC) for all control and stretch or RA (Sigma-Aldrich, St.Louis, MO) treatment samples. After 2 days of growth, cells were stretched. Flexcell FX-5000 was used to equibiaxially elongate cell-seeded elastic membrane against a loading post. Sinusoidal elongation at 10% strain and 0.25 Hz was applied to the cells for 7 days (120 min/d). Cell stretch was performed without or with RA exposure at 10 μM with media changed every 2 days. A six-well stretching plate was housed inside the regular incubator (37°C, 5% CO2), as we previously reported.11 Thus, four conditions (control with no stretch or RA, RA treatment alone, stretch alone, and stretch plus RA) were tested using the same culture protocol, period, and set up.
Neurite length and number measurements
Cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, St.Louis, MO)solution on day 7. From optical cell images, neurite length was measured by NeuronJ (ImageJ add-on software), and the number of neurites developed per cell was manually counted. Neurite length measurement followed our published protocol.8 Briefly, with the use of NeuronJ, the path of the neurite could be traced whether it was straight or curved (as shown by Poudel et al.8). Since no established criterion is available in the literature regarding where to begin neurite length measurement, the cellular extension process was quantified as a neurite from the point where a cell process has a width of <3.85 μm (following our previous study8). For assessing neurite number developed per cell, we simply counted all the neurites in the obtained image (as in Fig. 1A) and divided the neurite number by the total cell number. Three repeated cell culture assays were performed, and at least three different images were taken of each. From the images, a total of at least 100 neurites per condition were quantified for length and at least 200 cells per condition were used for neurite number counting.
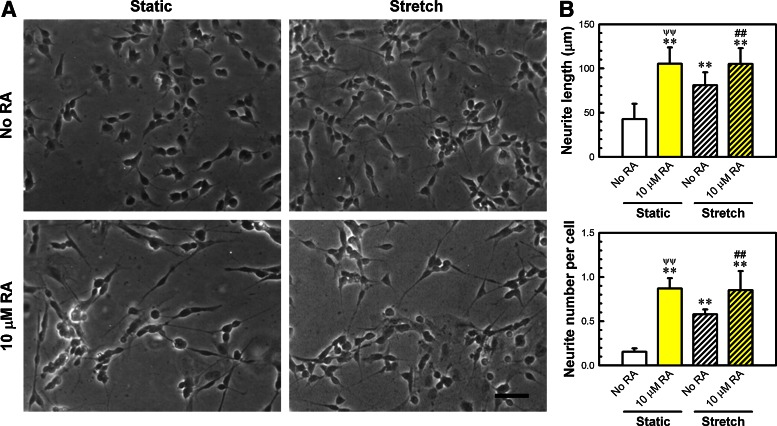
FIG. 1.
Cell stretching alone even without retinoic acid (RA) exposure produced significantly more and longer neurites relative to a non–RA-treated, static control. Human neuroblastoma (SH-SY5Y) cells were stretched (10% equibiaxial strain at 0.25 Hz, 120 min/d for 7 days) using a Flexcell device. Cells were stretched without or with 10 μM RA, a known soluble neurogenic factor. (A) Optical cell images shown with a scale bar of 50 μm. An objective lens with 10× magnification was used (eyepiece with 10×). (B) Neurite length was quantified by the tracing method and neurite number per cell was manually counted. Comparisons with non–RA-treated, static control are shown with **p<0.01. A comparison between no RA and 10 μM RA for stretched samples is shown with ##p<0.01. A comparison between RA alone and stretch alone is shown with ψψp<0.01.
Immunofluorescence and reverse-transcription polymerase chain reaction
Microtubule-associated protein 2 (MAP2) was visualized by immunofluorescence. On day 7 cells were fixed and permeabilized with 0.1% Triton X-100 solution (Sigma-Aldrich, St.Louis, MO). MAP2 was detected by chicken polyclonal primary antibody specific to MAP2 (Abcam, ab5392) at 1:10,000 dilution, tagged with fluorescein isothiocyanate (FITC)-conjugated anti-chicken IgY secondary antibody (Abcam, ab6749, Cambridge, MA) at 1:1000, and observed with a Leica DMI 4000B fluorescence microscope. Gene expression of several types of intermediate filaments specific to neuronal and astrocytic cells was assessed by reverse-transcription polymerase chain reaction (RT-PCR) using a published protocol.11
Statistics
Mean and standard deviation are presented. Statistical significance was tested by one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls tests.
Results
SH-SY5Y neuroblastoma cells were imaged after stretch and RA treatments (Fig. 1A). Almost no neurite development was observed for the non–RA-treated, static control. In the presence of a known neurogenic soluble factor (RA), well-developed neurites were seen. We used an optimal RA concentration (10 μM) that produces maximal neurite outgrowth.10,12 Interestingly, cell stretching alone induced noticeable neurite outgrowth even without RA exposure. Stretch and RA costimulation also induced well-developed neurites. These observations could be confirmed by quantified data (Fig. 1B). Most notably, cell stretching alone induced significantly longer neurites relative to the non–RA-treated, unstretched control (p<0.01). Under the given stretch conditions, however, neurite length for stretch alone was shorter than that of RA treatment alone, and RA plus stretch did not induce an additional increase in neurite length compared with RA alone. Almost identical trends were observed for the neurite number developed per cell.
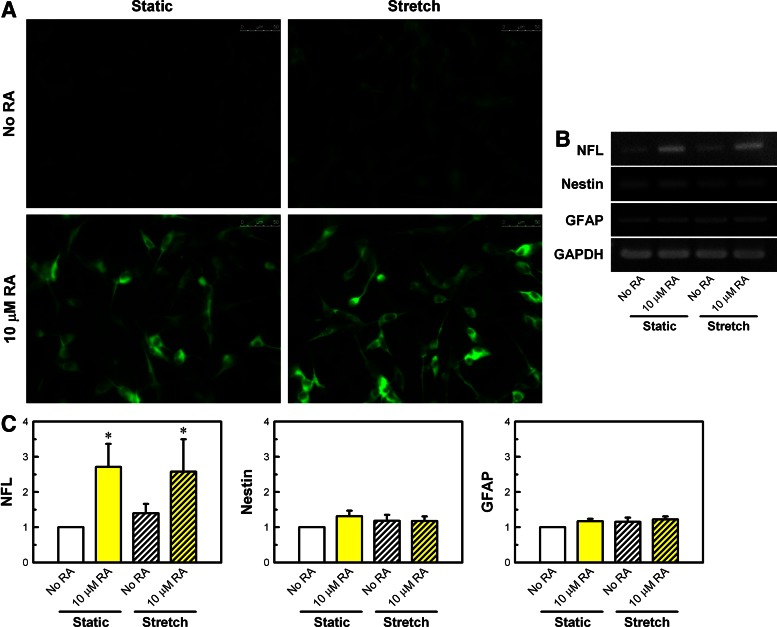
We tested whether stretch- and RA-induced changes in neurite outgrowth were reflected in changes in neuronal marker expression. Immunofluorescence of MAP2, an established neuronal differentiation marker,13 showed variations comparable to those of neurite length and number. RA, in the absence or presence of cell stretching, induced strong MAP2 immunostaining (Fig. 2A). For the sample treated with stretch alone, identifiable MAP2 could be seen, in contrast to no MAP2 staining being visible in the non–RA-treated, static control. Further, to examine potential molecular mechanosensor, several intermediate filament structures specific to neuronal and astrocytic cells were screened by RT-PCR (Fig. 2B, C). Gene expression of neurofilament light chain (NFL), the most abundant intermediate filament in neurons and axons and known to interact with MAP2,14 was up-regulated for two RA-treated samples without or with stretching. Stretch alone induced increased NFL expression relative to control (∼40% increase), but it did not reach statistical significance. Expression of nestin, another intermediate filament observed in nerve cells,15 did not change with respect to RA or stretch. Additionally, glial fibrillary acidic protein (GFAP), intermediate filament found in the other type of brain cells (astrocytes),16 did not vary with RA or stretch, either.
FIG. 2.
Microtubule-associated protein 2 (MAP2) immunofluorescence and intermediate filament gene expression in SH-SY5Y cells treated with stretch and RA. (A) MAP2 immunofluorescence with a scale bar of 50 μm. A 20× objective lens was used (eyepiece with 10×). (B, C) Gene expressions specific to neuronal intermediate filament (neurofilament light chain [NFL], nestin) and astrocytic intermediate filament (glial fibrillary acidic protein [GFAP]). Results from reverse-transcription polymerase chain reaction (RT-PCR) are presented with GAPDH as a loading control and by comparing with non–RA-treated, static control set at 1. NFL: sense ACC TCC TCA ACG TGA AGA TGG CTT, antisense ACT CTT CCT TGG CAGC TTC TTC CT; nestin: sense GCC CTG ACC ACT CCA GTT TA, antisense GGA GTC CTG GAT TTC CTT CC; GFAP: sense ACC AGG ACC TGC TCA ATG TC, antisense ATC TCC ACG GTC TTC ACC AC; GAPDH: sense CAT GAC CAC AGT CCA TGC CAT CAC T, antisense TGA GGT CCA CCA CCC TGT TGC TGT A. Comparisons with non–RA-treated, static control are shown with *p<0.05 (n=3). Other comparisons did not reach statistical significance.
Discussion
We demonstrated that neurite outgrowth, a key characteristic of cellular neurogenesis, could be stimulated by mechanical cell stretch. In particular, stretching of SH-SY5Y cells could induce neurite outgrowth even without the support from a soluble neurogenic factor (RA). A specific neuronal differentiation marker, MAP2 immunofluorescence, displayed variations comparable to those of neurite length and number. Among intermediate filament cytoskeletons, the NFL neuronal intermediate filament was relatively more mechanoresponsive than nestin or the astrocytic intermediate filament, GFAP. From these results, one may at least partly address one of the least known phenomena in neuronal regenerative medicine, mechanical induction of neurogenesis. Although findings are preliminary, it may be concluded that cell stretching under experimental conditions has anabolic effects on neuronal precursor cells in triggering neurite outgrowth and supporting neural differentiation, and this may be achieved potentially via mechanotransduction through NFL. This is, as far as we know, the first study to highlight the exclusive role of mechanical stretch in stimulating cellular neurogenesis.
We performed cell stretch experiments using the aforementioned stretch regimes based on reported cell stretch data. It is noteworthy that for neuronal cells, cell stretch has been used not to enhance neurogenesis, but rather to produce neuronal cell injury or death.17–19 These studies were intended to reproduce severe mechanical stretch conditions relevant to neuronal damage, such as that occurring in traumatic brain injury. For example, severe nonphysiological stretches (>30% strain and 10-Hz frequency or high strain rate such as 1% strain/ms) were used. So far, very little is known about stretch regimes that induce positive stimulatory effects in neurogenesis.20 The only available information is from the study by Haq et al.21 They performed uniaxial stretching of rat pheochromocytoma (PC12) cells and observed that mild stretch (4% at 1 Hz or 16% at 0.1 Hz) produced more and longer neurites, while relatively severe stretch (16% at 1 Hz) resulted in shorter neurites relative to the control. They did not test differentiation markers or mechanosensors. While their attempt covered ranges of strain and frequency, exclusive mechanical stretch effects could not be revealed since they completed all static and stretch test conditions in the presence of a soluble neurogenic factor. To address this, we attempted proof-of-concept assays. We chose a potentially stimulatory cell stretch condition (10%, 0.25 Hz) instead of testing all available combinations and demonstrated that stretch alone, even without a soluble neurogenic factor (RA), could induce neurite outgrowth and potentially support neuronal differentiation.
Knowing optimal stretch regimes for inducing maximized cellular neurogenesis will provide advanced strategy for neuronal regenerative medicine. Under the stretch regimes adopted, stretch alone resulted in less neurogenesis compared with RA treatment alone, and RA plus stretch did not additionally enhance neurogenesis relative to RA alone. This may be partly because the selected RA concentration was optimal, resulting in potentially saturated neurogenesis. This also suggests stretch conditions may be further optimized. Fine-tuning stretch conditions by systematically varying strain, frequency, duration, and resting period will provide optimized cell stretch conditions, which will be the focus of subsequent study.
It is noteworthy that another type of stretch study was undertaken by Loverde et al.22 They designed a device in which axons from dorsal root ganglia could be pulled from anchored points. The extension rate of axonal growth cone could be intentionally accelerated compared with the unpulled control. This study, based on data showing correlation between tension and axonal elongation/retraction,23,24 mimicked axon stretch growth during the embryonic maturation process. Although the stretch mechanisms and goals were different (i.e., direct pulling of developed axons from axonal anchoring points to accelerate axon growth cone extension versus stretching of neuronal precursor cells on stretchable membrane to induce cellular neurogenesis), both studies suggest a positive role of stretch signal in neurogenesis.
In conclusion, SH-SY5Y neuronal cells exposed to 10% equibiaxial strain at 0.25 Hz showed significant neurite outgrowth (both length and number) even without a soluble neurogenic factor. Specific neuronal differentiation and cytoskeletal markers, MAP2 and NFL, showed variations that were comparable with respect to stretch stimulation. Our data demonstrating anabolic effects of mechanical stretch for cellular neurogenesis suggest mechanotransduction may be pursued as a viable route in neuronal regenerative medicine.
Acknowledgment
This work was supported by AO Foundation Grant S-10-7L, NE DHHS Stem Cell Grant 2011-05, AHA Scientist Development Grant 12SDG12030109, and Osteology Foundation Grant 12-006 (all to J.Y.L.).
Author Disclosure
No competing financial interests exist.
References
- 1.Muramatsu R. Ueno M. Yamashita T. Intrinsic regenerative mechanisms of central nervous system neurons. Biosci Trends. 2009;3:179–183. [PubMed] [Google Scholar]
- 2.Bella AJ. Lin G. Lin CS, et al. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6(Suppl 3):347–352. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawson NE. LaMantia AS. Once and again: retinoic acid signaling in the developing and regenerating olfactory pathway. J Neurobiol. 2006;66:653–676. doi: 10.1002/neu.20236. [DOI] [PubMed] [Google Scholar]
- 4.Mey J. New therapeutic target for CNS injury? The role of retinoic acid signaling after nerve lesions. J Neurobiol. 2006;66:757–779. doi: 10.1002/neu.20238. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz A. Buzanska L. Gilliland D, et al. Micro-stamped surfaces for the patterned growth of neural stem cells. Biomaterials. 2008;29:4766–4774. doi: 10.1016/j.biomaterials.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Solanki A. Shah S. Memoli KA, et al. Controlling differentiation of neural stem cells using extracellular matrix protein patterns. Small. 2010;6:2509–2513. doi: 10.1002/smll.201001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Béduer A. Vieu C. Arnauduc F, et al. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012;33:504–514. doi: 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 8.Poudel I. Lee JS. Tan L. Lim JY. Micropatterning-retinoic acid co-control of neuronal cell morphology and neurite outgrowth. Acta Biomater. 2013;9:4592–4598. doi: 10.1016/j.actbio.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Huang J. Lu L. Zhang J, et al. Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PLoS One. 2012;7:e39526. doi: 10.1371/journal.pone.0039526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciofani G. Danti S. D'Alessandro D, et al. Enhancement of neurite outgrowth in neuronal-like cells following boron nitride nanotube-mediated stimulation. ACS Nano. 2010;4:6267–6277. doi: 10.1021/nn101985a. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS. Ha L. Park JH. Lim JY. Mechanical stretch suppresses BMP4 induction of stem cell adipogenesis via upregulating ERK but not through downregulating Smad or p38. Biochem Biophys Res Commun. 2012;418:278–283. doi: 10.1016/j.bbrc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K. Tamiya-Koizumi K. Hagiwara K, et al. Role of down-regulated neutral ceramidase during all-trans retinoic acid-induced neuronal differentiation in SH-SY5Y neuroblastoma cells. J Biochem. 2012;151:611–620. doi: 10.1093/jb/mvs033. [DOI] [PubMed] [Google Scholar]
- 13.Soltani MH. Pichardo R. Song Z, et al. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am J Pathol. 2005;166:1841–1850. doi: 10.1016/S0002-9440(10)62493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frappier T. Stetzkowski-Marden F. Pradel LA. Interaction domains of neurofilament light chain and brain spectrin. Biochem J. 1991;275:521–527. doi: 10.1042/bj2750521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guérette D. Khan PA. Savard PE. Vincent M. Molecular evolution of type VI intermediate filament proteins. BMC Evol Biol. 2007;7:164. doi: 10.1186/1471-2148-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandley MJ. Szebeni K. Szebeni A, et al. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J Psychiatry Neurosci. 2012;38:120110. doi: 10.1503/jpn.120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfister BJ. Weihs TP. Betenbaugh M. Bao G. An in vitro uniaxial stretch model for axonal injury. Ann Biomed Eng. 2003;31:589–598. doi: 10.1114/1.1566445. [DOI] [PubMed] [Google Scholar]
- 18.Geddes-Klein DM. Schiffman KB. Meaney DF. Mechanisms and consequences of neuronal stretch injury in vitro differ with the model of trauma. J Neurotrauma. 2006;23:193–204. doi: 10.1089/neu.2006.23.193. [DOI] [PubMed] [Google Scholar]
- 19.Hemphill MA. Dabiri BE. Gabriele S, et al. A possible role for integrin signaling in diffuse axonal injury. PLoS One. 2011;6:e22899. doi: 10.1371/journal.pone.0022899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bueno FR. Shah SB. Implications of tensile loading for the tissue engineering of nerves. Tissue Eng Part B Rev. 2008;14:219–233. doi: 10.1089/ten.teb.2008.0020. [DOI] [PubMed] [Google Scholar]
- 21.Haq F. Keith C. Zhang G. Neurite development in PC12 cells on flexible micro-textured substrates under cyclic stretch. Biotechnol Prog. 2006;22:133–140. doi: 10.1021/bp0501625. [DOI] [PubMed] [Google Scholar]
- 22.Loverde JR. Tolentino RE. Pfister BJ. Axon stretch growth: the mechanotransduction of neuronal growth. J Vis Exp. 2011;54:e2753. doi: 10.3791/2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidemann SR. Buxbaum RE. Mechanical tension as a regulator of axonal development. Neurotoxicology. 1994;15:95–107. [PubMed] [Google Scholar]
- 24.Heidemann SR. Lamoureux P. Buxbaum RE. Cytomechanics of axonal development. Cell Biochem Biophys. 1995;27:135–155. doi: 10.1007/BF02738107. [DOI] [PubMed] [Google Scholar]