Abstract
Elastic fibres have the unique ability to withstand large deformations and are found in numerous tissues, but their organization and structure have not been well defined in tendon. The objective of this study was to characterize the organization of elastic fibres in tendon to understand their function. Immunohistochemistry was used to visualize elastic fibres in bovine flexor tendon with fibrillin-1, fibrillin-2 and elastin antibodies. Elastic fibres were broadly distributed throughout tendon, and highly localized longitudinally around groups of cells and transversely between collagen fascicles. The close interaction of elastic fibres and cells suggests that elastic fibres are part of the pericellular matrix and therefore affect the mechanical environment of tenocytes. Fibres present between fascicles are likely part of the endotenon sheath, which enhances sliding between adjacent collagen bundles. These results demonstrate that elastic fibres are highly localized in tendon and may play an important role in cellular function and contribute to the tissue mechanics of the endotenon sheath.
Keywords: elastic fibres, elastin, fibrillin, tendon
Introduction
Tendon is a complex hierarchical tissue that transmits forces from muscles to bones, thus permitting joint locomotion. Tendon mechanics are dependent on the composition and organization of the extracellular matrix (ECM), which is primarily maintained by tenocytes. The ECM is mainly composed of type I collagen that forms bundles of increasing diameter from tropocollagen, to fibrils forming fibres, and fibres, which are organized into fascicles; the fascicles are enveloped by the endotenon sheath to form the tendon proper (Fig. 1). The structure and mechanics of collagen fibrils have been extensively investigated and well described through the nanostructural organization of collagen molecules (Fratzl & Weinkamer, 2007). However, tendon fibres contain ECM molecules in addition to type I collagen, which have an organization and function that is not fully understood.
Fig 1.
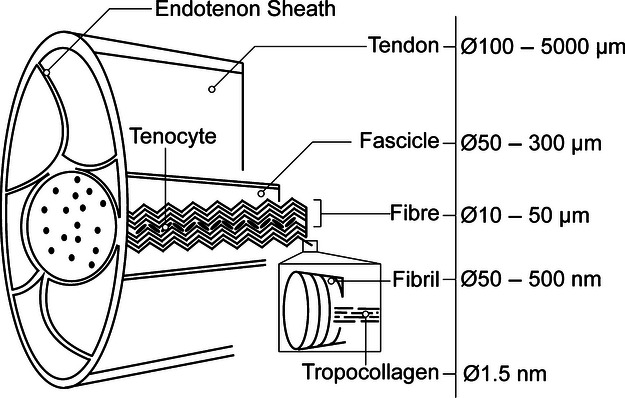
Structure of tendon modified from (Kastelic et al. 1978) showing hierarchical organization of collagen bundles. Research presented in this document focuses on the fibre level of organization.
Among the minor ECM components whose role in tendon is not well understood are elastic fibres, which have been reported to have a sparse distribution (Kannus, 2000). Although early studies in transmission electron microscopy (TEM) investigated the ultrastructure of elastic fibres in tendon (Parry & Craig, 1978; Ippolito et al. 1980; Caldini et al. 1990), little research has been conducted on their overall organization and function. Elastin, which has the unique ability to recover from deformations of 100% of its initial length (Fung, 1993), forms the core of the elastic fibre and has been reported to comprise 1–2% of the total dry weight of tendon (Kannus, 2000). Microfibrils, mainly composed of fibrillin-1 and fibrillin-2, form a scaffold around elastin (Mithieux & Weiss, 2005). During elastogenesis, tropoelastin is deposited onto pre-formed microfibril bundles and is stabilized by forming crosslinks through lysyl oxidase (Kielty, 2006). Mature elastic fibres have a diameter of 200–800 nm (Lorber, 1989) and an elastic modulus of 300–600 kPa (Mithieux & Weiss, 2005).
Elastic fibres can be categorized in terms of the amount of elastin present in their structure: mature elastic fibres contain a dense elastin core accounting for approximately 90% of the fibre, elaunin fibres contain an intermediate amount of elastin, and oxytalan fibres are composed entirely of microfibrils (Montes, 1996). Traditionally, elaunin and oxytalan fibres were thought to be immature elastic fibres that would undergo further tropoelastin deposition, but later studies have shown that all three forms exist in mature tissue specimens (Montes, 1996).
Given the unique ability of elastin to sustain large deformations, researchers have suggested that elastin provides tendon with elastic recoil and resilience (Butler et al. 1978), as observed in blood vessels and skin (Kielty et al. 2002). Moreover, microfibrils may contribute to tendon mechanics, as joint hypermobility and contractures have been found to be clinical features in patients with Marfan syndrome (OMIM-154700) and Beals syndrome (OMIM-121050), which are caused by the mutation for the gene encoding fibrillin-1 and fibrillin-2, respectively (Urban & Boyd, 2000; Gupta et al. 2002, 2004). Although elastic fibres have been suggested to contribute to tendon mechanics, a firm understanding of their organization will be required to elucidate their function.
The central motivation of this study was to investigate the detailed organization of elastic fibres in tendon through basic histology and immunohistochemistry as a basis for understanding their function.
Materials and methods
Sample collection and preparation
Ten bovine feet with no sign of tissue damage from young adult steers (18–24 months) were obtained from a local abattoir. Deep digital flexor tendons were excised from the myotendinous junction to the osteotendinous junction and 5-mm samples were dissected from the midpoint of the excised tendon as shown in Fig. 2. Tissue specimens for paraffin-embedded sections were fixed in 10% formalin for 24 h, dehydrated with a graded series of ethanol and then incubated in xylene to clear any remaining ethanol. The samples were embedded in paraffin wax, allowed to harden overnight, cut into 5-μm transverse and longitudinal sections with a microtome, and dried on slides at 65 °C for 24 h.
Fig 2.
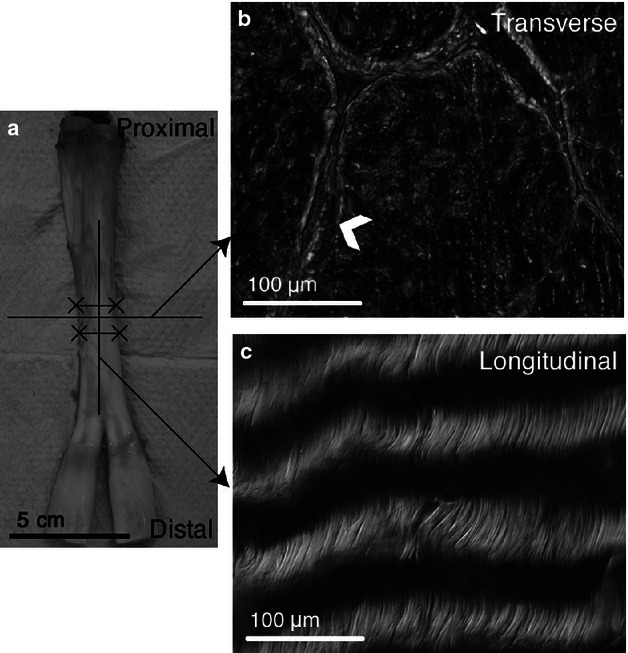
Dissection scheme for tendon organizational analysis. (a) Bovine flexor tendon with excised section marked by solid lines (X-X). Horizontal and vertical lines represent transverse and longitudinal cutting planes, respectively. (b) Transverse section clearly depicting the endotenon sheath (arrowhead) surrounding tendon fascicles. (c) Longitudinal section illustrating the collagen crimp structure aligned along the direction of loading.
Tissue specimens for fresh-frozen sections were immediately snap-frozen in hexane cooled by dry ice and embedded in OCT compound (Sakura Finetek, USA). A cryostat was used to cut 20-μm transverse and longitudinal sections, which were adhered to slides (VWR International Ltd, UK) and stored at −80 °C until immunostaining was conducted.
Histological staining and immunostaining of elastic fibres
Paraffin-embedded sections were rehydrated with a graded series of ethanol into phosphate-buffered saline (PBS) and serial sections were stained with haematoxylin and eosin (H&E) for general structure and Miller's stain for elastic fibres using standard techniques (Miller, 1971). Miller's stain highlights elastic fibres in black and collagen in red.
An enzyme treatment was used to improve immunodetection of elastic fibres that were deposited in a dense matrix to better understand the extent of their distribution. For each set of serial sectioned specimens, one section was pretreated with 4800 U mL−1 hyaluronidase (Sigma H6254, UK) at 37 °C overnight to degrade glycosaminoglycans.
Elastic fibres were dual immunostained with fibrillin-1 together with elastin or fibrillin-2 as previously described (Yu et al. 2007). Sections were rinsed with PBS for 3 min and blocked with 10% normal donkey serum (Stratech Scientific, UK) in a Tris buffer (50 mm plus 10 mm calcium acetate) to reduce non-specific hydrophobic bonding. After 30 min, the slides were drained of excess blocking reagent and incubated with the primary fibrillin-1 antibody (Table 1) at 4 °C overnight. Samples were rinsed with PBS and incubated with the secondary antibody for 40 min at room temperature. After washing with PBS, the sections were incubated with either the elastin or fibrillin-2 primary antibody at 4 °C overnight, then washed and incubated with the secondary antibody at room temperature for 40 min. After washing, sections were mounted with DAPI mounting media (Vector Laboratories, UK) to stain cell nuclei blue. Negative controls were incubated with normal serum in place of the primary antibody and positive controls were conducted on transverse bovine artery specimens.
Table 1.
Primary and secondary antibodies used for immunohistological detection
| Primary antibody | Secondary antibody | |
|---|---|---|
| Elastin | Anti-human alpha elastin raised in rabbit, 1 : 50 (AbD Serotec, UK; Cat. No. 4060-1054, Batch No. 20092751) | Dylight-488 conjugated donkey anti-rabbit IgG, 1 : 100 (Stratech Scientific, UK; Cat. No. 712-485-153) |
| Fibrillin-1 | Anti-bovine fibrillin-1 raised in mouse, 1 : 50 (Abcam, UK; Cat. No. ab3090) | Cy3-conjugated donkey anti-mouse IgG, 1 : 100 (Stratech Scientific, UK; Cat. No. 715-165-151) |
| Fibrillin-2 | Anti-human fibrillin-2 raised in rabbit, 1 : 50 (Elastin Products, USA; Cat. No. PR225) | Dylight-488 conjugated donkey anti-rabbit IgG, 1 : 100 (Stratech Scientific, UK; Cat. No. 712-485-153) |
Light and confocal microscopy
Miller's stained slides were analyzed using an upright light microscope (Zeiss, USA). A 100× oil immersion objective lens was used to capture images across the transverse and longitudinal sections and the images were processed using axiovision photo editing software (Zeiss, UK).
An LSM 710 inverted confocal laser-scanning microscope (Zeiss, UK) was used to analyze the elastic fibre organization of immunostained specimens. Oil immersion 40× and 63× objective lenses with an axial resolution of 0.4 μm were used to collect emission spectra for green, red and blue channels. Images were averaged over five frames to reduce background noise. A step size of 0.5 μm was used to create a z-stack over the entire 20-μm section and there were no signs of photo damage during imaging. The images were captured with zen 2010 software (Zeiss, UK) and analyzed in imaris 7.4.1 (Bitplane, USA). A 3D reconstruction of cell nuclei and elastic fibres was generated by volume rendering the red, green and blue channels. The maximum and minimum brightness and contrast intensities were adjusted using the Auto configuration setting.
Results
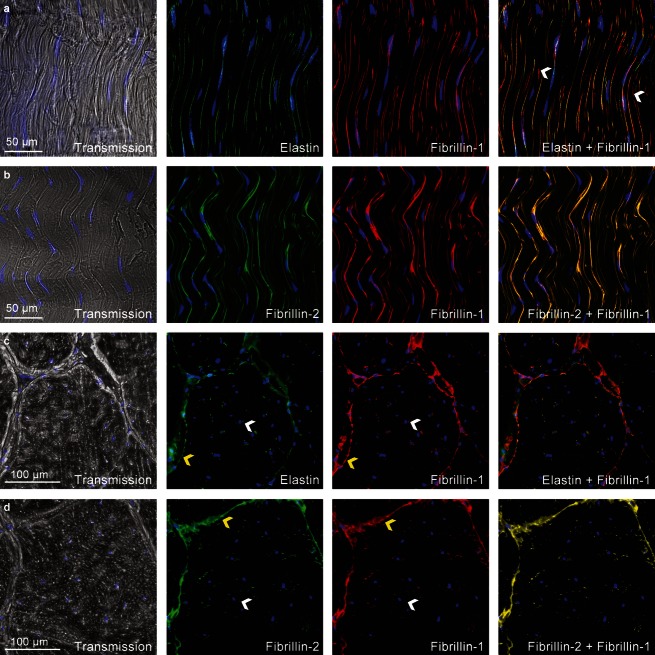
Immunohistochemistry indicated that elastic fibres ran longitudinally along tendon and conformed to collagen fibril crimp (Fig. 3). There were fewer elastic fibres found within collagen fibre bundles as compared with the dense distribution localized around tenocytes and between fascicles. Immunohistological results showed that fibrillin-1 and fibrillin-2 were strongly colocalized but fibrillin-1 was occasionally found independent of elastin (Fig. 3a, arrowheads). Immunostaining for elastin was weaker than fibrillin-1 and fibrillin-2 in both transverse and longitudinal sections. Little variation in elastic fibre organization was observed across the 10 bovine tendon samples.
Fig 3.
Elastin, fibrillin-1 and fibrillin-2 dual antibody immunostaining depicting overall elastic fibre organization in untreated specimens with cell nuclei highlighted in blue. (a) Longitudinal elastin and fibrillin-1 fibres conform to wavy tendon structure and are densely distributed around tenocytes. Merged image highlights the lack of colocalization between fibrillin-1 and elastin (white arrowheads). (b) Longitudinal fibrillin-1 and fibrillin-2 fibres following wavy tendon structure with a high level of colocalization. (c) Transverse elastin and fibrillin-1 depicting elastic fibres as points surrounding tenocytes (white arrowhead) and a concentrated distribution between fascicles (yellow arrowhead). (d) Transverse fibrillin-1 and fibrillin-2 microfibrils distributed around tenocytes (white arrowhead) and between fascicles (yellow arrowhead) showing a high level of colocalization.
Elastic fibres were observed as points surrounding tenocytes in transverse sections as fibres intersected the cutting plane (Fig. 4c). Similar to longitudinal sections, fibrillin-1 and fibrillin-2 were always colocalized but were occasionally detected independent of elastin. Transverse sections clearly indicated the presence of elastic fibres within the interfascicle region of tendon where they formed a loose mesh-like structure that was present throughout the region (Fig. 4d). The mesh-like structure enclosed blood vessels that were oriented longitudinally along tendon and present within the interfascicle space. Blood vessels between collagen fascicles were immunodetected by means of the elastin antibody, which immunostained vessels 10–70 μm in diameter (data not shown). Immunostaining was more prominent for fibrillin-1 and fibrillin-2 than for elastin between collagen bundles.
Fig 4.
Detailed fibrillin-1 organization in tendon showing longitudinally oriented fibres present along arrays of cells and transversely oriented fibres between collagen fascicles. (a) Longitudinal 3D reconstruction from a 20-μm z-stack showing multiple fibres localized around tenocytes. (b) Transverse section highlighting areas of dense microfibril distribution around cells and between adjacent fascicles. (c) High magnification image showing interaction of elastic fibres and cells in transverse section. (d) Interfascicle 3D reconstruction of loose mesh-like elastic fibre structure found between collagen fascicles.
Longitudinal and transverse sections indicated that elastic fibre density was greater around cells than between arrays of tenocytes. Multiple elastic fibres surrounded tenocytes and a 3D image reconstruction using z-stack images indicated that multiple fibres surrounded cells and occasionally had a branching structure (Fig. 5). Elastic fibres that were in close proximity to tenocytes almost always contained elastin, whereas fibres present between arrays of cells occasionally lacked elastin.
Fig 5.
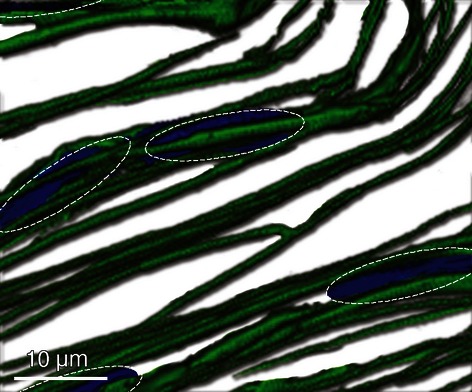
Three-dimensional reconstruction of elastic fibre organization (green) with outline of cell nuclei (blue) highlighted by dotted lines. Multiple elastic fibres surround groups of cells and have a branching structure. Fibres are also present between cells, but in fewer numbers.
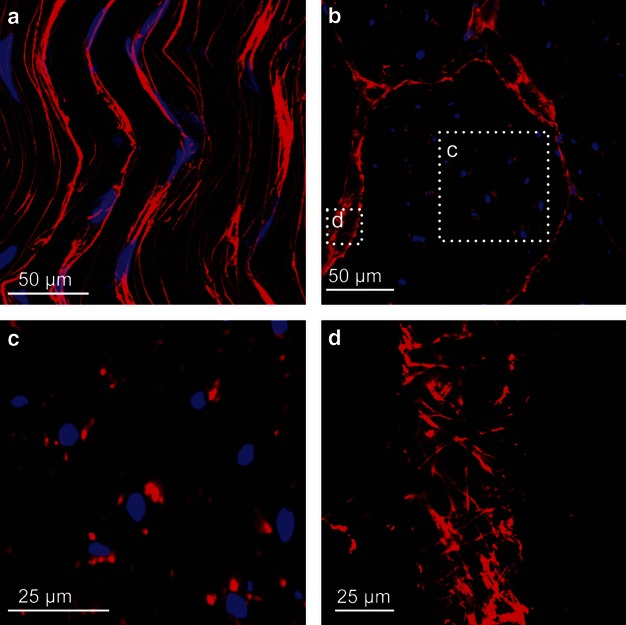
Miller's elastic stained specimens (Fig. 6b) revealed a sparse elastic fibre distribution consistent with previous histological findings (Smith et al. 2011). However, hyaluronidase-pretreated specimens immunostained with the elastin antibody exhibited a much more extensive network (Fig. 6d). However, the hyaluronidase pretreatment affected DAPI staining, as nuclei were not visible following the treatment. Moreover, the treated tissue had a disordered extracellular matrix organization (Fig. 6c) as compared with the untreated specimens (Fig. 3a,b) and the fibril crimp was difficult to distinguish.
Fig 6.
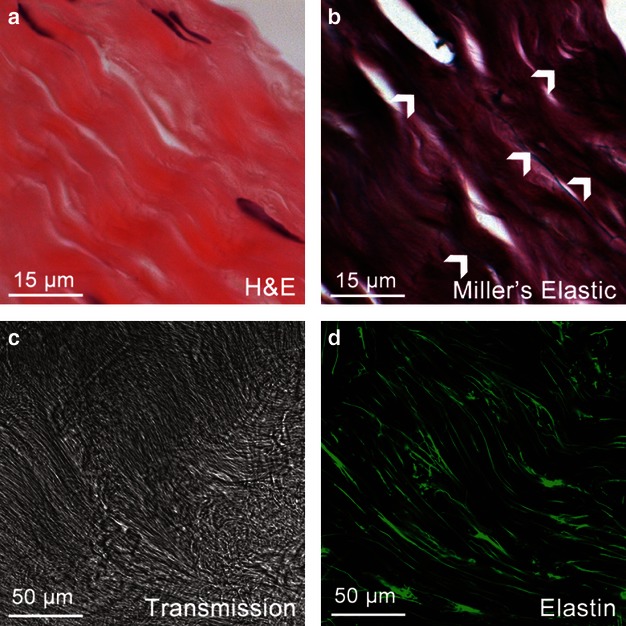
Comparison of basic histology and pretreated immunohistochemistry for detecting elastic fibres. (a) Basic structure of tendon stained with H&E showing wavy collagen structure and cells arranged in rows. (b) Sparse elastic fibre organization (white arrowheads) as observed with Miller's stain. (c) Structure of tendon following hyaluronidase pretreatment with disrupted organization and lack of cell nuclei staining. (d) Enhanced immunodetection of elastic fibres following pretreatment with hyaluronidase.
Discussion
This research has shown that elastic fibres are broadly distributed in tendon and are highly localized around tenocytes and between fascicles. Multiple elastic fibres surround groups of tenocytes and travel longitudinally along tendon, whereas fibres present between fascicles form a loose mesh-like organization oriented in the transverse direction. Fibrillin-1 and fibrillin-2 colocalize in tendon and are occasionally found independent of elastin.
There is little variation in the elastic fibre organization of tissue dissected from the midsection of bovine flexor tendon. The organization may vary as the tendon approaches the osteotendinous junction and into the fibrocartilage region, where the tissue is subjected to a different mechanical environment. Moreover, the elastic fibre organization may vary with age as the specimens examined in this study were from young animals. Future studies should be conducted on mature tendon specimens as well as human specimens to determine how the distribution changes with age and between species.
Observing the elastic fibre organization of dense connective tissues is difficult because of tightly packed collagen fibrils. Enzyme pretreatments, such as the hyaluronidase protocol used in this study, can be used to enhance visualization of elastic fibres. However, the hyaluronidase pretreatment disrupts the cell membrane of tenocytes and extracellular matrix organization. Therefore, the pretreated images can be used to draw conclusions about the extensiveness of the elastic fibre network, whereas untreated tissue can be used to gauge elastic fibre organization and their relationship with cells.
The close interaction of elastic fibres and tenocytes suggests that elastic fibres influence cellular function. The extracellular microenvironment is key to maintaining tissue homeostasis and disruption is thought to lead to a range of disorders (Ingber, 2003). Directly surrounding the cell is the pericellular matrix (PCM), whose constituents and organization are poorly understood. Results of the present study suggest that elastic fibres contribute to tendon PCM forming a continuous network around arrays of cells. The mechanical properties of the PCM play an important role in mechanotransduction mechanisms by altering forces sensed by cells, which are transmitted to the nuclear envelope through the cytoskeleton (Wang, 2006; Eyckmans et al. 2011). The inclusion of elastic fibres into tendon PCM is expected to have a significant effect on the cell-level forces experienced by tenocytes and therefore their mechanobiological response to load. Moreover, groups of cells have been shown to slide relative to each other during deformation (Screen et al. 2004) and elastic fibres may help return tenocytes to their unloaded configuration following the removal of load.
Given their close association with cells, elastic fibres may also play a role in cellular attachment. Type VI collagen contributes to 0.33% (wet weight) of tendon (Carvalho et al. 2006) and is a major constituent of the PCM (Ritty et al. 2003). Fibrillin-1 has been shown to bind to integrins and type VI collagen (Midwood & Schwarzbauer, 2002) and elastic fibres may therefore form an intermediate link between the cell and type VI collagen, which binds to collagen fibrils. It was found that perlecan and fibrillin-1 colocalize in a number of connective tissues, including the anterior cruciate ligament, and this may aid in the integration of elastic fibres into the surrounding matrix (Hayes et al. 2011).
In addition to the unique mechanical ability of elastic fibres to withstand large deformations, elastic fibres have been shown to regulate growth factor availability by binding cytokines, such as transforming growth factor (TGF)-β (Vehvilainen et al. 2009). When bound to fibrillin-1 these growth factors are protected from proteolytic attack and may be released when elastic fibres are damaged, initiating an important process for tissue remodelling. The clinical features associated with microfibril disorders such as Marfan syndrome and Beals syndrome may be attributed to the reduced ability of the tissue to bind cytokines.
Elastic fibres found in the interfascicle space are likely part of the endotenon sheath, which projects into tendon, dividing the structure into compartments, or fascicles. The organization of the sheath varies from that of tendon fascicles and enhances lubrication between collagen bundles during deformation. Blood vessels and nerves travel along the sheath and are interwoven into the elastic fibre network. A similar elastin mesh-like structure was reported in cruciate ligament (Smith et al. 2011) and Caldini et al. (1990) found that all three forms of elastic fibre exist in the endotenon sheath. Recent studies have shown that tendon deformation is governed by sliding of collagen bundles across the entire hierarchy, from fibrils to fascicles (Fratzl et al. 1998; Screen, 2009; Snedeker et al. 2009). The concentrated distribution of elastic fibres in the interfascicle space is likely to provide the sheath with elastic recoil, which experiences large shearing forces during deformation. Moreover, elastic fibres within the endotenon sheath may offer stress protection to blood vessels and nerves. Tendon is exposed to sustained periods of tensional loading and cells live in relatively avascular conditions (Sharma & Maffulli, 2006), and fibres may therefore provide additional elastic recoil to blood vessels, protecting tenocytes from oxygen deprivation, leading to ischaemia and necrosis.
Fibrillin-1 and fibrillin-2 strongly colocalize in the absence of elastin, suggesting that oxytalan fibres are present in tendon, which confirms previous findings in cruciate ligament (Smith et al. 2011) and flexor digitorum profundus tendon (Ritty et al. 2002). Whenever elastin is present in tendon it colocalizes with fibrillin-1, which is consistent with the process of elastogenesis in which microfibrils form the template for tropoelastin deposition (Fahrenbach et al. 1966). Microfibrils are two times stiffer and less extensible than elastin (Sherratt et al. 2003) but their ability to recover large deformations suggests that they may serve a similar mechanical role to that of elastic fibres (Baldock et al. 2001). The incorporation of three different types of fibre in tendon, each with different mechanical properties, may provide the tissue with the ability to address local mechanical requirements.
Previous studies have suggested that elastic fibres contribute to the compliance properties of tendon by maintaining collagen in a crimped configuration (Butler et al. 1978), which acts as a shock absorber when force is applied rapidly to the tissue. Results of the present study have shown a relatively sparse elastic fibre network that conforms to collagen fibril crimp. Further investigation into the function of elastic fibres will be required to determine whether they contribute to the macroscopic deformation of tendon as observed in other tissues, given their relatively sparse distribution.
In conclusion, elastic fibres are primarily deposited around groups of tenocytes and between collagen fascicles in tendon. We suspect that these fibres contribute to the pericellular matrix and endotenon sheath and will therefore affect the microenvironment of cells and the deformation mechanics of tendon. Further research is required to better understand the function of elastic fibres in tendon and the current work provides a detailed description of their organization for use in future studies.
Acknowledgments
We thank Clarence Yapp for his valuable technical assistance.
References
- Baldock C, Koster AJ, Ziese U, et al. The supramolecular organization of fibrillin-rich microfibrils. J Cell Biol. 2001;152:1045–1056. doi: 10.1083/jcb.152.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D, Grood E, Noyes F, et al. Biomechanics of ligaments and tendons. Exerc Sport Sci Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- Caldini EG, Caldini N, De-Pasquale V, et al. Distribution of elastic system fibres in the rat tail tendon and its associated sheaths. Acta Anat. 1990;139:341–348. doi: 10.1159/000147022. [DOI] [PubMed] [Google Scholar]
- Carvalho HF, Felisbino SL, Keene DR, et al. Identification, content, and distribution of type VI collagen in bovine tendons. Cell Tissue Res. 2006;325:315–324. doi: 10.1007/s00441-006-0161-0. [DOI] [PubMed] [Google Scholar]
- Eyckmans J, Boudou T, Yu X, et al. A hitchhiker's guide to mechanobiology. Dev Cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenbach WH, Sandberg LB, Cleary EG. Ultrastructural studies on early elastogenesis. Anat Rec. 1966;155:563–575. [Google Scholar]
- Fratzl P, Weinkamer R. Nature's hierarchical materials. Prog Mater Sci. 2007;52:1263–1334. [Google Scholar]
- Fratzl P, Misof K, Zizak I, et al. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1998;122:119–122. doi: 10.1006/jsbi.1998.3966. [DOI] [PubMed] [Google Scholar]
- Fung YC. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer-Verlag; 1993. [Google Scholar]
- Gupta PA, Putnam EA, Carmical SG, et al. Ten novel FBN2 mutations in congenital contractural arachnodactyly: delineation of the molecular pathogenesis and clinical phenotype. Hum Mutat. 2002;19:39–48. doi: 10.1002/humu.10017. [DOI] [PubMed] [Google Scholar]
- Gupta P, Wallis D, Chin T, et al. FBN2 mutation associated with manifestations of Marfan syndrome and congenital contractural arachnodactyly. J Med Genet. 2004;41:56–59. doi: 10.1136/jmg.2003.012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Lord MS, Smith SM, et al. Colocalization in vivo and association in vitro of perlecan and elastin. Histochem Cell Biol. 2011;136:437–454. doi: 10.1007/s00418-011-0854-7. [DOI] [PubMed] [Google Scholar]
- Ingber D. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ippolito E, Natali PG, Postacchini F. Morphological, immunochemical, and biochemical study of rabbit Achilles tendon at various ages. J Bone Joint Surg Am. 1980;62:583–598. [PubMed] [Google Scholar]
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Lorber M. Elastic fibers in the rat exorbital lacrimal gland duct system. Invest Ophthalmol Vis Sci. 1989;30:2002–2011. [PubMed] [Google Scholar]
- Midwood KS, Schwarzbauer JE. Elastic fibers: building bridges between cells and their matrix. Curr Biol. 2002;12:279–281. doi: 10.1016/s0960-9822(02)00800-x. [DOI] [PubMed] [Google Scholar]
- Miller PJ. An elastin stain. Med Lab Technol. 1971;28:148–149. [PubMed] [Google Scholar]
- Mithieux SM, Weiss AS. Elastin. Adv Protein Chem. 2005;70:437–461. doi: 10.1016/S0065-3233(05)70013-9. [DOI] [PubMed] [Google Scholar]
- Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27. doi: 10.1006/cbir.1996.0004. [DOI] [PubMed] [Google Scholar]
- Parry DAD, Craig AS. Collagen fibrils and elastic fibers in rat-tail tendon: an electron microscopic investigation. Biopolymers. 1978;17:843–855. doi: 10.1002/bip.1978.360170404. [DOI] [PubMed] [Google Scholar]
- Ritty T, Ditsios K, Starcher B. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat Rec. 2002;268:430–440. doi: 10.1002/ar.10175. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Roth R, Heuser JE. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure. 2003;11:1179–1188. doi: 10.1016/s0969-2126(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Screen HR. Hierarchical approaches to understanding tendon mechanics. J Biomec Sci Eng. 2009;4:481–499. [Google Scholar]
- Screen HRC, Bader DL, Lee DA, et al. Local strain measurement within tendon. Strain. 2004;40:157–163. [Google Scholar]
- Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- Sherratt MJ, Baldock C, Haston JL, et al. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J Mol Biol. 2003;332:183–193. doi: 10.1016/s0022-2836(03)00829-5. [DOI] [PubMed] [Google Scholar]
- Smith KD, Vaughan-Thomas A, Spiller DG, et al. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J Anat. 2011;218:600–607. doi: 10.1111/j.1469-7580.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedeker JG, Pelled G, Zilberman Y, et al. An analytical model for elucidating tendon tissue structure and biomechanical function from in vivo cellular confocal microscopy images. Cells Tissues Organs. 2009;190:111–119. doi: 10.1159/000189211. [DOI] [PubMed] [Google Scholar]
- Urban Z, Boyd CD. Elastic-fiber pathologies: primary defects in assembly and secondary disorders in transport and delivery. Am J Hum Genet. 2000;67:4–7. doi: 10.1086/302987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehvilainen P, Hyytiainen M, Keski-Oja J. Matrix association of latent TGF-beta binding protein-2 (LTBP-2) is dependent on fibrillin-1. J Cell Physiol. 2009;221:586–593. doi: 10.1002/jcp.21888. [DOI] [PubMed] [Google Scholar]
- Wang JHC. Mechanobiology of tendon. J Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Yu J, Tirlapur U, Fairbank J, et al. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat. 2007;210:460–471. doi: 10.1111/j.1469-7580.2007.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]