Abstract
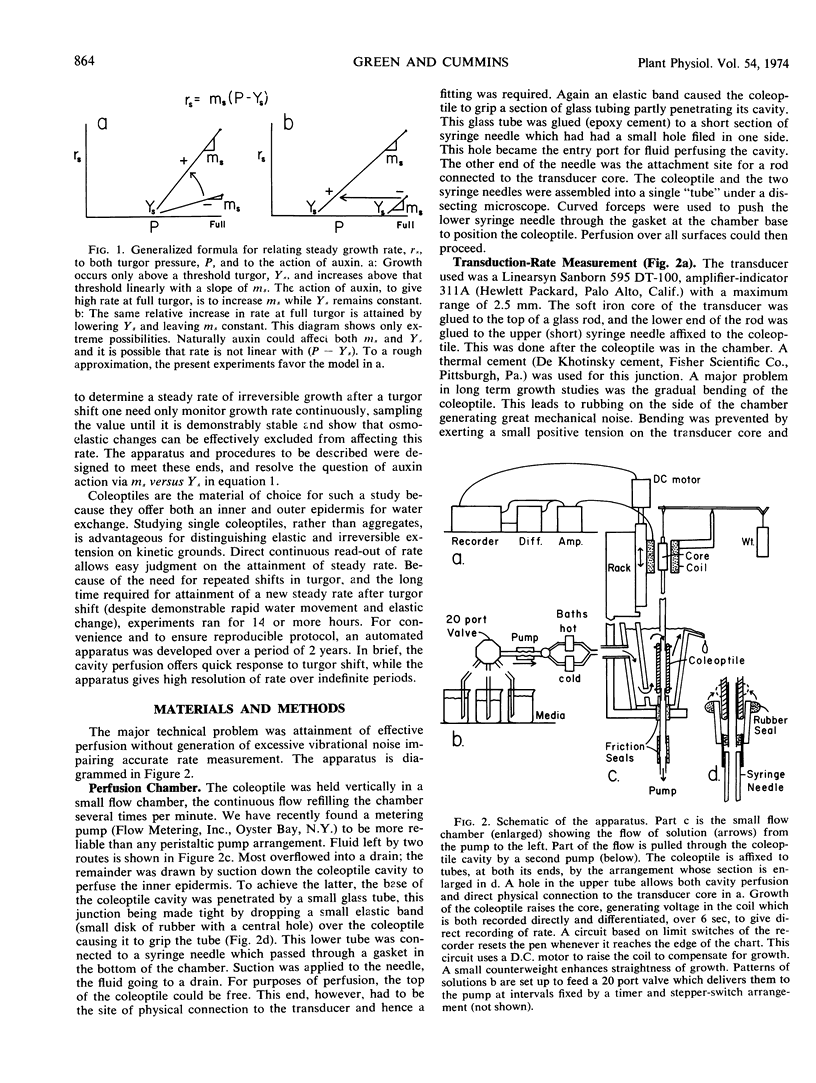
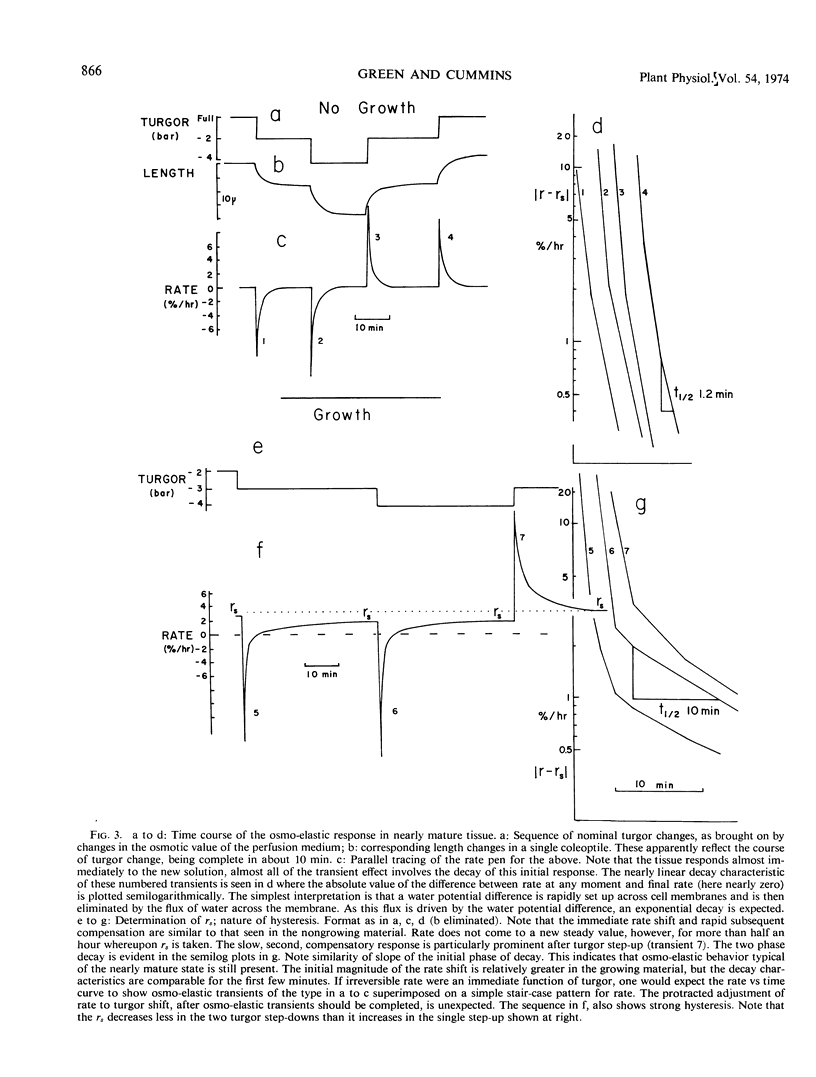
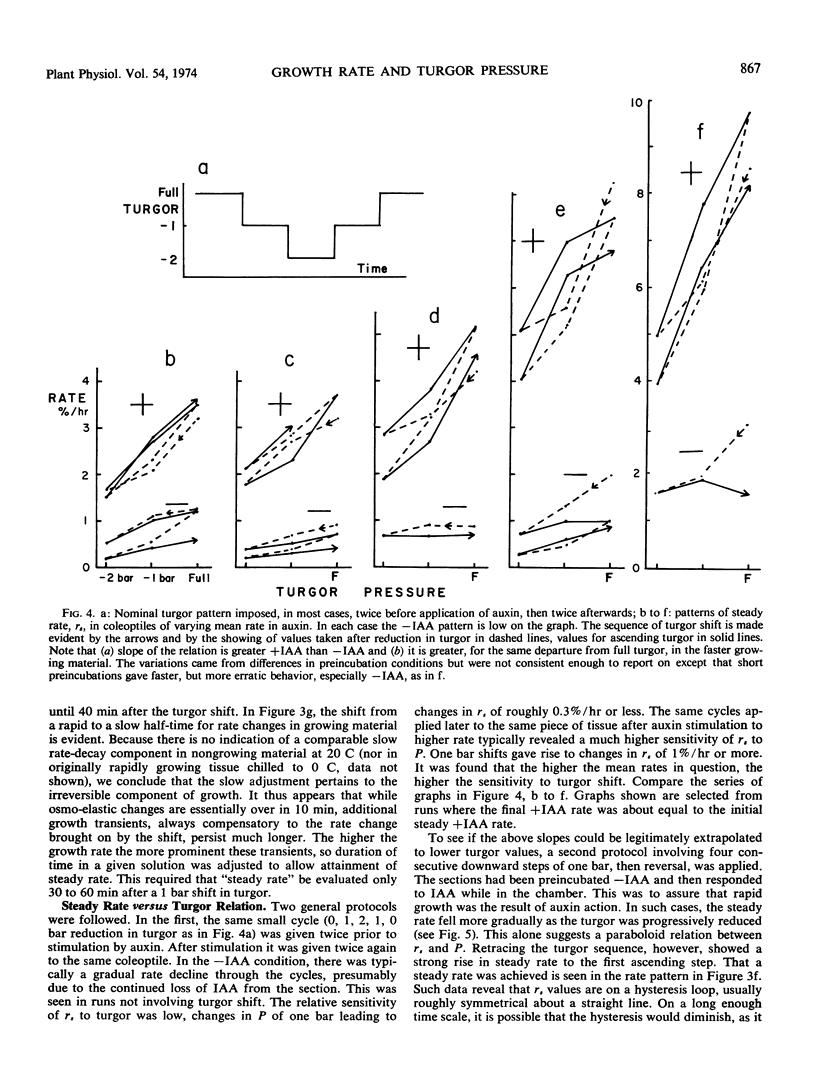
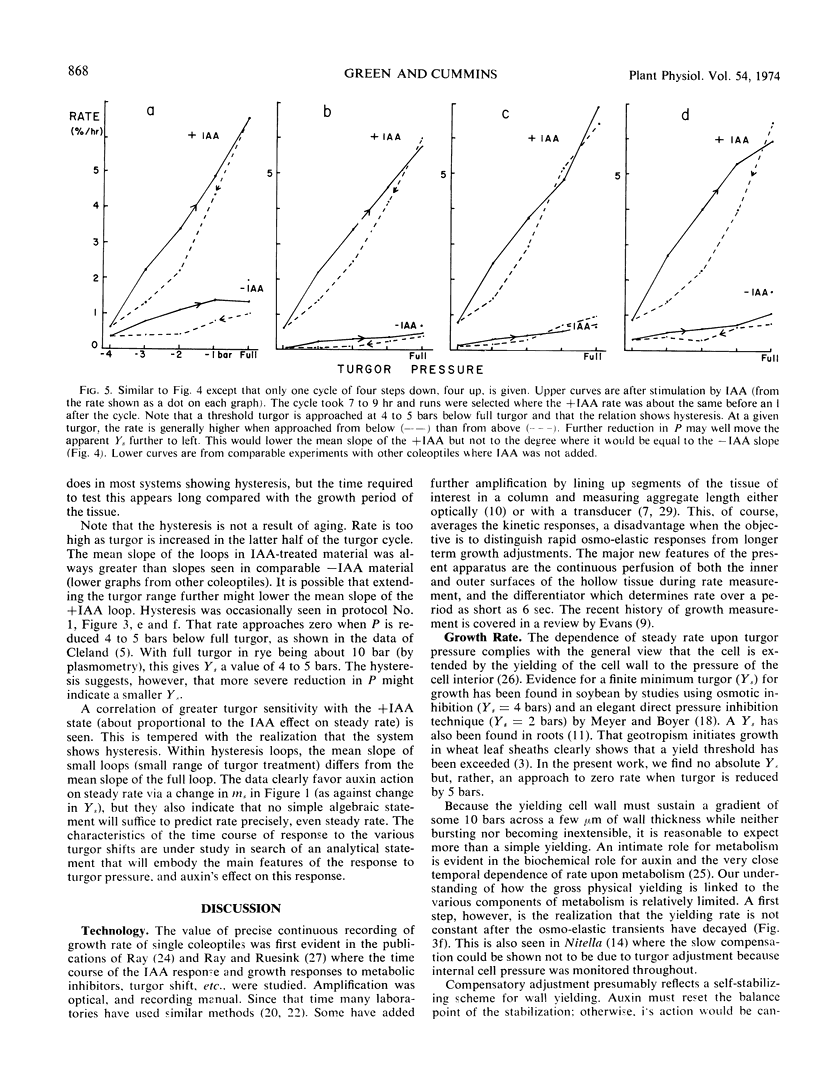
Because turgor pressure is regarded as the driving force for cell extension, any general theory of plant growth requires quantitative information on the relationship between steady irreversible growth rate and turgor pressure. To investigate contrasting views of this relation an automated apparatus was constructed which perfused both the outer and inner epidermis of a single coleoptile while its growth rate was continuously recorded. Turgor was altered abruptly by perfusing with solutions of varying tonicity. With specially grown rye coleoptiles the half-time of the osmo-elastic response was reduced to 2 minutes or less. After decay of this response, however, rate continued to change (so as to partially compensate the effects of the turgor shift in question) for 30 to 60 minutes. Only then could a steady rate be taken. A characterization of steady rate versus turgor covering five turgor values for a single coleoptile thus required many hours. The conclusions are as follows. (a) The change in steady rate, per unit change in turgor, was much greater +IAA than −IAA. (b) Both auxin and turgor act to reset an apparent stabilizing system whose presence is shown in the partial compensation of the initial response to turgor shifts. The above “extensibility” changes are operational only. They need not reflect changes in the immediate physical extensibility of the wall; they could reflect changes in a process acting on the wall. (c) The growth rate versus turgor relation shows some hysteresis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J. Mechanism of osmotic regulation of hydrolase synthesis in aleurone cells of barley: inhibition of protein synthesis. Biochem Biophys Res Commun. 1973 Jul 2;53(1):99–104. doi: 10.1016/0006-291x(73)91406-x. [DOI] [PubMed] [Google Scholar]
- Dela Fuente R. K., Leopold A. C. Time course of auxin stimulations of growth. Plant Physiol. 1970 Aug;46(2):186–189. doi: 10.1104/pp.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greacen E. L., Oh J. S. Physics of root growth. Nat New Biol. 1972 Jan 5;235(53):24–25. doi: 10.1038/newbio235024a0. [DOI] [PubMed] [Google Scholar]
- Green P. B., Erickson R. O., Buggy J. Metabolic and physical control of cell elongation rate: in vivo studies in nitella. Plant Physiol. 1971 Mar;47(3):423–430. doi: 10.1104/pp.47.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. Physical nature of irreversible deformation of plant cells. Plant Physiol. 1967 Nov;42(11):1545–1552. doi: 10.1104/pp.42.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W. H., Nevins D. J. Turgor-dependent Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1973 Sep;52(3):248–251. doi: 10.1104/pp.52.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordin L., Applewhite T. H., Bonner J. Auxin-Induced Water Uptake by Avena Coleoptile Sections. Plant Physiol. 1956 Jan;31(1):44–53. doi: 10.1104/pp.31.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D. H., Berger L. R. Inhibition of metabolism by hydrostatic pressure: what limits microbial growth? Arch Mikrobiol. 1973 Nov 19;93(4):367–370. doi: 10.1007/BF00427933. [DOI] [PubMed] [Google Scholar]
- Rehm M. M., Cline M. G. Rapid growth inhibition of Avena coleoptile segments by abscisic Acid. Plant Physiol. 1973 Jan;51(1):93–96. doi: 10.1104/pp.51.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]



