Abstract
Stem cells used for clinical tissue regeneration therapy should have the capacity of self-renewal, high proliferation, and differentiation and be able to be transplanted in large numbers. Although high concentrations of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) may induce the differentiation of stem cells, these factors have been widely used to enhance the propagation of stem cells, including adipose-derived mesenchymal stem cells (ASCs). However, the effects of low concentrations of EGF and bFGF on stem cells need to be evaluated carefully. This study illustrates that low concentrations of EGF (5 ng/mL) and bFGF (10 ng/mL) increase the proliferative ability of ASCs and induce the typical spindle-shaped cell morphology. EGF and bFGF added to medium promoted neural lineage differentiation and impaired the mesodermal differentiation ability of ASCs. This study demonstrates that even low concentrations of EGF and bFGF may limit the differentiation ability of stem cells during stem cell expansion in vitro. EGF and bFGF supplementation should be carefully considered in stem cells for clinical applications.
Introduction
Many previous studies have shown that stem cells are ideal candidates for the fields of tissue engineering and regenerative medicine (Bianco et al., 2001b; Reya et al., 2001; Vogel 2002). Stem cell replacement therapies have become modern therapeutic approaches, as stem cells function as “stem cell plasticity” (Bianco et al., 2001a; Wagers and Weissman, 2004). Mesenchymal stem cells (MSCs) for transplantation to either autologous or allogeneic hosts should have the ability of high self-renewal, proliferation, and differentiation (Belluzzi et al., 2007; Pittenger et al., 1999). A small percentage of abnormal cells within the population may potentially have a tumorigenic lineage and pose a risk of cancer upon transplantation, and this was shown in previous studies in which MSCs grew slowly and differentiated easily or become senescent in vitro (Chamberlain et al., 2007; Ksiazek, 2009). Thus, to obtain large numbers of more useful autologous MSCs in vitro, the optimal nutrient supplements and growth factors for optimal cell culture media have been investigated extensively (Caterson et al., 2002; Doucet et al., 2005; Gstraunthaler, 2003; Horwitz et al., 2002; Kuznetsov et al., 2000; Nimura et al., 2008; Shahdadfar et al., 2005). Basic fibroblast growth factor (bFGF) belongs to the heparin-binding growth factor family. Epidermal growth factor (EGF) participates in tissue repair and cellular viability in the central nervous system (Schmidt et al., 2006; Skaletz-Rorowski et al., 2005; Tassi et al., 2001; Vallier et al., 2005; Vassaux et al., 1994). Previous evidence has indicated that EGF and bFGF are effective cell mitogens, and these factors have been added to the media of stem cell cultures at different concentrations (Ito et al., 2007; Kelly et al., 2005; Quarto et al., 2006; Quarto et al., 2008; Tsutsumi et al., 2001; Wang et al., 2012a). According to previous studies, maintenance of human MSCs or embryonic stem cells at low concentrations (<10 ng/mL) of EGF and bFGF, alone or in combination with other factors, can stimulate cell proliferation and sustain high cell density without compromising their stem cell nature. Therefore, 5 ng/mL EGF and 10 ng/mL bFGF are added routinely to the media for in vitro stem cell culture (Kang et al., 2005; Krampera et al., 2005; Lu et al., 2006; Sotiropoulou et al., 2005; Weiss et al., 1996).
Adipose-derived stem cells (ASCs) may be useful “seed” cells for cellular therapy applications because they are more readily acquired, relatively safe, and easy to expand in vitro (Gimble et al., 2007; Gimble et al., 2011; Lindroos et al., 2011; Majka et al., 2011; Zuk et al., 2001). Previous studies have shown that 5 ng/mL EGF or 10 ng/mL bFGF can promote the expansion of ASCs (Baer et al., 2009; Hauner et al., 1995; Lee et al., 2004; Tapp et al., 2009). However, little is known about the effect of these pro-survival and pro-proliferative concentrations of EGF and bFGF on the differentiation of ASCs.
To investigate whether EGF and bFGF can influence the stemness and differentiative ability of ASCs when enhancing the proliferation of ASCs, we cultured ASCs in medium supplemented with 5 ng/mL EGF and 10 ng/mL bFGF. The results of this study suggest that EGF and bFGF, even at low concentrations, can direct the fate of ASCs toward a neural lineage in vitro, which may limit the usage of stem cells in specific clinical trials.
Materials and Methods
Isolation and culture of ASCs
ASCs were obtained from 4-week-old female Sprague-Dawley rats (weight, 100–130 grams, n=9), as previously described (Zuk et al., 2001). Briefly, inguinal fat tissue was washed with sterile phosphate-buffered saline (PBS) and digested with 0.1% collagenase I in a water bath at 37°C for 1 h, and the cells were centrifuged at 800 rpm for 5 min. The cells were resuspended in either (1) unsupplemented basal medium (UN medium) only composed of Dulbecco's modified Eagle medium (DMEM; Thermo Fisher Scientific, West Sussex, UK), 5% fetal bovine serum (FBS; Thermo Fisher Scientific, West Sussex, UK), and 1% penicillin/streptomycin (Invitrogen Corp., Carlsbad, CA, USA); or (2) EF medium, which consisted of basal medium containing 5 ng/mL EGF (Gibco Lab., NY, USA) and 10 ng/mL bFGF (Peprotech, NJ, USA). The cells were seeded into T25 flasks and cultured at 37°C in 5% CO2. Approximately 1 day after initial plating, the media were removed and replaced with fresh media to remove nonadherent cells. On day 7 (D7), the cells were washed with PBS, incubated in 0.25% trypsin (Gibco Lab., NY, USA) at 37°C for 2 min, and subcultured at 1:3. The cells were routinely subcultured every 3 days thereafter. All experimental procedures were conducted according to Southeast University, Medical Faculty ethic committee approval.
Analysis of ASC morphology, proliferation, and cell cycle distribution
On D20, the morphology of ASCs cultured in UN or EF medium at 90% confluence was assessed by bright-field light microscopy (Zeiss Ti-S Germany, Oberkochen, Germany). Then, the same cells were incubated in 0.25% trypsin and plated into 12-well plates at 1×103 cells/mL; the cell numbers were counted daily for 8 continuous days. The cell numbers were calculated in the log phase of the resulting growth curves. Cell cycle analysis was performed using propidium iodide (PI; Sunshine, Nanjing, China) staining and flow cytometry (Accuri C6; BD, Michigan, USA). The cells were detached, centrifuged, fixed with 70% cold (4°C) ethanol for 0.5 h, and 1 mL of 50 μg/mL PI and 20 μg/mlL RNase A (Sunshine, Nanjing, China) were added. The cells were incubated for 0.5 h in the dark and filtered through a 100-μm nylon mesh to remove clumps. Data analysis was performed using C-Flow software (BD, Michigan, USA) and FlowJo analysis software (FlowJo; Ashland, OR, USA). All experiments were performed in triplicate.
Osteogenic and neural differentiation
On D20, ASCs cultured in UN or EF medium were seeded into 12-wells plate at a density of 1×103 cells/ mL. After 1–2 days, the media were replaced with osteogenic differentiation medium, which was replaced every 3 days for a period of 15 days. The osteogenic differentiation medium was UN or EF medium supplemented with 10 mmol/L glycerol phosphate disodium salt hydrate, 10 nmol/L dexamethasone, 50 μmol/L L-ascorbic acid sodium salt, 300 mg/L L-glutamine, and 10 nmol/L 1α,25-dihydroxyvitamin D3 (Sigma-Aldrich, St. Louis, MO, USA).
After 7 days induction, an alkaline phosphatase (AP) detection kit (Amresco, Solon, OH, USA) with 5-bromo-4-chloro-3-indolyl phosphate/p-nitroblue tetrazolium chloride (BCIP/NBT) as a substrate was used to assess osteogenic differentiation. The cells were fixed with 4% paraformaldehyde for 30 min, rinsed three times with PBS, and BCIP/NBT was added. The cells were incubated for 30 min, and the samples were rinsed once with water and observed using bright-field light microscopy. Neural differentiation was also assessed after 15 days induction using immunofluorescent staining and PCR, as described below.
Neural differentiation was performed in a similar manner over a period of 15 days using UN or EF medium supplemented with 100 ng/mL retinoic acid (RA; Sigma-Aldrich, St. Louis, MO, USA). Neural differentiation was assessed after 15 days induction using immunofluorescent staining and PCR, as described below.
Immunofluorescent analysis
Immunofluorescent analysis was employed to detect the expression of stemness markers in cells cultured in UN or EF medium. After 20 days of culture, the cells were fixed with 0.5% formaldehyde containing 0.2% Triton X-100 in PBS buffer at pH 7.4 for 5 min at room temperature, rinsed once with PBS, fixed again in 4% formaldehyde in PBS for 20 min, and rinsed three times with PBS. The samples were incubated with rabbit anti-rat primary antibodies against the stemness markers Oct4 and Sox2 (1:100, Santa Cruz Biotechnology, CA, USA) in 1% bovine serum albumin (BSA; Sunshine, Nanjing, China) at 4°C for at least 12 h.
After 15 days of induction in UN and EF osteogenic differentiation medium, ASCs were subjected to immunofluorescent staining using rabbit anti rat osteocalcin (Ocn) primary antibody (1:100; in 1% BSA; Santa Cruz Biotechnology, CA, USA) to detect differentiated osteogenic cells. After 15 days induction in UN and EF neural differentiation media, ASCs were subjected to immunofluorescent staining using rabbit anti-rat nestin and glial fibrillary acidic protein (Gfap) primary antibodies (1:100 in 1% BSA, Santa Cruz Biotechnology, CA, USA) to detect differentiated neural cells.
After washing with PBS, the samples were incubated with the appropriate secondary antibody (1:200 in 1% BSA; goat anti-rabbit Alexa-Fluor 488 or donkey anti-rabbit Alexa-Fluor 647; Invitrogen Corp., Carlsbad, California, USA) for 1 h at 37°C while protected from light. Then, the samples were washed twice with PBS, the nuclei were detected by staining with 10 μg/mL Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) for 30 min while protected from light, and images were obtained using a Revolution XD confocal laser scanning microscope (Andor, Belfast, Northern Ireland).
RNA isolation, reverse transcription polymerase chain reaction, and real-time quantitative fluorescence PCR
ASCs cultured in EF or UN medium were harvested on D10, D20, and D30, and the expression of Oct4, Sox2, Klf4, Nanog, and Lin28a was measured to assess the changes in ASC stemness markers. After induction with osteogenic medium for 15 days, the expression of Alp, Ocn, core binding factor alpha (Cbfa), and collagen type I (Col) was quantified to assess osteogenic differentiation. After induction with neural medium for 15 days, the expression of Nestin, Gfap, and microtubule-associated protein-2 (Map2) was quantified to assess neural differentiation. Glycerol-3-phosphate dehydrogenase (Gapdh) was used as a control.
Total cellular RNA was isolated using the TRIzol method (Invitrogen Corp., Carlsbad, California, USA). DNase-treated total RNA (20 μL total volume) was incubated with 1 μL of 50 mM oligo(dT18) (TaKaRa, Dalian, China). After denaturation, 5× buffer, dNTPs, RNase inhibitor, and PrimeScript Reverse Transcriptase (TaKaRa, Dalian, China) were added, as specified by the manufacturer's protocol. Reverse transcription was performed for 60 min at 42°C, followed by 5 min at 85°C to inactivate the reverse transcriptase.
Reverse transcription polymerase chain reaction (RT-PCR), and real-time quantitative fluorescence PCR (qRT-PCR) were performed using standard protocols. Specific primers were designed for each gene based on the GenBank sequences; the primer sequences are listed in Table 1. RT-PCR was carried out at 95°C for 1 min, 95°C for 15 sec, 57°C/60°C for 40 sec, and 72°C for 40 sec (28–37 cycles). qRT-PCR was performed on the Applied Biosystems 7500 Sequence Detection System (BD, Michigan, USA). Briefly, 1 μL cDNA was added to 10 μL of 2× SYBR Green PCR master mix (TaKaRa, Dalian, China) and 200 nM of each primer in a total volume of 20 μL. The reactions were amplified over 40 cycles of 95°C for 15 sec and 60°C for 1 min. Afterward, a thermal denaturation protocol was performed to determine the number of products present in each reaction. The reactions were typically run in triplicate. The cycle number at which the reaction crossed an arbitrarily placed threshold (Ct) was determined for each gene. Target gene expression was normalized to the expression of Gapdh in each sample. Data were analyzed using the 2−ΔΔCt method(Livak et al., 2001).
Table 1.
List of Primers Used for RT-PCR and qRT-PCR
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Gapdh | CGATCCCGCTAACATCAAAT | GGATGCAGGGATGATGTTCT |
| Oct4 | AGGCAGGAGCACGAGTGGA | CGAAGCGGCAGATGGTTGT |
| Sox2 | CAGGGAGTTCGCAAAAGTCT | AAACCCAGCAAGAACCCTTT |
| Klf4 | CAGACCTGGAAAGTGGTGG | ACCTGTGTTGCCCGCAGCC |
| Lin28a | GGAGGGTGGGAAAGTGTGTACT | CCAGGGAAGAGAAGGGAAGGTA |
| Nanog | TCTCCTCCGCCTTCCTCT | TTGCCTCTGAAACCTATCCTTG |
| Alp | CTCCGGATCCTGACAAAGAA | ACGTGGGGGATGTAGTTCTG |
| Ocn | AAAGCCCAGCGACTCT | CTAAACGGTGGTGCCATAGAT |
| Cbfa | GCCGGGAATGATGAGAACTA | GGACCGTCCACTGTCACTTT |
| Col | TAAAGGGTCATCGTGGCTTC | ACTCTCCGCTCTTCCAGTCA |
| Nestin | TGGAGGTGGCTACATACAGG | TGGGAGGATAGCAGAAGAAC |
| Gfap | ATTCCGCGCCTCTCCCTGTCTC | GCTTCATCCGCCTCCTGTCTGT |
| Map2 | AATTGCCTTCCTCATTCGC | TGTCTTCCAGGTTGGTACCG |
Statistical analysis
All data were expressed as the mean &plumn; standard deviation (SD). Differences were compared using the Student t-test; p values <0.05 were considered statistically significant (*p<0.05, **p<0.01).
Results
EGF and bFGF enhance the proliferation of ASCs
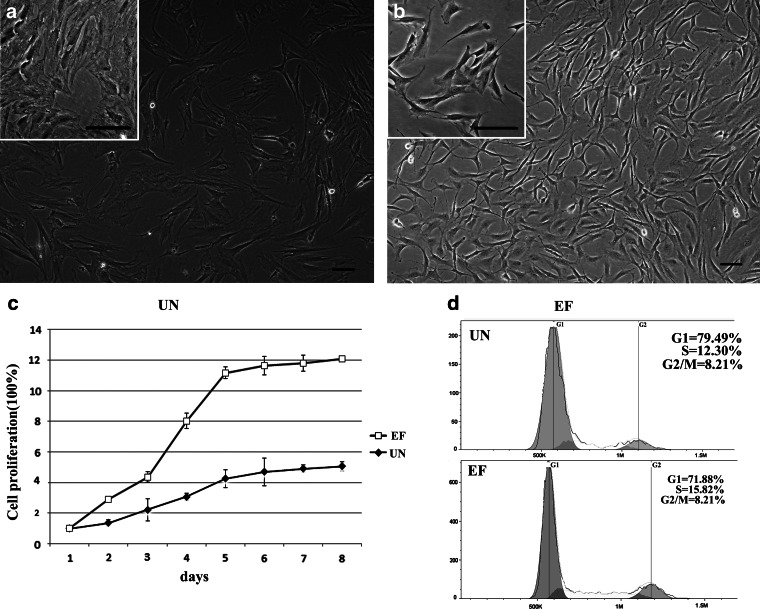
ASCs were isolated from adipose tissue, according to a previously published protocol, and the influence of EFG and bFGF on cell morphology, proliferation, and cell cycle distribution was analyzed. ASCs grew in colonies and continuously expanded until they approached confluency; the cells displayed stable and rapid growth in vitro. The expression of ASC surface markers was confirmed by flow cytometry (data not shown). ASCs cultured in UN medium displayed a heterogeneous morphology; most of the cells had a broad, flattened morphology and extended pseudopods (Fig. 1a). In contrast, ASCs cultured in EF medium displayed evident central nucleoli, their cytoplasm contained elongated and spindle-shaped processes, and short protrusions appeared on the surface of the cell bodies (Fig. 1b).
FIG. 1.
Effects of EGF and bFGF on the morphology, proliferation and cell cycle distribution of ASCs. (a) Photomicrograph of ASCs cultured in UN medium for 20 days. (b) Photomicrograph of ASCs cultured in EF medium for 20 days. Scale bars, 50 μm. (c) Growth curve analysis of ASCs cultured in UN and EF media. Induction of percentage of cells in each phase of cell cycle of ASCs cultured by UN and EF media. (d) ASCs cultured in the UN medium were analyzed by PI staining and FlowJo analysis software. (e) ASCs cultured in the EF medium were analysed by PI staining and FlowJo analysis software.
To investigate the biological effects of EGF and bFGF on ASCs, the number of cells was recorded every day for 8 days and cell cycle distribution was analyzed by flow cytometry. ASCs displayed logarithmic growth curves in both UN and EF media. After an initial lag or stationary period, the cells expanded rapidly in a logarithmic manner, until they reached a plateau. However, ASCs grew significantly faster in the presence of EGF and bFGF (Fig. 1c). On day 8, the number of cells in the EF group was three-fold higher than the UN group.
The cell cycle analysis (Fig. 1d, e) indicated that, compared to UN medium, the ratio of G1-phase ASCs in the EF medium decreased from 79.49% to 71.88% (p<0.05), and the ratio of G2/M phase increased from 8.21% to 12.30% (p<0.05). These data demonstrate that EGF and bFGF stimulate the growth of ASCs and enhance their proliferative capacity.
EGF and bFGF alter the pluripotency of ASCs
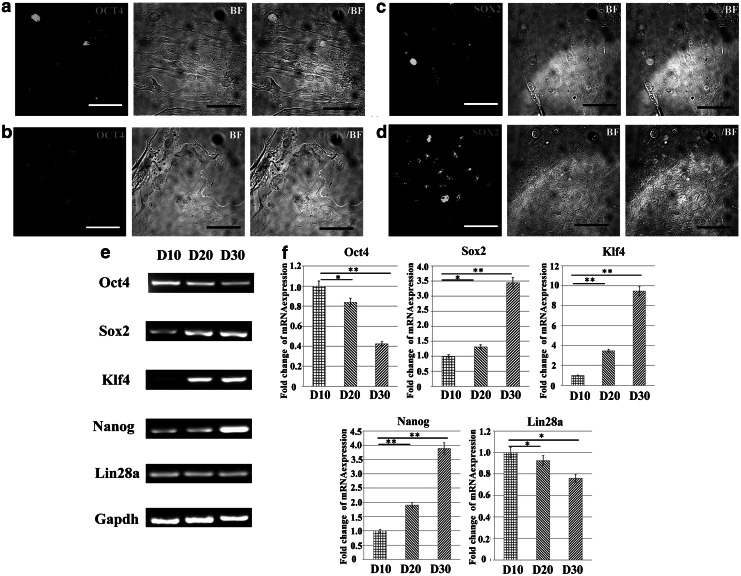
Alhough EGF and bFGF can stimulate ASC proliferation, the effect of these growth factors on the maintenance of stemness should be carefully investigated. Oct4 and Sox2, markers of undifferentiated stem cells, are critically involved in self-renewal (Driessens et al., 2011; Wang et al., 2012b). Immunofluorescent staining was used to detect the expression of both Oct4 and Sox2 in ASCs cultured in UN and EF media for 20 days (Fig. 2a, b, c, d). The level of Oct4 expression in ASCs cultured in EF medium was lower than in UN medium (Fig. 2 a, b). However, the expression of Sox2 was higher in ASCs cultured in EF medium (Fig. c, d).
FIG. 2.
EGF and bFGF affect the pluripotency of ASCs. Immunofluorescent staining and bright-field micrographs (BF) of ASCs after 20 days of culture in UN or EF medium. (a and b) Expression of Oct4 in ASCs cultured in UN medium (a) or EF medium (b). (c and d) Expression of Sox2 in ASCs cultured in UN medium (c) or EF medium (d). Scale bars, 20 μm. (e and f) Analysis of stemness markers in undifferentiated ASCs cultured in EF medium on three time points, D10, D20, and D30 by RT-PCR (e) and qRT-PCR (f). Gapdh was used as a loading control. (*) p<0.05; (**) p<0.01.
RT-PCR and qRT-PCR were used to assess the pluripotency of ASCs cultured in EF medium on D10, D20, and D30. As shown in Figure 2e, the levels of Sox2, Klf4, and Nanog initially increased by D20 and were further upregulated by D30. The expression of Sox2, Klf4, and Nanog increased 3.45-fold, 9.47-fold, and 3.89-fold by D30 compared to D10 (Fig. 2f). In contrast, the expression of Oct4 and Lin28a was downregulated 2.38-fold and 1.76-fold, respectively (Fig. 2f). These results indicate that culture in medium supplemented with 5 ng/mL EGF and 10 ng/mL bFGF may affect the pluripotent state of ASCs.
EGF and bFGF impair the osteogenic differentiation potential of ASCs
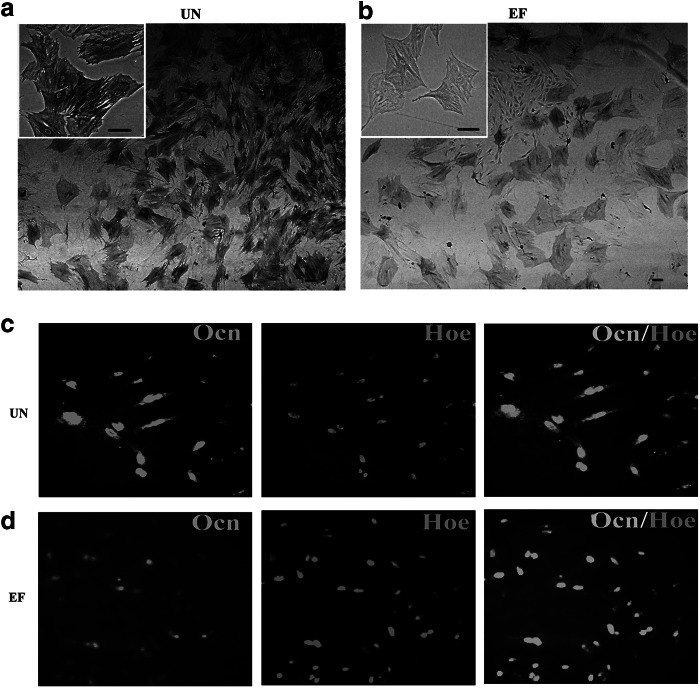
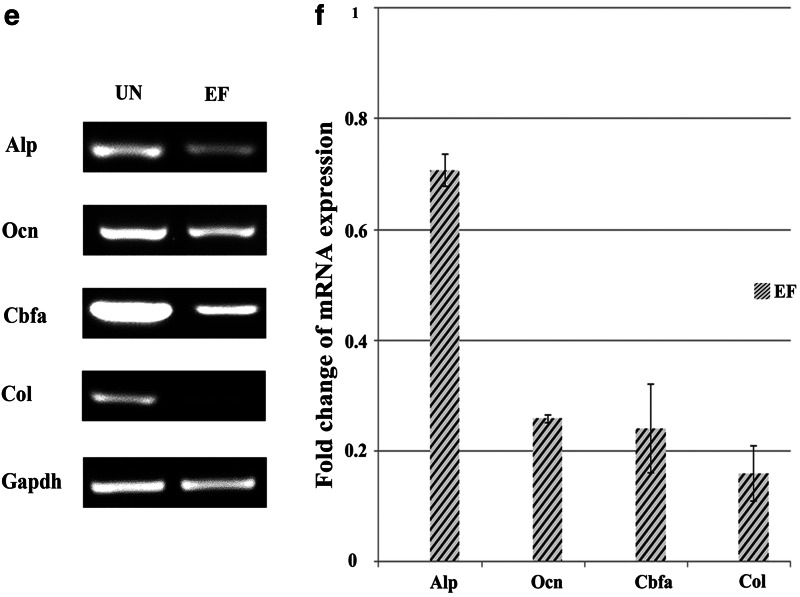
The results in this study demonstrated that EGF and bFGF increased the expression of Sox2 and decreased the expression of Oct4 in ASCs, which indicates that EGF and bFGF may induce ASCs to undergo neural lineage differentiation and impair their ability to undergo osteogenic differentiation. To investigate this, osteogenic differentiation was induced by culturing ASCs in UN or EF medium containing glycerol phosphate disodium salt hydrate, dexamethasone, L-ascorbic acid sodium salt, L-glutamine, and 1α, 25-dihydroxyvitamin D3. As shown in Figure 3, a and b, ALP activity significantly reduced after 7 days in the cells cultured in osteogenic EF medium, compared to ASCs cultured in osteogenic UN medium. By D15, the level of Ocn, a late osteogenic marker, was markedly attenuated in ASCs cultured in osteogenic EF medium (Fig. 3c, d). RT-PCR confirmed that ASCs cultured in osteogenic EF medium expressed significantly lower levels of the osteogenic markers Alp, Ocn, Col, and Cbfa (Fig. 3e). qRT-PCR revealed that expression of Alp, Ocn, Col, and Cbfa decreased 1.4-fold, 3.8-fold, 4.1-fold, and 6.2-fold in ASCs cultured in osteogenic EF medium, compared to ASCs cultured in osteogenic UN medium (Fig. 3f). The results suggested that the osteogenic potential of ASCs was impaired by culture in EGF and bFGF medium.
FIG. 3.
EGF and bFGF reduce ASC osteogenic differentiation. (a and b) ALP staining of ASCs cultured for 7 days in UN medium containing osteogenic induction factors (a) or EF medium containing osteogenic induction factors (b). (c and d) Immunofluorescent staining for Ocn in ASCs cultured for 15 days in UN medium containing osteogenic induction factors (c) or EF medium containing osteogenic induction factors (d). Scale bars, 50 μm. Nuclei are stained with Hoechst. (e and f) Expression of osteogenic markers by ASCs cultured in UN medium containing osteogenic induction factors or EF medium containing osteogenic induction factors for 15 days, by RT-PCR (e) and qRT-PCR (f). Gapdh was used as a loading control. Values in f are expressed relative to ASCs cultured UN medium containing osteogenic induction factors.
EGF and bFGF enhance the neural differentiation potential of ASCs
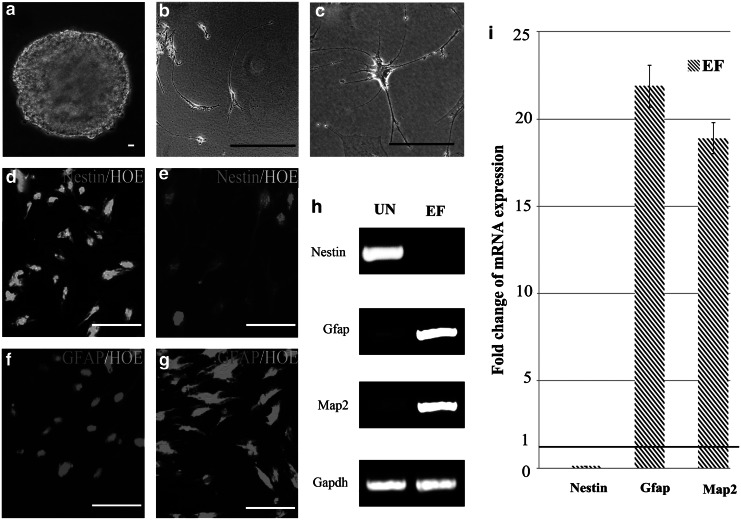
Next, the ability of ASCs cultured in UN or EF medium to differentiate into the neural lineage after the addition of RA was investigated. Morphologically, ASCs cultured in medium containing RA retracted continuously to form a sphere-shaped group of cells (Fig. 4a). Subsequently, the cells migrated from the sphere and formed simple bipolar-shaped cells. ASCs cultured in UN medium containing RA for 15 days expressed Nestin, an early neuronal marker, but did not express detectable levels of Gfap (Fig. 4b, d, f). Conversely, ASCs cultured in EF medium containing RA displayed retractile cell bodies with highly branched, complex multipolar structures and expressed Gfap and low levels of Nestin (Fig. 4c, e, g). Immunofluorescent staining confirmed that these markers were continuously expressed for at least 2 weeks, which indicated that ASCs cultured in EF neural differentiation medium terminally differentiated into neural lineage cells. In contrast, the mature neural proteins Gfap and Map2 were barely detectable in the cells cultured in UN neural differentiation medium. Nevertheless, the ASCs derived from EF neural differentiation medium expressed Gfap and Map2, but not Nestin. Quantification revealed that expression of Nestin decreased 9-fold, whereas Gfap and Map2 were upregulated 21.9-fold and 18.9-fold, respectively, in ASCs derived from EF neural differentiation medium, compared to UN neural differentiation medium (Fig. 4i).
FIG. 4.
EGF and bFGF promote ASC neural differentiation. (a) Photomicrograph of a stem cell sphere; (b) photomicrograph of mature neurons cell in UN medium; (c) photomicrograph of mature glial cell in EF medium. (d and e) Immunofluorescent staining for Nestin in ASCs cultured for 15 days in UN medium containing RA (d) or EF medium containing RA (e). (f and g) Immunofluorescent staining for Gfap in ASCs cultured for 15 days in UN medium containing RA (f) or EF medium containing RA (g). Scale bars, 20 μm. Nuclei are stained with Hoechst. (e and f) Expression of osteogenic markers by ASCs cultured UN medium containing RA or EF media containing RA for 15 days, by RT-PCR (e) and qRT-PCR (f). Gapdh was used as a loading control. Values in f are expressed relative to ASCs cultured UN media containing RA.
These data suggested that ASCs cultured in neural differentiation medium containing EGF and bFGF successfully transdifferentiated into mature neural cells, whereas the cells induced in UN neural differentiation medium only generated neural progenitors. These results show that ASCs cultured in EGF and bFGF will differentiate into neural cells rather than osteocyte lineage cells when treated with RA.
Discussion
Extracellular growth factors are involved in a number of biological signal–regulated kinase signaling and processes, which may affect cell adhesion, migration, proliferation, and differentiation (Discher et al., 2009; Lee et al., 2009). EGF and bFGF participate in a complex network of cellular processes, and are commonly added into stem cell cultures to enhance their proliferation capacity, with the expectation of maintaining the stem cells' ability for multipotent differentiation. Kakudo et al. and Kalyani et al. reported that high concentrations of EGF or bFGF may act as either positive or negative regulators of stem cell gene expression, as well as adipocyte, osteogenic, and chondrogenic differentiation (Kakudo et al., 2007; Kalyani et al., 1999). Meanwhile, other research indicated that incubation with low concentrations of EGF or bFGF significantly enhanced cell proliferation and migration, but did not affect the undifferentiated state of ESCs (Park et al., 2011; Vassaux et al., 1994). On the basis of these studies, the effects of low concentrations of a combination of EGF and bFGF on the proliferation and differentiation of ASCs during stem cell expansion in vitro were investigated.
ASC morphology, growth curves, and cell cycle analysis indicated that the addition of EGF and bFGF significantly increased the proliferative capacity of ASCs, and that these potent mitogenic activity factors were able to produce a greater quantity of ASCs (Hebert et al., 2009; Mydlo et al., 1998; Neubauer et al., 2004; Vassaux et al., 1994; Zuk 2010).
Oct4 and Sox2, in concert with other factors (Klf4, Nanog, Lin28a), are involved in the maintenance of stem cell self-renewal and pluripotency (Chen et al., 2008; Driessens et al., 2011; Thomson et al., 2011; Wang et al., 2012b). Immunofluorescent staining and PCR revealed that EGF and bFGF enhanced Sox2 expression and downregulated Oct4 expression, and that EGF and bFGF could affect the expression of stem cell–related genes in ASCs. There is ample evidence to demonstrate that downregulation of Oct4 and upregulation of Sox2 can affect the pluripotency of stem cells, stimulate differentiation to the ectoderm lineage, and improve induction efficiency (Thomson et al., 2011; Wang et al., 2012b).
The results in this study revealed that EGF and bFGF may induce ASCs to transinduce into an ectodermal lineage rather than a mesodermal lineage. Osteogenic differentiation assays confirmed that ASCs cultured with EGF and bFGF had weaker AP activity and lower levels of Ocn, Cbfa, and Col compared to the cells treated without EGF and bFGF. Moreover, the neurogenic differentiation assay proved that ASCs treated with EGF and bFGF preferred to transdifferentiate into mature neurons, even in the presence of low concentrations of EGF and bFGF. Expression of Gfap in ASCs induced in the presence of EGF and bFGF indicated that the cells had partially differentiated into glial cells.
This study demonstrates that EGF and bFGF play an important role in the proliferation and stem cell plasticity of ASCs. Low concentrations of EGF (5 ng/mL) and bFGF (10 ng/mL) not only enhanced the proliferation of ASCs, but also affected their differentiation. EGF and bFGF, even at low concentrations, may limit the differentiative ability of stem cells during stem cell expansion in vitro; therefore, the supplementation of stem cell cultures with EGF and bFGF should be carefully considered during in vitro expansion for clinical applications.
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (973 Program) (no. 2013CB932902), and the National Natural Science Foundation of China (NSFC) (no. 61071047)
Author Disclosure Statement
The authors declare that there is no conflict of interest.
References
- Baer P.C. Schubert R. Bereiter-Hahn J., et al. Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur. J. Cell Biol. 2009;88:273–283. doi: 10.1016/j.ejcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Belluzzi A. Lanzoni G. Roda G., et al. Mesenchymal stem cells from the gastrointestinal stroma in inflammatory bowel disease (IBD) and colon cancer: Isolation, in vitro expansion and characterization. Gastroenterology. 2007;132:A553–A554. [Google Scholar]
- Bianco P. Riminucci M. Gronthos S., et al. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001a;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- Bianco P. Robey P.G. Stem cells in tissue engineering. Nature. 2001b;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- Caterson E. Nesti L. Danielson K.G., et al. Human marrow-derived mesenchymal progenitor cells—Isolation, culture expansion, and analysis of differentiation. Mol. Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- Chamberlain G. Fox J. Ashton B., et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Chen X. Xu H. Yuan P., et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Discher D.E. Mooney D.J. Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet C. Ernou I. Zhang Y.Z., et al. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- Driessens G. Blanpain C. Kabiri A. Long live Sox2: Sox2 lasts a lifetime. Cell Stem Cell. 2011;9:283–284. doi: 10.1016/j.stem.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J.M. Bunnell B.A. Chiu E.S., et al. Concise review. Adipose-derived stromal vascular fraction cells and stem cells: Let's not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- Gstraunthaler G. Alternatives to the use of fetal bovine serum: Serum-free cell culture. Altex-Altern. Tierexp. 2003;20:275–281. [PubMed] [Google Scholar]
- Hauner H. Rohrig K. Petruschke T. Effects of epidermal growth-factor (EGF), platelet-derived growth-factor (PDFG) Eur. J. Clin. Invest. 1995;25:90–96. doi: 10.1111/j.1365-2362.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Hebert T.L. Wu X.Y. Yu G., et al. Culture effects of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) on cryopreserved human adipose-derived stromal/stem cell proliferation and adipogenesis. J. Tiss. Eng. Regen. Med. 2009;3:553–561. doi: 10.1002/term.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz E.M. Gordon P.L. Koo W.K.K., et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T. Sawada R. Fujiwara Y., et al. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta 2. Biochem. Biophys. Res. Commun. 2007;359:108–114. doi: 10.1016/j.bbrc.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Kakudo N. Shimotsuma A. Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2007;359:239–244. doi: 10.1016/j.bbrc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Kalyani A.J. Mujtaba T. Rao M.S. Expression of EGF receptor and FGF receptor isoforms during neuroepithelial stem cell differentiation. J. Neurobiol. 1999;38:207–224. [PubMed] [Google Scholar]
- Kang H.B. Kim J.S. Kwon H.J., et al. Basic fibroblast growth factor activates ERK and induces c-Fos in human embryonic stem cell line MizhES1. Stem Cells Dev. 2005;14:395–401. doi: 10.1089/scd.2005.14.395. [DOI] [PubMed] [Google Scholar]
- Kelly C.M. Tyers P. ter Borg M., et al. EGF and FGF-2 responsiveness of rat and mouse neural precursors derived from the embryonic CNS. Brain Res. Bull. 2005;68:83–94. doi: 10.1016/j.brainresbull.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Krampera M. Pasini A. Rigo A., et al. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- Ksiazek K. A Comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009;12:105–116. doi: 10.1089/rej.2009.0830. [DOI] [PubMed] [Google Scholar]
- Kuznetsov S.A. Mankani M.H. Robey P.G. Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation. 2000;70:1780–1787. doi: 10.1097/00007890-200012270-00018. [DOI] [PubMed] [Google Scholar]
- Lee O.K. Kuo T.K. Chen W.M., et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Lee S.Y. Lim J. Khang G., et al. Enhanced ex vivo expansion of human adipose tissue-derived mesenchymal stromal cells by fibroblast growth factor-2 and dexamethasone. Tissue Eng. Part A. 2009;15:2491–2499. doi: 10.1089/ten.tea.2008.0465. [DOI] [PubMed] [Google Scholar]
- Lindroos B. Suuronen R. Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- Livak K.J. Schmittgen T.D. La Russa V. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu L.L. Liu Y. Yang S.G., et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- Majka S.M. Barak Y. Klemm D.J. Concise review: Adipocyte origins: Weighing the possibilities. Stem Cells. 2011;29:1034–1040. doi: 10.1002/stem.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mydlo J.H. Kral J.G. Macchia R.J. Preliminary results comparing the recovery of basic fibroblast growth factor (FGF-2) in adipose tissue and benign and malignant renal tissue. J. Urol. 1998;159:2159–2163. doi: 10.1016/S0022-5347(01)63298-1. [DOI] [PubMed] [Google Scholar]
- Neubauer M. Fischbach C. Bauer-Kreisel P., et al. Basic fibroblast growth factor enhances PPAR gamma ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett. 2004;577:277–283. doi: 10.1016/j.febslet.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Nimura A. Muneta T. Koga H., et al. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: Comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501–410. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- Park Y. Kim J.H. Lee S.J., et al. Human feeder cells can support the undifferentiated growth of human and mouse embryonic stem cells using their own basic fibroblast growth factors. Stem Cells Dev. 2011;20:1901–1910. doi: 10.1089/scd.2010.0496. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F. Mackay A.M. Beck S.C., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quarto N. Longaker M.T. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng. 2006;12:1405–1418. doi: 10.1089/ten.2006.12.1405. [DOI] [PubMed] [Google Scholar]
- Quarto N. Wan D.C. Longaker M.T. Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs) Bone. 2008;42:1040–1052. doi: 10.1016/j.bone.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Reya T. Morrison S.J. Clarke M.F., et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Schmidt A. Ladage D. Schinkoethe T., et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- Shahdadfar A. Frønsdal K. Haug T., et al. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- Skaletz-Rorowski A. Eschert H. Leng J., et al. PKC delta-induced activation of MAPK pathway is required for bFGF-stimulated proliferation of coronary smooth muscle cells. Cardiovasc. Res. 2005;67:142–150. doi: 10.1016/j.cardiores.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou P.A. Perez S.A. Salagianni M., et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2005;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- Tapp H. Hanley E.N., Jr Patt J.C., et al. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. 2009;234:1–9. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]
- Tassi E. Al-Attar A. Aigner A., et al. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J. Biol. Chem. 2001;276:40247–40253. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]
- Thomson M. Liu S.J. Zou L.N., et al. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S. Shimazu A. Miyazaki K., et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Vallier L. Alexander M. Pedersen R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Vassaux G. Negrel R. Ailhaud G., et al. Proliferation and differentiation of rat adipose precursor cells in chemically-defined medium—differential action of anti-adipogenic agents. J. Cell. Physiol. 1994;161:249–256. doi: 10.1002/jcp.1041610209. [DOI] [PubMed] [Google Scholar]
- Vogel G. Stem cell research. Studies cast doubt on plasticity of adult cells. Science. 2002;295:1989–1991. doi: 10.1126/science.295.5562.1989. [DOI] [PubMed] [Google Scholar]
- Wagers A.J. Weissman I.L. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wang Y. Xu C. Wang H., et al. Efficient derivation of human embryonic stem cell lines from discarded embryos through increases in the concentration of basic fibroblast growth factor. Human Cell. 2012a;25:16–23. doi: 10.1007/s13577-011-0039-7. [DOI] [PubMed] [Google Scholar]
- Wang Z. Oron E. Nelson B., et al. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell. 2012b;10:440–454. doi: 10.1016/j.stem.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Weiss S. Reynolds B.A. Vescovi A.L., et al. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- Zuk P.A. The adipose-derived stem cell: Looking back and looking ahead. Mol. Biol. Cell. 2010;21:1783–1787. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk P.A. Zhu M. Mizuno H., et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]