Summary
Mammalian LGR4, 5 and 6 are seven-transmembrane receptors that are important for diverse physiological processes. These receptors are orthologous to DLGR2, a Drosophila receptor activated by the burs/pburs heterodimer important for morphogenesis. Although recent studies indicated that four R-spondin proteins are cognate ligands for LGR4, 5 and 6 receptors, several BMP antagonists in vertebrates have been postulated to be orthologous to burs and pburs. Using newly available genome sequences, we showed that norrin is a vertebrate ortholog for insect burs and pburs and stimulates Wnt signaling mediated by LGR4, but not by LGR5 and 6, in mammalian cells. Although norrin could only activate LGR4, binding studies suggested interactions between norrin and LGR4, 5 and 6. Norrin, the Norrie disease gene product, is also capable of activating Wnt signaling mediated by the Frizzled4 receptor and serves as a BMP antagonist. Mutagenesis studies indicated that different norrin mutations found in patients with Norrie disease can be categorized into subgroups according to defects for signaling through the three distinct binding proteins. Thus, norrin is a rare ligand capable of binding three receptors/binding proteins that are important for BMP and Wnt signaling pathways.
Key words: Norrin, LGR4, Wnt signaling, BMP antagonist, Ligand–receptor interaction
Introduction
LGRs (leucine-rich repeat-containing, G-protein-coupled receptors) are an evolutionarily conserved group of seven-transmembrane receptors consisting of three subgroups in mammals (Hsu et al., 2000). LGRs in subgroup A interact with glycoprotein hormones, whereas those in subgroup C are receptors for relaxin and INSL3 (Hsu et al., 2002). As for the subgroup B, LGR5 has a well-established role of as a marker for adult stem cells (Barker and Clevers, 2010), and LGR4 is expressed in proliferating cells of diverse tissues, including adult stem cells and progenitor cells. LGR4-null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality (Mazerbourg et al., 2004). The ortholog to mammalian LGR4, 5 and 6 in Drosophila, DLGR2, is important for cuticle hardening and morphogenesis (Luo et al., 2005).
Proteins with a cystine knot motif are usually secreted by cells and play important roles in extracellular signaling in multicellular metazoans (Avsian-Kretchmer and Hsueh, 2004). DLGR2 interacts with the heterodimeric bursicon, consisting of two cystine knot-containing ligands burs and pburs, leading to increases in cAMP production (Luo et al., 2005). In contrast, vertebrate LGR4, 5 and 6 were recently found to associate with Wnt receptors and mediate R-spondin signaling (Carmon et al., 2011; de Lau et al., 2011; Glinka et al., 2011). The four R-spondin proteins contain two cysteine-rich, furin-like repeats and a single thrombospondin domain (de Lau et al., 2012) distinct from burs and pburs proteins. Although vertebrate cystine knot-containing BMP antagonists have been proposed as orthologs for the fly burs and pburs (Luo et al., 2005), no studies have demonstrated the ability of BMP antagonists to interact with vertebrate LGR4/5/6 receptors.
In humans, norrin mutations were found in patients with Norrie disease, an X-linked disorder characterized by hypovascularization of the retina and a severe loss of visual function. Vascular defects, sensori-neural deafness and blindness were also found in norrin-null mice (Rehm et al., 2002). More than 70 norrin mutations have been identified in patients with Norrie disease, and several more norrin mutations have been found in familial exudative vitroretinopathy, Coates disease, and retinopathy of prematurity (Berger et al., 1992).
With the completion of more genome sequencing projects, we were able to trace the evolution of genes related to burs and pburs and found vertebrate norrin to be an ortholog of invertebrate burs and pburs genes. In contrast to the stimulation of G-protein coupling by the heterodimeric burs/pburs, norrin stimulates Wnt signaling mediated by LGR4, but not LGR5 and LGR6, in mammalian cells. Combined with the known roles of norrin as a ligand for Frizzled4 (Fzl4) (Xu et al., 2004) and as a BMP antagonist (Xu et al., 2012), our studies indicated that norrin interacts with three distinct receptors/binding proteins.
Results
Norrin is the mammalian ortholog for fly burs and pburs
Based on sequence homology, the cystine-knot-containing BMP antagonists gremlin and DAN in vertebrates have been found to be closely related to insect burs or pburs genes (Avsian-Kretchmer and Hsueh, 2004). We identified gremlin and DAN orthologs in insects (gremlin-Tc, DAN-Tc and DAN-Ap) and other invertebrates (gremlin-Nv, gremlin-like-Hm and gremlin-Hm; Fig. 1A, solid circles). After alignment of these proteins with vertebrate cystine-knot-containing proteins, it became evident that norrin is a burs/pburs ortholog in vertebrates, because of both high sequence similarity and the conserved 11 cysteine structure distinct from the 9 or 10 cysteine structure found in gremlin/DAN subfamilies (Fig. 1A; supplementary material Fig. S1). Based on the ability of fly burs/pburs heterodimers to activate the cAMP pathway mediated by fly DLGR2 (Luo et al., 2005), we investigated potential G protein signaling mediated by LGR4/5/6. As shown in supplementary material Fig. S2, norrin is not capable of stimulating different G proteins (Gs, Gi, Gq and G12) mediated by LGR4, 5 or 6 (as determined by CRE-, SRE-, NFAT- and SRF-RE reporter assays) (Cheng et al., 2010). Because LGR4, 5 and 6 are known to mediate Wnt signaling (de Lau et al., 2011), we further tested the ability of norrin to activate Wnt signaling mediated by these receptors. HEK293T cells were transfected with a luciferase reporter under the control of seven TCF (T cell factor)/LEF-1 (lymphoid enhancer factor 1) binding sites (Super TOPFLASH) and LGR4, together with those for norrin and related genes (gremlin, gremlin2 and DAN). As shown in Fig. 1B, overexpression of increasing amounts of norrin, but not gremlin, gremlin2 or DAN, stimulated Wnt signaling mediated by LGR4, as reflected by increases in TOP-luciferase reporter activity. We also showed the expression of gremlin, gremlin2 and DAN after transfection into HEK293T cells (supplementary material Fig. S3). On the basis of the ability of norrin to increase β-catenin levels mediated by Fzl4 in mouse L cells (Xu et al., 2004), we further demonstrated increases in β-catenin levels after transfection of L cells with plasmids for both norrin and LGR4 (supplementary material Fig. S4).
Fig. 1.

Norrin is a ligand for LGR4. (A) Phylogenetic relationship of norrin, burs, pburs, gremlin, and DAN. Genes with cystine-knot structures homologous to burs and pburs were categorized into three subfamilies by the ClustalW program (Thompson et al., 1994). The phylogenetic tree was built with Mega 4 (Tamura et al., 2007) using the Neighbor-Joining method and 1000 bootstrap replications. The numbers at interior branches refer to the bootstrap values (in percentages). Sk, Saccoglossus kowalevskii; Ce, Caenorhabditis elegans; Hm, Hydra magnipapillata; Dm, Drosophila melanogaster; Nv, Nematostella vectensis; Xt, Xenopus tropicalis (Silurana); Mm, Mus musculus; Hs, Homo sapiens; Ap, Acyrthosiphon pisum; Tc, Tribolium castaneum. (B) Norrin, but not gremlin or DAN, stimulated Wnt signaling in HEK293T cells expressing LGR4. HEK293T cells were transfected with TOPFLASH, LGR4, together with increasing amounts of plasmids encoding norrin, gremlin, gremlin2 or DAN before luciferase assays.
Norrin is a cognate ligand for LGR4
The specificity of norrin activation of LGR4 was further tested in cells transfected with increasing amounts of norrin, together with different receptors. As shown in Fig. 2A, cells expressing human (h) or mouse (m) LGR4, but not human LGR5 or LGR6, responded to norrin with increases in Wnt signaling. Because the leucine-rich repeat-containing ectodomains of LGR proteins are capable of binding ligands whereas the seven-transmembrane and C-terminal tails are important for signaling (Kudo et al., 1996), we constructed chimeric receptors by swapping different domains. Overexpression of the chimeric receptor LGR4/5, consisting of the ectodomain of LGR4 and transmembrane and C-tail of LGR5, resulted in Wnt signaling by norrin (Fig. 2A). In contrast, overexpression of chimeric LGR5/4, consisting of the LGR5 ectodomain and transmembrane and C-tail of LGR4 did not increase Wnt signaling. Consistent with earlier findings (Carmon et al., 2011; de Lau et al., 2011), comparable stimulation of Wnt signaling by Wnt3A and R-spondin2, was observed for all LGR receptors tested. These findings suggest that LGR4, 5 and 6 are all capable of signaling through the Wnt pathway; however, norrin can only activate LGR4.
Fig. 2.
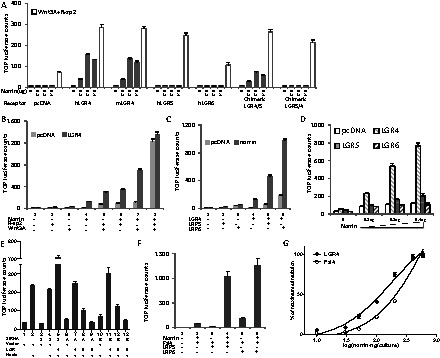
Norrin activates Wnt signaling mediated by LGR4 and the augmenting actions of Wnt3A and LRP proteins. (A) Norrin stimulation of Wnt signaling in HEK293T cells overexpressing LGR4 but not LGR5 and 6. HEK293T cells were transfected with plasmids encoding TOPFLASH and norrin together with human (h) or mouse (m) LGR4, hLGR5 or hLGR6 for 6 hours. After medium changes and culturing for 24 hours in serum-containing medium, cells were incubated in serum-free medium for 16 hours before luciferase assays. Some cells were transfected with plasmids encoding chimeric LGR4/5 or LGR5/4. To demonstrate receptor functionality, cells were treated with R-spondin2 and Wnt3A (open bars). (B) Co-treatment with Wnt3A or R-spondin2 amplified the stimulatory effects of norrin on LGR4-mediated Wnt signaling. HEK293T cells were transfected with TOPFLASH with or without LGR4 and/or norrin before luciferase analyses. Some cells were also treated with a maximal dose of Wnt3A (10 nM) and/or R-spondin2 (1 nM). (C) Norrin stimulation of LGR4-mediated Wnt signaling is augmented by LRP5 and LRP6. HEK293T cells were transfected with plasmids encoding TOPLFLASH with or without norrin, LGR4, LRP5 and/or LRP6, as described above, before luciferase assays. (D) Norrin, together with Wnt3A, stimulated Wnt signaling mediated by LGR4, but minimally by LGR5 and 6. HEK293T cells were transfected with increasing amounts of norrin plasmids together with TOPFLASH and LGR receptors for 6 hours. Following recovery in serum-containing medium for 24 hours, cells were treated with serum-free medium containing Wnt3A for 16 hours before luciferase assays. (E) Knockdown of endogenous LGR4 in HEK293T cells decreased basal norrin stimulation of Wnt signaling whereas overexpression of LGR4 enhanced norrin signaling. HEK293T cells were transfected with plasmids encoding TOPFLASH and LRP5 with or without norrin, two different siRNAs (A and B) against LGR4 or scrambled (S) siRNA as indicated. Some cells were also transfected with LGR4, 5, 6, or the empty vector. (F) Stimulation of Fzl4-mediated Wnt signaling by norrin: augmentation by LRP5 and 6. HEK293T cells were transfected with different plasmids for 6 hours. After culturing for another 24 hours, cells were incubated in serum-free medium for 16 hours before luciferase assay. (G) Potentiation of Wnt signaling by LGR4 and Fzl4 in response to increasing dosages of norrin plasmids. Although the normalized data are based on transfection of 50 ng plasmid/well, a range of receptor concentrations (10–250 ng/well) produced similar dose–response curves for both LGR4 and Fzl4.
Because LGR4 associates with Wnt receptors and mediates Wnt and R-spondin signaling augmented by the low-density-lipoprotein-related receptor LRP6 (Glinka et al., 2011; Carmon et al., 2011; de Lau et al., 2011), we further tested norrin signaling in the presence of Wnt3A, R-spondin2 and LRP5/6 co-receptors. As shown in Fig. 2B, co-treatment with Wnt3A augmented norrin stimulation of Wnt signaling mediated by LGR4 (lanes 3 and 4 versus lane 7). In contrast, co-treatment with R-spondin2 minimally potentiated norrin-stimulated Wnt signaling mediated by LGR4 (Fig. 2B, lane 4 versus 6). In contrast, transfection with LGR4 did not further augment norrin actions when cells were treated with maximal doses of Wnt3a and R-spondin2 (lane 8). We further co-transfected cells with LRP5 or LRP6 plasmids. As shown in Fig. 2C, co-expression of either LRP protein augmented norrin stimulation of Wnt signaling in LGR4-expressing cells, with LRP6 showing a stronger effect than LRP5 (lane 5 versus lane 6). Consistent with the ability of DKK1 to antagonize canonical Wnt signaling by competitively interfering with Wnt binding to LRP6 (Bafico et al., 2001), we found that co-treatment with DKK1 reduced Wnt signaling stimulated by norrin in cells overexpressing LGR4 (supplementary material Fig. S5). We further tested receptor specificity under optimal Wnt3A stimulation. As shown in Fig. 2D, increasing concentrations of norrin stimulated Wnt signaling mediated by LGR4. However, only minor increases were found after overexpression of high concentrations of LGR5 and 6.
Because norrin stimulated Wnt signaling in the presence of Wnt3A in HEK293T cells even without overexpression of LGR4, we tested the roles of endogenous LGR4 by using two different siRNAs, as reported earlier (de Lau et al., 2011). These LGR4 siRNAs block endogenous LGR4 expression by binding to the 3′-end of the endogenous LGR4 transcripts. They do not interfere with the transfected LGR4 plasmid containing only the open reading frame missing the 3′-end region. As shown in Fig. 2E, knockdown of LGR4 decreased basal Wnt signaling induced by norrin (lane 2 versus lanes 6 and 10). In contrast, co-transfection with the LGR4 plasmid restored norrin-induced Wnt signaling (lanes 7 and 11 versus lanes 6 and 10). However, transfection with plasmids for LGR5 or LGR6 showed minimal effects (lanes 6 versus lanes 8 and 9; lane 10 vs. lanes 12 and 13). Measurement of LGR4 transcripts in siRNA-treated HEK293T cells confirmed decreases in LGR4 expression (supplementary material Fig. S6).
Norrin activates Wnt signaling mediated by LGR4 and Fzl4
Norrin, a secreted protein of 133 residues, is a known ligand for Fzl4, also capable of activating the Wnt signaling pathway (Xu et al., 2004). As shown in Fig. 2F, norrin stimulation of Wnt signaling, mediated by Fzl4, is dependent on LRP5 or 6. We further compared norrin stimulation of LGR4 and Fzl4 under optimal conditions. HEK293T cells were transfected with Fzl4, norrin and LRP5 to evaluate Fzl4 signaling; Wnt3A was also included to evaluate optimal LGR4 signaling. Under these conditions, transfection with increasing amounts of the norrin plasmid indicated that LGR4 (EC50: 0.15 µg/culture) is 1.6-fold more responsive to norrin than Fzl4 (EC50: 0.24 µg/culture) in stimulating TOP-luciferase activities (Fig. 2G).
Alkaline phosphatase–norrin binds to LGR4, LGR5 and LGR6
Because secreted norrin forms disulfide-linked oligomers to associate with the extracellular matrix (Perez-Vilar and Hill, 1997), earlier studies used an alkaline phosphatase (AP)–norrin chimera to evaluate norrin binding to Fzl4 (Xu et al., 2004). The AP–norrin consists of the norrin molecule with alkaline phosphatase appended at its N-terminal end to facilitate secretion of the soluble protein and for easy detection. As shown in Fig. 3A, incubation with increasing levels of AP–norrin led to dose-dependent binding to HEK293T cells expressing LGR4. Although norrin does not activate cells expressing LGR5 and 6, dose-dependent binding of AP–norrin to cells overexpressing LGR5 and 6 was also evident (Fig. 3C). Scatchard plot analyses (Fig. 3B,D) indicated equilibrium binding constants (Kd; LGR4: 20 nM; LGR5: 21 nM; and LGR6: 19 nM) that were higher than the Kd value for Fzl4 (3.5 nM). These data suggested that LGR5 and 6 are capable of binding norrin but not mediating biological responses. Consistent with ligand-binding analyses, immunocytochemical staining studies also indicated the binding of AP–norrin to cells overexpressing Fzl4, LGR4, LGR5 and LGR6 (supplementary material Fig. S7).
Fig. 3.
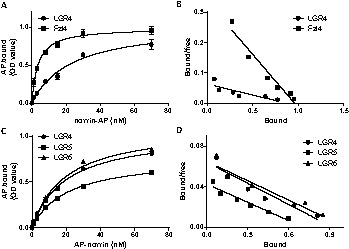
Direct binding of norrin to LGR4, 5 and 6. (A) Saturation curves for AP–norrin binding to LGR4 and Fzl4. After knocking down endogenous LGR4, HEK293T cells were transfected with empty vector or plasmids encoding human LGR4 or Fzl4 for 6 hours. After culturing for 2 days, cells were incubated with AP–norrin for 90 minutes at 23°C before determination of alkaline phosphatase activities. Specific binding was calculated by subtracting the values from cells transfected with the empty plasmids. (B) Scatchard analysis of AP–norrin binding to HEK293T cells expressing LGR4 or Fzl4. (C) Binding of AP–norrin to LGR4, 5 and 6 after knocking down endogenous LGR4. (D) Scatchard analysis of AP–norrin binding to HEK293T cells expressing LGR4, 5 or 6.
Different regions of norrin are important for interactions with LGR4, Fzl4 and BMP4
Studies in Xenopus indicated that norrin promotes formation of anterior neural tissue in embryos and is a potent antagonist of BMP- and Nodal/activin-induced functions mediated by direct binding to these ligands (Xu et al., 2012). We tested the BMP antagonistic properties of norrin in mammalian cells. COS-7 cells were transfected with plasmids encoding norrin and the BRE–luciferase reporter, before incubation with BMP2 or 4. As shown in supplementary material Fig. S8, norrin suppressed BRE–luciferase activity induced by BMP2 or 4 in a dose-dependent manner, demonstrating norrin as a BMP antagonist in mammals. Consistent with a lack of non-specific effects of the norrin plasmid, transfection of norrin plasmid did not alter BRE–luciferase activity (supplementary material Fig. S8, right part). Also, transfection with increasing doses of norrin or control plasmids did not alter cell viability (supplementary material Fig. S9).
More than 70 distinct Norrie disease point mutations have been identified in patients with the Norrie disease (Xu et al., 2004). From a knowledge of the crystal structure of follicle stimulating hormone-β, 6 of the 11 cysteine residues in the norrin protein form a cystine-knot structure (Fig. 4A). In addition, there are four β-sheets and one α-helix in norrin. We constructed 16 norrin point mutants (15 single mutants and 1 double mutant) that were based on those found in patients with Norrie disease (GenBank) and that occurred at key cysteine residues and specific domains (Fig. 4A). The expression of wild-type norrin and its mutants were examined in the extracellular matrix and conditioned media of transfected HEK293T cells (supplementary material Fig. S11). Although several mutants were present in higher levels in the conditioned media than in the matrix, all mutants were expressed at comparable levels as compared to wild-type norrin.
Fig. 4.
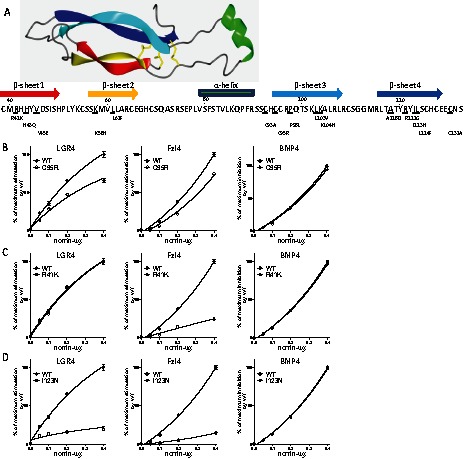
Structure-functional analyses of interactions between multi-functional norrin and LGR4, Fzl4 or BMP4. (A) Predicated three-dimensional structure of norrin with 4 β-sheets and 1 α-helix. The location of norrin mutants is also shown. (B) Different Norrie disease mutants located in key structural domains were generated, using mutagenesis kits, and transfected into HEK293T cells as described in the Materials and Methods. A representative cysteine mutant (C95R) in norrin causes loss of both LGR4 and Fzl4 signaling without affecting BMP4 interactions. (C) A representative mutant (R41K) in β-sheets 1 and 2 of norrin causes defective Fzl4 signaling without affecting LGR4 or BMP4 signaling. (D) A representative mutant (I123N) in β-sheets 3 and 4 of norrin causes defective signaling for both LGR4 and Fzl4 without affecting BMP4 interactions. All results were normalized as the percentage of maximal stimulation of TOPFlash reporter activities by wild-type norrin for LGR4 and Fzl4 receptors and as the percentage of maximal inhibition of BRE reporter activities by wild-type norrin for BMP4 antagonism. WT, wide type.
We checked their ability to activate LGR4 and Fzl4 as well as their antagonism of BMP4 actions (supplementary material Fig. S8). Of interest, all but two mutants showed no obvious loss of BMP antagonist function compared with wide-type norrin, with mutants L61F and R121G showing 40% and 36% loss of BMP antagonist activity, respectively (supplementary material Fig. S10, asterisks). These results suggest that the entire norrin protein is probably important for BMP binding; mutations of one or two residues cannot change the overall protein structure for binding to BMP4. Also, four cysteine mutants (C93A, C95R, C131A, 95R+C131A; Fig. 4B; supplementary material Fig. S10A) showed defective Wnt signaling mediated by either LGR4 or Fzl4, but the degrees of signaling defects varied, suggesting disulfide bonds are important for binding these two receptors. Furthermore, four out of the five mutations (Fig. 4C; supplementary material Fig. S10B) found in the β-sheets 1 and 2 (R41K, H43Q, V45E and L61F) caused major defects in Fzl4 signaling but had minimal impact on LGR4 signaling. In contrast, five of the seven mutations (Fig. 4D; supplementary material Fig. S10C) in β-sheets 3 and 4 (P98L, A118D, R121G, I123N and L124F) caused similar defects in signaling by both LGR4 and Fzl4.
Discussion
Wnt/Frizzled signaling is involved in a variety of developmental processes including cell fate determination, cell polarity, tissue patterning and control of cell proliferation (Wodarz and Nusse, 1998). The canonical Wnt signaling pathway culminates in the accumulation of β-catenin, a transcriptional activator for the TCF/LEF-1 family of DNA binding proteins. Norrin is a small, cystine-knot containing, secreted protein with sequence homology to mammalian BMP antagonists (Avsian-Kretchmer and Hsueh, 2004). In addition to activating LGR4, norrin is also the ligand for the Fzl4 receptor, activating Wnt signaling together with the co-receptor LRP5 and an auxiliary membrane protein, Tspan12 (Ye et al., 2010) (Fig. 5). Although our data indicated that norrin is more potent in activating Wnt signaling mediated by LGR4 than Fzl4 (Fig. 2G), the observed lower affinity of AP–norrin to LGR4 than Fzl4 (Xu et al., 2004) could be due to interference by the large N-terminal AP domain. The other possibility is that the affinity of norrin for LGR4 is not particularly high. Consistent with in vivo studies using Xenopus embryos (Xu et al., 2012), our data showed that norrin, like closely related gremlin (Khokha et al., 2003) and DAN (Sudo et al., 2004), is an antagonist to BMP2/4, capable of blocking the SMAD1/5/8 pathway. Thus, norrin is at the junction of two important intracellular signaling pathways (Fig. 5). Although mediated through different domains, norrin could bind to either LGR4 or Fzl4 to activate Wnt signaling. It could also antagonize the actions of BMP2 and 4 proteins, by preventing the activation of serine kinase receptors for these extracellular ligands. Although most polypeptide ligands interact with one receptor or, in some cases, several paralogous receptors with similar evolutionary origins (Ben-Shlomo and Hsueh, 2005), the present findings provide a unique example of a polypeptide ligand interacting with more than two phylogenetically unrelated receptors/binding proteins.
Fig. 5.
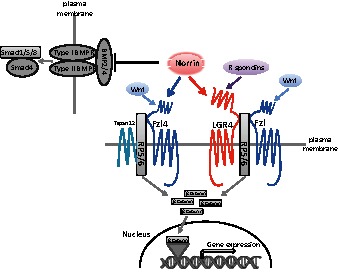
Diagram of norrin as a multifunctional ligand for three receptors/binding proteins. Norrin binds to LGR4, which is also the receptor for R-spondin proteins. LGR4 presumably cooperates with Frizzled (Fzl) receptors activated by Wnt ligands to promote the internalization of LRP5/6, leading to increases in β-catenin levels and the expression of downstream Wnt pathway genes. Norrin also interacts directly with Fzl4 and an auxiliary membrane protein Tspan12 to promote LRP5/6 internalization and to increase β-catenin levels. In addition, norrin binds to BMP2/4 to block the activation of type I and II BMP receptors, leading to decreases in downstream Smad activities. Mutations in humans associated with Norrie disease result in diverse phenotypes probably because of differential activation of Wnt signaling mediated by LGR4 or Fzl4 as well as differential BMP antagonistic activities.
In addition to the defects identified in studies using LGR4-null mice (Mazerbourg et al., 2004), a broad range of defects have been found in hypomorphic LGR4 mutant mice, including intestinal stem cell maintenance and Paneth cell differentiation (Mustata et al., 2011), bone formation and remodeling (Luo et al., 2009), kidney organogenesis (Kato et al., 2006), gall bladder and cystic duct development (Yamashita et al., 2009), hair placode formation (Mohri et al., 2008), keratinocyte proliferation (Wang et al., 2012; Kato et al., 2007), definite erythropoiesis (Song et al., 2008), male reproductive tract development (Mendive et al., 2006) and mammary gland morphogenesis (Oyama et al., 2011). Similar to phenotypes found in norrin-null mice (Rehm et al., 2002), LGR4-null mice also showed reduced keratinocyte motility in the eye (Kato et al., 2007) and ocular anterior segment dysgenesis (Weng et al., 2008). Furthermore, conditional deletion of both LGR4 and 5 genes in the mouse gut results in the rapid demise of intestinal crypts (de Lau et al., 2011). When cultured ex vivo, LGR4-deficient crypts or progenitors, but not LGR5-deficient progenitors, die rapidly with marked downregulation of Wnt target genes (Mustata et al., 2011). In addition, LGR4 expression is increased in human colon cancers, with higher levels associated with lymph node metastasis (Gao et al., 2006b). The present identification of norrin as a specific ligand for LGR4 opens new avenues to study LGR4 and norrin physiology.
The evolutionary relationship of subgroup B LGR receptors and their ligands is becoming clearer. Norrin in vertebrates and burs and pburs in invertebrates (insects and members of the Radiata) all contain 11 cysteine residues (supplementary material Fig. S1). In contrast, gremlin and DAN from vertebrates and invertebrates have either 9 or 10 cysteine residues (missing C95 and C131, or missing C95 only). Our data also showed that C95, important for multimerization (Perez-Vilar and Hill, 1997) is important for Wnt signaling by norrin through either LGR4 or Fzl4, consistent with earlier findings suggesting norrin multimers are likely to be the functional ligand (Perez-Vilar and Hill, 1997). Heterodimeric bursicon activates the Gs protein in insects (Luo et al., 2005). However, we could not detect G-protein coupling mediated by the norrin–LGR4 pair. Because elevated basal cAMP levels have been found for constitutively active LGR4 mutants (Gao et al., 2006a; Luo et al., 2009), future studies on potential G-protein signaling mediated by LGR4 are of interest. Unlike four R-spondin ligands capable of activating Wnt signaling mediated by LGR4, 5 and 6, norrin specifically activates LGR4 to stimulate Wnt signaling. Because the cephalochordate Ciona intestinalis and insects all have one LGR-4-like gene (supplementary material Fig. S12), LGR5 and 6 probably emerged during vertebrate evolution. Although norrin signals through LGR4 but not LGR5 and 6, AP–norrin is capable of binding to all three LGRs. Assuming the common ancestor of LGR4/5/6 is capable of binding both norrin and R-spondin, LGR5 and 6 could retain norrin binding ability but lose their ability to mediate norrin signaling. However, one cannot rule out the possibility that norrin signaling through LGR5/6 requires co-factors missing in the HEK293T cells.
Consistent with the widespread expression of LGR4, norrin is also expressed in many tissues other than the eye. Abnormalities associated with Norrie disease can be diverse, including mental retardation, deafness, seizures, behavioral problems, hypogonadism and delayed development; specific abnormalities and their severity depend on the type and location of the norrin mutation. Our mutational analysis indicated few mutations affected norrin interaction with BMP4 but the functional domains of norrin required for LGR4 and Fzl4 activation are overlapping and distinct, thus probably explaining the diverse phenotypes found in Norrie disease patients. The important role of β-sheets 1 and 2 of norrin for Fzl4 activation is consistent with earlier studies showing the role of the extended β-sheet of norrin facing away from the interface of norrin homodimers for Fzl4 recognition (Smallwood et al., 2007). It is likely that patients with mutations in β-sheets 3 and 4, which affect both LGR4 and Fzl4 activation, could exhibit more severe phenotypes.
Materials and Methods
Materials
Full-length cDNAs for norrin, gremlin, gremlin2 and DAN were subcloned into the pBudCE 4.1 plasmid and verified by DNA sequencing. Chimeric LGR4/5 and LGR5/4 were constructed by exchanging the ectodomains of mouse LGR4 and human LGR5 based on overlapping PCR using identical sequences preceding the first transmembrane domain. Super TOPFLASH plasmid and pSV-β-galactosidase control vector were purchased from the Promega. Recombinant Wnt3A, R-spondin 2 and BMP2 and 4 were from R&D Systems.
Phylogenetic analysis
Protein sequences were aligned by ClustalW (Thompson et al., 1994) as implemented with MEGA 4 (Tamura et al., 2007) using the Neighbor-Joining method and 1000 bootstrap replications.
Luciferase assays
TOPFLASH–luciferase assays were performed by transfecting norrin plasmid as described previously with slight modifications (Xu et al., 2004). Briefly, HEK293T cells were placed into 24-well plates, and 24 hours later were transfected with empty vector or receptor plasmids (0.03 µg), pSV-β-Gal (0.01 µg), LRP5/6 (0.01 µg) and/or increasing amounts of plasmids encoding norrin, gremlin, gremlin-2 and DAN using Lipofectamine™ 2000 (Invitrogen). At 24 hours after transfection, cells were treated for another 16 hours in serum-free medium with or without Wnt3A or R-spondin2. Luciferase activities were determined using luciferase assay kits (Promega) and normalized using β-galactosidase activities. All experiments were performed at least three times in triplicate. For the BRE–luciferase assay, COS-7 cells were placed into 24-well plates and 24 hours later, transfected with 0.02 µg BRE–luciferase reporter plasmid and 0.01 µg pSV-β-Gal plasmid, together with empty vector or different doses of the norrin plasmid. Six hours after transfection, the medium was changed to fresh serum-containing medium and the cells were cultured for 24 hours. BMP4/2 (20 ng/ml) was then added to the serum-containing medium and the cells cultured for another 16 hours before luciferase assay.
Binding assays
The assay for the production of alkaline phosphatase (AP)–norrin proteins and affinity measurements were previously described (Xu et al., 2004). Briefly, the AP-norrin plasmid was transiently transfected into HEK293T cells and at 24 hours later, cells were cultured in serum-free medium for 2 days. The supernatant containing AP–norrin was concentrated and then diluted in PBS. The concentration of AP–norrin was quantified by AP activity assay (Genhunter). For the cell-based binding assay, endogenous LGR4 in HEK293T cells were first knocked down using siRNA-A as previously described (de Lau et al., 2011). Cells were then culture in 24-well plates and transiently transfected with plasmids (0.3 µg) for Fzl4, LGR4, LGR5, LGR6 or empty plasmids. At 48 hours after transfection, cells were grown to near confluence before incubation with AP–norrin for 90 minutes at room temperature. At the end of incubation, cells were washed with HBSS containing 0.5 mg/ml BSA and 20 mM Hepes (pH 7.0), and bound AP activities were determined by using AP assay reagents (GenHunter). Data were analyzed using Graphpad Prism 5.0 and Kd values were calculated.
Generation of norrin mutants
Different Norrin mutants were generated by introducing point mutations using the QuikChange II site directed mutagenesis kit (Agilent Technologies). Briefly, overlapping primers with the desired point mutations were used to amplify wild-type norrin. The parental plasmid was digested using the DpnI enzyme and the newly synthesized plasmid was used as a template for PCR amplification of norrin mutants before sub-cloning into the pBudCE 4.1 plasmid. To generate double mutants, the single mutants were used as templates to introduce the second point mutation. The clones with the desired point mutations were selected and verified by sequencing.
Modeling norrin structure
The three-dimensional norrin structure was modeled using the Phyre server (Kelley and Sternberg, 2009), based on the crystal structure of human follicle stimulating hormone-β (PDB id: 1xwd). The high resolution norrin structure image was created in CCP4MG (Potterton et al., 2004).
Quantitative RT-PCR
Two siRNAs directed at the 3′-untranslated region of human LGR4 were made by Thermo Scientific Dharmacon. Sense sequences: A, 5′-gaaaguaaacuguggucaauu-3′; B, 5′-gguaagaaacuccuaauuauu-3′; scrambled (S) Non-Targeting Pool from Dharmacon as a negative control. The total RNAs were isolated using a RNA extraction kit (Qiagen) and eluted with RNase-free, DEPC-treated water before treatment with DNase. After reverse transcription using the Sensiscript RT kit (Qiagen), quantitative PCR were performed in triplicate using iTaq SYBR Green Supermix kit (Bio-Rad). Expression levels for LGR4 were normalized to GAPDH levels. The q-PCR primers were: qPCRhLGR4-5′: CTTTGTTTGCCATTTCCTA; qPCRhLGR4-3′: CTAGTGAGTTTAATAGCACTAA.
Immunocytochemical staining
Transfected HEK293T cells were stained with conditioned medium containing AP–norrin as described before (Xu et al., 2004). Cells growing on coverslips (six-well plates) were transfected with pcDNA, LHR, LGR4, hLGR5, hLGR6 or Fzl4 (3 µg/well) for 48 hours. Transfected cells were then cultured in serum-free medium with 50 nM AP–norrin at room temperature for 1 hour. After binding, cells were washed three times with the binding buffer (0.5 mg/ml BSA in Hank’s balanced salt solution with 20 mM Hepes, pH 7.0) and then fixed for 30 seconds in ice-cold HEPES buffer (20 mM HEPES, 60% acetone, 3% formaldehyde, pH 7.0) before washing twice. Fixed cells were then incubated in 20 mM Hepes (pH 7.0), 150 mM NaCl at 65°C for 100 minutes, washed once in alkaline phosphate washing buffer (0.1 M Tris-HCl, pH 9.4) before staining at room temperature overnight with the BCIP/NBT alkaline phosphatase substrate.
Sequences of cystine-knot proteins and LGR-B receptors for phylogenetic analysis
The protein sequences were retrieved from the GenBank database. The GenBank accession numbers for cystine-knot proteins are: pburs-Sk, XP_002732831; gremlin-like-Sk, NP_001164702; gremlin-Ce, NP_001256342; burs-like-Hm, XP_002156629; gremlin-Hm, XP_002162057; gremlin-like-Hm, XP_002160711; pburs-Dm, XP_001961531; gremlin-Nv, ABF06564; norrin-Xt, NP_001154869; norrin-Mm, NP_035013; norrin-Hs, NP_000257; gremlin-Hs, NP_037504; gremlin2-Hs, AAH46632; DAN-Hs, CAM45836; gremlin2-Mm, NP_035955; gremlin-Mm, BAE22388; DAN-Mm, NP_032701; DAN-Xt, NP_001006826; gremlin-Xt, NP_001083746; burs-Dm, NP_650983; DAN-Sk, NP_001158417; gremlin-Sk, NP_001161561; DAN-Ap, BAH71755; pburs-Ap, XP_001946298; burs-Ap, XP_001946341; burs-Tc, NP_001107779; gremlin-Tc, XP_973724; DAN-Tc, XP_972176; pburs-Tc, NP_001107780; burs-Sp, NP_001103719; pburs-Sp, NP_001103717. Sequences of LGR-B receptors were also retrieved from the GenBank: NP_476702, DLGR2-Dm; XP_425441,LGR5-Ga; XP_003642780, LGR6-Gg; XP_426162, LGR4-Gg; XP_782167, DLGR2-Sp; NP_003658, LGR5-Hs; BAD92980, LGR4-Hs; NP_001017403, LGR6-Hs; NP_034325, LGR5-Mm; NP_001028581, LGR6-Mm; NP_766259, LGR4-Mm; XP_002123281, LGR4-Ct; XP_002169896, DLGR2-Hm; and XP_001635321, DLGR2-Nv. Dm, Drosophila melanogaster; Ga, Gallus gallus; Sp, Strongylocentrotus purpuratus; Hs, Homo sapiens; Mm, Mus musculus; Ct, Ciona intestinalis; Hm, Hydra magnipapillata; Nv, Nematostella vectensis; Xt, Xenopus tropicalis: Sk, Saccoglossus kowalevskii; Ap, Acyrthosiphon pisum; Tc, Tribolium castaneum.
Analysis of secreted gremlin, gremlin 2 and DAN
HEK293T cells were transfected with gremlin, gremlin or DAN plasmid or the empty vector. One day later, cells were cultured in serum-free medium for another 2 days. The concentrated medium Centricon was used for immunoblotting analysis with v5 or myc antibody (Cell Signaling), respectively.
β-Catenin stabilization assay
Mouse L-cells grown in 10% serum in a six-well plate were transfected with empty vector or plasmids encoding norrin and/or LGR4 using Lipofectamine 2000 (Invitrogen). Thirty hours after transfection, cells were incubated in serum-free medium for 8 hours and then lysed in 500 µl lysis buffer [150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1 mM EGTA, 1× protease inhibitor cocktail (EDTA-free; Roche), 1% NP-40, 0.1% N-dodecyl-β-D-maltoside (Calbiochem), 3 mM MgCl2 and 30 U/ml DNaseI] on ice for 1 hour. After centrifugation to remove pellets, 400 µl of the supernatant were incubated for 1 hour at 4°C with Concanavalin-A beads to adsorb junctional β-catenin (Xu et al., 2004). Supernatants were cleared by centrifugation before immunoblotting using anti-β-catenin (Cell Signaling) and anti-GAPDH (Cell Signaling) antibodies.
MTT assay
HEK293T cells were transfected with different amounts of norrin or empty vector using Lipofectamine 2000 (Invitrogen). After 48 hours culture in 10% FBS, the number of viable cells was determined using the Promega Cell Titer 96 MTT assay method.
Expression analysis of norrin and its mutants in extracellular matrix and conditional medium
HEK293T cells cultured in six-well plates were transfected with norrin and its mutants using Lipofectamine 2000 (Invitrogen). One day later, cells were culture in serum-free medium for 3 days. Conditioned medium was then concentrated (∼30–50×) using Centricon before immunoblotting analysis. For the detection of norrin bound to the extracellular matrix (ECM), culture plates were washed once with 5 ml PBS to remove detached cells. After removing residual medium, 200 µl of buffer containing 6 M guanidine hydrochloride were added and culture plates put on ice. After 5 minutes, plates were scraped many times to collect cell-matrix-bound norrin and its mutants for immunoblotting analysis (Perez-Vilar and Hill, 1997).
Supplementary Material
Acknowledgments
We thank Dr Hans Clevers for human LGR4 and six plasmids; Dr QingYun Liu for plasmids encoding mouse LGR4 and human LGR5; and Dr Jeremy Nathans for AP–norrin, human Fzl4 and LRP5/6 plasmids.
Footnotes
Author contributions
C.D. and A.J.W.H. designed research; C.D., P.R., Y.C., C.-W.L., C.-L.H., performed research; C.D. and A.J.W.H. analyzed data; and C.D. and A.J.W.H. wrote the paper.
Funding
This work was supported by the National Institutes of Health [grant number R21DK081898 to A.H.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.123471/-/DC1
References
- Avsian-Kretchmer O., Hsueh A. J. (2004). Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol. Endocrinol. 18, 1–12 10.1210/me.2003-0227 [DOI] [PubMed] [Google Scholar]
- Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S. A. (2001). Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3, 683–686 10.1038/35083081 [DOI] [PubMed] [Google Scholar]
- Barker N., Clevers H. (2010). Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138, 1681–1696 10.1053/j.gastro.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo I., Hsueh A. J. (2005). Three’s company: two or more unrelated receptors pair with the same ligand. Mol. Endocrinol. 19, 1097–1109 10.1210/me.2004-0451 [DOI] [PubMed] [Google Scholar]
- Berger W., Meindl A., van de Pol T. J., Cremers F. P., Ropers H. H., Döerner C., Monaco A., Bergen A. A., Lebo R., Warburg M. et al. (1992). Isolation of a candidate gene for Norrie disease by positional cloning. Nat. Genet. 1, 199–203 10.1038/ng0692-199 [DOI] [PubMed] [Google Scholar]
- Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011). R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z., Garvin D., Paguio A., Stecha P., Wood K., Fan F. (2010). Luciferase Reporter Assay System for Deciphering GPCR Pathways. Curr. Chem. Genomics 4, 84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M. et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- de Lau W. B., Snel B., Clevers H. C. (2012). The R-spondin protein family. Genome Biol. 13, 242 10.1186/gb-2012-13-3-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Kitagawa K., Shimada M., Uchida C., Hattori T., Oda T., Kitagawa M. (2006a). Generation of a constitutively active mutant of human GPR48/LGR4, a G-protein-coupled receptor. Hokkaido Igaku Zasshi 81, 101–105, 107, 109 [PubMed] [Google Scholar]
- Gao Y., Kitagawa K., Hiramatsu Y., Kikuchi H., Isobe T., Shimada M., Uchida C., Hattori T., Oda T., Nakayama K. et al. (2006b). Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res. 66, 11623–11631 10.1158/0008-5472.CAN-06-2629 [DOI] [PubMed] [Google Scholar]
- Glinka A., Dolde C., Kirsch N., Huang Y. L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C. M., Niehrs C. (2011). LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 10.1038/embor.2011.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. Y., Kudo M., Chen T., Nakabayashi K., Bhalla A., van der Spek P. J., van Duin M., Hsueh A. J. (2000). The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol. Endocrinol. 14, 1257–1271 10.1210/me.14.8.1257 [DOI] [PubMed] [Google Scholar]
- Hsu S. Y., Nakabayashi K., Nishi S., Kumagai J., Kudo M., Sherwood O. D., Hsueh A. J. (2002). Activation of orphan receptors by the hormone relaxin. Science 295, 671–674 10.1126/science.1065654 [DOI] [PubMed] [Google Scholar]
- Kato S., Matsubara M., Matsuo T., Mohri Y., Kazama I., Hatano R., Umezawa A., Nishimori K. (2006). Leucine-rich repeat-containing G protein-coupled receptor-4 (LGR4, Gpr48) is essential for renal development in mice. Nephron Exp. Nephrol. 104, e63–e75 10.1159/000093999 [DOI] [PubMed] [Google Scholar]
- Kato S., Mohri Y., Matsuo T., Ogawa E., Umezawa A., Okuyama R., Nishimori K. (2007). Eye-open at birth phenotype with reduced keratinocyte motility in LGR4 null mice. FEBS Lett. 581, 4685–4690 10.1016/j.febslet.2007.08.064 [DOI] [PubMed] [Google Scholar]
- Kelley L. A., Sternberg M. J. (2009). Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 10.1038/nprot.2009.2 [DOI] [PubMed] [Google Scholar]
- Khokha M. K., Hsu D., Brunet L. J., Dionne M. S., Harland R. M. (2003). Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat. Genet. 34, 303–307 10.1038/ng1178 [DOI] [PubMed] [Google Scholar]
- Kudo M., Osuga Y., Kobilka B. K., Hsueh A. J. (1996). Transmembrane regions V and VI of the human luteinizing hormone receptor are required for constitutive activation by a mutation in the third intracellular loop. J. Biol. Chem. 271, 22470–22478 10.1074/jbc.271.37.22470 [DOI] [PubMed] [Google Scholar]
- Luo C. W., Dewey E. M., Sudo S., Ewer J., Hsu S. Y., Honegger H. W., Hsueh A. J. (2005). Bursicon, the insect cuticle-hardening hormone, is a heterodimeric cystine knot protein that activates G protein-coupled receptor LGR2. Proc. Natl. Acad. Sci. USA 102, 2820–2825 10.1073/pnas.0409916102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zhou W., Zhou X., Li D., Weng J., Yi Z., Cho S. G., Li C., Yi T., Wu X. et al. (2009). Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136, 2747–2756 10.1242/dev.033571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerbourg S., Bouley D. M., Sudo S., Klein C. A., Zhang J. V., Kawamura K., Goodrich L. V., Rayburn H., Tessier-Lavigne M., Hsueh A. J. (2004). Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol. Endocrinol. 18, 2241–2254 10.1210/me.2004-0133 [DOI] [PubMed] [Google Scholar]
- Mendive F., Laurent P., Van Schoore G., Skarnes W., Pochet R., Vassart G. (2006). Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev. Biol. 290, 421–434 10.1016/j.ydbio.2005.11.043 [DOI] [PubMed] [Google Scholar]
- Mohri Y., Kato S., Umezawa A., Okuyama R., Nishimori K. (2008). Impaired hair placode formation with reduced expression of hair follicle-related genes in mice lacking Lgr4. Dev. Dyn. 237, 2235–2242 10.1002/dvdy.21639 [DOI] [PubMed] [Google Scholar]
- Mustata R. C., Van Loy T., Lefort A., Libert F., Strollo S., Vassart G., Garcia M. I. (2011). Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 12, 558–564 10.1038/embor.2011.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama K., Mohri Y., Sone M., Nawa A., Nishimori K. (2011). Conditional knockout of Lgr4 leads to impaired ductal elongation and branching morphogenesis in mouse mammary glands. Sex Dev. 5, 205–212 10.1159/000329476 [DOI] [PubMed] [Google Scholar]
- Perez-Vilar J., Hill R. L. (1997). Norrie disease protein (norrin) forms disulfide-linked oligomers associated with the extracellular matrix. J. Biol. Chem. 272, 33410–33415 10.1074/jbc.272.52.33410 [DOI] [PubMed] [Google Scholar]
- Potterton L., McNicholas S., Krissinel E., Gruber J., Cowtan K., Emsley P., Murshudov G. N., Cohen S., Perrakis A., Noble M. (2004). Developments in the CCP4 molecular-graphics project. Acta Crystallogr. D Biol. Crystallogr. 60, 2288–2294 10.1107/S0907444904023716 [DOI] [PubMed] [Google Scholar]
- Rehm H. L., Zhang D. S., Brown M. C., Burgess B., Halpin C., Berger W., Morton C. C., Corey D. P., Chen Z. Y. (2002). Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J. Neurosci. 22, 4286–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood P. M., Williams J., Xu Q., Leahy D. J., Nathans J. (2007). Mutational analysis of Norrin-Frizzled4 recognition. J. Biol. Chem. 282, 4057–4068 10.1074/jbc.M609618200 [DOI] [PubMed] [Google Scholar]
- Song H., Luo J., Luo W., Weng J., Wang Z., Li B., Li D., Liu M. (2008). Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. J. Biol. Chem. 283, 36687–36697 10.1074/jbc.M800721200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S., Avsian-Kretchmer O., Wang L. S., Hsueh A. J. (2004). Protein related to DAN and cerberus is a bone morphogenetic protein antagonist that participates in ovarian paracrine regulation. J. Biol. Chem. 279, 23134–23141 10.1074/jbc.M402376200 [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Jin C., Li H., Li C., Hou Q., Liu M., Dong Xda. E., Tu L. (2012). GPR48-Induced keratinocyte proliferation occurs through HB-EGF mediated EGFR transactivation. FEBS Lett. 584, 4057–4062 10.1016/j.febslet.2010.08.028 [DOI] [PubMed] [Google Scholar]
- Weng J., Luo J., Cheng X., Jin C., Zhou X., Qu J., Tu L., Ai D., Li D., Wang J. et al. (2008). Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc. Natl. Acad. Sci. USA 105, 6081–6086 10.1073/pnas.0708257105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Nusse R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 10.1146/annurev.cellbio.14.1.59 [DOI] [PubMed] [Google Scholar]
- Xu Q., Wang Y., Dabdoub A., Smallwood P. M., Williams J., Woods C., Kelley M. W., Jiang L., Tasman W., Zhang K. et al. (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 10.1016/S0092-8674(04)00216-8 [DOI] [PubMed] [Google Scholar]
- Xu S., Cheng F., Liang J., Wu W., Zhang J. (2012). Maternal xNorrin, a canonical Wnt signaling agonist and TGF-β antagonist, controls early neuroectoderm specification in Xenopus. PLoS Biol. 10, e1001286 10.1371/journal.pbio.1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita R., Takegawa Y., Sakumoto M., Nakahara M., Kawazu H., Hoshii T., Araki K., Yokouchi Y., Yamamura K. (2009). Defective development of the gall bladder and cystic duct in Lgr4- hypomorphic mice. Dev. Dyn. 238, 993–1000 10.1002/dvdy.21900 [DOI] [PubMed] [Google Scholar]
- Ye X., Wang Y., Nathans J. (2010). The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 16, 417–425 10.1016/j.molmed.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


