Abstract
Human hematopoietic stem cells (hHSCs) cannot be maintained in vitro for extended time periods because they rapidly differentiate or die. To extend in vitro culture time, researchers have made attempts to use human mesenchymal stem cells (hMSCs) to create feeder layers that mimic the stem cell niche. We have conducted an array of experiments including adipocytes in these feeder layers that inhibit hHSC differentiation and by that prolong stem cell survival in vitro. The amount of CD34+ cells was quantified using flow cytometry. In a first experiment, feeder layers of undifferentiated hMSCs were compared with feeder layers differentiated toward osteoblasts or adipocytes using minimal medium, showing the highest survival rate where adipocytes were included. The same conclusion was drawn in a second experiment in comparing hMSCs with adipogenic feeder cells, using a culture medium supplemented with a cocktail of hHSC growth factors. In a third experiment, it was shown that direct cell–cell contact is necessary for the supportive effect of the feeder layers. In a fourth and fifth experiment the amount of adipocytes in the feeder layers were varied, and in all experiments a higher amount of adipocytes in the feeder layers showed a less rapid decay of CD34+ cells at later time points. We therefore concluded that adipocytes assist in suppressing hHSC differentiation and aid in prolonging their survival in vitro.
Key words: cell culture, cytokines, stem cells, tissue engineering
Introduction
Many bone marrow–related diseases have limited treatment options and generally require a transplant for a full cure. To foster progress in the field, in vitro tissue models of the bone marrow microenvironment are needed in order to test novel drugs. A major issue in developing these models is the difficulty in maintaining human hematopoietic stem cells (hHSCs) in vitro.1 This is largely due to the fact that the cues to maintain both self-renewal and differentiation of the hHSCs can only be partially controlled.2 Using a cocktail of growth factors, researchers have been able to stimulate rapid self-renewal divisions of hHSCs,3 which can be quantified by their expression of the surface protein CD34.4 Though this rapid expansion may have aided in increasing the percentage of hHSCs for transplants, it is not necessarily beneficial in long-term maintenance of the stem cells in vitro. Rapid expansion is accompanied by differentiation,5 ultimately resulting in depletion of the stem cell pool. A more natural stem cell proliferation was achieved by culturing the bone marrow cells on a layer of feeder cells of mesenchymal origin (human mesenchymal stem cells [hMSCs]).6–9 hMSCs are present in the bone marrow stem cell niche and help reconstitute the natural environment by providing the appropriate growth factors.10 Though the pure hMSC feeder layers have shown an improvement in initial proliferation, cultures are never sustained beyond 2–3 weeks, leading to the conclusion that they do not mimic a niche that maintains quiescence of hHSCs. A comparison of current methods reveals that they all attempt to mimic the highly complex red bone marrow, where hHSCs not only self-replicate, but also rapidly differentiate to perform hematopoiesis. If instead an environment were created that suppresses differentiation, one could prolong the survival of hHSCs in vitro.
For this we suggest including adipocytes in the feeder layers, thus mimicking yellow bone marrow (YBM). Anatomically, YBM is closely related to red bone marrow, with the former containing an abundance of adipocytes. In newborn mammals there is no YBM; however, the number of adipocytes increases with age in the marrow. In this respect a high abundance of adipocytes in the bone marrow is generally considered a negative property, since down-regulation of hematopoiesis results.11 However, there is a lot of debate regarding the functionality of YBM.12–14 Even in healthy humans at 30 years of age, most of the femoral cavity is already occupied by adipose tissue. This natural process of bone marrow adipogenesis can be reversed, however, through long-term physical training, likely due to the increased demand in blood cells.15 Similarly, during the process of a bone marrow transplant, adipocytes fill the bone marrow cavity after radiation. These adipocytes then gradually diminish, allowing the bone marrow cavity to slowly regain hematopoietic activity.16 Therefore, though the presence of adipocytes does suppress hematopoiesis, the stem cells in the YBM are more quiescent and have shown to provide a significantly higher multi-lineage engraftment, which leads to the conclusion that adipocytes help preserve the stem cell pool.11
With this in mind the adipocytes can be used as a “tool” to relieve the initial stress of differentiation on the hHSCs in vitro. Certain studies have even suggested that the presence of adipocytes in the niche is necessary to support hHSCs.17,18 Ultimately the goal is to verify the hypothesis that in vitro cultures of hHSCs in the presence of adipocytes allow for a prolonged hHSC survival.
Materials and Methods
Extracting hMSCs for feeder layers
For each experiment hMSCs were extracted from a new human bone marrow aspirate to create new feeder layers. Aspirates were obtained commercially (Lonza, Walkersville, MD) and shipped overnight for next day processing. Donors were male, under 25 years of age and disease-free. For hMSC extraction the aspirate was diluted with Dulbecco's modified Eagle's medium (DMEM) and plated on tissue-culture plastic (TCP). After 14 days the nonadherent cells were washed away and the adherent cells kept in medium for an additional 7 days to reach confluence. These cells were passaged using 0.25% trypsin-EDTA, seeded at 200,000 cells per well in a six-well plate and expanded for 7 days to reach confluence. In general, feeder layers were formed using twice-passaged (P2) hMSCs, unless otherwise noted. Inclusion of adipocytes or osteoblasts was achieved by subjecting the hMSC feeder layers to a differentiation medium, as will be detailed.
Feeder cells were not irradiated for several reasons. First, irradiation inhibits mitotic activity of the feeder cells, and the inability to proliferate eventually leads to apoptosis and depletion of cytokines released by the feeder layer cells. Therefore, experiments using irradiated feeder layers rarely go beyond 14 days.8,9 Second, it has been shown that only an untreated stroma provides discrete niches for long-term repopulating cells.19 Third, little has been documented about the effects of irradiation on adipocytes. Therefore, we wanted to minimize any alterations for the in vitro experiments.
Seeding bone marrow mononuclear cells on feeder layers
After preparation of the feeder layers, bone marrow mononuclear cells (BMMNCs) were seeded on top. For each experiment new BMMNCs were obtained from a freshly isolated human bone marrow aspirate. The entire BMMNC population was seeded, rather than isolated CD34+ fractions, to more closely mimic the marrow cavity and also because it had been shown previously that the optimal culture output was achieved using unmanipulated BMMNCs.19 BMMNCs were extracted over a Ficoll density gradient and each well received a plating of 1×106 BMMNCs. Half medium exchanges were performed biweekly, reducing the loss of nonadherent cells. Also, cultures were kept in a 5% oxygen environment to mimic the lower oxygen tension in native bone marrow and to further improve maintenance of a quiescent state.20,21
Flow cytometric quantification of CD34+ cells
At frequent intervals, all the cells were extracted from six wells of each condition using 0.25% trypsin-EDTA to extract all adherent cells. The cells were resuspended in a FACS buffer and stained with CD34–fluorescein isothiocyanate antibody (BD Biosciences, San Diego, CA) to label the hHSCs. After incubation, the cells were washed twice and finally resuspended in FACS buffer for flow cytometric analysis on a FACSCalibur (BD Biosciences, San Jose, CA).
Data analysis was performed using FlowJo analytical software (Tree Star, Inc., Ashland, OR). To allow for better comparison between the multiple experiments, the data were plotted as the percentage of seeded CD34+ cells remaining in each well at each time point, with 100% being the initial amount of CD34+ cells in the fresh bone marrow.
Effects of feeder cells alone
In an initial experiment the feeder layers were analyzed alone for CD34+ cells to determine any amount of hHSCs remaining in the feeder layers. This was especially important because the feeder layers were extracted from fresh bone marrow and with low passages may have trapped hHSCs.
For the first experiment, four different cultures were set up: besides a control of no feeder cells, BMMNCs were also seeded on undifferentiated hMSCs as well as hMSCs differentiated towards adipocytes or osteoblasts. MSC feeder layers were kept undifferentiated using a maintenance medium consisting of DMEM:F12 supplemented with 3% fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL fungizone). For adipogenic differentiation, this maintenance medium was additionally supplemented with 33 μM biotin, 17 μM D-pantothenic acid hemicalcium salt, 1 μM human insulin, 1 μM dexamethasone, 50 mM 3-isobutyl-1-methylxanthine, and 5 μM 2,4-thiazolidinedione. For osteogenic differentiation the maintenance medium was instead supplemented with 10 mM glycerol-2-phosphate disodium salt hydrate, 400 μM L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate, 10 nM 1α,25-dihydroxyvitamin D3, 10 nM dexamethasone, and 10% FBS instead of 3% FBS.
The feeder layers were kept for 3 weeks in maintenance or differentiation medium, respectively, and replaced with a hematopoietic medium after plating the BMMNCs. In this experiment the cultures were kept in a minimal hematopoietic medium consisting of Iscove's modified Dulbecco's medium (IMDM) supplemented with 2% FBS and antibiotics. The reasoning behind the minimal medium was to not mask the effects of cytokines released by the feeder cells with a cocktail of growth factors that are generally added to hHSC expansion media.
Combination of adipocytes and growth factors
In a second experiment the hMSC feeder layers were compared with just adipogenic feeder layers, including the cocktail of growth factors in the culture medium. The omission of the no-feeder and osteogenic feeder layer was based on two reasons. First, the amount of BMMNCs available in each bone marrow aspirate limited the amount of wells available to test different culture conditions. Second, MSC feeder layers are the current gold standard in in vitro hHSC culture, and therefore the hypothesis was compared against them. The hMSCs were prepared as described in the previous experiment, and after seeding BMMNCs of a new human bone marrow aspirate, each condition was cultured in either a minimal or hematopoietic expansion medium. The minimal medium consisted of IMDM supplemented with 15% FBS and antibiotics. The expansion medium was additionally supplemented with 55 μM β-mercaptoethanol, 10 ng/mL Flt-3, 10 ng/mL interleukin (IL)-6, 10 ng/mL stem cell factor, and 2 ng/mL IL-3.
Contact requirement of feeder cells
A transwell study was performed in a third experiment to determine if direct contact was necessary for the observed effects. MSC and adipogenic feeder layers were created as previously described. For direct co-cultures BMMNCs were seeded directly on to the feeder layers. For indirect co-cultures BMMNCs were seeded into a transwell-insert with a polyethylene terephthalate filter membrane of 0.4-μm pore size (Millipore, Billerica, MA) and kept in hematopoietic expansion medium.
Varying the amount of adipocytes
To further test the hypothesis, we varied the amount of adipocytes in the feeder layer to determine whether the survival rate would also vary. Since varying the quantities of supplements in the differentiation medium does not exactly control the amount of adipocytes that form in a layer of hMSCs, we had to create a different strategy. In a fourth experiment we first prepared either undifferentiated or adipogenic differentiated feeder layers as previously described. Cells of each condition were then extracted, pooled, and reseeded as either undifferentiated MSC feeder cells, adipogenically differentiated feeder cells, or a 1:1 mixture of both cell types (MSC/adipo), with the expectation that the mixed cultures would have half the amount of adipocytes as purely adipogenic cultures. After seeding BMMNCs, the cultures were kept in hematopoietic expansion medium.
Using unpassaged feeder cells
In a final experiment we made an attempt to more closely re-create a native stem cell niche in vitro by using unpassaged (P0) hMSCs. To form P0 feeder layers, 100 μL of fresh human bone marrow aspirate was plated per well in six-well plates, and hMSCs were expanded to confluence and differentiated directly in the wells as described previously. After seeding BMMNCs, the cultures were kept in hematopoietic expansion medium.
Statistical analysis
All data are expressed as means±SD, and a minimum of three replicates were performed for each assay. Using GraphPad Prism (GraphPad Software, San Diego, CA) a Student t-test was used to compare between two groups, and a two-way analysis of variance (ANOVA) was used for statistical analysis between multiple groups. Differences between experimental groups were considered to be statistically significant when the p value was<0.05.
Results
Effects of feeder cells alone
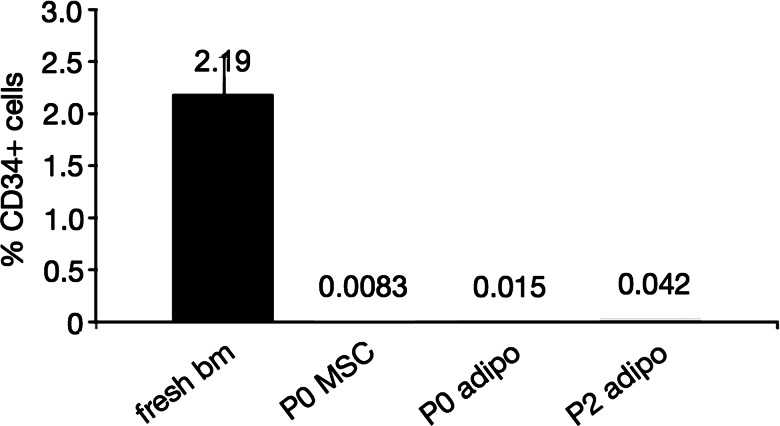
Flow cytometric analysis of the feeder layers alone showed a very low percentage of CD34+ cells compared with the initial percentage in fresh bone marrow of 2.19%±0.4% (Fig. 1). Additionally, the difference was very small between P0 and P2 cells as well as undifferentiated and differentiated feeder layers. Therefore the amount of CD34+ cells contributing from the feeder layers alone was minimal and could be neglected in the time-lapsed quantification.
FIG. 1.
Percentage of CD34+ cells in passage (P)0- and P2-derived feeder layers alone, compared with fresh bone marrow (bm). All values obtained by flow cytometric analysis of >200,000 events from a single sample, except for the fresh bone marrow (n=3, data displayed as mean±SD). MSC, mesenchymal stem cell; adipo, adipogenic.
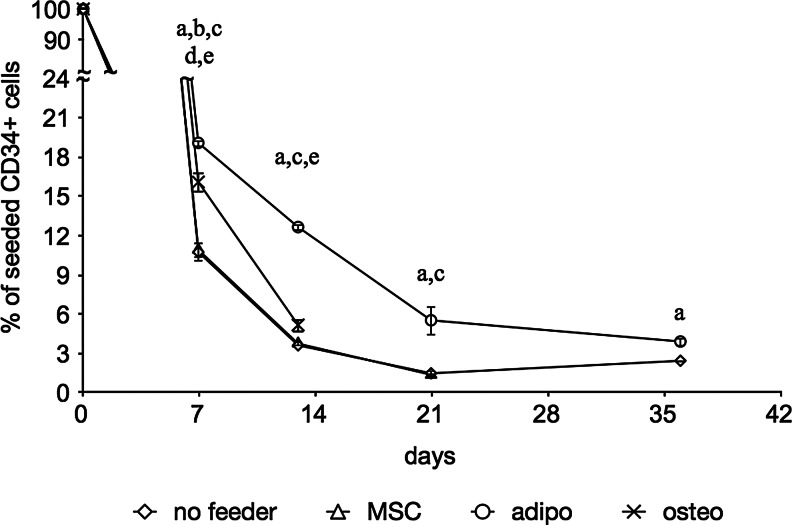
In cultures using a minimal medium, the percentage of CD34+ cells dropped rapidly to less than 20% after 7 days, regardless of the type of feeder layer (Fig. 2). This decrease continued gradually, though less rapidly over time. The MSC feeder layer had no significant difference over TCP, and the osteogenic feeder layer showed a slight improvement over both TCP and MSC feeder layers with statistical significance at day 7. Adipogenic feeder layers had a CD34+ cell survival of 19% after 7 days, down to 4% after 35 days. This was a significant improvement over MSC and no feeder layers throughout the experiment, with an averagely twofold higher CD34+ cell survival. Due to contamination, measurement of the osteogenic feeder layers was halted after 2 weeks.
FIG. 2.
Flow cytometric quantification of CD34+ cells in feeder cultures in minimal medium. MSC, adipogenic (adipo), and ostegenic (osteo) feeder layers are compared with tissue-culture plastic (TCP) alone. Data are given as means±SD (n ≥3), and statistical significance is denoted by a letter above the respective time point: ano feeder vs. adipo feeder; bno feeder vs. osteo feeder; cMSC vs. adipo feeder; dMSC vs. osteo feeder; eadipo vs. osteo feeder.
Combination of adipocytes and growth factors
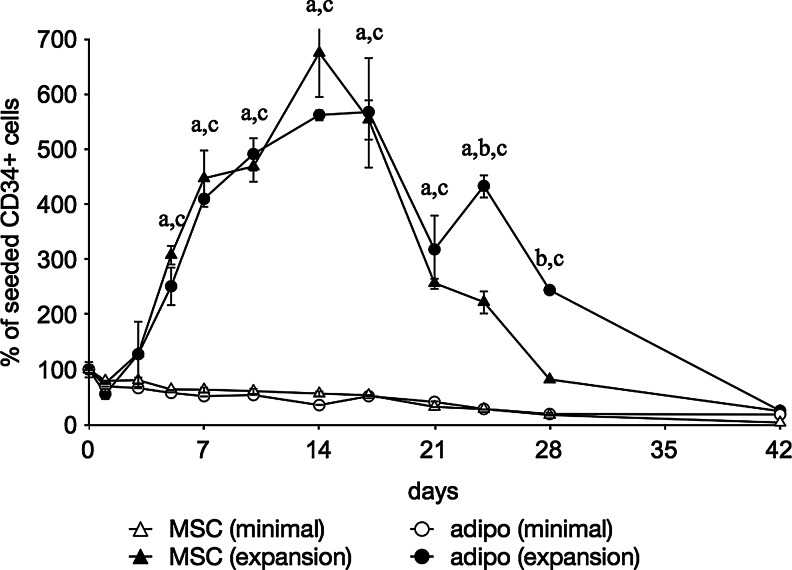
Cultures in minimal medium again showed a gradual decrease in CD34+ cell survival, however the addition of growth factors induced a highly significant (p<0.0001) increase in the CD34+ cell proliferation, reaching a maximum at 2 weeks (Fig. 3). After 3 weeks, the remaining CD34+ cell population was significantly higher in the presence of adipocytes than with undifferentiated hMSCs. Ultimately, the CD34+ population diminished almost completely after 42 days. It was observed that the edges of all feeder layer types started peeling off the wells after 3 weeks and started rolling up towards the center of the well, with some layers contracting to a ball around 5–6 weeks (Fig. 4).
FIG. 3.
Flow cytometric quantification of CD34+ cells in both MSC as well as adipogenic feeder cultures. Feeder cultures were additionally maintained in either minimal medium (minimal) or a medium supplemented with a cocktail of growth factors (expansion). Data are given as means±SD (n ≥3) and a statistically significant difference is denoted by a letter above the respective time point: aMSC (minimal) vs. MSC (expansion); bMSC (expansion) vs. adipo (expansion); cadipo (minimal) vs. adipo (expansion).
FIG. 4.
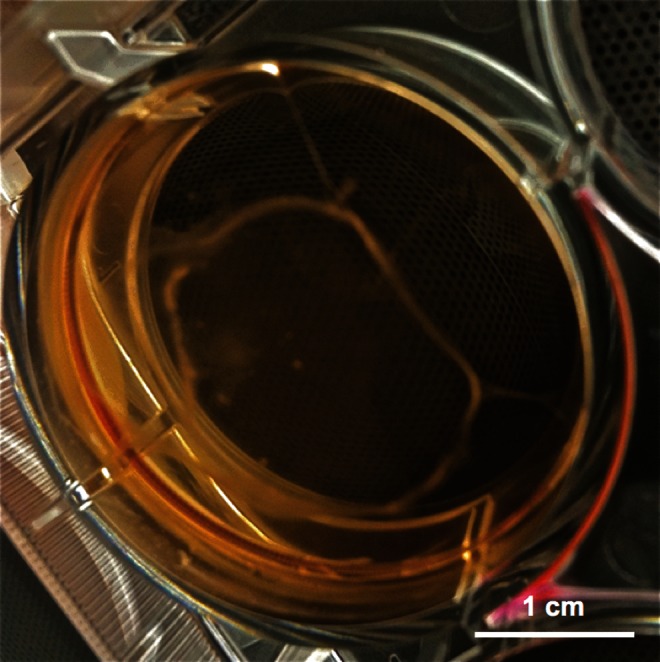
Photograph of a well with feeder cells contracting. The picture was taken 6 weeks post seeding of the bone marrow mononuclear cells and is representative for all feeder layer types. Scale bar is 1 cm.
Contact requirement of feeder cells
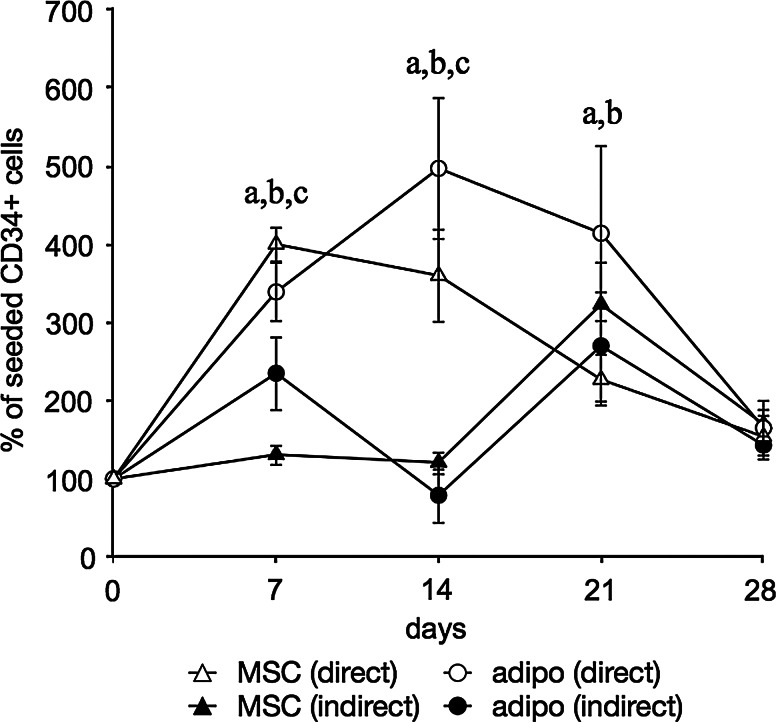
Direct co-cultures showed the rapid expansion of CD34+ cells that peaked at 2 weeks and declined again thereafter (Fig. 5). Here again the adipogenic feeder cells had a significantly higher amount of CD34+ cells at later time points. In the transwell cultures, the initial expansion was observed delayed at 3 weeks. As in the previous experiment, it was observed that at this time the feeder layers started peeling off the well and in this case started attaching to the bottom side of the transwell membrane.
FIG. 5.
Flow cytometric quantification of CD34+ cells in both MSC as well as adipogenic feeder cultures. Feeder cultures were additionally maintained in either direct co-culture with hHSCs or indirectly using transwells. Data are given as means±SD (n ≥3) and a statistically significant difference is denoted by a letter above the respective time point: aMSC (direct) vs. adipo (direct); bMSC (direct) vs. MSC (indirect); cadipo (direct) vs. adipo (indirect).
Varying the amount of adipocytes
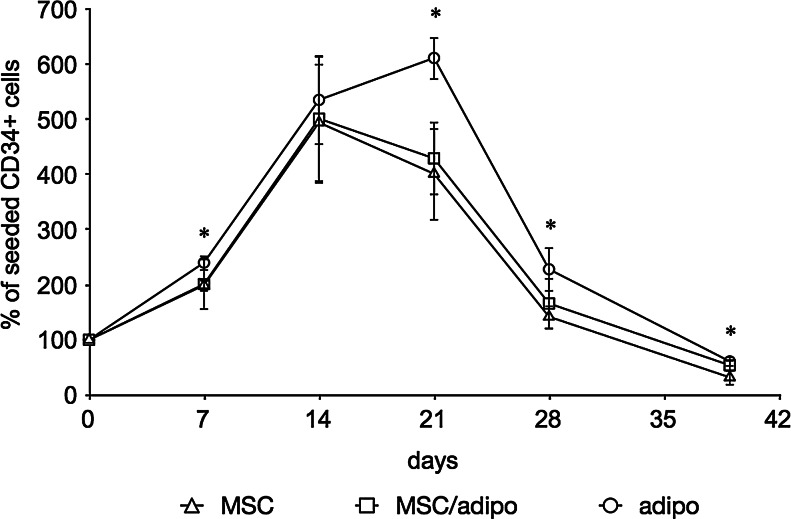
Cultures showed again rapid expansion of CD34+ cells that peaked at 2–3 weeks and declined thereafter (Fig. 6). Adipogenic feeder layers had a significantly higher amount of CD34+ cells that was at least 50% higher than undifferentiated feeder layers at all time points after 3 weeks. The mean values of the 1:1 mixture of undifferentiated and adipogenic differentiated cells appeared between the values of nonmixed feeder cultures; however, large standard deviations of all cultures yielded no statistical significance. This was confirmed by performing a two-way ANOVA test.
FIG. 6.
Flow cytometric quantification of CD34+ cells in feeder cultures with varying amounts of adipocytes. Feeder cells were first kept either undifferentiated or differentiated towards adipocytes then detached, pooled, and replated as either undifferentiated cells (MSC), adipogenic differentiated cells (adipo), or a 1:1 mixture of each cell type (MSC/adipo). Data are given as means±SD (n≥6) and a statistically significant difference between MSC and adipo feeder layer is denoted by an asterisk above the respective time point.
Using unpassaged feeder cells
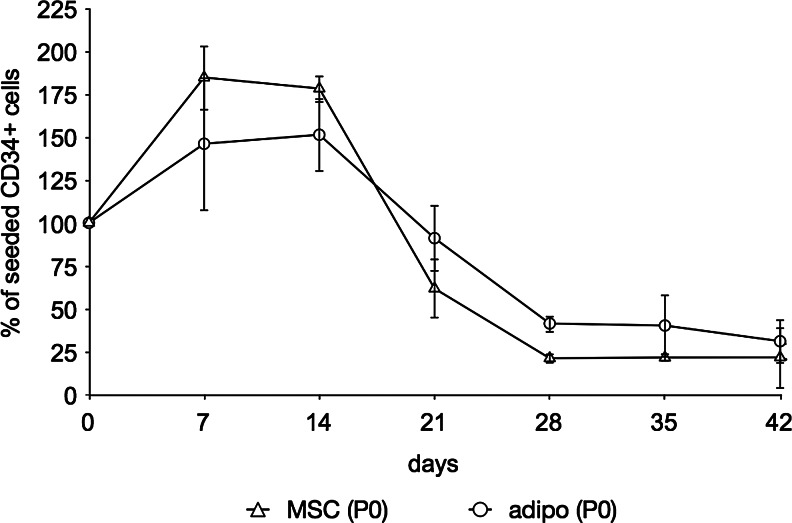
When compared with P2 feeder layers, cultures using P0 hMSCs showed a lower initial expansion in both undifferentiated as well as adipogenic differentiated feeder layers (Fig. 7). Though statistical significance diminished, the same trend was noted based on the mean values: The initial proliferation of the CD34+ cells was slightly higher on P0 hMSC than on P0 adipogenic feeder layers, but after 3 weeks until the end of the experiment the mean CD34+ cell population was again higher in the presence of adipocytes than with undifferentiated hMSCs. As in the previous experiments the feeder layers started peeling off the wells at 3 weeks.
FIG. 7.
Flow cytometric quantification of CD34+ cells cultured on P0 MSC feeder layers as well as adipogenic feeder layers derived from both P0 and P2 hMSCs. Data are given as means±SD (n ≥3). Differences between feeder layers were not statistically significant.
Discussion
The goal of this study was to assess the ability of adipocytes to inhibit hHSC differentiation and therefore prolong hHSC survival in long-term in vitro cultures. This is in stark contrast to the many experiments that are focused on rapid short-term expansion of hHSCs in vitro. Therefore, the rate of expansion was of less interest, and instead the duration of stem cell survival in vitro was the focus of the hypothesis.
The first evidence of this occurred in the experiment comparing no feeder, undifferentiated, osteogenic, and adipogenic feeder layers in a minimal medium. The adipogenic feeder layers showed a significantly higher CD34+ cell survival at all time points. Not adding any additional cytokines to the culture medium allowed an unmasked demonstration of the feeder cells alone; however, the lack of sufficient serum in the medium was likely detrimental to the proliferative capacity of the feeder cells. This is especially visible in that the hMSC feeder layers performed as poorly as the cultures without any feeder cells, which is in contrast to experiments showing improved hHSC proliferation with hMSC feeder layers.8,9 The lack of sufficient growth factors stimulating proliferation is likely also the reason behind the rapid decline in hHSC survival.
In the second experiment, the cultures using a minimal medium showed a similar trend, however, with a much less rapid decline. The biggest difference was the addition of 15% instead of 2% FBS in the culture medium. This led to the conclusion that a higher FBS content is necessary for long-term hHSC survival, supposedly due to an enhanced proliferative capacity of the feeder cells. More FBS, however, only reduced the rate of decay. For an increase of CD34+ cells, supplementation with a cocktail of growth factors was required. This result was expected because the composition of the expansion medium closely resembled commercially available hHSC expansion media and coincided with current cultures using these commercial media, with which maximum expansion was achieved at 2 weeks.
In the context of the goal of this project, the lack of statistical difference in initial proliferation on both the hMSC and adipogenic feeder layers indicates that the presence of adipocytes does not significantly inhibit hHSC expansion under the given culture conditions, but does reduce the rate of CD34+ cell decay, which is visible after 3 weeks. This decay in CD34 expression can result from either apoptosis or differentiation. This observation coincides with in vivo studies that show that adipocytes inhibit hematopoiesis but may also preserve a pool of stem cells.11
Interestingly, the indirect co-cultures did not show the initial expansive peak that had been previously observed. If one assumes that this effect is not influenced by the material of the culture membrane, one can safely state that direct co-culture is necessary for the observed effects. This coincides with observations made by Koller et al.19 who confirmed the presence of soluble signals released by feeder cells; however, the conditioned medium did not completely substitute for direct contact. The late increase in the indirect co-cultures at 3 weeks coincides with the observation that the feeder layers started detaching from the well and instead started attaching to the bottom side of the transwell. This likely converted the transwell cultures to direct co-cultures.
When varying the amounts of adipocytes in the feeder layer, we showed again the improved maintenance of CD34+ cells when using a feeder layer containing adipocytes. Although the mean values of the mixed cultures followed the trend by lying between the values of the MSC and adipo cultures, the large variances do not allow one to draw a confident conclusion that the amount of adipocytes correlate to the in vitro survival rate of the HSCs. The large variance likely has to do with a few observations. First, given that we started differentiation on P2 hMSCs, we did not have a very high percentage of adipocytes within the feeder layers to start with. Therefore, by cutting the amount of adipocytes in half, the percentage was even lower (approx. 5% adipocytes in mixed cultures). Second, obtaining a mixed population required an additional passaging step, using trypsin and centrifugation. This additional step lowered the total amount of cells available for reseeding, which was likely biased against adipocytes because these are more fragile than undifferentiated hMSCs. Third, although the two different cell conditions were mixed at a 1:1 ratio, there were still differences in adipogenic differentiation visible between cultures of the mixed populations.
Not considering the difference between feeder layer cells derived from different donors, the lower initial expansion when using P0 feeder cells leads to the assumption that the use of unpassaged hMSCs creates a more “natural” stem cell niche. According to the niche theory, hHSCs anchor to the niche cells and are provided with the appropriate signals to remain in a quiescent state.10,22 The assumption that the P0 feeder cells re-create the niche better than the P2 cells leads to the conclusion that the initial expansion of CD34+ cells, stimulated by the cytokines in the culture medium, is inhibited in P0 cultures by the presence of a quiescent stem cell niche. Comparing the undifferentiated hMSC and the adipogenic feeder layers derived from P0 cells showed no statistically significant difference between the two. However, the mean values of the adipogenic feeder layers showed a lower initial expansion up to 2 weeks and a less rapid decay of CD34+ cells after 3 weeks.
Therefore, in all cases, the CD34+ cell survival was prolonged by using feeder layers that included adipocytes. Given that this observation was present in all experiments and that the cells for each experiment were derived from different donors, it appears that this observation is not an artifact of a single donor. A possible explanation can be found in that adipocytes might help to preserve the pool of stem cells through reduced production of growth factors such as granulocyte–macrophage colony-stimulating factor and granulocyte colony-stimulating factor, but also by the secretion of tumor necrosis factor-alpha and adiponectin.23,24
Considering the current belief that the niche is likely more plastic than originally suggested and that osteoblasts, adipocytes, and sinusoidal endothelial and stromal cells interplay dynamically,25 we propose that future experiments should include co-cultures of osteoblasts, adipocytes, hMSCs, and possibly also endothelial cells to re-create a more complete stem cell niche. Though our observed effect was significant, prolonged in vitro survival gradually diminished in all cultures post 5 weeks. As noted in the results, the feeder layers cells started to detach after 3 weeks and contract, which likely led to this decay in survival. This decay is inevitable in proliferative two-dimensional cultures. Future plans should therefore include the use of a three-dimensional scaffold biomaterial that would not only better replicate the bone marrow niche but also avoid the peeling effects of the feeder layers.
Acknowledgments
The authors thank Nicholas Bayhi, Kasey Mitchell, Kristina Papa, and Emily Shaw (Tufts University) for assistance in cell culture, and Stephen Kwok and Allen Parmelee (Tufts Laser Cytometry, Medford, MA) for technical support on the flow cytometer. This work was supported by the NIH Tissue Engineering Resource Center (P41 EB002520).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Clark BR. Jamieson C. Keating A. Human long-term bone marrow culture. Methods Mol Biol. 1997;75:249–256. doi: 10.1385/0-89603-441-0:249. [DOI] [PubMed] [Google Scholar]
- 2.Seita J. Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640–653. doi: 10.1002/wsbm.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray LJ. Young JC. Osborne LJ, et al. Thrombopoietin, flt3, and kit ligands together suppress apoptosis of human mobilized CD34+ cells and recruit primitive CD34+ Thy-1+ cells into rapid division. Exp Hematol. 1999;27:1019–1028. doi: 10.1016/s0301-472x(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 4.Kondo M. Wagers AJ. Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 5.Boitano AE. Wang J. Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goncalves R. Lobato da Silva C. Cabral JM, et al. A Stro-1(+) human universal stromal feeder layer to expand/maintain human bone marrow hematopoietic stem/progenitor cells in a serum-free culture system. Exp Hematol. 2006;34:1353–1359. doi: 10.1016/j.exphem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y. Chai C. Jiang XS, et al. Co-culture of umbilical cord blood CD34+ cells with human mesenchymal stem cells. Tissue Eng. 2006;12:2161–2170. doi: 10.1089/ten.2006.12.2161. [DOI] [PubMed] [Google Scholar]
- 8.Jang YK. Jung DH. Jung MH, et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85:212–225. doi: 10.1007/s00277-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeister CC. Zhang J. Knight KL, et al. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39:11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A. Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 11.Naveiras O. Nardi V. Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–539. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen CJ. Ackert-Bicknell C. Rodriguez JP, et al. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimble JM. Zvonic S. Floyd ZE, et al. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 15.Gurevitch O. Slavin S. Feldman AG. Conversion of red bone marrow into yellow—cause and mechanisms. Med Hypotheses. 2007;69:531–536. doi: 10.1016/j.mehy.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 16.Casamassima F. Ruggiero C. Caramella D, et al. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood. 1989;73:1677–1681. [PubMed] [Google Scholar]
- 17.Gimble JM. The function of adipocytes in the bone marrow stroma. New Biol. 1990;2:304–312. [PubMed] [Google Scholar]
- 18.Gimble JM. Robinson CE. Wu X, et al. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- 19.Koller MR. Manchel I. Palsson BO. Importance of parenchymal:stromal cell ratio for the ex vivo reconstitution of human hematopoiesis. Stem Cells. 1997;15:305–313. doi: 10.1002/stem.150305. [DOI] [PubMed] [Google Scholar]
- 20.Koller MR. Bender JG. Papoutsakis ET, et al. Beneficial effects of reduced oxygen tension and perfusion in long-term hematopoietic cultures. Ann NY Acad Sci. 1992;665:105–116. doi: 10.1111/j.1749-6632.1992.tb42578.x. [DOI] [PubMed] [Google Scholar]
- 21.Yin T. Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L. Xie T. Stem cell niche: structure and function. Annu Rev Cell Develop Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. Harada A. Bluethmann H, et al. Tumor necrosis factor (TNF) is a physiologic regulator of hematopoietic progenitor cells: increase of early hematopoietic progenitor cells in TNF receptor p55-deficient mice in vivo and potent inhibition of progenitor cell proliferation by TNF alpha in vitro. Blood. 1995;86:2930–2937. [PubMed] [Google Scholar]
- 24.DiMascio L. Voermans C. Uqoezwa M, et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- 25.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]