Abstract
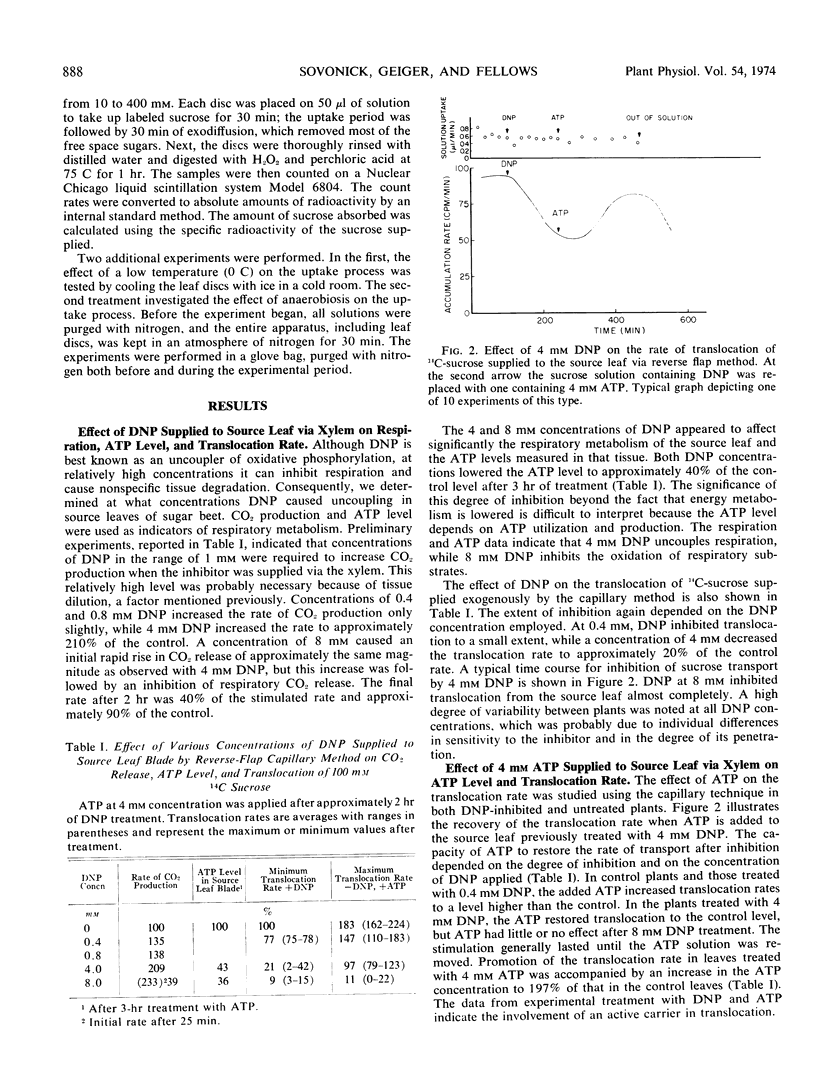
Phloem loading in source leaves of sugar beet (Beta vulgaris, L.) was studied to determine the extent of dependence on energy metabolism and the involvement of a carrier system. Dinitrophenol at a concentration of 4 mm uncoupled respiration, lowered source leaf ATP to approximately 40% of the level in the control leaf and inhibited translocation of exogenously supplied 14C-sucrose to approximately 20% of the control. Dinitrophenol at a concentration of 8 mm inhibited rather than promoted CO2 production, indicating a mechanism of inhibition other than uncoupling of respiration. The 8 mm dinitrophenol also reduced ATP to approximately 40% of the level in the control source leaf and reduced translocation of exogenous sucrose to approximately 10% of the control. Application of 4 mm ATP to an untreated source leaf promoted the translocation rate by approximately 80% over the control, while in leaves treated with 4 mm dinitrophenol, 4 mm ATP restored translocation to the control level. No recovery of translocation was observed when ATP was applied to leaves treated with 8 mm dinitrophenol. The results indicate an energy-requiring process for both phloem loading and translocation in the source leaf.
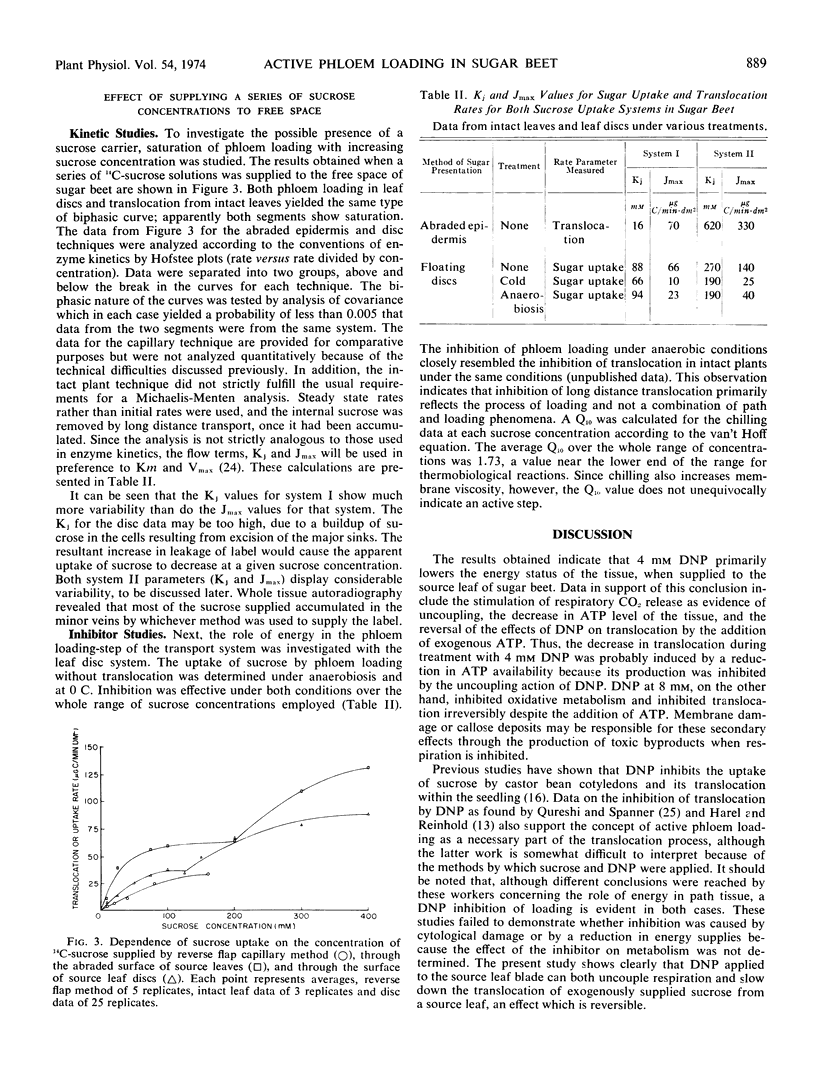
Application of 14C-sucrose solutions in a series of concentrations through the upper surface of a source leaf produced a biphasic isotherm for translocation out of the fed region. A similar dual isotherm was obtained for phloem loading with leaf discs floated on 14C-sucrose solutions. The first and possibly the second phases were attributed to active, carrier-mediated accumulation in the minor vein phloem. Autoradiography of the tissue confirmed that most of the sucrose was localized in the minor veins. Data from uptake through the abraded surface of intact leaves, the most reliable method, were analyzed by the Hofstee method. Kinetic parameters, analogous to Km and Vmax of enzyme studies, were calculated to be: Kj = 16 mm and Jmax = 70 μg C/min dm2 or 490 nmoles sucrose/min·dm2. Rates for phloem loading and translocation of exogenous sucrose are equal to or greater than those observed for compounds derived from photosynthetically fixed CO2. The data indicate that a free space sucrose concentration in the region of the minor vein phloem of approximately 20 mm can support translocation at the rates commonly observed for photosynthetically produced sugars.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cataldo D. A. Vein Loading: The Role of the Symplast in Intercellular Transport of Carbohydrate between the Mesophyll and Minor Veins of Tobacco Leaves. Plant Physiol. 1974 Jun;53(6):912–917. doi: 10.1104/pp.53.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Cataldo D. A. Leaf structure and translocation in sugar beet. Plant Physiol. 1969 Jan;44(1):45–54. doi: 10.1104/pp.44.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Christy A. L. Effect of sink region anoxia on translocation rate. Plant Physiol. 1971 Feb;47(2):172–174. doi: 10.1104/pp.47.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Giaquinta R. T., Sovonick S. A., Fellows R. J. Solute distribution in sugar beet leaves in relation to Phloem loading and translocation. Plant Physiol. 1973 Dec;52(6):585–589. doi: 10.1104/pp.52.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Evaluation of Selected Parameters in a Sugar Beet Translocation System. Plant Physiol. 1965 Sep;40(5):942–947. doi: 10.1104/pp.40.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Sucrose Translocation in the Sugar Beet. Plant Physiol. 1965 Jul;40(4):685–690. doi: 10.1104/pp.40.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriedemann P., Beevers H. Sugar uptake and translocation in the castor bean seedling I. Characteristics of transfer in intact and excised seedlings. Plant Physiol. 1967 Feb;42(2):161–173. doi: 10.1104/pp.42.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask J., Laties G. G. Multiphasic absorption of glucose and 3-o-methyl glucose by aged potato slices. Plant Physiol. 1973 Feb;51(2):289–294. doi: 10.1104/pp.51.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Geiger D. R. Effects of light intensity and oxygen on photosynthesis and translocation in sugar beet. Plant Physiol. 1974 Oct;54(4):575–578. doi: 10.1104/pp.54.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroya M. Comparison of upward and downward translocation of C from a single leaf of sunflower. Plant Physiol. 1968 Oct;43(10):1605–1610. doi: 10.1104/pp.43.10.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. M. Reduction of water permeability in potato tuber slices by cyanide, ammonia, 2,4-dinitrophenol, and oligomycin and its reverse by adenosine 5'-triphosphate and cytidine 5'-triphosphate. Plant Physiol. 1973 Mar;51(3):485–488. doi: 10.1104/pp.51.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]



