INTRODUCTION
Sexual dysfunction is common in patients taking antipsychotics- more than twice as common as in healthy controls 1;2, and greater in patients taking antipsychotics than unmedicated schizophrenic patients and patients with affective disorders 3-5. The CATIE study has recently highlighted that sexual function is commonly impaired in patients taking atypical as well as typical antipsychotic treatment 6. A number of factors may play a role in impairing sexual dysfunction in patients taking antipsychotic treatment, including ‘negative’ and co-morbid depressive symptoms, the anticholinergic and adrenergic effects of antipsychotics, and higher rates of smoking and physical illness2;7. It has long been supposed, however, that prolactin elevation and low gonadal hormone levels are significant factors underlying the high rates of sexual dysfunction seen in patients treated with antipsychotics8-10. Prolactin may impair sexual function through its actions on the hypothalamic-pituitary-gonadal axis altering sex hormone release. Hyperprolactinemia is associated with hypogonadism and sexual dysfunction11, although a preliminary naturalistic study of women taking antipsychotics found high but similar rates of reproductive dysfunction in normoprolactinemic and hyperprolactinemic groups 12. Low sex hormone levels have been reported in patients taking antipsychotics 13-16. We have previously reported high rates of sexual dysfunction and high rates of hypogonadism in patients taking antipsychotics 1, but no previous studies have investigated the association between the two. We hypothesised that low sex hormone levels are associated with sexual dysfunction in patients taking antipsychotics.
We therefore set out to substantiate the finding that rates of sexual dysfunction are higher in patients taking antipsychotics than healthy controls in a larger sample and to test the hypothesis that sexual dysfunction is associated with prolactin, and gonadal hormone levels in patients taking antipsychotic treatment for schizophrenia or schizo-affective disorder.
METHOD
Study design and setting
A cross-sectional design was used. The Institute of Psychiatry Research Ethics committee approved the study. After complete description of the study, subjects gave written informed consent.
Sample
Patients were recruited by direct approach of the investigators (OH, SS) from an inner-city community mental health team (catchment area n= 272 000). Consecutive outpatient attendees were invited to participate (sixteen declined, three were excluded and 103 participated, mean age: 46.2 (SD: 12.9), 51.5% male). Inclusion criteria were: aged 18-65 years, diagnosed with schizophrenia, or schizoaffective disorder according to DSM-IV criteria and stabilised on antipsychotic medication for greater than six months. Consecutive attendees at a general practice clinic (n=62, mean age: 36.1 (SD: 9.6), 55% male) and a general hospital sexual dysfunction clinic (n=57, mean age: 39.1 (SD: 10.7, 79% male)), both within the same catchment area served by the mental health team, were used as ‘healthy’ and ‘ill’ controls respectively for the assessment of sexual functioning, as previously described1. The controls did not receive the hormonal measures. Exclusion criteria for all groups were: medical/physiological/psychiatric cause of gonadal or sexual dysfunction (e.g.: depression, vascular disease related to smoking or other causes, renal failure, hypothyroidism, pregnancy or lactation, Cushing’s disease, diabetes mellitus, drug treatments (eg: antidepressants, exogenous hormones), and for the control groups any history of abnormal menses, or endocrine disorder. All groups gave smoking, substance and alcohol histories, and subjects were excluded if dependent on a substance other than nicotine (pathology related to smoking that might affect sexual dysfunction was an exclusion criterion). The sexual dysfunction clinic controls received a full assessment to exclude endocrine causes of sexual dysfunction.
Procedure
Demographic and clinical data, including menstrual function using the STRAW guidelines17, was obtained by a semi-structured assessment as previously described18. Antipsychotic doses were converted to chlorpromazine equivalents using established criteria19;20. Subjects completed the short Sexual Functioning Questionnaire (SFQ), a self-report structured instrument that has been previously validated in patients with psychotic disorders1. The SFQ includes subscales assessing libido, physical arousal, erectile function, orgasm and ejaculatory function. Higher scores indicate greater impairment, and a total SFQ score ≥ 8 is the cut-off indicating sexual dysfunction. A psychiatrist (OH) rated psychopathology and side-effects as these may influence sexual function 1 using the following measures, having been previously trained in their application:
Structured Clinical Interview for the Positive and Negative Syndrome Scale (PANSS) 21
Abnormal and Involuntary Movement Scale (AIMS)22
Simpson Angus Scale (SAS)23
Anticholinergic and adrenergic side-effects section of the UKU side-effect rating scale24
Barnes Akathisia scale25
Calgary Depression Scale26
Hormone measurement
Blood samples were taken between 11am and midday, 30 minutes after insertion of a cannula (to reduce transient stress effects). Subjects omitted all medication for at least twelve hours, or seven days in the case of depot medications, prior to assessment. Menstruating women were tested during the luteal phase of their cycle. Serum levels of estradiol, progesterone, follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, sex hormone binding globulin (SHBG), thyroid stimulating hormone (TSH), and testosterone were measured using chemiluminescent immunoassays (Bayer ADVIA Centaur, and Diagnostic Products Corporation, Los Angeles, CA). Thyroid stimulating hormone levels were measured as high levels of TSH may alter SHBG and sex hormone levels 27. Intra- and inter-assay variation was <12.5% for progesterone, TSH, sex hormone binding globulin and estradiol, and <7% for the other assays across all the concentration ranges studied. Free testosterone levels were derived using the method described by Nanjee and Wheeler 28.
STATISTICAL ANALYSIS
Males and females were analysed separately. All statistical tests were two-tailed.
Analysis of sexual function across groups
The analysis compared sexual function (measured using the SFQ) between the healthy controls, sexual dysfunction controls and patients. The SFQ total and sub-scale scores were compared across groups using an ANCOVA, adjusting for age, and menopausal status in women, as these variables are recognised determinants of sexual function29-31. Where the F-test showed an overall group difference, pair wise post-hoc comparisons were carried out to evaluate individual group differences. A conservative 1% significance level was applied because of the multiple tests, and this was made more stringent for the post-hoc tests by dividing the significance level by the number of post-hoc tests (Bonferroni correction).
Analysis of the relationship between sexual function and hormonal measures in patients
Hormonal abnormalities were defined in accordance with the hospital laboratory reference range (prolactin >480 mIU/L indicating hyperprolactinemia, total testosterone<9nmol/L in men indicating hypotestosteronism, and, in women, estradiol levels<365pmol/L indicating hypoestrogenism, and progesterone levels<30nmol/L indicating hypoprogesteronism), and are presented separately for post-menopausal and pre-menopausal women, using the STRAW criteria for determining menopausal status17. Linear regression was used to test the hypothesis that sexual function was related to hormonal factors, using SFQ total score as the dependent variable, and age, and hormonal levels as the independent variables. Side-effect and psychopathology levels, and menstrual status were entered as additional independent variables.
RESULTS
Demographic and clinical variables
The mean ages (SD) of the healthy controls, sexual dysfunction clinic controls, and patients were 36.1 (9.6), 39.1 (10.7), and 46.2 (12.9) years respectively. As sexual dysfunction is more common in older people, comparisons between groups were adjusted for age. Patients were taking the following antipsychotics: amisulpride (n=11), chlorpromazine (n=5), flupentixol (n=23), fluphenazine (n=5), haloperidol (n=9), pimozide (n=1), pipotiazine (n=2), risperidone (n=8), sulpiride (n=3), trifluoperazine (n=7), and zuclopenthixol (n=9), olanzapine (n=18), quetiapine (n=1), and ziprasidone (n=1). The mean dose of antipsychotic treatment expressed as chlorpromazine equivalents was 355mg (SD=321), and median treatment duration was 3.3 years (interquartile range=8.5). Patients were taking the following additional medication: anticholinergics (procyclidine (n=28), orphenadrine (n=8), benzhexol (n=1)), mood stabilisers (lithium (n=4), valproate (n=7), carbamazepine (n=4)), and hypnotics (diazepam (n=10), zopiclone (n=1)). In the patient group, the mean PANSS total and negative subscale and Calgary Depression Scale scores were 44.1 (11.9), 12.8 (5.4), and 2.6 (3.8) respectively, indicating good symptom control. Their mean Barnes, Angus Simpson, AIMS, and UKU scale (anticholinergic and adrenergic section) side-effect scores were 2.5 (3.8), 2,4 (5.0), 1.9 (3.8), and 5.6 (5) respectively, indicating low levels of these side-effects. Fifty one per cent (n=52) of the patients were smokers (median consumption= 15cigs/ day, interquartile range=10-30), forty-seven per cent (n=48) drank alcohol (median 6.0 units/ week, interquartile range= 2-13.5), and mean BMI was 29m2/kg (SD=9.8). In the women (n=50), the lifetime median number of pregnancies was 2 (interquartile range 0-4), total median time breastfeeding was 0 months (interquartile range 0-3), and none were currently pregnant or lactating. Twenty-one were post-menopausal, and twenty-nine were pre-menopausal, of whom six were oligomenorrheic. All of the controls were pre-menopausal and none reported menstrual disturbance.
Sexual Function
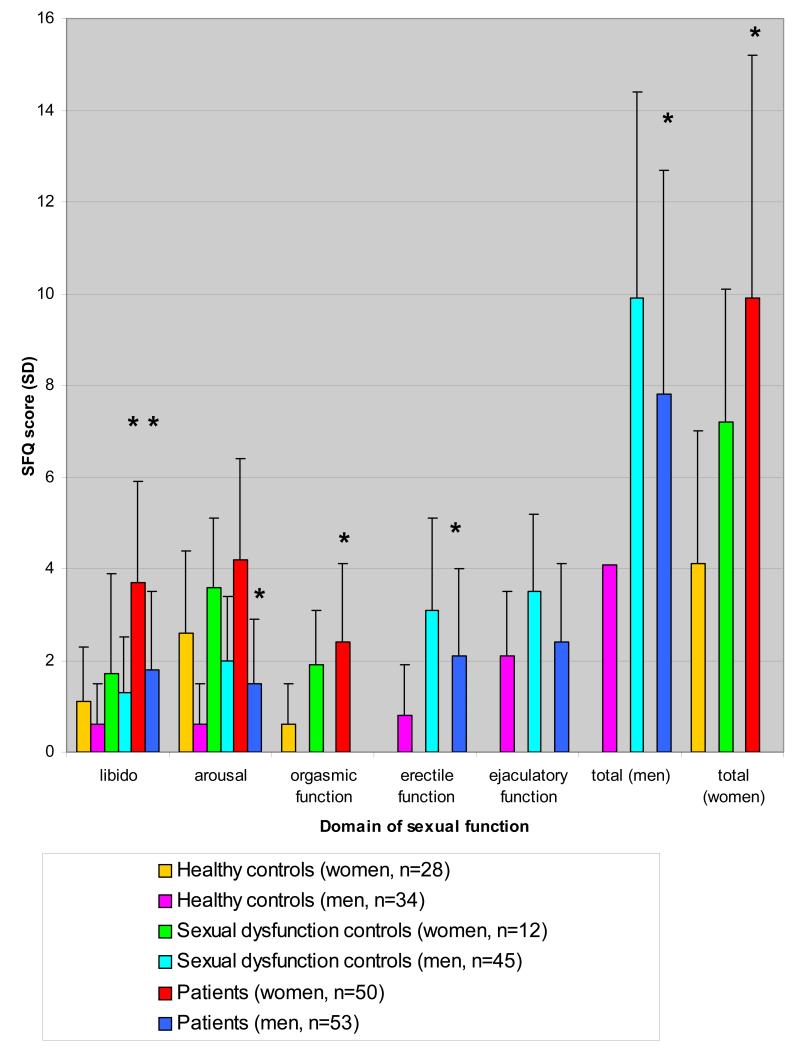
Mean SFQ scores were higher, indicating poorer sexual functioning, in the female and male patients compared with healthy and sexual dysfunction clinic controls (figure 1). Table 1 shows the group analysis, indicating that there was a significant effect of group on sexual function for men and women across all the domains of sexual function. Post-hoc comparisons in the men indicate that sexual function scores were significantly higher in the patient group compared with the healthy controls across all domains (mean difference (95% confidence interval) for libido= 1.2 (0.5 to 1.8) p<0.001; erectile function= 1.4 (0.6 to 2.1) p<0.001; arousal=1.02 (0.4 to 1.6) p=0.001; total score: 3.5 (1.5 to 5.4) p=0.001) except for ejaculatory function (0.3 (−0.4 to 1.03) p=0.5). In the women sexual function scores were higher across all domains (mean difference (95% confidence interval) for libido=2.6 (1.3 to 3.8), p<0.001; orgasmic function= 1.4 (0.4 to 2.4) p=0.006; and total score=5.8 (2.3 to 9.3) p=0.002) except arousal (0.6 (−0.3 to 1.5) p=0.2). At the corrected significance level, there was no significant difference between patients and sexual dysfunction clinic controls in the men (mean difference (95% confidence interval) in total score= −2.2 (4 to 0.3), p=0.02) or women (total score= 2.7 (−1.4 to 6.9), p=0.19).
Figure 1. Sexual function (mean SFQ (SD)) in patients taking antipsychotics, healthy controls, and sexual dysfunction controls.
Higher SFQ score indicates greater dysfunction. Compared to healthy controls patients show significantly greater (*p<0.01) sexual dysfunction across all domains except arousal (female) and ejaculatory function (male), and similar SFQ scores to sexual dysfunction controls
Table 1. Analysis of sexual function (SFQ total and sub–domain scores) by group showing a significant effect of group across all domains for men and women.
| Sexual function |
Df | F-statistic | p-value |
|---|---|---|---|
|
| |||
| Libido: men | 2, 124 | 6.8 | 0.002 |
| women | 2, 67 | 7.9 | 0.001 |
|
| |||
| Arousal- men | 2, 122 | 10.4 | <0.001 |
| women | 2, 66 | 5.6 | 0.006 |
|
| |||
| Erectile function-men |
2, 121 | 15.8 | <0.001 |
|
| |||
| Ejaculatory function-men |
2, 114 | 7.9 | 0.001 |
|
| |||
| Orgasmic function- women |
2, 62 | 7.5 | 0.001 |
|
| |||
| Total: men | 2, 112 | 16.6 | <0.001 |
| women | 2, 51 | 7.0 | 0.002 |
Using the SFQ cut-off for sexual dysfunction, sixty-eight per cent of female patients and 52% of male patients showed sexual dysfunction, compared to 13.6% and 22% of controls respectively. The unadjusted odds ratio of patients having sexual dysfunction compared to healthy controls was 15.2 (95% CI=3.84-60.2) for women and 3.7 (95% CI=1.7-13.9) for men.
Hormonal measures
Mean TSH levels (males (SD)=1.3 mU/L (0.85), and females (SD)=1.8 mU/L (2.0)) were within the normal range and none indicated hypothyroidism.
The distributions of gonadal hormones showed a positive skew in the women, and are therefore presented as median values (table 2). Of pre-menopausal women, twelve (48%) were hyperprolactinemic, seventeen (70.8%) showed hypoestrogenism, and nine (37.5%) showed severe hypoestrogenism (estradiol< 74 pmol/L). Twenty-three (92%) showed low progesterone levels (indicating intermittent anovulatory cycles), and fourteen (56%) showed markedly low progesterone levels (<2 nmol/L, indicating completely anovulatory cycles). Excluding those with oligomenorrhea, 76% of pre-menopausal women reporting regular menses showed low estradiol levels (median estradiol level= 68.2 pmol/L [interquartile range, IQR=123.4]), much less than the lower limit of the range in the luteal phase (365-1100) and even lower than the range in the follicular phase (74-365). Similarly, they had median progesterone levels of 1.4 nmol/L (IQR=4.22), much less than the lower limit of the range in the luteal phase (30-100) and even lower than the range in the follicular phase (2-8), and 90% showed low progesterone levels. In post-menopausal women, eleven (52.6%) showed hyperprolactinemia, although progesterone and estradiol levels were within normal post-menopausal ranges.
Table 2. Hormone levels in patients with the laboratory reference range (results: outside of the reference range are indicated in bold).
| Prolactin (mIU/L) |
Estradiol (pmol/L) |
Progesterone (nmol/L) |
FSH (IU/L) |
LH (IU/L) |
Total testosterone (nmol/l) |
Free testosterone (pmol/L) |
|
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pre- menopausal women |
480 (240- 1563) |
142 (26.5- 324.3) |
1.3 (0.8-17.1) |
4.4 (3.2- 11.7) |
5.9 (2.2- 11.3) |
1.3 (0.8-1.7) |
19.2 (8.9-23) |
| (n=29): Median(IQR) |
|||||||
| reference range: |
<480 | 365- 1100 |
30-100 | 2.6- 9.1 |
1.1- 12.1 |
0.5-2.5 | 10-50 |
|
| |||||||
| Post- menopausal women |
505 (193- 2160) |
37 (17.3- 60) |
1.2 (0.8-1.5) |
49.7 (15.0- 65.7) |
24.2 (8.2- 30.6) |
1.6 (1.1-1.8) |
21.8 (9.2-33.5) |
| (n=21): Median(IQR) |
|||||||
| reference range: |
<480 | <174 | <2.5 | 43.7- 106 |
13.2- 45.7 |
0.5-2.5 | 10-50 |
|
| |||||||
| Men (n=53): | 360 | 56.7 | - | 4.1 | 4.2 | 13.8 | 352.3 |
| Mean(SD) | (258) | (36.8) | (2.3) | (2.2) | (6.8) | (154.5) | |
| reference range: |
<480 | <174 | 1.8- 8.6 |
0.8- 6.1 |
9.0-30.0 | 134-844 | |
Gonadal hormone levels were normally distributed in the men (table 2). Hyperprolactinemia was present in nine (19.1%) men. Thirteen men (27.7%) had total testosterone levels below the lower limit of the normal range, and two (4.2%) had free testosterone levels below this range.
The relationship between hormonal measures and sexual function in patients
Table 3 shows the results of the linear regression analysis testing the hypothesis that sexual function is associated with prolactin and gonadal hormones. The analysis indicates that there is no association between total SFQ score and prolactin or the gonadal hormones measured in male or female patients. This remained the case after adjusting for side-effect and psychopathology levels, BMI, and antipsychotic class (typical/ atypical). In women, entering menopausal status as a covariate or analysing pre-menopausal women with regular menses as a separate group made no difference.
Table 3. The results of the linear regression of hormonal measures on total sexual function score in men and women showing that hormonal level has no significant effect on SFQ score (adjusted for multiple comparisons).
| Hormonal measure | Df | F statistic | Significance |
|---|---|---|---|
|
| |||
| Prolactin | |||
| Men | 2, 32 | 1.2 | 0.3 |
| Women | 2, 30 | 0.5 | 0.6 |
|
| |||
| Free testosterone | |||
| Men | 2, 32 | 1.7 | 0.2 |
| Women | 2, 31 | 0.3 | 0.8 |
|
| |||
| Total testosterone | |||
| Men | 2, 32 | 1.5 | 0.2 |
| Women | 2, 31 | 0.9 | 0.4 |
|
| |||
| Estradiol | |||
| Men | 2, 32 | 1.6 | 0.2 |
| Women | 2, 31 | 1.3 | 0.3 |
|
| |||
| Progesterone | |||
| Women | 2, 31 | 0.5 | 0.6 |
DISCUSSION
This study is the first study we are aware of to examine the relationship between sexual function and gonadal hormone levels in patients taking antipsychotics, and the largest to systematically assess sexual function in patients taking antipsychotic drugs. High rates of sexual dysfunction were found in patients, and their sexual function was impaired compared to healthy controls and indeed was similar to that seen in attendees at a sexual dysfunction clinic, indicating that clinically significant sexual dysfunction is common in patients taking antipsychotics. High rates of hyperprolactinemia and hypogonadism were found in males and females. Ninety-two per cent of pre-menopausal women and 27.7% of men showed biochemical hypogonadism. Estradiol, and progesterone levels were found to be much lower than the normal reference range in pre-menopausal women and approached those of the post-menopausal women, indicating impaired ovulation and fertility. Of the pre-menopausal women, over half showed progesterone levels that were very low: below the lower limit of the progesterone range in the follicular phase; and over a third showed estradiol levels below the lower limit of the range in the follicular phase. This is particularly striking as samples were taken in the luteal phase when progesterone and estradiol levels should be higher than the follicular phase. These results are in keeping with other studies indicating that sexual dysfunction and low gonadal hormone levels are common in patients taking antipsychotics 2;13;16, and extend earlier research by finding that there is no relationship between sexual function and prolactin or gonadal hormone levels in this group.
Methodological considerations
The large size and representativeness of the sample are strengths of the study. However, a number of methodological issues need to be considered. The study is cross-sectional so the direction of causality cannot be determined, and a number of factors that we were unable to control for may contribute to the observation of high rates of hypogonadism, and sexual dysfunction. Hypogonadism and sexual dysfunction may be intrinsic to schizophrenia, although normal prolactin, sex hormone levels and sexual function have been found in unmedicated patients with schizophrenia 3;14;32;33. The finding of low estrogen in women reporting regular menstrual cycles should be treated cautiously, as there is a possibility of errors in recording menstrual diaries (meaning higher rates of oligo/amenorhea), although women with apparently regular menstruation can show anovulatory cycles associated with low estrogen levels spontaneously or in association with hyperprolactinemia34. Longitudinal studies would be useful to evaluate this further. Testosterone shows a diurnal variation, peaking in healthy controls between 7-9am35. In this study samples were taken between 11am-midday and may have missed peak levels, although this was done because subjects typically slept until mid-morning, and schizophrenia is associated with reduced diurnal variation36-38. Current depressive disorder was excluded and levels of depressive symptoms were low, indicating that the high rates of sexual dysfunction are unlikely to be secondary to depression. Antimuscarinic medication and mood stabilisers may affect sexual function, and could have contributed to the higher rates in our subjects, although these are unlikely to result in hypogonadism. Obesity and smoking may affect sexual function and sex hormone levels, and may be more common in patients than the general population. However, as smoking tends to increase testosterone and reduce estradiol levels, and obesity has the opposite influences, the net effect is unlikely to explain the high rates of hypogonadism we observed 39-41. The CATIE trial has recently highlighted the high rates of hypertension and diabetes in patients taking antipsychotics, both associated with sexual dysfunction42. We excluded hypertension and diabetes in our sample through clinical assessment and blood pressure measurement, which may indicate that rates of sexual dysfunction will be higher in unselected patients. However, pre-diabetic states may have been missed and could be contributing to the high rate of sexual dysfunction in our patients, through microvascular damage. It is possible that perimenopausal women were misclassified. However, repeating the analyses in the group of pre-menopausal women with regular menses made no difference, indicating that mis-assignment of menopausal status is unlikely to explain the high rates of hypogonadism or the absence of a relationship between hormonal levels and SFQ scores. It is possible that the null hypothesis has been falsely accepted and there is in fact an association between sex function and sex hormones, or prolactin (“type II error”). However, the power calculation indicates the sample size has over 95% power to detect a relationship explaining 30% or more of the variance, and 80% power to detect a relationship explaining 17% or more of the variance when α=0.05. It is possible the association is non-linear, for example showing a threshold effect, but this was not apparent when the data was plotted and there was no evidence of threshold effects (for example, an association in a hyperprolactinemic sub-group only).
Clinical and research implications
The high rates of hypogonadism are of particular concern given the association between low sex hormone levels and osteoporosis, infertility and cardiovascular disease43. Low sex hormone levels have also been linked with exacerbation of psychotic symptoms in patients with schizophrenia 15;16;44.
Sexual dysfunction and side-effects are a major concern to patients, although often neglected by clinicians, and linked to non-adherence to antipsychotic treatment 29;45. The low psychopathology, and side-effect rating scores in the sample demonstrate that sexual dysfunction may be present even in stable patients treated with low-moderate doses of antipsychotics. The absence of an association between hormone levels and SFQ scores highlights that the etiology of sexual dysfunction in patients taking antipsychotics is likely to be complex, and suggests a model involving the interaction of multiple psychological, social and pharmacological factors is needed. Antipsychotics could contribute directly through adrenergic and cholinergic mechanisms and indirectly by increasing the likelihood of other risk factors for sexual dysfunction, such as diabetes mellitus.
Conclusions
Our findings confirm that clinically significant sexual dysfunction and hypogonadism are common in patients taking antipsychotics and indicate that prolactin and gonadal hormone levels are unlikely to be major etiological factors. The high rates of hypogonadism suggest that patients are at increased risk of cardiovascular disease, and osteoporosis. Clinicians are advised to enquire about sexual dysfunction, and monitor prolactin and gonadal hormone levels in patients taking antipsychotics. Future research, ideally randomising drug naïve patients to treatment with antipsychotics showing different dopaminergic, cholinergic and adrenergic activity profiles, is needed to further tease apart the pharmacological, psychological and social etiological factors and guide treatment choice for the large proportion of patients taking antipsychotics who show sexual dysfunction and low sex hormone levels.
Reference List
- 1.Smith SM, O’Keane V, Murray R. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry. 2002;181:49–55. doi: 10.1192/bjp.181.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald S, Halliday J, MacEWAN T, et al. Nithsdale Schizophrenia Surveys 24: sexual dysfunction. Case-control study. Br J Psychiatry. 2003;182:50–56. doi: 10.1192/bjp.182.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Kockott G, Pfeiffer W. Sexual disorders in nonacute psychiatric outpatients. Compr Psychiatry. 1996;37:56–61. doi: 10.1016/s0010-440x(96)90052-8. [DOI] [PubMed] [Google Scholar]
- 4.Aizenberg D, Zemishlany Z, Dorfman-Etrog P, et al. Sexual dysfunction in male schizophrenic patients. J Clin Psychiatry. 1995;56:137–141. [PubMed] [Google Scholar]
- 5.Blair JH, Simpson GM. Effect of antipsychotic drugs on reproductive functions. Dis Nerv Syst. 1966;27:645–647. [PubMed] [Google Scholar]
- 6.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 7.Knegtering H, van der Moolen AE, Castelein S, et al. What are the effects of antipsychotics on sexual dysfunctions and endocrine functioning? Psychoneuroendocrinology. 2003;28(Suppl 2):109–123. doi: 10.1016/s0306-4530(02)00130-0. [DOI] [PubMed] [Google Scholar]
- 8.Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J Nerv Ment Dis. 1982;170:463–467. doi: 10.1097/00005053-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64:2291–2314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cutler AJ. Sexual dysfunction and antipsychotic treatment. Psychoneuroendocrinology. 2003;28(Suppl 1):69–82. doi: 10.1016/s0306-4530(02)00113-0. [DOI] [PubMed] [Google Scholar]
- 11.Bancroft J. Endocrinology of sexual function. Clin Obstet Gynaecol. 1980;7:253–281. [PubMed] [Google Scholar]
- 12.Canuso CM, Goldstein JM, Wojcik J, et al. Antipsychotic medication, prolactin elevation, and ovarian function in women with schizophrenia and schizoaffective disorder. Psychiatry Res. 2002;111:11–20. doi: 10.1016/s0165-1781(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 13.Smith S, Wheeler MJ, Murray R, et al. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol. 2002;22:109–114. doi: 10.1097/00004714-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Baptista T, Reyes D, Hernandez L. Antipsychotic drugs and reproductive hormones: relationship to body weight regulation. Pharmacol Biochem Behav. 1999;62:409–417. doi: 10.1016/s0091-3057(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 15.Huber TJ, Tettenborn C, Leifke E, et al. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30:111–114. doi: 10.1016/j.psyneuen.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Huber TJ, Borsutzky M, Schneider U, et al. Psychotic disorders and gonadal function: evidence supporting the oestrogen hypothesis. Acta Psychiatr Scand. 2004;109:269–274. doi: 10.1046/j.1600-0447.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 17.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 18.Howes OD, Wheeler MJ, Meaney AM, et al. Bone mineral density and its relationship to prolactin levels in patients taking antipsychotic treatment. J Clin Psychopharmacol. 2005;25:259–261. doi: 10.1097/01.jcp.0000162798.87249.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 20.Rey MJ, Schulz P, Costa C, et al. Guidelines for the dosage of neuroleptics. I: Chlorpromazine equivalents of orally administered neuroleptics. Int Clin Psychopharmacol. 1989;4:95–104. doi: 10.1097/00004850-198904000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Smith JM, Kucharski LT, Oswald WT, et al. A systematic investigation of tardive dyskinesia in inpatients. Am J Psychiatry. 1979;136:918–922. doi: 10.1176/ajp.136.7.918. [DOI] [PubMed] [Google Scholar]
- 23.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 24.Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 25.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 26.Addington D, Addington J, Maticka-Tyndale E, et al. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. doi: 10.1016/0920-9964(92)90003-n. [DOI] [PubMed] [Google Scholar]
- 27.Meikle AW. The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid. 2004;14(Suppl 1):S17–S25. doi: 10.1089/105072504323024552. [DOI] [PubMed] [Google Scholar]
- 28.Nanjee MN, Wheeler MJ. Plasma free testosterone--is an index sufficient? Ann Clin Biochem. 1985;22(Pt 4):387–390. doi: 10.1177/000456328502200410. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson RW. Sexual medicine: why psychiatrists must talk to their patients about sex. Can J Psychiatry. 2004;49:673–677. doi: 10.1177/070674370404901004. [DOI] [PubMed] [Google Scholar]
- 30.Avis NE. Sexual function and aging in men and women: community and population-based studies. J Gend Specif Med. 2000;3:37–41. [PubMed] [Google Scholar]
- 31.Guay A, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16:112–120. doi: 10.1038/sj.ijir.3901178. [DOI] [PubMed] [Google Scholar]
- 32.Ozcan ME, Banoglu R. Gonadal hormones in schizophrenia and mood disorders. Eur Arch Psychiatry Clin Neurosci. 2003;253:193–196. doi: 10.1007/s00406-003-0424-7. [DOI] [PubMed] [Google Scholar]
- 33.Segal M, Avital A, Rojas M, et al. Serum prolactin levels in unmedicated first-episode and recurrent schizophrenia patients: a possible marker for the disease’s subtypes. Psychiatry Res. 2004;127:227–235. doi: 10.1016/j.psychres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Fraser IS, Michie EA, Wide L, et al. Pituitary gonadotropins and ovarian function in adolescent dysfunctional uterine bleeding. J Clin Endocrinol Metab. 1973;37:407–414. doi: 10.1210/jcem-37-3-407. [DOI] [PubMed] [Google Scholar]
- 35.Diver MJ. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem. 2006;43:3–12. doi: 10.1258/000456306775141803. [DOI] [PubMed] [Google Scholar]
- 36.Boivin DB. Influence of sleep-wake and circadian rhythm disturbances in psychiatric disorders. J Psychiatry Neurosci. 2000;25:446–458. [PMC free article] [PubMed] [Google Scholar]
- 37.Duncan E, Bollini AM, Sanfilipo M, et al. Diurnal variation in plasma homovanillic acid in patients with schizophrenia and healthy controls. Schizophr Res. 2006;81:323–326. doi: 10.1016/j.schres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Martin JL, Jeste DV, ncoli-Israel S. Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res. 2005;39:251–259. doi: 10.1016/j.jpsychires.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Sowers MF, Beebe JL, McConnell D, et al. Testosterone concentrations in women aged 25-50 years: associations with lifestyle, body composition, and ovarian status. Am J Epidemiol. 2001;153:256–264. doi: 10.1093/aje/153.3.256. [DOI] [PubMed] [Google Scholar]
- 40.Jensen TK, Andersson AM, Jorgensen N, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 41.Trummer H, Habermann H, Haas J, et al. The impact of cigarette smoking on human semen parameters and hormones. Hum Reprod. 2002;17:1554–1559. doi: 10.1093/humrep/17.6.1554. [DOI] [PubMed] [Google Scholar]
- 42.Meyer JM, Nasrallah HA, McEvoy JP, et al. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Haddad PM. Antipsychotics and diabetes: review of non-prospective data. Br J Psychiatry Suppl. 2004;47:S80–S86. doi: 10.1192/bjp.184.47.s80. [DOI] [PubMed] [Google Scholar]
- 44.Huber TJ, Rollnik J, Wilhelms J, et al. Estradiol levels in psychotic disorders. Psychoneuroendocrinology. 2001;26:27–35. doi: 10.1016/s0306-4530(00)00034-2. [DOI] [PubMed] [Google Scholar]
- 45.Finn SE, Bailey JM, Schultz RT, et al. Subjective utility ratings of neuroleptics in treating schizophrenia. Psychol Med. 1990;20:843–848. doi: 10.1017/s0033291700036539. [DOI] [PubMed] [Google Scholar]