Abstract
High mobility group box 1 (HMGB1) was originally discovered as a chromatin-binding protein several decades ago. It is now increasingly evident that HMGB1 plays a major role in several disease conditions such as atherosclerosis, diabetes, arthritis, sepsis, and cancer. It is intriguing how deregulation of HMGB1 can result in a myriad of disease conditions. Interestingly, HMGB1 is involved in cell proliferation, angiogenesis, and metastasis during cancer progression. Furthermore, HMGB1 has been demonstrated to exert intracellular and extracellular functions, activating key oncogenic signaling pathways. This paper focuses on the role of HMGB1 in prostate cancer development and highlights the potential of HMGB1 to serve as a key target for prostate cancer treatment.
1. Introduction
Current treatment methods for prostate cancer (PCa) such as radical prostatectomy, chemotherapy, radiation therapy, or hormonal therapy are used to effectively manage this disease. However, majority of patients undergoing androgen deprivation therapy develop castration resistant PCa [1]. Hence, there is a great interest in understanding the molecular events that are critical for the development of this disease. If characterized, the genes that play a crucial role in PCa progression or hormone resistance PCa will result in development of novel strategies for treating PCa. Recent evidences strongly suggest that high mobility group box 1 (HMGB1) plays a pivotal role in the development of several cancer types including PCa [2–4]. It is found to be associated with all the hallmarks of cancer development such as cell proliferation, anchorage-independent growth, angiogenesis, migration, and invasion [3].
HMGB1 is a DNA binding protein involved in DNA replication and DNA repair process [5]. Outside the cell, it functions as a proinflammatory cytokine [6]. The extracellular receptors of HMGB1 include RAGE and TLR4, with RAGE being implicated as a major receptor for HMGB1 in tumor development. Deregulation of HMGB1 has been shown to be associated with several inflammation associated diseases such as atherosclerosis [7, 8], arthritis [9], and sepsis [10]. Moreover, HMGB1 is also shown to promote tumorigenesis by inducing inflammation [11, 12]. Inflammation is one of the key risk factors implicated in prostate carcinogenesis [13–15]. Based on the recent published evidences, we highlight and speculate on the role of HMGB1 in PCa development and the potential strategies to target HMGB1 for PCa treatment.
2. HMGB1 Expression in Prostate Cancer Cells: Preclinical and Clinical Samples
HMGB1 is known to be consistently overexpressed in cancer cells compared to normal cell types [3, 16–18]. Similarly, HMGB1 is also reported to be highly expressed in PCa cells [4, 19, 20]. Interestingly, androgen deprivation resulted in the secretion of HMGB1 in prostatic stromal cells and found to be associated with metastatic PCa [21]. This finding support the notion that androgen deprivation therapy may upregulate the expression of HMGB1 leading to either hormone resistance or metastatic disease.
Studies conducted by He et al. [22] employing transgenic adenocarcinoma mouse prostate (TRAMP) model demonstrated that HMGB1 promotes invasive carcinoma in this experimental setting. Furthermore, their study also showed that HMGB1 is released in the serum during tumor progression correlating with severity of disease pathology. Previous studies have shown that serum HMGB1 can serve as a biomarker for variety of cancers such as pancreatic ductal adenocarcinoma [23], colorectal carcinoma [24], malignant mesothelioma [25], canine lymphoma [26], non-small-cell lung cancer [27, 28], gastric cancer [29], and hepatocellular carcinoma [30]. However, a study conducted by Mengus et al. [31] to determine the circulating levels of cytokines in early stage prostate cancer (1 to 2c) showed that HMGB1 levels were not found to be significant when compared to control benign hyperplastic prostate (BPH) samples. These results combined with serum levels of HMGB1 in the TRAMP mouse PCa model may suggest that HMGB1 be a marker for advanced stages of PCa.
Expression of HMGB1 in clinical samples was first reported by Kuniyasu et al. [21] in a pilot study where they found that HMGB1 is expressed in tumor (27%) and stromal cells (63%) of metastatic patients. Interestingly, they also observed that HMGB1 was not expressed (0%) in tumors of nonmetastatic cases, while only 11% of patients with nonmetastases expressed HMGB1 in stromal cells. In subsequent study, Ishiguro et al. [19] using real-time quantitative PCR showed that HMGB1 and its cognate receptor, RAGE, are significantly expressed in primary PCa and refractory samples compared to normal control prostate samples. More recently, Li et al. [20] determined the correlation pattern of HMGB1 expression with clinical characteristics of PCa. Their findings showed that about 60% (101/168) of PCa cases were positive for HMGB1 expression. Specifically, this study revealed that HMGB1 expression correlated with stage of cancer (pT), Gleason grade, preoperative prostate specific antigen, biochemical recurrence, and poor survival rates. Thus, these in vitro, preclinical and clinical evidences strongly point that HMGB1 may have a pivotal role in the progression of PCa.
3. HMGB1 Interacting Genes/Proteins in Prostate Cancer
HMGB1 has been reported to transactivate sex steroid hormone receptors such as androgen receptor, mineralocorticoid receptor, progesterone receptor, and glucocorticoid receptor [32, 33]. In PCa, transactivation of androgen receptor (AR) by HMGB1 [33] may have clinical significance. AR is a crucial gene required for PCa survival and PCa progression [34, 35]. In addition, AR activation is also known to play a major role in the development of androgen-independent PCa [34–36]. Activation or expression of AR is shown to be regulated by many signaling pathways [36–38]. Our recent publication showed that targeting receptor for advanced glycation end products (RAGEs) downregulated the expression of prostate specific antigen (PSA), the downstream target gene of AR [39], suggesting that RAGE may have a role in the regulation of AR in PCa cells. Interestingly, previous study by Ishiguro et al. [19] showed that both RAGE and HMGB1 are coexpressed in PCa samples and suggested that they may have cooperative role in the progression of PCa. Thus, HMGB1 may regulate AR either by acting as co-activator of AR or indirectly associating with RAGE signaling in prostate oncogenesis.
HGMB1 and RAGE have been shown to interact in many types of tumor cells but not in normal cells [40]. In extracellular milieu, HMGB1 may interact with RAGE receptor in PCa cells. This notion is supported by our recent work [39], which showed that silencing RAGE expression by RNAi approach abrogated the cell proliferative effects of extracellular recombinant HMGB1 on PCa cells. Interestingly, HMGB1 is also shown to enhance DNA binding activity of ETS transcription factor in regulating peroxiredoxin-1 and -5 expression in combating oxidative stress in PCa cells [41]. That HMGB1 directly interact with ETS to enhance its target gene transcriptional activity may have significant implications in PCa disease progression, as ETS is known to play a major role in PCa progression, androgen independence, and metastatic progression [42–46]. Given the recent findings that HMGB1 can facilitate gene recombination [5, 47, 48] and the fact that frequent gene rearrangements of ETS derived transcription factors are detected in PCa [42, 49], the possibility of ETS gene recombination driven by HMGB1 may favor promotion of aggressive PCa.
4. Possible HMGB1 and Inflammation Link in PCa
Risk factors for PCa include genetic factors, hormonal changes, chronic inflammation, and dietary differences [14, 50]. Among these etiological factors, there is growing evidence for the role of inflammation in the prostate carcinogenesis [15, 51, 52], in particular chronic inflammation [14, 15, 53]. The role of inflammation in the prostate carcinogenesis is now widely accepted [15, 51, 54]. HMGB1-RAGE axis plays a major role in inflammation induced carcinogenesis [55, 56]. Evidence for the role of HMGB1 in PCa inflammation can be inferred from a recent study by He et al. [22] using TRAMP animal model of PCa. In this study, they showed that targeting HMGB1 disrupts tumor progression by inhibiting activation of T-cells and reducing infiltration of macrophages, which are considered to be key inflammatory cells in promoting variety of cancers including PCa [57–60]. Thus, inflammation may be one mechanism by which HMGB1 may accelerate PCa as depicted in Figure 1. In our recent work [61], we also showed that HMGB1 is one of the target inflammatory gene for 18-alpha glycyrrhetinic acid in PCa cells. Our study thus suggests that inflammation associated genes such as HMGB1 may play a vital role in the multistep process of PCa development.
Figure 1.
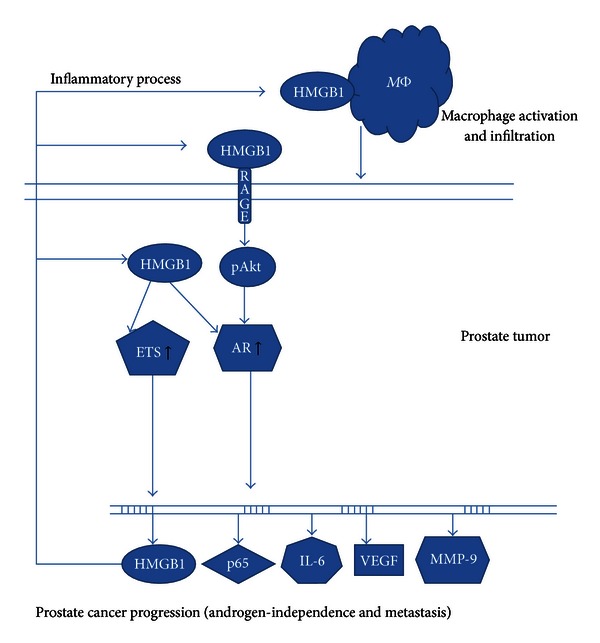
Proposed model of HMGB1 mediated prostate cancer progression.
5. Can HMGB1 Be a Viable Target for PCa Treatment?
HMGB1 is suggested to be a potential target gene for various diseases such as atherosclerosis, inflammation, sepsis, and arthritis [7, 8]. Accumulating evidences suggest that HMGB1 can also serve as a target for various cancer types including PCa [4, 62–65]. Some potential HMGB1 targeting strategies are highlighted here.
6. Gene Targeting
Antisense and RNA interference (RNAi) technology are most commonly used strategies to eliminate or silence the expression of target genes [66]. These technologies are also used as a tool to study gene function in cancer cells [67]. Interestingly, several preclinical and early clinical trials have shown great promise of these strategies for use in PCa treatment [68–72].
Studies performed by Kuniyasu et al. [21] showed that antisense targeting of HMGB1 in PC-3 cells significantly inhibited the invasive potential of these cells in vitro. In our recent work [4], we showed that targeting HMGB1 by RNAi resulted in the inhibition of PCa cell proliferation and apoptotic elimination of PCa cells. Furthermore, our additional work also revealed that targeting RAGE by RNAi prevented HMGB1-mediated cell proliferation of PCa cells and reduction of HMGB1 levels in the RAGE RNAi transfected cells. This also led to growth inhibition of androgen-dependent and -independent prostate tumor in nude mice model. Targeting HMGB1 by RNAi is also shown to inhibit osseous metastasis of PC cells in an experimental metastases model [73]. Thus antisense and RNAi strategies represent promising methods to target HMGB1 expression to achieve therapeutic effects against PCa.
7. Antibody (Ab) Based Targeting
Ab based treatment approach is a promising strategy to target PCa. For example, anti-VEGF antibody treatment has yielded encouraging results in clinical trials [74]. However, the anti-VEGF Ab may primarily target angiogenesis process of tumor growth. In this case, HMGB1 may be a desirable target for prostate PCa, as it is involved in cell proliferation, apoptosis regulation, angiogenesis, and metastases [3]. In support of this, studies by He et al. [22] showed that administration of anti-HMGB1 significantly inhibited the prostate tumor progression in TRAMP mouse PCa model. In another study [75], anti-HMGB1 was shown to inhibit HMGB1-enforced angiogenic process of colon cancer cells. Furthermore in a malignant mesothelioma study, targeting HMGB1 by mAb also showed to effectively inhibit the matrigel invasion of malignant mesothelial cells in vitro, hinder the tumor growth, and extend the survival of nude mice. The potential of anti-HMGB1 to inhibit colon cancer development was also recently demonstrated [76, 77]. Collectively these studies attest to the therapeutic utility of anti-HMGB1 antibody for cancer treatment in general, which can also be developed for PCa treatment.
8. Use of Natural Products
Naturally occurring agents such as glycyrrhizin, glycyrrhetinic acid, ethyl pyruvate, and green tea phenols have been shown to target HMGB1 expression in variety of cell/disease models [61, 78–84]. Specifically, we showed that 18-alpha glycyrrhetinic acid, a derivative of glycyrrhizin that is abundantly present in the licorice root, can decrease HMGB1 gene expression and result in the therapeutic effects in PCa cells [61]. Other natural compounds known to target HMGB1 include some cholinergic agonists [85, 86], thrombomodulin [87–89], and low molecular-weight heparin [90]. All these natural agents could be potentially tested against PCa cells/tumors that display high levels of HMGB1 expression.
9. Other Potential HMGB1 Targeting Therapies
Recent studies advocate that peptide (A-Box) derived from HMGB1 can be used to effectively antagonize the functions of HMGB1 [91, 92]. This A-Box has been shown to downregulate the inflammatory activity of HMGB1 [91]. It has also been postulated to inhibit tumor angiogenesis by disrupting HMGB1-RAGE signaling [93]. Although, the anti-cancer potential of A-Box is yet to be evaluated against PCa, it may offer novel treatment strategy for PCa given the fact that A-Box can be used as gene delivery agent [94, 95].
Targeting extracellular HMGB1 represents another strategy to combat PCa as we recently showed that addition of rHMGB1 promotes PCa cell proliferation [39]. Secreted HMGB1 can be neutralized by administering soluble RAGE as discussed for controlling atherosclerosis [8] and inflammation [96]. Interestingly, the endogenous levels of soluble RAGE are downregulated in patients with liver cancer [97], colorectal adenoma [98], pancreatic cancer [99, 100], lung cancer [101], and breast carcinoma [102]. However, the status of soluble RAGE in PCa patients is not yet known and warrants further investigation for PCa treatment.
Developing HMGB1 as a vaccine for PCa is a feasible immunotherapeutic strategy as the previous study strongly support that peptides derived from HMGB1 engrafted in liposomes induced potent antigen-specific and tumor specific immunity against B16-OVA melanoma model [103]. A follow-up study also demonstrated that HMGB1 antigenic peptides can act as an adjuvant for subunit cancer vaccines [104]. These HMGB1 derived peptides could also be tested as adjuvant for enhancing the efficacy of PCa vaccines that are currently under development [105–107].
10. Concluding Remarks
The evidence for role of HMGB1 in cancer progression is now rapidly accumulating. Studies from others and our group suggest that HMGB1 may also have a prominent role in PCa development. The interaction of HMGB1 with AR, RAGE, and ETS point to a central role of HMGB1 in PCa progression. Clinical studies also support that HMGB1 is overexpressed in PCa patients and may serve as a novel prognostic marker for BCR-free survival for prostate cancer patients after undergoing radical prostatectomy. Importantly, several preclinical studies highlighted in this paper suggest that HMGB1 can be targeted by variety of approaches, which may ultimately lead to the development of effective therapy for PCa patients.
Conflicts of Interests
The authors have no conflict interests to declare.
Acknowledgment
Some of the author's published studies cited in this paper were partially supported by the American Cancer Society, IL Division (Grant no. 141287) and Excellence in Academic Medicine (EAM) award.
References
- 1.Divrik RT, Turkeri L, Sahin AF, et al. Prediction of response to androgen deprivation therapy and castration resistance in primary metastatic prostate cancer. Urologia Internationalis. 2012;88:25–33. doi: 10.1159/000334539. [DOI] [PubMed] [Google Scholar]
- 2.Ellerman JE, Brown CK, De Vera M, et al. Masquerader: high mobility group box-1 and cancer. Clinical Cancer Researchearch. 2007;13(10):2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 3.Tang D, Kang R, Zeh HJ, Lotze MT. High-mobility group box 1 and cancer. Biochimica et Biophysica Acta. 2010;1799(1-2):131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnanasekar M, Thirugnanam S, Ramaswamy K. Short hairpin RNA (shRNA) constructs targeting high mobility group box-1 (HMGB1) expression leads to inhibition of prostate cancer cell survival and apoptosis. International Journal of Oncology. 2009;34(2):425–431. [PubMed] [Google Scholar]
- 5.Štros M. HMGB proteins: interactions with DNA and chromatin. Biochimica et Biophysica Acta. 2010;1799(1-2):101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Czura CJ, Wang H, Tracey KJ. Dual roles for HMGB1: DNA binding and cytokine. Journal of Endotoxin Research. 2001;7(4):315–321. doi: 10.1177/09680519010070041401. [DOI] [PubMed] [Google Scholar]
- 7.Naglova H, Bucova M. HMGB1 and its physiological and pathological roles. lBratislavské Lekárske Listy. 2012;113:163–171. doi: 10.4149/bll_2012_039. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Yoon SJ, Tae HJ, Shim CY. RAGE and cardiovascular disease. Frontiers in Bioscience. 2011;16(2):486–497. doi: 10.2741/3700. [DOI] [PubMed] [Google Scholar]
- 9.Maillard-Lefebvre H, Boulanger E, Daroux M, Gaxatte C, Hudson BI, Lambert M. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology. 2009;48(10):1190–1196. doi: 10.1093/rheumatology/kep199. [DOI] [PubMed] [Google Scholar]
- 10.Andersson U, Tracey KJ. HMGB1 in sepsis. Scandinavian Journal of Infectious Diseases. 2003;35(9):577–584. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Ray R, Rajeswari MR. Overexpression of high mobility group (HMG) B1 and B2 proteins directly correlates with the progression of squamous cell carcinoma in skin. Cancer Investigation. 2008;26(8):843–851. doi: 10.1080/07357900801954210. [DOI] [PubMed] [Google Scholar]
- 12.Huttunen HJ, Rauvala H. Amphoterin as an extracellular regulator of cell motility: from discovery to disease. Journal of Internal Medicine. 2004;255(3):351–366. doi: 10.1111/j.1365-2796.2003.01301.x. [DOI] [PubMed] [Google Scholar]
- 13.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. American Journal of Pathology. 1999;155(6):1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urologic Oncology. 2007;25(5):398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nature Reviews Cancer. 2007;7(4):256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao X, Zhao G, Yang H, Hong X, Bie L, Liu G. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. Journal of Cancer Researchearch and Clinical Oncology. 2010;136(5):677–684. doi: 10.1007/s00432-009-0706-1. [DOI] [PubMed] [Google Scholar]
- 17.Yan W, Chang Y, Liang X, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–1875. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong H, Zuliani P, Komuravelli A, Faeder JR, Clarke EM. Analysis and verification of the HMGB1 signaling pathway. BMC Bioinformatics. 2010;11(supplement 7):p. S10. doi: 10.1186/1471-2105-11-S7-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64(1):92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Gui Y, Yuan T, et al. Overexpression of high mobility group box 1 with poor prognosis in patients after radical prostatectomy. British Journal of Urology International. 2012;110:E1125–E1130. doi: 10.1111/j.1464-410X.2012.11277.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuniyasu H, Chihara Y, Kondo H, Ohmori H, Ukai R. Amphoterin induction in prostatic stromal cells by androgen deprivation is associated with metastatic prostate cancer. Oncology reports. 2003;10(6):1863–1868. [PubMed] [Google Scholar]
- 22.He Y, Zha J, Wang Y, Liu W, Yang X, Yu P. Tissue damage-associated “danger signals” influence T-cell responses that promote the progression of preneoplasia to cancer. Cancer Researchearch. 2013;73:629–639. doi: 10.1158/0008-5472.CAN-12-2704. [DOI] [PubMed] [Google Scholar]
- 23.Chung HW, Lim JB, Jang S, Lee KJ, Park KH, Song SY. Serum high mobility group box-1 is a powerful diagnostic and prognostic biomarker for pancreatic ductal adenocarcinoma. Cancer Science. 2012;103:1714–1721. doi: 10.1111/j.1349-7006.2012.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Song M, Shin N, et al. Diagnostic significance of serum HMGB1 in colorectal carcinomas. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034318.e34318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jube S, Rivera ZS, Bianchi ME, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Research. 2012;72:3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer A, Eberle N, Bullerdiek J, Nolte I, Simon D. High-mobility group B1 proteins in canine lymphoma: prognostic value of initial and sequential serum levels in treatment outcome following combination chemotherapy. Veterinary and Comparative Oncology. 2010;8(2):127–137. doi: 10.1111/j.1476-5829.2010.00216.x. [DOI] [PubMed] [Google Scholar]
- 27.Shang GH, Jia CQ, Tian H, et al. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respiratory Medicine. 2009;103(12):1949–1953. doi: 10.1016/j.rmed.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Naumnik W, Nilklińska W, Ossolińska M, Chyczewska E. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochemica et Cytobiologica. 2009;47(4):703–709. doi: 10.2478/v10042-009-0025-z. [DOI] [PubMed] [Google Scholar]
- 29.Chung H, Lee SG, Kim H, et al. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. Journal of Translational Medicine. 2009;7, article 38 doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng BQ, Jia CQ, Liu CT, et al. Serum high mobility group box chromosomal protein 1 is associated with clinicopathologic features in patients with hepatocellular carcinoma. Digestive and Liver Disease. 2008;40(6):446–452. doi: 10.1016/j.dld.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Mengus C, Le Magnen C, Trella E, et al. Elevated levels of circulating IL-7 and IL-15 in patients with early stage prostate cancer. Journal of Translational Medicine. 2011;9:p. 162. doi: 10.1186/1479-5876-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melvin VS, Roemer SC, Churchill MEA, Edwards DP. The C-terminal extension (CTE) of the nuclear hormone receptor DNA binding domain determines interactions and functional response to the HMGB-1/-2 co-regulatory proteins. Journal of Biological Chemistry. 2002;277(28):25115–25124. doi: 10.1074/jbc.M110400200. [DOI] [PubMed] [Google Scholar]
- 33.Verrijdt G, Haelens A, Schoenmakers E, Rombauts W, Claessens F. Comparative analysis of the influence of the high-mobility group box 1 protein on DNA binding and transcriptional activation by the androgen, glucocorticoid, progesterone and mineralocorticoid receptors. Biochemical Journal. 2002;361(1):97–103. doi: 10.1042/0264-6021:3610097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Current Cancer Drug Targets. 2007;7(6):591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 35.Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth. Cancer Researchearch. 2005;65(6):2287–2295. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 36.Jain G, Cronauer MV, Schrader M, Moller P, Marienfeld RB. NF-kappaB signaling in prostate cancer: a promising therapeutic target? World Journal of Urology. 2012;30:303–310. doi: 10.1007/s00345-011-0792-y. [DOI] [PubMed] [Google Scholar]
- 37.Reebye V, Frilling A, Habib NA, Mintz PJ. Intracellular adaptor molecules and AR signalling in the tumour microenvironment. Cellular Signalling. 2011;23(6):1017–1021. doi: 10.1016/j.cellsig.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. Journal of Clinical Oncology. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 39.Elangovan I, Thirugnanam S, Chen A, et al. Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochemical and Biophysical Research Communications. 2012;417:1133–1138. doi: 10.1016/j.bbrc.2011.12.060. [DOI] [PubMed] [Google Scholar]
- 40.Todorova J, Pasheva E. High mobility group B1 protein interacts with its receptor RAGE in tumor cells but not in normal tissues. Oncology Letters. 2012;3:214–218. doi: 10.3892/ol.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiota M, Izumi H, Miyamoto N, et al. Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high-mobility group protein B1. Cancer Scienceence. 2008;99(10):1950–1959. doi: 10.1111/j.1349-7006.2008.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianchini D, Zivi A, Sandhu S, de Bono JS. Horizon scanning for novel therapeutics for the treatment of prostate cancer. Annals of Oncology. 2010;21(supplement 7):vii43–vii55. doi: 10.1093/annonc/mdq369. [DOI] [PubMed] [Google Scholar]
- 43.Hahne JC, Okuducu AF, Sahin A, Fafeur V, Kiriakidis S, Wernert N. The transcription factor ETS-1: its role in tumour development and strategies for its inhibition. Mini-Reviews in Medicinal Chemistry. 2008;8(11):1095–1105. doi: 10.2174/138955708785909934. [DOI] [PubMed] [Google Scholar]
- 44.Turner DP, Moussa O, Sauane M, Fisher PB, Watson DK. Prostate-derived ETS factor is a mediator of metastatic potential through the inhibition of migration and invasion in breast cancer. Cancer Researchearch. 2007;67(4):1618–1625. doi: 10.1158/0008-5472.CAN-06-2913. [DOI] [PubMed] [Google Scholar]
- 45.Findlay VJ, Turner DP, Yordy JS, et al. Prostate-derived ETS factor regulates epithelial-to-mesenchymal transition through both SLUG-dependent and independent mechanisms. Genes and Cancer. 2011;2(2):120–129. doi: 10.1177/1947601911410424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clinical Cancer Researchearch. 2006;12(20):6296s–6300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 47.Dai Y, Wong B, Yen YM, Oettinger MA, Kwon J, Johnson RC. Determinants of HMGB proteins required to promote RAG1/2-recombination signal sequence complex assembly and catalysis during V(D)J recombination. Molecular and Cellular Biology. 2005;25(11):4413–4425. doi: 10.1128/MCB.25.11.4413-4425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Numata M, Nagata K. Synergistic requirement of orphan nonamer-like elements and DNA bending enhanced by HMGB1 for RAG-mediated nicking at cryptic 12-RSS but not authentic 12-RSS. Genes to Cells. 2011;16(8):879–895. doi: 10.1111/j.1365-2443.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- 49.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mettlin C. Recent developments in the epidemiology of prostate cancer. European Journal of Cancer A. 1997;33(3):340–347. doi: 10.1016/s0959-8049(97)89003-x. [DOI] [PubMed] [Google Scholar]
- 51.Narayanan NK, Nargi D, Horton L, Reddy BS, Bosland MC, Narayanan BA. Inflammatory processes of prostate tissue microenvironment drive rat prostate carcinogenesis: preventive effects of celecoxib. Prostate. 2009;69(2):133–141. doi: 10.1002/pros.20862. [DOI] [PubMed] [Google Scholar]
- 52.Vasto S, Carruba G, Candore G, Italiano E, Di Bona D, Caruso C. Inflammation and prostate cancer. Future Oncology. 2008;4(5):637–645. doi: 10.2217/14796694.4.5.637. [DOI] [PubMed] [Google Scholar]
- 53.Sugar LM. Inflammation and prostate cancer. Canadian Journal of Urology. 2006;13(supplement 1):46–47. [PubMed] [Google Scholar]
- 54.Chaturvedi AK, Moore SC, Hildesheim A. Invited commentary: circulating inflammation markers and cancer risk—implications for epidemiologic studies. American Journal of Epidemiology. 2013;177:14–19. doi: 10.1093/aje/kws357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gebhardt C, Riehl A, Durchdewald M, et al. RAGE signaling sustains inflammation and promotes tumor development. Journal of Experimental Medicine. 2008;205(2):275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taguchi A, Blood DC, Del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 57.Lucia MS, Torkko KC. Inflammation as a target for prostate cancer chemoprevention: pathological and laboratory rationale. Journal of Urology. 2004;171(2):S30–S34. doi: 10.1097/01.ju.0000108142.53241.47. [DOI] [PubMed] [Google Scholar]
- 58.Dubey S, Vanveldhuizen P, Holzbeierlein J, Tawfik O, Thrasher JB, Karan D. Inflammation-associated regulation of the macrophage inhibitory cytokine (MIC-1) gene in prostate cancer. Oncology Letters. 2012;3:1166–1170. doi: 10.3892/ol.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clegg NJ, Couto SS, Wongvipat J, et al. MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017449.e17449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong CP, Bray TM, Ho E. Induction of proinflammatory response in prostate cancer epithelial cells by activated macrophages. Cancer Letters. 2009;276(1):38–46. doi: 10.1016/j.canlet.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shetty AV, Thirugnanam S, Dakshinamoorthy G, et al. 18alpha-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. International Journal of Oncology. 2011;39:635–640. doi: 10.3892/ijo.2011.1061. [DOI] [PubMed] [Google Scholar]
- 62.Liu L, Yang M, Kang R, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25(1):23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Liu K, Yu Y, et al. Targeting HMGB1-mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy. 2012;8:275–277. doi: 10.4161/auto.8.2.18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Cheng Y, Ren X, et al. NAC1 modulates sensitivity of ovarian cancer cells to cisplatin by altering the HMGB1-mediated autophagic response. Oncogene. 2011;31:1055–1064. doi: 10.1038/onc.2011.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang R, Tang D. Autophagy in pancreatic cancer pathogenesis and treatment. American Journal of Cancer Research. 2012;2:383–396. [PMC free article] [PubMed] [Google Scholar]
- 66.Makinen PI, Yla-Herttuala S. Therapeutic gene targeting approaches for the treatment of dyslipidemias and atherosclerosis. Current Opinion in Lipidology. 2013;24(2):116–122. doi: 10.1097/MOL.0b013e32835da13c. [DOI] [PubMed] [Google Scholar]
- 67.Huschka R, Barhoumi A, Liu Q, Roth JA, Ji L, Halas NJ. Gene silencing by gold nanoshell-mediated delivery and laser-triggered release of antisense oligonucleotide and siRNA. ACS Nano. 2012;6:7681–7691. doi: 10.1021/nn301135w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Cresce C, Koropatnick J. Antisense treatment in human prostate cancer and melanoma. Current Cancer Drug Targets. 2010;10(6):555–565. doi: 10.2174/156800910791859452. [DOI] [PubMed] [Google Scholar]
- 69.Miyake H, Hara I, Gleave ME. Antisense oligodeoxynucleotide therapy targeting clusterin gene for prostate cancer: vancouver experience from discovery to clinic. International Journal of Urology. 2005;12(9):785–794. doi: 10.1111/j.1442-2042.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 70.Ke N, Zhou D, Chatterton JE, et al. A new inducible RNAi xenograft model for assessing the staged tumor response to mTOR silencing. Experimental Cell Research. 2006;312(15):2726–2734. doi: 10.1016/j.yexcr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Kaur P, Nagaraja GM, Asea A. Combined lentiviral and RNAi technologies for the delivery and permanent silencing of the hsp25 gene. Methods in Molecular Biology. 2011;787:121–136. doi: 10.1007/978-1-61779-295-3_10. [DOI] [PubMed] [Google Scholar]
- 72.Bisanz K, Yu J, Edlund M, et al. Targeting ECM-integrin interaction with liposome-encapsulated small interfering RNAs inhibits the growth of human prostate cancer in a bone xenograft imaging model. Molecular Therapy. 2005;12(4):634–643. doi: 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 73.Song DX, Chen AM, Guo FJ, et al. Differential proteomic analysis and function study of human prostate carcinoma cells with different osseous metastatic tendency. National Medical Journal of China. 2008;88(17):1197–1201. [PubMed] [Google Scholar]
- 74.Kerr DJ. Targeting angiogenesis in cancer: clinical development of bevacizumab. Nature Clinical Practice Oncology. 2004;1(1):39–43. doi: 10.1038/ncponc0026. [DOI] [PubMed] [Google Scholar]
- 75.van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, Hautvast P, Buurman WA, Griffioen AW. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene. 2013;32:363–374. doi: 10.1038/onc.2012.49. [DOI] [PubMed] [Google Scholar]
- 76.Ohmori H, Luo Y, Fujii K, et al. Dietary linoleic acid and glucose enhances azoxymethane-induced colon cancer and metastases via the expression of high-mobility group box 1. Pathobiology. 2010;77(4):210–217. doi: 10.1159/000296305. [DOI] [PubMed] [Google Scholar]
- 77.Luo Y, Chihara Y, Fujimoto K, et al. High mobility group box 1 released from necrotic cells enhances regrowth and metastasis of cancer cells that have survived chemotherapy. European Journal of Cancer. 2013;49:741–751. doi: 10.1016/j.ejca.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Smolarczyk R, Cichon T, Matuszczak S, et al. The role of Glycyrrhizin, an inhibitor of HMGB1 protein, in anticancer therapy. Archivum Immunologiae et Therapiae Experimentalis. 2012;60:391–399. doi: 10.1007/s00005-012-0183-0. [DOI] [PubMed] [Google Scholar]
- 79.Ohnishi M, Katsuki H, Fukutomi C, et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61:975–980. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 80.Cavone L, Muzzi M, Mencucci R, et al. 18β-Glycyrrhetic acid inhibits immune activation triggered by HMGB1, a pro-inflammatory protein found in the tear fluid during conjunctivitis and blepharitis. Ocular Immunology and Inflammation. 2011;19(3):180–185. doi: 10.3109/09273948.2010.538121. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi H, Kidachi Y, Kamiie K, Noshita T, Umetsu H. Structural insight into the ligand-receptor interaction between glycyrrhetinic acid (GA) and the high-mobility group protein B1 (HMGB1)-DNA complex. Bioinformation. 2012;8:1147–1153. doi: 10.6026/97320630081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai B, Deitch EA, Ulloa L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators of Inflammation. 2010;2010:10 pages. doi: 10.1155/2010/642462.642462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang X, Chavez ARDV, Schapiro NE, et al. Ethyl pyruvate administration inhibits hepatic tumor growth. Journal of Leukocyte Biology. 2009;86(3):599–607. doi: 10.1189/jlb.0908578. [DOI] [PubMed] [Google Scholar]
- 84.Saiwichai T, Sangalangkarn V, Kawahara KI, et al. Green tea extract supplement inhibition of HMGB1 release in rats exposed to cigarette smoke. Southeast Asian Journal of Tropical Medicine and Public Health. 2010;41(1):250–258. [PubMed] [Google Scholar]
- 85.Li F, Chen Z, Pan Q, et al. The protective effect of PNU-282987, a selective alpha7 nicotinic acetylcholine receptor agonist, on the hepatic ischemia-reperfusion injury is associated with the inhibition of high-mobility group box 1 protein expression and nuclear factor kappaB activation in mice. Shock. 2013;39:197–203. doi: 10.1097/SHK.0b013e31827aa1f6. [DOI] [PubMed] [Google Scholar]
- 86.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Molecular Medicine. 2008;14(9-10):567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai CH, Shi GY, Lee FT, et al. Recombinant human thrombomodulin suppresses experimental abdominal aortic aneurysms induced by calcium chloride in mice. doi: 10.1097/SLA.0b013e31827df7cb. Annals of Surgery. In press. [DOI] [PubMed] [Google Scholar]
- 88.Hagiwara S, Iwasaka H, Goto K, et al. Recombinant thrombomodulin prevents heatstroke by inhibition of high-mobility group box 1 protein in sera of rats. Shock. 2010;34(4):402–406. doi: 10.1097/SHK.0b013e3181d492e4. [DOI] [PubMed] [Google Scholar]
- 89.Iba T, Aihara K, Watanabe S, et al. Recombinat thrombomodulin improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat LPS model. Thrombosis Research. 2012 doi: 10.1016/j.thromres.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 90.Li LF, Yang CT, Huang CC, Liu YY, Kao KC, Lin HC. Low-molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B. Respiratory Research. 2011;12:p. 90. doi: 10.1186/1465-9921-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H, Yao Y, Su Z, et al. Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-α/JNK. American Journal of Physiology. 2011;300(3):H913–H921. doi: 10.1152/ajpheart.00703.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang CL, Shu MG, Qi HW, Li LW. Inhibition of tumor angiogenesis by HMGB1 A box peptide. Medical Hypotheses. 2008;70(2):343–345. doi: 10.1016/j.mehy.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 94.Kim HA, Park JH, Cho SH, Lee M. Lung epithelial binding peptide-linked high mobility group box-1 A box for lung epithelial cell-specific delivery of DNA. Journal of Drug Targeting. 2011;19(7):589–596. doi: 10.3109/1061186X.2010.547584. [DOI] [PubMed] [Google Scholar]
- 95.Jee SH, Kim K, Lee M. A high mobility group B-1 box A peptide combined with an artery wall binding peptide targets delivery of nucleic acids to smooth muscle cells. Journal of Cellular Biochemistry. 2009;107(1):163–170. doi: 10.1002/jcb.22112. [DOI] [PubMed] [Google Scholar]
- 96.Nogueira-Machado JA, Volpe CMDO, Veloso CA, Chaves MM. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opinion on Therapeutic Targets. 2011;15(8):1023–1035. doi: 10.1517/14728222.2011.575360. [DOI] [PubMed] [Google Scholar]
- 97.Moy KA, Jiao L, Freedman ND, et al. Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology. 2013 doi: 10.1002/hep.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiao L, Chen L, Alsarraj A, Ramsey D, Duan Z, El-Serag HB. Plasma soluble receptor for advanced glycation end-products and risk of colorectal adenoma. International Journal of Molecular Epidemiology and Genetics. 2012;3:294–304. [PMC free article] [PubMed] [Google Scholar]
- 99.Jiao L, Weinstein SJ, Albanes D, et al. Evidence that serum levels of the soluble receptor for advanced glycation end products are inversely associated with pancreatic cancer risk: a prospective study. Cancer Research. 2011;71(10):3582–3589. doi: 10.1158/0008-5472.CAN-10-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krechler T, Jáchymová M, Mestek O, Žák A, Zima T, Kalousová M. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clinical Biochemistry. 2010;43(10-11):882–886. doi: 10.1016/j.clinbiochem.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Jing R, Cui M, Wang J, Wang H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma. 2010;57(1):55–61. doi: 10.4149/neo_2010_01_055. [DOI] [PubMed] [Google Scholar]
- 102.Tesařová P, Kalousová M, Jáchymová M, Mestek O, Petruzelka L, Zima T. Receptor for advanced glycation end products (RAGE)—soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Investigation. 2007;25(8):720–725. doi: 10.1080/07357900701560521. [DOI] [PubMed] [Google Scholar]
- 103.Faham A, Bennett D, Altin JG. Liposomal Ag engrafted with peptides of sequence derived from HMGB1 induce potent Ag-specific and anti-tumour immunity. Vaccine. 2009;27(42):5846–5854. doi: 10.1016/j.vaccine.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 104.Saenz R, Souza CDS, Huang CT, Larsson M, Esener S, Messmer D. HMGB1-derived peptide acts as adjuvant inducing immune responses to peptide and protein antigen. Vaccine. 2010;28(47):7556–7562. doi: 10.1016/j.vaccine.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sonpavde G, Agarwal N, Choueiri TK, Kantoff PW. Recent advances in immunotherapy for the treatment of prostate cancer. Expert Opinion on Biological Therapy. 2011;11(8):997–1009. doi: 10.1517/14712598.2011.575357. [DOI] [PubMed] [Google Scholar]
- 106.DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM™) in patients with prostate cancer. Journal of Translational Medicine. 2006;4, article 1 doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madan R, Gulley J. The current and emerging role of immunotherapy in prostate cancer. Clinical Genitourinary Cancer. 2010;8(1):10–16. doi: 10.3816/CGC.2010.n.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


