Abstract
The advent of microprocessed “metabolic carts” and rapidly incremental protocols greatly expanded the clinical applications of cardiopulmonary exercise testing (CPET). The response normalcy to CPET is more commonly appreciated at discrete time points, for example, at the estimated lactate threshold and at peak exercise. Analysis of the response profiles of cardiopulmonary responses at submaximal exercise and recovery, however, might show abnormal physiologic functioning which would not be otherwise unraveled. Although this approach has long been advocated as a key element of the investigational strategy, it remains largely neglected in practice. The purpose of this paper, therefore, is to highlight the usefulness of selected submaximal metabolic, ventilatory, and cardiovascular variables in different clinical scenarios and patient populations. Special care is taken to physiologically justify their use to answer pertinent clinical questions and to the technical aspects that should be observed to improve responses' reproducibility and reliability. The most recent evidence in favor of (and against) these variables for diagnosis, impairment evaluation, and prognosis in systemic diseases is also critically discussed.
1. Introduction
Cardiopulmonary exercise testing (CPET) provides a means of unraveling abnormal physiologic functioning which may not be apparent at rest [1, 2]. The advent of microprocessed CPET systems [3] increased our technical capabilities in recording several variables throughout a single exercise bout—even of a relatively “short” duration of 10 minutes [4, 5]. The response normalcy to rapidly incremental CPET is more commonly judged by comparing the observed values at discrete time points (e.g., at the estimated lactate threshold (LT) and at peak exercise) with those previously obtained in apparently healthy subjects [6, 7]. It should be noted, however, that relying only in such discrete analysis leads to substantial loss of physiologic information given by the observation of the responses profiles during submaximal exercise and recovery [8–11].
In this context, authoritative textbooks [2, 12] and guidelines [13, 14] advocated that the trending of certain variables is a crucial component of the interpretative strategy as they might show substantial abnormalities even when the discrete values are still within the expected range [15–17]. Moreover, the response dynamics are highly reproducible [8–11], encompassing a range of exercise intensities which are likely to be faced by the patients in daily life [18–26]. Although the scientific foundations supporting their use have long been established, [8–17] they are still not routinely assessed and clinically valued in practice.
The purpose of this brief review, therefore, is to emphasize the practical usefulness of analyzing the response profiles of selected variables during rapidly-incremental CPET. Special care is taken to physiologically justify their use to answer relevant clinical questions and to the technical details that should be observed to improve responses' reproducibility and reliability. The response profiles to be discussed, however, are applicable to ramp-incremental [4] cycle ergometry, and the practitioner should be aware that different patterns of response can be anticipated if other ergometers (e.g., treadmill) and protocols (e.g., step-like) are used.
2. Metabolic Responses
2.1. Estimated Lactate Threshold
2.1.1. Physiological Background
The rate at which arterial lactate anions [Lac−]a and the associated proton (H+) accumulate as exercise progresses is directly related to the ratio between lactic acid (LA) release as a final byproduct of muscle anaerobic glycolysis and LA clearance by metabolism and buffering [29–31]. Although there seems to exist a period of time—not a discrete time point—in which LA production exceeds its rate of clearance, the term LA “threshold” (LT) [32, 33] is widely used. LA production increases as tissue O2 delivery diminishes [34] though some LA can be produced without any evidence of tissue hypoxia [35]. This justifies the notion that LA release during exercise is a reasonably sensitive (albeit non-specific) [36] marker of tissue anaerobiosis.
LA dissociates fast in Lac− and H+ in the physiological pH; that is, it is a strong acid. Plasma bicarbonate (HCO3 −) is the main buffer of lactic acidosis leading to the formation of carbonic acid (H2CO3) which in turn dissociates into carbon dioxide (CO2) and water; that is,
| (1) |
Although this reaction has the advantage to turn a fixed acid into a volatile gas, the “extra-CO2” (approximately 22–26 mL of additional CO2 is produced from each mEq decrease of [HCO3 −]) [31] derived from buffering of Lac−-associated protons will not only accelerate CO2 output relative to O2 uptake but also stimulate ventilation . These phenomena underlie the techniques for a noninvasive estimation of the LT.
2.1.2. Technical Considerations
As LA is buffered by HCO3 −, increases (1) out of proportion of , and a plot between these variables will show a discernible breakpoint; that is, the - relationship evidences an increased slope at the point of [Lac−]a increase. This is more commonly referred as the gas exchange threshold and determined by the V-slope method (Figure 1(a)) [37]. Increase in will drive in its direct proportion leading the latter to increase faster than . The consequent increase in (and the end-tidal partial pressure for O2, P ETO2) with a stable (and P ETCO2) establishes the so-called ventilatory threshold (Figure 1(b)) [38]. It should be noted that despite reflecting the same phenomenon (LA buffering), the gas exchange threshold slightly precedes the ventilatory threshold (VT) (Figure 1). After the LT, and P ET CO2 remain stable for a variable period of time during the “isocapnic buffering”. However, as more H+ is released with further increases in work rate, eventually increases out of proportion to at the respiratory compensation point (RCP) thereby leading to alveolar hyperventilation and progressive reductions in P ET CO2 towards the end of the test (Figure 1(b)).
Figure 1.
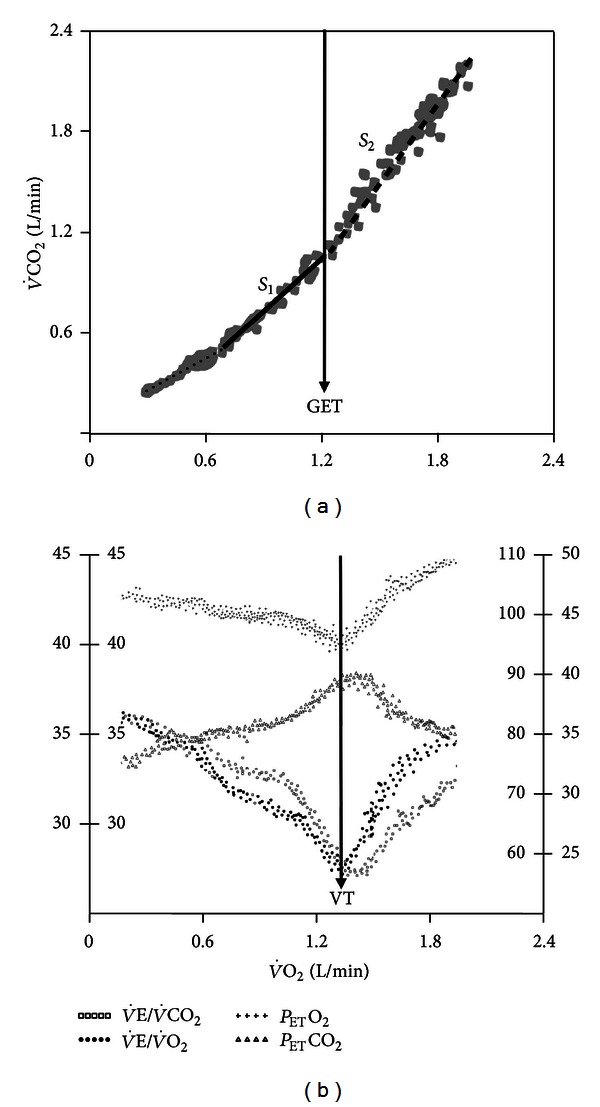
Noninvasive estimation of the lactate threshold by the V-slope method (gas exchange threshold (GET), panel (a)) and the ventilatory method (ventilatory threshold (VT), panel (b)) in a normal subject. Note that the GET slightly precedes the VT as the later depends on the ventilatory response to the “extra-CO2” generated by buffering of H+ associated with (lactate) increase. S 1 and S 2 refer to the two sequential slopes (before and after the GET) with S 2 being characteristically steeper than S 1 (i.e., slope inclination >1.)
Irrespective of the denomination, the following technical aspects for the LT estimation should be noted:
automatic estimations (by the CPET software) should be viewed with caution and routinely double-checked with manually determined values;
if an unitary tangent is used to estimate the LT in the V-slope plot, the range of and values should be the same as any discrepancy would invalidate its underlying mathematical (and physiological) principles [37] (Figure 1(b));
use of discrete R () values (i.e., > 1 from tabular data) as indicative of the LT might lead to erroneous estimations;
at any particular WR during a ramp-incremental test is lower than the steady-state value at that same WR due to a variable kinetics delay. As a result, the WR corresponding to LT precedes the WR in which the LT was identified by approximately 30–45 s (or even more in patients) [4]. Accordingly, if one is interested in exercising a subject at the LT, the selected WR should lead the WR-LT by this timeframe;
a given change in has a greater effect on CO2 release than O2 uptake by the lungs; consequently, preexercise hyperventilation may deplete the amount of CO2 stored in the body without major effects on O2 stores [39]. As the body capacitance for CO2 increases during the early phase of the ramp, repletion of the CO2 stores slows relative to ; that is, - slope in this region becomes shallow (“S 1” in Figure 1(a)). As the body CO2 reservoirs are filled in with exercise progression, the rate of CO2 storage will decrease thereby accelerating relative to [40]. This might mistakenly suggest the onset of lactic acidosis, that is, a “pseudo-LT” [41]. Precautions should therefore be taken to avoid hyperventilation prior to the noninvasive estimation of LT by the V-slope method;
LT should always be expressed relative to predicted peak not to the attained peak, especially in patient populations where the latter procedure might create a false concept of preserved (or even increased) LT, and
peak declines with senescence at a steeper rate than LT; that is, LT (% peak) increases as a function of age in both genders [41–43].
2.1.3. Clinical Usefulness
The physiologic changes associated with [Lac−]a and H+ accumulation (e.g., metabolic acidosis, impaired muscle contraction, hyperventilation, and altered kinetics) are important to document clinically as they are associated with reduced cardiopulmonary performance. An early LT is a marker of impaired aerobic metabolism [44–49] due to insufficient O2 delivery, increased recruitment of fast-twitch type II fibers which are metabolically less efficient than the slow-twitch type I fibers (i.e., have a greater O2/ATP ratio), and/or mitochondrial enzymatic dysfunction. The isolated analysis of the LT does not allow the differentiation of cardiovascular limitation from sedentarity though a severely decreased LT (e.g., <40% predicted peak) [6] is more frequently found in patients. A low LT has been found useful to predict an increased risk of post-operatory complications in the elderly [50, 51], worse prognosis in chronic heart failure (CHF) [52], and disease severity in pulmonary arterial hypertension (PAH) [53]. On the other hand, improvements in LT after pharmacological and nonpharmacological interventions have been associated with increased functional performance in a range of clinical populations [54–69]. Although there is lack of evidence that training at (or above) the LT is essential to improve exercise capacity in patients with CHF, coronary artery disease (CAD), and chronic obstructive pulmonary disease (COPD), training at higher intensities elicits larger physiological adaptations in less severe patients who are able to tolerate such regimens [54, 70, 71]. Training at the LT also seems to reduce the risk of complications during early phases of cardiac rehabilitation [72, 73]. In patients with COPD, however, LT cannot always be identified (even using the V-slope method), and when identified it varies widely as expressed in % peak [74]. In fact, important subjective improvements after rehabilitation can be found despite the lack of measurable physiological effects [75] which casts doubt on its usefulness to target exercise training intensity in these patients.
2.2. Δ Oxygen Uptake Work Rate (WR)
2.2.1. Physiological Background
From a relatively constant value of 500 mL/min at unloaded pedaling, increases linearly as exercise progresses during a rapidly-incremental exercise test [4]. The slope of the relationship, therefore, is an index of the overall gain of the response, and normal values would indicate adequate metabolic cost for the production of a given power output [4, 8].
2.2.2. Technical Considerations
For an accurate calculation of the slope, any delay in increase at the start of the ramp or any eventual plateau near the end of exercise should be discarded (Figures 2(a) and 4). Considering that the LT can potentially distort the response's linearity [157–160], it is advisable to check if there is an inflection point in the at the LT. If this is discernible, the slope should be calculated over the sub-LT range.
Figure 2.
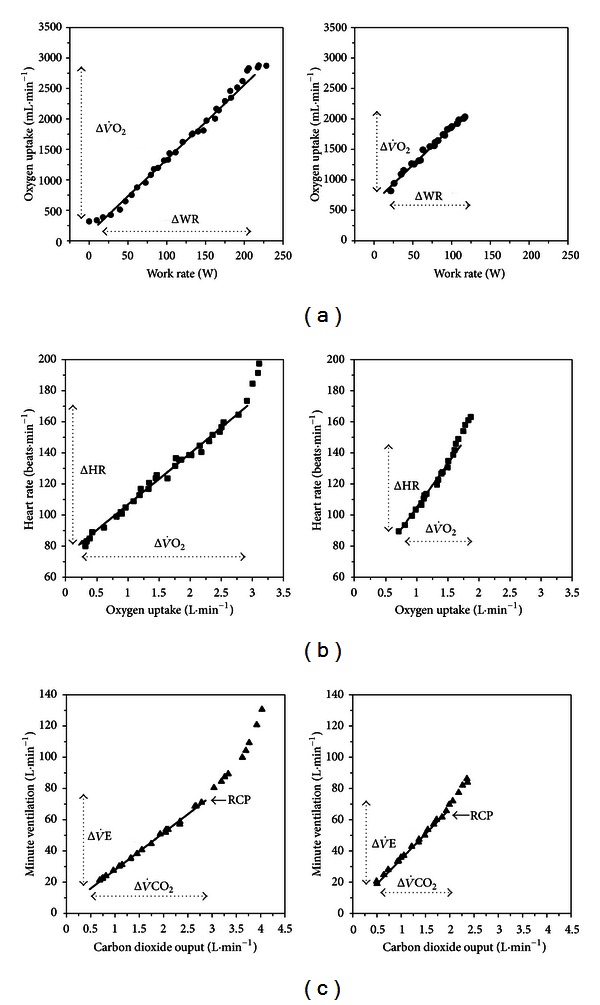
Procedures to establish 3 dynamic submaximal relationships by simple linear regression during incremental CPET in young (24-yr-old, left panels) and old (70-yr-old, right panels) subjects. (a) Δ oxygen uptake work rate (WR); (b) Δ heart rate (c) Δ minute ventilation carbon dioxide output . The arrows show the range of values considered for analysis. RCP is the respiratory compensation point. (Modified with permission from [10].)
Figure 4.
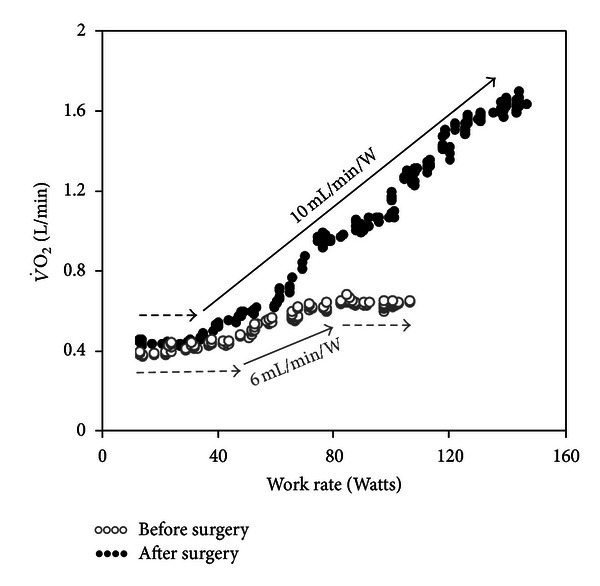
Oxygen uptake work rate (WR) relationship during ramp-incremental CPET before and after pulmonary endarterectomy in a 21-year-old male with thromboembolic occlusion of the left pulmonary artery. Note that after the surgery, peak increased not only due to a higher peak WR but also owing to a large improvement in .
2.2.3. Interpretative Issues
is not significantly influenced by the training status, ageing, or gender (Figure 3(a)) [2, 10, 12–14]. A shallow over the entire range of values and/or a shift from a linearly increasing profile to a shallower rate of change has been shown to be indicative of circulatory dysfunction [77–80] (Figure 4) and severe impairment in mitochondrial function [81]. The latter pattern of response has been found to enhance ECG sensitivity to detect myocardial ischemia [82–86], and some studies suggested that it might be useful to unravel early abnormalities in the coronary microcirculation [87, 88].
Figure 3.
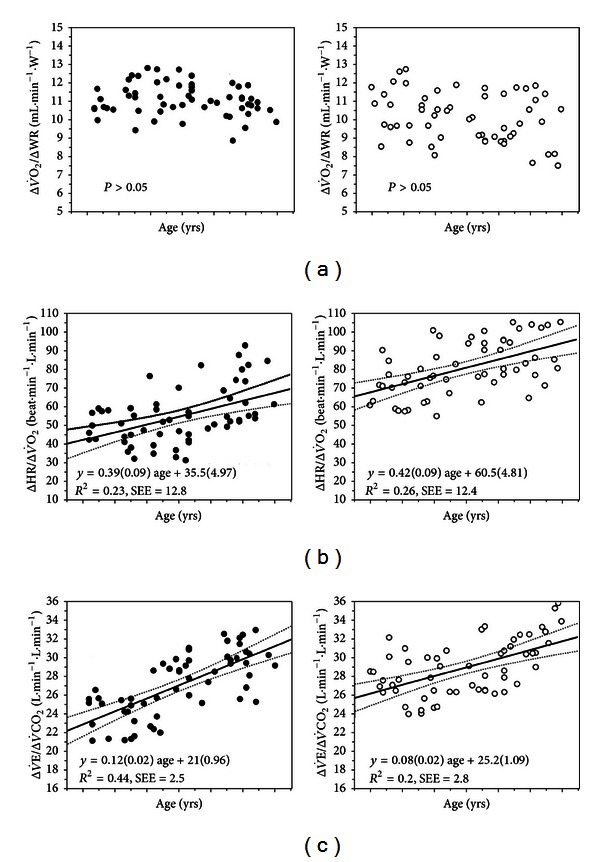
The submaximal relationships depicted in Figure 2 as a function of age in males (left panels) and females (right panels). Regression lines are shown with their respective 95% confidence intervals for those relationships in which the variables were influenced by age. Regression coefficients and intercepts of the linear prediction equations are depicted with their respective standard error of the estimate (SEE). (Modified with permission from [10]).
2.3. Efficiency
2.3.1. Physiological Background
increases curvilinearly relative to in response to a ramp-incremental exercise test. At least in theoretical grounds, several variables known to interfere with both and would bear an influence in this relationship; that is, it is deemed to be modulated by cardiovascular, pulmonary, and muscular factors [161–168]. Most authors have expressed the - relationship with as the dependent variable [89, 165, 169]. In this construct, higher values (or steeper rates of change) for a given would indicate a more “efficient” O2 uptake by the lungs. It should be emphasized, however, that exercise is more closely related to than [170] which makes the concept of efficiency prone to misinterpretation (see Section 2.3.3).
2.3.2. Technical Considerations
Baba and coworkers [165] proposed a logarithmic transformation of over the entire exercise period to “linearize” this relationship, the so-called efficiency slope (OUES) (Figure 5(a)). More recently, Sun et al. [89, 169] expressed the OUE as a ratio ( in mL/L) over time which, as expected, gives a mirror image of the ventilatory equivalent for O2. The authors proposed the term OUE plateau (OUEP) to the 90 s-average of the highest consecutive measurements; that is, the values just before the LT (Figure 5(b)). Although they reported that OUEP was more reproducible than OUES, this was not yet independently confirmed. It has been claimed that both relationships are independent of interobserver variability and effort [90, 164, 171–173]. However, Williamson et al. [173] recently found that there was a significant increase in OUES as exercise moved from low to moderate intensity with a peak value at an RER value of 1.0. Oscillatory breathing (see Section 3.3) has been found to interfere little with OUE estimations [89]. It should be recognized that both OUES and OUEP require separate computation though some commercially available CPET systems allow logarithmic transformations for OUES calculation.
Figure 5.
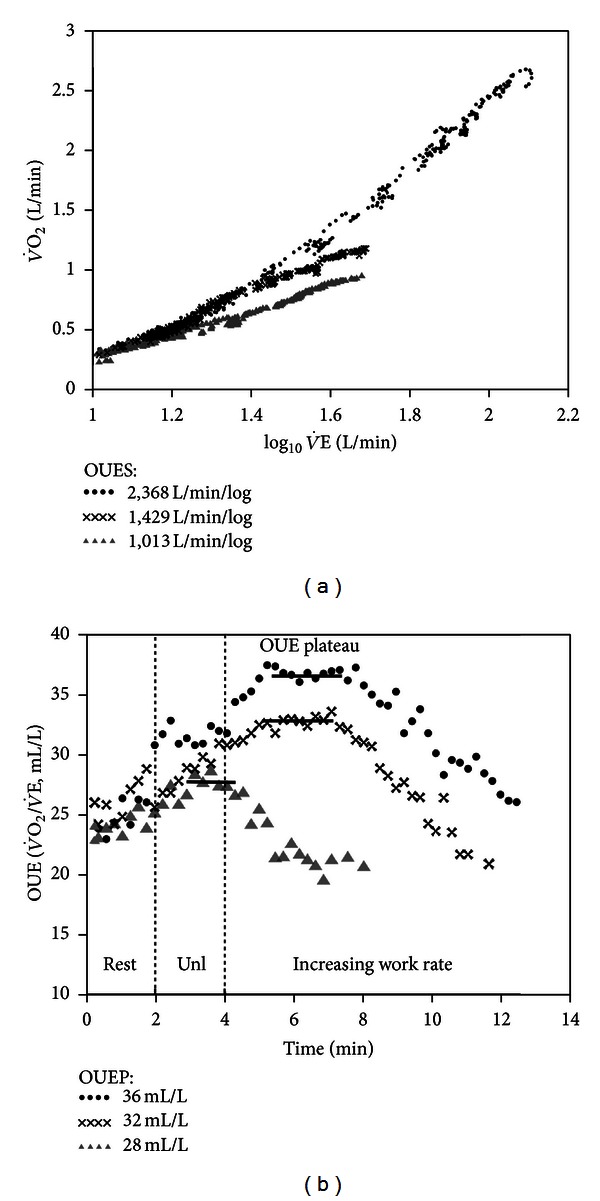
Relationship between oxygen uptake and minute ventilation during incremental exercise in a healthy subject (∙∙∙∙) and patients with mild (xxxx) and severe (▲▲▲▲) CHF. (a) The slope of upon is the oxygen uptake efficiency slope (OUES) which gives the rate of increase in for a 10-fold rise in . (b) The highest ratio is the efficiency slope (OUEP) which is the average of values just prior to the estimated lactate threshold. Unl is unloaded pedaling.
2.3.3. Interpretative Issues
It is well established that exercise hyperpnea is under stronger influence of P aCO2 and pHa (rather than P aO2) [170]. As detailed later (Section 3.1), changes in CO2 set-point and ventilatory “efficiency” control the rate of CO2 clearance. This brings substantial uncertainty on the exact physiological meaning of a disturbed relationship between and . Nevertheless, the literature pertaining to the clinical usefulness of OUES is rather vast in CHF [90, 164, 165, 167, 171, 172], and interest in this relationship has been spread to other populations (cystic fibrosis, and surgical candidates) [174, 175]. A number of studies have found that OUES is strongly correlated with peak [90, 164, 165, 167, 171, 172, 176, 177] and may hold prognostic value in CHF [18, 89–94]. However, the prognostic advantage of OUES over slope remains unclear [178, 179]. In the pediatric group, mixed results were reported and at least one study found that OUES determined at different WRs differed significantly within patients with cystic fibrosis and correlated only moderately with peak and VT [180]. Interestingly, OUES showed to be more sensitive to the effects of training than slope in patients with CHF [96], a finding correlated with enhanced cerebral and muscle hemodynamics in another study [95]. On a single investigation from the group which proposed OUEP, this relationship either on isolation or in combination with oscillatory breathing was prognostically superior to traditional key CPET parameters in CHF [89]. Predicting equations for OUES and OUEP have been recently published [169].
2.4. Postexercise
2.4.1. Physiological Background
After ramp-incremental exercise, does not decline immediately towards the resting level. The traditional view is that there would be a “debt payment” of energy deficit contracted at the start of effort (O2 deficit). Indeed, the time course of recovery after a moderate, constant test has been found to track the rate of phosphocreatine resynthesis [181]. At early recovery, replenishment of local O2 sources in muscles (oxymyoglobin and dissolved O2) and reloading of haemoglobin are also needed [182]. At later stages, lactate metabolism (oxidation or gluconeogenesis) and increased cathecolamines and temperature also interfere with the dynamics of decrease [183, 184].
2.4.2. Technical Considerations
during recovery has been evaluated by (a) the ratio between total during exercise and recovery [185], (b) the time constant of decay (i.e., time to reach 63% of the lowest value as obtained by fitting a decreasing monoexponential function) [182, 186, 187], (c) t 1/2 (time required for to decrease to half of its peak value) [185, 188–190], and (d) -slope (the response slope during the first minute of recovery by linear regression) [188, 189]. A further increase in during recovery [191] (i.e., a “overshoot”) has been found indicative of severe hemodynamic dysfunction as it reflects prolonged kinetics [192, 193]. Importantly, the level of effort seems not critical for a valid analysis of post-exercise dynamics [190].
2.4.3. Interpretative Issues
Delayed recovery has been related to functional impairment in CHF [188, 189, 192, 194], myocardial ischemia [195], COPD [196], and functional impairment in several conditions, including cystic fibrosis [197], diabetes [198], deconditioning [199], and obstructive sleep apnea [139]. Impairment in cardiovascular responses to exercise as indicated by a delayed recovery of cardiac output was closely associated with slower off-exercise kinetics in CHF [200]. Improvements in O2 delivery might be expected to speed the rate of O2 recovery in cardiovascular diseases (Figure 6) [201].
Figure 6.
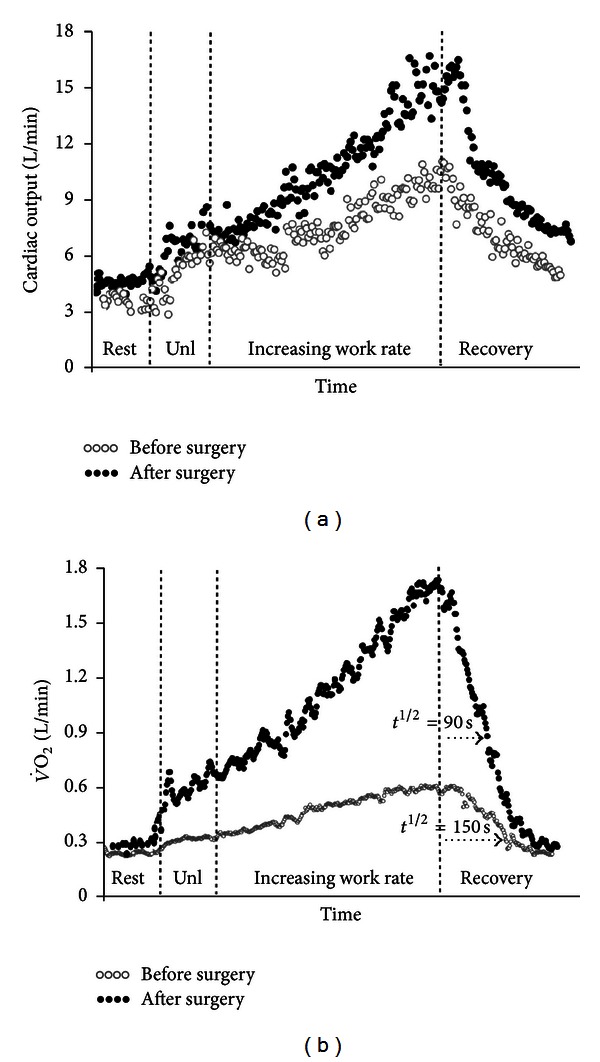
Incremental cycle ergometer exercise tests in the same patient of Figure 4 with chronic thromboembolic pulmonary hypertension. After pulmonary endarterectomy (closed symbols), haemodynamic improvement (panel (a)) led to a higher oxygen uptake at peak exercise and a faster (lower half-time (t 1/2) post-exercise decrease in (panel (b)). Cardiac output was noninvasively estimated by impedance cardiography and the tests were time-aligned by total exercise duration. Unl is unloaded pedaling.
3. Ventilatory Responses
3.1. Excess Exercise Ventilation
3.1.1. Physiological Background
Adequate increases in alveolar ventilation () are paramount to wash out metabolically produced CO2. Exercise for a given is inversely related to the prevailing level at which P aCO2 is regulated (the CO2 “set-point”) and the dead space (V D)/tidal volume (V T) ratio; that is,
| (2) |
Consequently, the largest values will be found in those who chronically hyperventilate (low CO2 “set-point”) and have the large V D coupled with a low V T [202–206]. In the clinical literature, an increased slope of the - relationship has been termed ventilatory “inefficiency” though it could be argued that there is no “inefficiency” when increased results from alveolar hyperventilation. “Excess exercise ventilation” seems therefore a more appropriated description of a greater-than-expected ventilatory response to metabolic demand [205].
3.1.2. Technical Considerations
There are a number of alternatives to express the - relationship during progressive exercise: (1) as a ratio () at peak exercise, at the VT (Figure 1(b)), and as the lowest (nadir) value and (2) as a slope of versus from the beginning of exercise to the RCP () (Figure 2(c)) or, alternatively, up to peak exercise () (Figure 7) [25, 26, 207]. Sun et al. reported that the had the least variability with the advantage that choosing the lowest value does not require VT identification [26]. However, might not decline at all during early exercise in some patients with severe cardiopulmonary disease (Figure 8) which might preclude LT identification. P aCO2 is relatively constant up to the RCP, and, as described (2), a steeper-than-normal can be explained by a higher V D/V T and/or a low CO2 set point. is expected to be even steeper than (Figure 7(a)) because the former adds a component of hyperventilation to lactic acidosis and/or to other sources of stimuli at near maximum exercise [26, 207]. It should be emphasized, however, that there are interpretational pitfalls of using as a single linear characterization of a relationship which is characteristically curvilinear (Figure 7). is equal to when the slope has an y-intercept of zero. However, has a positive y-intercept in normal subjects [208] which explains why is usually greater than the slope. will also exceed the slope if the VT is a low value (i.e., in less fit subjects) [10]. On the other hand, a very steep would produce a negative y-intercept thereby making it greater than [205].
Figure 7.
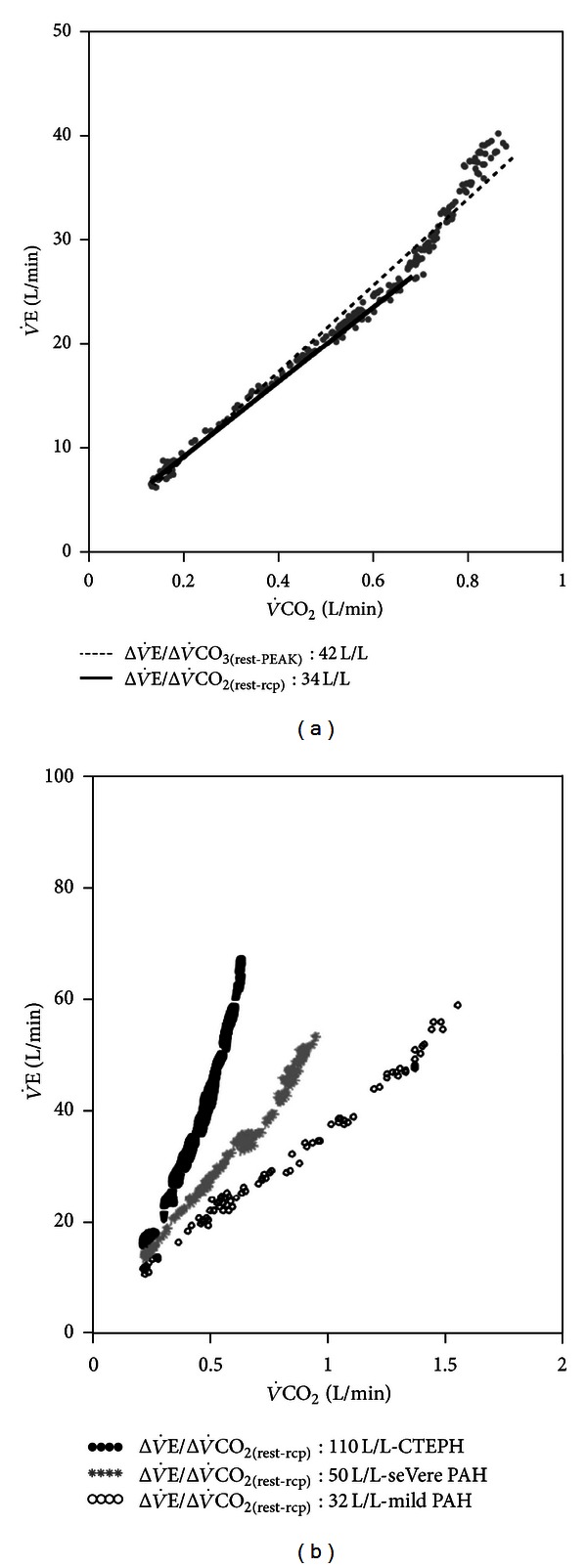
(a) Minute ventilation carbon dioxide output () relationship from the beginning of exercise to the respiratory compensation point (solid line) or up to peak exercise (dashed line) in a patient with CHF. Note that is steeper than because it adds a component of hyperventilation to lactic acidosis and/or other stimuli after the respiratory compensation point. (b) as a function of disease severity in pulmonary arterial hypertension (PAH). Higher values, however, are usually found in chronic thromboembolic pulmonary hypertension (CTEPH) due to pronounced increases in tidal volume ratio.
Figure 8.
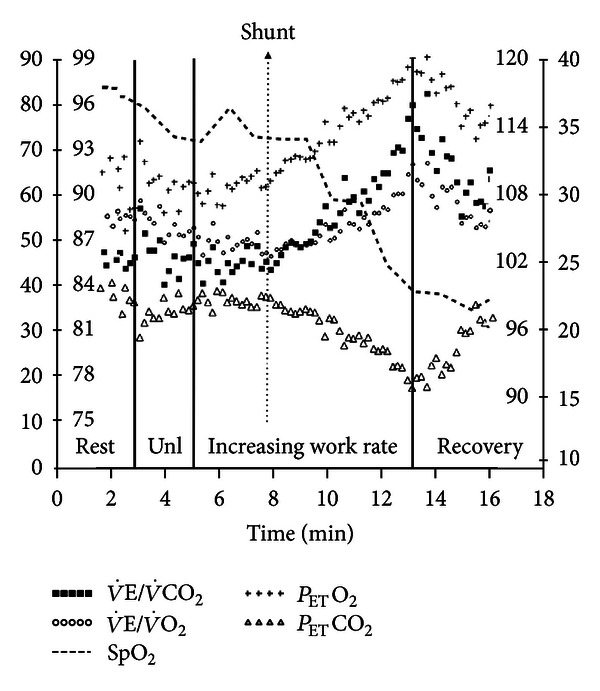
Exercise-induced right-to-left shunt as suggested by sudden decrease in oxyhemoglobin saturation by pulse oximetry (SpO2) and abrupt increases in the ventilatory equivalents for CO2 and O2 ( and ) associated with a sustained decrease in the end-tidal partial pressure for CO2 (P ETCO2) with a concomitant increase in P ETO2 in a patient with pulmonary arterial hypertension. Shunting of systemic venous blood in the arterial circulation stimulated the peripheral chemoreceptors thereby leading to this pattern of ventilatory and gas exchange responses. Unl is unloaded pedaling.
3.1.3. Interpretative Issues
in healthy young males is approximately 30 [25, 26]; however, it increases with age probably as a result of larger V D/V T in older subjects [10, 11]. Females have lower V T for a given than males independent of senescence which might explain their higher across all age ranges (Figure 3(c)) [10, 11]. There is plenty of evidence that is clinically useful as a prognostic marker in CHF [52, 108, 109, 163, 209–212] and, more recently, in PAH [97, 98, 213] with more discriminatory information than peak. The prognostic value in CHF persisted in patients on β-blockers [99, 100]. Interestingly, has been found better than to predict 1-year cardiac mortality and hospitalization in these patients [207]. As expected, composite scores adding to other cardiopulmonary variables improved even further their prognostic value [211]. A single study found that coexistence of COPD tends to “normalize” in CHF patients which casts doubt on its prognostic usefulness in this specific subpopulation [214].
In patients with PAH, and (at rest, VT, and peak) are higher compared to CHF [215]. > 37 plus P ETCO2VT < 30 mmHg increased the probability of pulmonary vascular disease [111]. In those with idiopathic PAH, higher and (VT and nadir) were related to clinical [53] and hemodynamic impairment [104]. Importantly, these indexes improved with specific treatment [104, 105] and after pulmonary endarterectomy [106]. Although to date there is a lack of evidence that indices of excess exercise ventilation in PAH hold the same prognostic importance as in CHF, Deboeck et al. recently described that (and the 6-min walking distance) were independent predictors of death [98]. Oudiz et al., however, found that was valuable to prognosis assessment only when exercise-induced right-to-left shunt (Figure 8) was absent [119]. Although is particularly disturbed in chronic thromboembolic pulmonary hypertension (CTEPH) (Figure 7(b)), thrombotic vessels occlusion increases V D/V T and excess exercise ventilation to levels which may not be proportionately related to hemodynamic impairment [216].
In patients with other chronic respiratory diseases, > 34 increased the risk of post-operative complications after lung resection surgery with better prediction power than peak and predicted post-operative peak [110]. It could also be empirically expected that a low would be rarely associated with increased V D/V T whereas the opposite would be likely at very high . In fact, Roman and coworkers recently described that when was ≤28 and within 29–32, 96% and 83% of subjects had normal V D/V T. On the other hand, V D/V T was abnormal in 87% of the cases when was ≥39. Unfortunately, intermediate values were not useful to discriminate the underlying mechanisms. Interestingly, 95% of the patients with an obstructive ventilatory defect (FEV1/FVC < 0.7) and ≥ 39 had increased V D/V T [217].
3.2. End-Tidal Partial Pressure for CO2
3.2.1. Physiological Background
Expired CO2 concentration increases as air from the serial (“anatomic”) V D is progressively enriched with CO2 from the gas exchanging areas. Consequently, the largest partial pressures for CO2 are found at the end of tidal expiration (P ETCO2). However, P ETCO2 is influenced not only by the metabolic rate (i.e., the rate of increase in mixed venous P CO2) but also by the deepness of the previous inspiration (i.e., VT) and the duration of the exhalation. P ETCO2 reflects poorly P aCO2, (ideal alveolar) as there are significant regional variations in alveolar P CO2 (P ACO2) and -to-perfusion ratios—even in normal subjects [2, 16]. It should also be recognized that P ETCO2 becomes systematically greater than P aCO2 during incremental exercise as the first is the peak of the intrabreath oscillation of P ACO2 and P aCO2 measured in peripheral arterial blood is an average of the oscillation over several breaths [2, 16].
3.2.2. Technical Considerations
P ETCO2 increases from rest to LT (which is proportional to decrease in) in this time range, followed by a stable phase during the isocapnic buffering period, and then a fall after the RCP (Figures 1(b) and 9(a)). As mentioned, P aCO2 underestimation by P ETCO2 is roughly proportional to V D/V T; consequently, computing V D/V T using P ETCO2 instead of P aCO2 overestimates V D/V T in normal subjects and underestimates it in patients [218].
Figure 9.
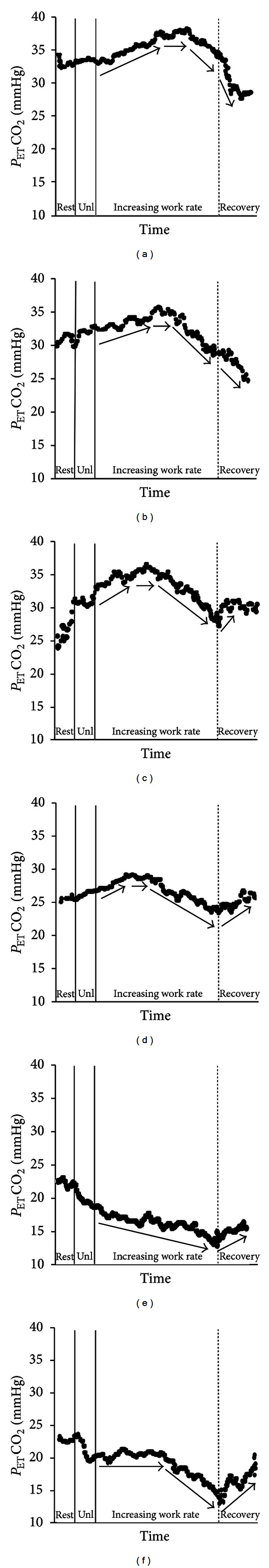
Time course of end-tidal partial pressure for carbon dioxide (P ETCO2) during incremental exercise and early recovery in a healthy control (panel (a)) and five patients with pulmonary arterial hypertension of progressing severity (panels (b) to (f)). Note that P ETCO2 becomes lower and even fails to increase as disease progresses. Moreover, it frequently increases (instead of diminishing) during recovery. Panel (f), in particular, depicts a severely impaired patient showing abrupt and sustained decrease in P ETCO2 concomitant with the opening of a forame ovale (Figure 8). Unl is unloaded pedaling.
3.2.3. Interpretative Issues
P ETCO2 differs from P aCO2 as a result of ventilation-to-perfusion inhomogeneities, right-to-left shunt, and changes in breathing pattern [2, 16]. However, arterial blood gases are not routinely measured during clinical CPET. Consequently, interpretation of a reduced P ETCO2 is complex in the absence of P aCO2 measurements as it might be related to abnormal gas exchange, alveolar hyperventilation, or a tachypneic and shallow pattern of breathing. Regardless of the exact mechanism, abnormally low values at the LT have been found useful for the diagnosis of pulmonary vascular diseases in patients with unexplained dyspnea [111]. There is now established evidence that P ETCO2 at rest [112–114], LT [115], and peak exercise [116] are valuable for prognosis estimation and disease severity assessment in CHF [219, 220]. Low P ETCO2 values have also been found in PAH (see also later) [97, 111, 117, 118]. Decreased P ETCO2 at rest and during exercise seems to track the blunted cardiac output response to exercise in cardiovascular disease [219, 221]. Accordingly, exercise training after acute myocardial infarction increases both P ETCO2 and cardiac output [120]. In addition to reduced cardiac output, an augmented ventilatory drive may also account for a reduction in P ETCO2 whereas altered breathing pattern seems to have a minor role in CHF [204].
P ETCO2 is typically lower in PAH than CHF [111, 219]. In fact, Yasunobu and co-workers suggested that observation of an unusually low P ETCO2 at the LT (<30 mmHg or, in particular, <20 mmHg) in a patient with exertional dyspnea of unknown cause without evidence of acute hyperventilation (ie, normal R) should prompt the hypothesis of pulmonary vasculopathy [111]. P ETCO2 response profile is also informative as failure to increase below the LT or progressive decreases from the start of exercise are associated with worsening clinical and hemodynamic impairment (Figures 9(b) to 9(e)) [111] and are rarely found in CHF [112–116]. Based on (2), it might be expected that if P ETCO2 changed parallel to P ACO2, a hyperbolic relationship between and P ETCO2 at the LT would result. As this was observed by Yasunobu et al. [111] and confirmed by others [104, 216], it seems that alveolar hyperventilation is an important contributing mechanism to the excess exercise ventilation in PAH. Moreover, sharp decreases in P ETCO2 may indicate exercise-induced intracardiac shunt, a finding with ominous consequences (Figures (8) and 9(f)) [119]. Additionally, an abnormal increase in P ETCO2 during early recovery has been described in PAH (Figure 9(c)), even in mildly-impaired patients [111].
3.3. Exertional Oscillatory Ventilation (EOV)
3.3.1. Physiological Background
An abnormal pattern of ventilation consisting of cyclic hyperpnea and hypopnea without interposed apneas can be detected by CPET in some patients with advanced CHF. The EOV might occur throughout the test, but the oscillations frequently dampen as exercise progresses [121, 222–224]. The pathophysiological mechanisms are multifactorial including low cardiac output leading to a prolonged time of pulmonary venous blood to reach the central or peripheral chemoreceptors, low lung volume, pulmonary congestion, augmented chemoreceptor sensitivity, and the narrow difference between the eupneic P aCO2 and the apneic (or hypoventilatory) threshold [27, 122, 123, 225–235].
3.3.2. Technical Considerations
Different criteria for EOV might help explaining why its prevalence has been found to vary from 12% to 50% in CHF [123, 124, 236–238]. A widely used definition is as follows (Figure 10): (1) three or more regular oscillations (i.e., clearly discernible from inherent data noise); (2) standard deviation of three consecutive cycle lengths (time between 2 consecutive nadirs) within 20% of the average; (3) minimal average amplitude of oscillation of 5 L/min (peak value minus the average of two in-between consecutive nadirs) [27]. Alternative definitions require: (i) criteria for persistence of the EOV pattern (three or more consecutive cyclic oscillations) for at least 60% of exercise at an amplitude ≥ 15% of the average resting value [122, 239–241] or (ii) 3 or more consecutive cyclic fluctuations with amplitude exceeding 30% of mean and oscillatory cycle within 40 to 140 s in 3 or more gas exchange/ventilatory variables [124].
Figure 10.
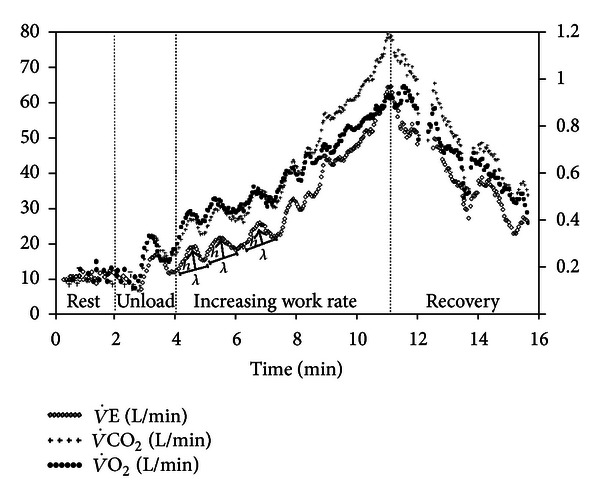
Exertional oscillatory ventilation (EOV) during incremental CPET in a 56-yr-old male with severe CHF. EOV was defined by regular (standard deviation of three consecutive cycle lenghts (λ) within 20% of their average) and ample (minimal h of 5 L/min) cycles of ventilatory oscillations [27]. A similar oscillatory pattern is also seen in oxygen uptake and carbon dioxide output .
3.3.3. Clinical Usefulness
There is now well-established evidence that EOV holds important negative prognostic implications in patients with CHF [27, 124, 222, 236, 239], being related to worsening clinical status [121, 122, 124], severe hemodynamic dysfunction [123], and reduced functional capacity [125, 126]. Unfortunately, EOV may preclude an adequate identification of the LT by either the V-slope or the ventilatory equivalent methods [242]. EOV is highly reproducible regardless of the CHF aetiology [121]. Interestingly, several interventions including inotropics [237], exercise and inspiratory muscle training [243–245], and transplantation [237] lessened of even abolished EOV. Future larger trials should establish whether EOV might add independent information to commonly used outcomes for interventional studies in CHF.
4. Cardiovascular Responses
4.1. Δ Heart Rate (HR)/Δ Oxygen Uptake
4.1.1. Physiological Background
Increases in HR with progressive exercise are initially mediated by parasympathetic tonus withdrawal and, subsequently, by increased sympathetic activity [246]. There is an effectively linear increase in HR as a function of during ramp-incremental exercise [3, 24, 25] though departs from linearity might occur at higher exercise intensities (Figure 2(b)) [247]. According to the Fick principle, reduced stroke volume (SV) and/or diminished C(a–v)O2 would lead to a steeper slope. Consequently, cardiac dysfunction, decreased arterial O2 content (anemia and hypoxemia), and impaired muscle aerobic capacity (e.g., deconditioning, mitochondrial dysfunction) can potentially increase . On the other hand, training has a flattening effect on (Figure 11).
Figure 11.
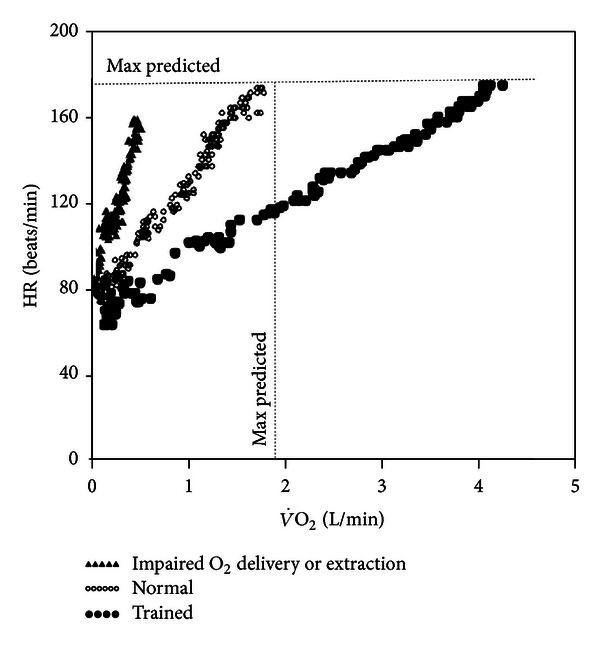
Heart rate (HR) response as a function of O2 uptake in 3 males of same age: a patient with abnormal O2 delivery and/or extraction (severe pulmonary arterial hypertension, = 158 beats/L), a normal sedentary subject ( = 65 beats/L), and a triathlete ( = 26 beats/L).
4.1.2. Technical Considerations
Although is the appropriate dependent variable, this relationship has been traditionally described with HR on the y-axis [3, 24, 25]. Linearity of the HR response throughout the test duration should be firstly established. In event of late departures from linearity, the slope should be calculated only over the initial linear phase response (Figure 2(b)). As detailed later, pronounced changes in linearity may hold important clinical implications.
4.1.3. Clinical Usefulness
increases with age being consistently higher in females than males (Figure 3(b)) [10]. As expected, cardiovascular and muscular diseases which are known to impair O2 delivery and/or utilization have been found to increase both the slope and the intercept of the relationship [127–130]. Some specific conditions, however, may prevent HR to increase even in the presence of disease: (a) patients under β-blocker therapy [248], (b) ischemic involvement of the sinusal node artery [249], and (c) advanced CHF [250]. The so-called O2 pulse ( ratio) is a commonly used derivation of . As the primary -HR relationship has a negative y-intercept, O2 pulse increases hyperbolically [16] towards an asymptotic value at end-exercise (Figure 13(a)) which might reflect the SV response [131]. However, all pathologic conditions known to increase (including desaturation, anemia, and impaired O2 extraction) will also diminish peak O2 pulse. Moreover, early exercise termination due to symptom limitation (including breathlessness in patients with COPD) (Figure 13(b)) and/or submaximal effort would decrease peak O2 pulse in the absence of cardiovascular limitation. In these cases, however, a normal is reassuring. A more clinically useful pattern of response relates to abrupt increases in slope to an extent that the relationship goes through its origin or becomes with a negative y-intercept; that is, O2 pulse turns flat (Figure 12) or even decreases (Figure 13(d)). This suggests that the HR response became the sole mechanism for cardiac output increase due to a severely impaired SV response. In practical grounds, there is limited evidence that as myocardial perfusion is reduced in patients with coronary artery disease, there is reversible left ventricle dysfunction thereby steepening (Figure 12(a)) and flattening (Figure 12(b)) (or even decreasing) (Figure 13(d)) O2 pulse [88, 132, 133].
Figure 13.
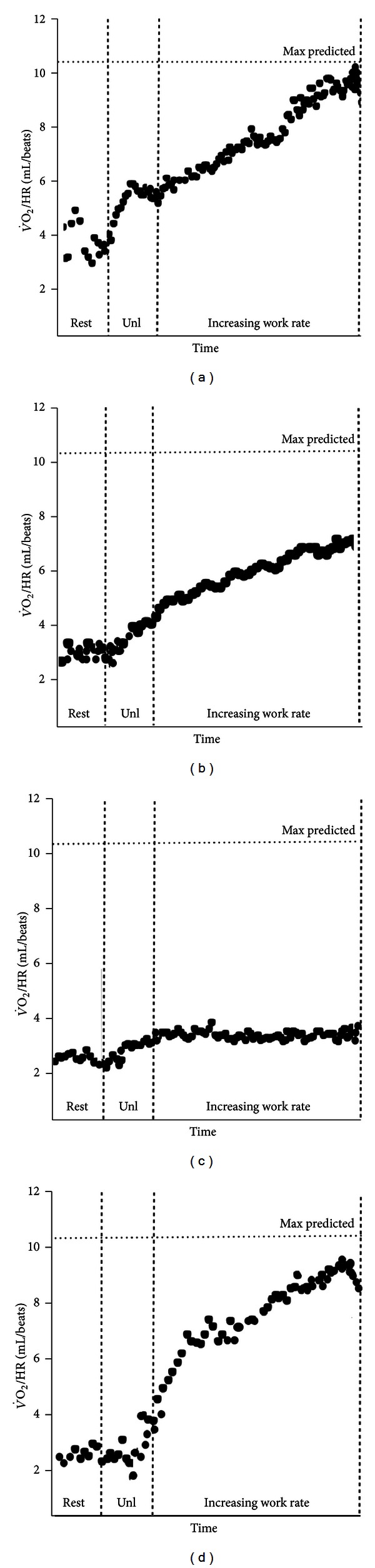
O2 pulse as a function of time during incremental exercise. (a) Curvilinear increase up to a normal predicted value in a healthy subject; (b) abnormally low peak values due to ventilatory limitation and early exercise cessation in a patient wirh COPD; (c) failure to increase and early plateau in a patient with end-stage pulmonary arterial hypertension; (d) decrease at near maximum exercise in a patients with concomitant electrocardiographic abnormalities indicative of coronary artery disease. Unl is unloaded pedaling.
Figure 12.
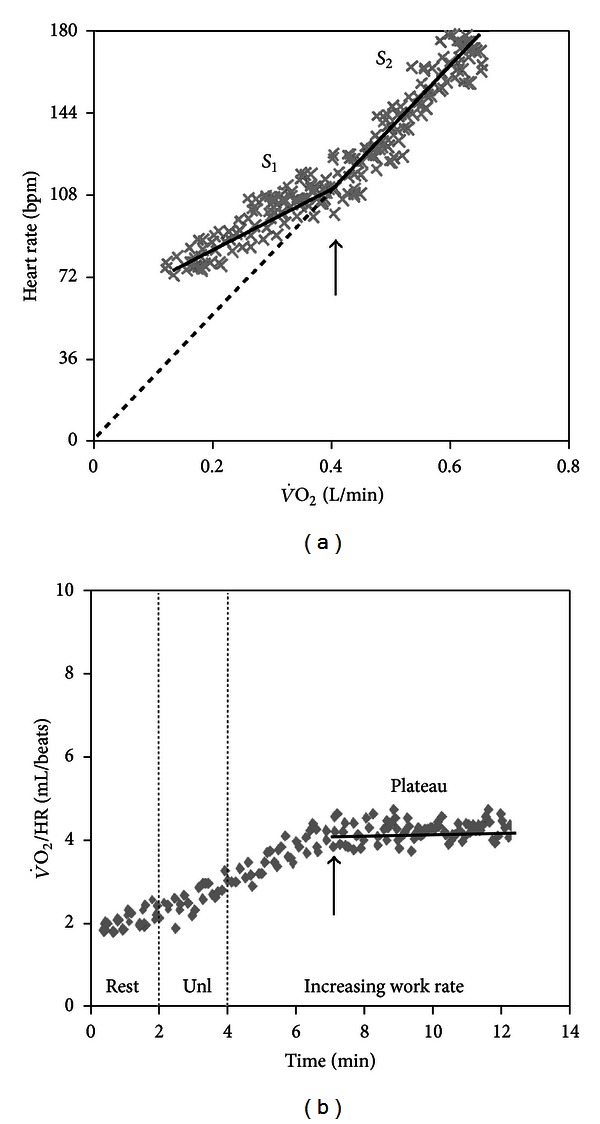
Change in Δ heart rate (HR)/Δ oxygen uptake (arrow) slope (arrow) during incremental CPET in a patient with severe cardiovascular limitation to exercise (panel (a)). Note that this led to a plateau in O2 pulse ( ratio) as the y-intercept becomes zero; that is, the relationship passes through its origin (panel (b)). Unl is unloaded pedaling.
4.2. Heart Rate Recovery (HRR)
4.2.1. Physiological Background
At the start of exercise, HR increases as a result of early parasympathetic withdrawal and subsequent sympathetic activation [246]. After effort cessation, vagal reactivation (with opposition of the sympathetic drive) is primarily responsible for the return to baseline conditions [251], especially during the first 30 seconds of recovery [252]. Consequently, autonomic imbalance (increased sympathetic stimuli and/or impaired parasympathetic activity) might slow post-exercise HR decay.
4.2.2. Technical Considerations
HRR is the difference between peak HR and HR at selected time points after exercise (e.g., 30 sec and every minute thereafter). HRR analysis may be performed independent of the mode of exercise (treadmill [134, 135, 140, 152, 253], cycle ergometer [28, 254–256], or field tests [257]), and a cool-down period at the end of maximal effort seems not to interfere with its prognostic value [28, 134, 150].
4.2.3. Interpretative Issues
HRR has been found a simple and inexpensive prognostic marker in healthy populations [134], CHF [135], CAD [151, 258], PAH [28] (Figure 14), diabetes mellitus [136], and COPD [137]. Abnormal HRR has also been demonstrated in other systemic disorders such as metabolic syndrome [138], obstructive sleep apnea [139], sarcoidosis [140], rheumatological diseases [141, 142], polycystic ovary syndrome [143], polycystic kidney disease [144], and HIV infection [145]. Of note, it has been useful for risk stratification in CHF patients with mildly reduced peak [259]. HRR seems to be responsive to exercise training in some disorders [146–149], probably due to effects of exercise on autonomic regulation [260, 261]. Interestingly, these modifications were related to increased survival after rehabilitation in patients with previous myocardial infarction [262, 263].
Figure 14.
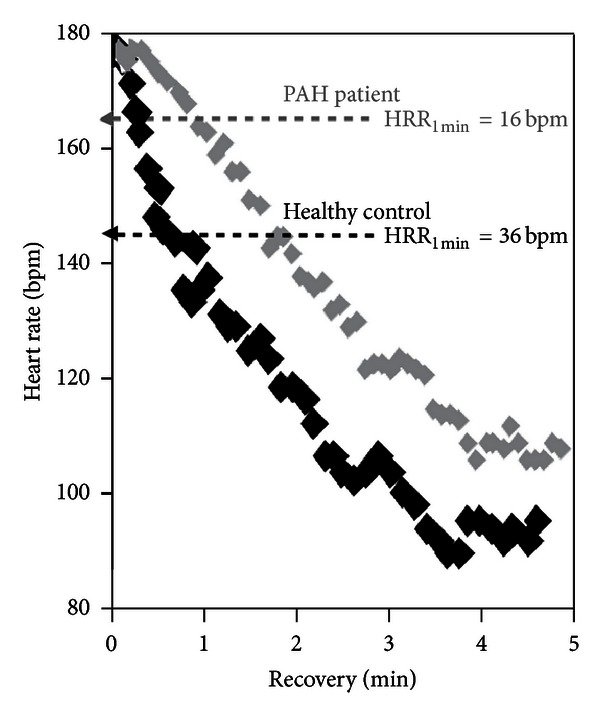
Heart rate (HR) response after incremental exercise in a healthy control and a patient with pulmonary arterial hypertension (PAH) of same age and gender (both females aged 31). Note the delayed HR recovery (HRR) up to the 5th minute after-exercise in the patient compared to the control. HRR1 min ≤ 18 bpm after cycle ergometer exercise test has recently been found an independent predictor of mortality in these patients [28].
5. Conclusions
Interpretation of incremental CPET is best performed by a judicious analysis of all available physiological information provided by the procedure (and by previous testing) taking into consideration the underlying clinical question(s). Although a considerable lack of information on the individual diagnostic and prognostic value of the dynamic sub-maximal relationships still persists, the bulk of evidence is reassuring in relation to their practical usefulness. Large-scale, multicentric studies, however, are urgently needed to validate the suggested cutoffs of abnormality (Table 1) in different clinical scenarios and disease populations.
Table 1.
Clinical usefulness and suggested cutoffs of selected dynamic responses to rapidly incremental CPET.
| Variable | Clinical usefulness | Cutoffs/patterns of abnormality |
|---|---|---|
| Metabolic | ||
|
| ||
| Estimated lactate threshold (LT) | (i) Prognosis in CHF [52] | (i) LT < 40% predicted peak [2] |
| (ii) Marker of disease severity in PAH [53] | (ii) Influenced by age, gender, and fitness [4, 7, 42, 76] | |
| (iii) Risk predictor of postoperatory complications in the elderly [50, 51] | ||
| (iv) Guide exercise training intensity [72, 73] | ||
| (v) Responsive to rehabilitation in less impaired patients with chronic cardiopulmonary diseases [54, 70] | ||
|
| ||
| /Δ work rate (mL/min/W) | (i) Indicative of impaired O2 delivery and/or utilization [77–81] | (i) <lower limit of normality (<8.5 mL/min/W) [4, 8] |
| (ii) Adjunct for the diagnosis of myocardial ischemia [82–88] | (ii) Decrease in slope (or plateau) as exercise progresses [77–81] | |
|
| ||
| efficiency slope (OUES) | (i) Functional impairment and prognosis in CHF [18, 89–94] | Mortality in CHF <1.05 L/min/log (L/min) or <65% predicted [89] |
| (ii) Response to interventions in CHF [95] | ||
| (iii) More sensitive to training than the / slope in CHF [96] | ||
|
| ||
| efficiency plateau (OUEP) | Functional impairment and prognosis in CHF [89] | Mortality in CHF <25 mL/L or <65% predicted [89] |
|
| ||
| Ventilatory | ||
|
| ||
| Excess exercise ventilation | (i) Prognosis in PAH [97, 98] and CHF, even under β-blocker therapy (CHF) [99, 100] | <age—and gender-specific lower limits of normality [10, 11] |
| (ii) Responsive to therapy in CHF [101–103], PAH [104, 105], and CTEPH [106] | ||
| (iii) Responsive to exercise training [107] | Mortality in CHF | |
|
/≥ 34 [108] ≥ 45 [109] |
||
| Mortality in PAH | ||
| / ≥ 52 [97] | ||
| LT ≥ 54 [98] | ||
| / ≥ 62 [98] | ||
| / ≥ 48 [97] | ||
| Postoperative complications of lung resection | ||
| / ≥ 34 [110] | ||
|
| ||
| End-tidal partial pressure for CO2 (P ETCO2) | (i) Adjunct for the diagnosis of PVD [111] | Diagnosis of PVD [111] |
| (ii) Prognosis in CHF [112–116] | “likely” = ≤ 30 mmHg at the LT | |
| (iii) Marker of disease severity in PAH [97, 111, 117, 118] | “very likely” = ≤ 20 mmHg at the LT | |
| (iv) Diagnosis of a patent forame ovale in PAH [119] | progressive reductions as exercise increases | |
| (v) Responsive to drug therapy in PAH[105] and CHF [101] | sudden increase with exercise cessation | |
| (vi) Responsive to exercise training [120] | ||
| Mortality in CHF | ||
| ≤33 mmHg at rest [112, 114] | ||
| ≤36 mmHg at the LT [115] | ||
| <31 mmHg at peak [116] | ||
|
| ||
| Exertional oscillatory ventilation | (i) Indicative of worsening clinical status, severe hemodynamic dysfunction, and reduced functional capacity in CHF [121–126] | Three or more regular oscillations (standard deviation of three consecutive cycle lengths within 20% of their average), with minimal average amplitude of ventilatory oscillation of 5 L/min [27] |
| (ii) Responsive to interventions in CHF [101] | ||
|
| ||
| Cardiovascular | ||
|
| ||
| ΔHeart rateO2 (beat/L) | (i) Indicative of abnormal cardiovascular response to exercise [127–130] | <age—and gender-specific lower limits of normality [9, 10] |
| (ii) Adjunct for the diagnosis of myocardial ischemia [88, 131–133] | Changes in linearity with increases in steepness [88, 132, 133] | |
|
| ||
| Heart rate recovery (HRR) (beats/min) | (i) Prognosis in asymptomatic subjects referred for exercise testing [134], CHF [135], PAH [28], Type 2 diabetes [136], and COPD [137] | Mortality in patients referred for exercise testing |
| (ii) Disease severity in metabolic syndrome [138], obstructive sleep apnea [139], sarcoidosis [140], rheumatological diseases [141, 142], polycystic ovary syndrome [143], polycystic kidney disease [144], and HIV infection [145] | Treadmill, cooldown: HRR1 min ≤ 12 [134, 150, 151] |
|
| (iii) Responsive to aerobic training in CHF, COPD, obstructive sleep apnea, and systemic lupus erythematosus [146–149] | Treadmill, no cooldown: HRR1 min ≤ 18 [135] HRR2 min ≤ 22 [152] |
|
| Treadmill, no cooldown: HRR2 min ≤ 42 [153] |
||
| Mortality in CHF | ||
| Treadmill, cooldown: | ||
| HRR1 min < 6.5 [154] | ||
| Treadmill, no cooldown: HRR1 min ≤ 12 [155] |
||
| Bike, cooldown: HRR1 min < 17 [156] |
||
| Mortality in PAH Bike, cooldown: HRR1 min ≤ 18 [28] |
||
| Mortality in COPD Bike, cooldown: HRR1 min ≤ 14 [137] |
||
| Mortality in Type 2 diabetes | ||
| Treadmill, cooldown: | ||
| HRR1
min < 12 HRR2 min < 28 [136] |
||
: oxygen uptake; : carbon dioxide output; : minute ventilation; COPD: chronic obstructive pulmonary disease; CHF: chronic heart failure; PAH: pulmonary arterial hypertension; PVD: pulmonary vascular disease; RCP: respiratory compensation point.
Abbreviations
- CAD:
Coronary artery disease
- CHF:
Chronic heart failure
- COPD:
Chronic obstructive pulmonary disease
- CPET:
Cardiopulmonary exercise testing
- CTEPH:
Chronic thromboembolic pulmonary hypertension
- EOV:
Exertional oscillatory ventilation
- FEV1:
Forced expiratory volume in one second
- FVC:
Forced vital capacity
- GET:
Gas exchange threshold
- HR:
Heart rate
- HRR:
Heart rate recovery
- LA:
Lactic acid
- LT:
Lactate threshold
- OUES:
Oxygen uptake efficiency slope
- OUEP:
Oxygen uptake efficiency plateau
- PAH:
Pulmonary arterial hypertension
- Pa:
Arterial partial pressure
- PA:
Alveolar pressure
- PET:
End-tidal partial pressure
- PVD:
Pulmonary vascular disease
- R:
Respiratory exchange ratio
- RCP:
Respiratory compensation point
- SpO2:
Pulse oxygen saturation
- Unl:
Unloaded pedaling
- :
Carbon dioxide output
- VD/VT:
Dead space to tidal volume ratio
- :
Alveolar ventilation
- :
Minute ventilation
- :
Ventilatory equivalent for O2
- :
Ventilatory equivalent for CO2
- :
Oxygen uptake
- VT:
Ventilatory threshold
- WR:
Work rate.
References
- 1.Wasserman K, Whipp BJ. Exercise physiology in health and disease. The American Review of Respiratory Disease. 1975;112(2):219–249. doi: 10.1164/arrd.1975.112.2.219. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman K, Hansen JE, Sue DY, et al. Principles of Exercise Testing and Interpretation. 5th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 3.Beaver WL, Wasserman K, Whipp BJ. On-line computer analysis and breath-by-breath graphical display of exercise function tests. Journal of Applied Physiology. 1973;34(1):128–132. doi: 10.1152/jappl.1973.34.1.128. [DOI] [PubMed] [Google Scholar]
- 4.Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1981;50(1):217–221. doi: 10.1152/jappl.1981.50.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Buchfuhrer MJ, Hansen JE, Robinson TE, et al. Optimizing the exercise protocol for cardiopulmonary assessment. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1983;55(5):1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. The American Review of Respiratory Disease. 1984;129(2):S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 7.Koch B, Schäper C, Ittermann T, et al. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. European Respiratory Journal. 2009;33(2):389–397. doi: 10.1183/09031936.00074208. [DOI] [PubMed] [Google Scholar]
- 8.Hansen JE, Casaburi R, Cooper DM, Wasserman K. Oxygen uptake as related to work rate increment during cycle ergometer exercise. European Journal of Applied Physiology and Occupational Physiology. 1988;57(2):140–145. doi: 10.1007/BF00640653. [DOI] [PubMed] [Google Scholar]
- 9.Fairbarn MS, Blackie SP, McElvaney NG, Wiggs BR, Pare PD, Pardy RL. Prediction of heart rate and oxygen uptake during incremental and maximal exercise in healthy adults. Chest. 1994;105(5):1365–1369. doi: 10.1378/chest.105.5.1365. [DOI] [PubMed] [Google Scholar]
- 10.Neder JA, Nery LE, Peres C, Whipp BJ. Reference values for dynamic responses to incremental cycle ergometry in males and females aged 20 to 80. American Journal of Respiratory and Critical Care Medicine. 2001;164(8 I):1481–1486. doi: 10.1164/ajrccm.164.8.2103007. [DOI] [PubMed] [Google Scholar]
- 11.Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. American Journal of Respiratory and Critical Care Medicine. 2002;166(11):1443–1448. doi: 10.1164/rccm.2202033. [DOI] [PubMed] [Google Scholar]
- 12.Steinacker J, Ward SA. The Physiology and Pathophysiology of Exercise Toleranceedition. 1st edition. New York, NY, USA: Plenum Press; 1984. [Google Scholar]
- 13.Weisman IM, Marciniuk D, Martinez FJ. ATS/ACCP Statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 14.Palange P, Ward SA, Carlsen KH, et al. Recommendations on the use of exercise testing in clinical practice. European Respiratory Journal. 2007;29(1):185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 15.Whipp BJ, Wagner PD, Agusti A. Factors determining the response to exercise in healthy subjects. European Respiratory Monograph. 1997;2(6):3–31. [Google Scholar]
- 16.Whipp BJ, Wagner A PD. Determinants of the physiological systems responses to muscular exercise in healthy subjects. In: Ward SA, Palange P, Agusti A, editors. Clinical Exercise Testing. Vol. 36. European Respiratory Monograph; 2007. pp. 1–35. [Google Scholar]
- 17.Ward SA. Discriminating features of responses in cardiopulmonary exercise testing. In: Ward SA, Palange P, editors. Clinical Exercise Testing. Vol. 36. European Respiratory Monograph; 2007. pp. 36–68. [Google Scholar]
- 18.Grant S, McMurray J, Aitchison T, et al. The reproducibility of symptoms during a submaximal exercise test in chronic heart failure. British Journal of Clinical Pharmacology. 1998;45(3):287–290. doi: 10.1046/j.1365-2125.1998.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kettner A, Goldberg A, Hagberg J. Cardiovascular and metabolic responses to submaximal exercise in hemodialysis patients. Kidney International. 1984;26(1):66–71. doi: 10.1038/ki.1984.135. [DOI] [PubMed] [Google Scholar]
- 20.Lipkin DP, Bayliss J, Poole-Wilson PA. The ability of a submaximal exercise test to predict maximal exercise capacity in patients with heart failure. European Heart Journal. 1985;6(10):829–833. doi: 10.1093/oxfordjournals.eurheartj.a061768. [DOI] [PubMed] [Google Scholar]
- 21.Clark AL, Rafferty D, Arbuthnott K. Exercise dynamics at submaximal workloads in patients with chronic heart failure. Journal of Cardiac Failure. 1997;3(1):15–19. doi: 10.1016/s1071-9164(97)90004-x. [DOI] [PubMed] [Google Scholar]
- 22.Belardinelli R, Zhang YY, Wasserman K, Purcaro A, Agostoni PG. A four-minute submaximal constant work rate exercise test to assess cardiovascular functional class in chronic heart failure. The American Journal of Cardiology. 1998;81(10):1210–1214. doi: 10.1016/s0002-9149(98)00093-9. [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Nodari S, Raccagni D, et al. Maximal and submaximal exercise testing in heart failure. Journal of Cardiovascular Pharmacology. 1998;32(1):S36–S45. doi: 10.1097/00005344-199800003-00007. [DOI] [PubMed] [Google Scholar]
- 24.Faggiano P, Gualeni A. Methodology of exercise test in patients with heart failure. Maximal test and submaximal test. Italian Heart Journal. 2000;1:313–319. [PubMed] [Google Scholar]
- 25.Bittner V. Exercise testing in heart failure: maximal, submaximal, or both? Journal of the American College of Cardiology. 2003;42(1):123–125. doi: 10.1016/s0735-1097(03)00501-1. [DOI] [PubMed] [Google Scholar]
- 26.Woods PR, Bailey KR, Wood CM, Johnson BD. Submaximal exercise gas exchange is an important prognostic tool to predict adverse outcomes in heart failure. European Journal of Heart Failure. 2011;13(3):303–310. doi: 10.1093/eurjhf/hfq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leite JJ, Mansur AJ, De Freitas HFG, et al. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. Journal of the American College of Cardiology. 2003;41(12):2175–2181. doi: 10.1016/s0735-1097(03)00460-1. [DOI] [PubMed] [Google Scholar]
- 28.Ramos RP, Arakaki JS, Barbosa P, et al. Heart rate recovery in pulmonary arterial hypertension: relationship with exercise capacity and prognosis. American Heart Journal. 2012;163(4):580–588. doi: 10.1016/j.ahj.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Wasserman K, Whipp BJ, Koyal SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. Journal of Applied Physiology. 1973;35(2):236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- 30.Green HJ, Hughson RL, Orr GW, Ranney DA. Anaerobic threshold, blood lactate, and muscle metabolites in progressive exercise. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1983;54(4):1032–1038. doi: 10.1152/jappl.1983.54.4.1032. [DOI] [PubMed] [Google Scholar]
- 31.Beaver WL, Wasserman K, Whipp BJ. Bicarbonate buffering of lactic acid generated during exercise. Journal of Applied Physiology. 1985;60(2):472–478. doi: 10.1152/jappl.1986.60.2.472. [DOI] [PubMed] [Google Scholar]
- 32.Green HJ, Hughson RL. Anaerobic threshold: review of the concept and directions for future research. Medicine and Science in Sports and Exercise. 1985;17(5):621–624. [PubMed] [Google Scholar]
- 33.Hopker JG, Jobson SA, Pandit JJ. Controversies in the physiological basis of the ‘anaerobic threshold’ and their implications for clinical cardiopulmonary exercise testing. Anaesthesia. 2011;66(2):111–123. doi: 10.1111/j.1365-2044.2010.06604.x. [DOI] [PubMed] [Google Scholar]
- 34.Wasserman K, Beaver WL, Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation. 1990;81(supplement 1):II14–II30. [PubMed] [Google Scholar]
- 35.Wasserman K, Koike A. Is the anaerobic threshold truly anaerobic? Chest. 1992;101(supplement 5):211S–218S. doi: 10.1378/chest.101.5_supplement.211s. [DOI] [PubMed] [Google Scholar]
- 36.Brooks GA. Current concepts in lactate exchange. Medicine and Science in Sports and Exercise. 1991;23(8):895–906. [PubMed] [Google Scholar]
- 37.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. Journal of Applied Physiology. 1986;60(6):2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 38.Reinhard U, Muller PH, Schmulling RM. Determination of anaerobic threshold by the ventilation equivalent in normal individuals. Respiration. 1979;38(1):36–42. doi: 10.1159/000194056. [DOI] [PubMed] [Google Scholar]
- 39.Ward SA, Whipp BJ, Koyal S, Wasserman K. Influence of body CO2 stores on ventilatory dynamics during exercise. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1983;55(3):742–749. doi: 10.1152/jappl.1983.55.3.742. [DOI] [PubMed] [Google Scholar]
- 40.Ozcelik O, Ward SA, Whipp BJ. Effect of altered body CO2 stores on pulmonary gas exchange dynamics during incremental exercise in humans. Experimental Physiology. 1999;84(5):999–1011. doi: 10.1111/j.1469-445x.1999.01868.x. [DOI] [PubMed] [Google Scholar]
- 41.Whipp BJ. Mechanisms dissociating pulmonary CO2 and O2 exchange dynamics during exercise. Experimental Physiology. 2007;92(2):347–355. doi: 10.1113/expphysiol.2006.034363. [DOI] [PubMed] [Google Scholar]
- 42.Neder JA, Nery LE, Castelo A, et al. Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: a randomised study. European Respiratory Journal. 1999;14(6):1304–1313. doi: 10.1183/09031936.99.14613049. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman K. The anaerobic threshold measurement to evaluate exercise performance. The American Review of Respiratory Disease. 1984;129(2):S35–S40. doi: 10.1164/arrd.1984.129.2P2.S35. [DOI] [PubMed] [Google Scholar]
- 44.Wasserman K. Determinants and detection of anaerobic threshold and consequences of exercise above it. Circulation. 1987;76(6):I-29–I-39. [PubMed] [Google Scholar]
- 45.Wasserman K. The anaerobic threshold: definition, physiological significance and identification. Advances in Cardiology. 35(1):1–23. [PubMed] [Google Scholar]
- 46.Wasserman K. Reduced aerobic enzyme activity in skeletal muscles of patients with heart failure. A prelimary defect or a result of limited cardiac output? Circulation. 1991;84(4):1868–1870. doi: 10.1161/01.cir.84.4.1868. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan MJ, Cobb FR. The anaerobic threshold in chronic heart failure. Relation to blood lactate, ventilatory basis, reproducibility, and response to exercise training. Circulation. 1990;81(1):II47–II58. [PubMed] [Google Scholar]
- 48.Sullivan MJ, Green HJ, Cobb FR. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991;84(4):1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 49.Koike A, Hiroe M, Adachi H, et al. Anaerobic metabolism as an indicator of aerobic function during exercise in cardiac patients. Journal of the American College of Cardiology. 1992;20(1):120–126. doi: 10.1016/0735-1097(92)90147-f. [DOI] [PubMed] [Google Scholar]
- 50.Older P, Smith R, Courtney P, Hone R. Preoperative evaluation of cardiac failure and ischemia in elderly patients by cardiopulmonary exercise testing. Chest. 1993;104(3):701–704. doi: 10.1378/chest.104.3.701. [DOI] [PubMed] [Google Scholar]
- 51.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116(2):355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 52.Gitt AK, Wasserman K, Kilkowski C, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106(24):3079–3084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 53.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104(4):429–435. doi: 10.1161/hc2901.093198. [DOI] [PubMed] [Google Scholar]
- 54.Patessio A, Carone M, Ioli F, Donner CF. Ventilatory and metabolic changes as a result of exercise training in COPD patients. Chest. 1992;101(5):274S–278S. doi: 10.1378/chest.101.5_supplement.274s. [DOI] [PubMed] [Google Scholar]
- 55.Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. The American Review of Respiratory Disease. 1991;143(1):9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 56.Kavanagh T, Myers MG, Baigrie RS, Mertens DJ, Sawyer P, Shephard RJ. Quality of life and cardiorespiratory function in chronic heart failure: effects of 12 months' aerobic training. Heart. 1996;76(1):42–49. doi: 10.1136/hrt.76.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99(9):1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 58.Gimenez M, Servera E, Vergara P, Bach JR, Polu JM. Endurance training in patients with chronic obstructive pulmonary disease: a comparison of high versus moderate intensity. Archives of Physical Medicine and Rehabilitation. 2000;81(1):102–109. doi: 10.1016/s0003-9993(00)90229-6. [DOI] [PubMed] [Google Scholar]
- 59.Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109(11):1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 60.Klocek M, Kubinyi A, Bacior B, Kawecka-Jaszcz K. Effect of physical training on quality of life and oxygen consumption in patients with congestive heart failure. International Journal of Cardiology. 2005;103(3):323–329. doi: 10.1016/j.ijcard.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 61.Zainuldin R, Mackey MG, Alison JA. Intensity and type of exercise for lower limb endurance training to optimise exercise capacity for people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2009;(4) doi: 10.1002/14651858.CD008008.pub2.CD008008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hambrecht R, Neibauer J, Fiehn E, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. Journal of the American College of Cardiology. 1995;25(6):1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 63.Desai SA, Channick RN. Exercise in patients with pulmonary arterial hypertension. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28(1):12–16. doi: 10.1097/01.HCR.0000311502.57022.73. [DOI] [PubMed] [Google Scholar]
- 64.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114(14):1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 65.Uchi M, Saji T, Harada T. Feasibility of cardiopulmonary rehabilitation in patients with idiopathic pulmonary arterial hypertension treated with intravenous prostacyclin infusion therapy. Journal of Cardiology. 2005;46(5):183–193. [PubMed] [Google Scholar]
- 66.Grunig E, Ehlken N, Ghofrani A, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration. 2011;81(5):394–401. doi: 10.1159/000322475. [DOI] [PubMed] [Google Scholar]
- 67.Arena R. Exercise testing and training in chronic lung disease and pulmonary arterial hypertension. Progress in Cardiovascular Diseases. 2011;53(6):454–463. doi: 10.1016/j.pcad.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Grunig E, Lichtblau M, Ehlken N, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. European Respiratory Journal. 2012;40(1):84–92. doi: 10.1183/09031936.00123711. [DOI] [PubMed] [Google Scholar]
- 69.Grunig E, Maier F, Ehlken N, et al. Exercise training in pulmonary arterial hypertension associated with connective tissue diseases. Arthritis Research and Therapy. 2012;14(3):p. R148. doi: 10.1186/ar3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. The American Review of Respiratory Disease. 1991;143(1):9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 71.Vogiatzis I, Terzis G, Stratakos G, et al. Effect of pulmonary rehabilitation on peripheral muscle fiber remodeling in patients with COPD in GOLD stages II to IV. Chest. 2011;140(3):744–752. doi: 10.1378/chest.10-3058. [DOI] [PubMed] [Google Scholar]
- 72.Meyer T, Görge G, Schwaab B, et al. An alternative approach for exercise prescription and efficacy testing in patients with chronic heart failure: a randomized controlled training study. American Heart Journal. 2005;149(5):926.e1–926.e7. doi: 10.1016/j.ahj.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Curnier D, Galinier M, Pathak A, et al. Rehabilitation of patients with congestive heart failure with or without β-blockade therapy. Journal of Cardiac Failure. 2001;7(3):241–248. doi: 10.1054/jcaf.2001.26565. [DOI] [PubMed] [Google Scholar]
- 74.Zacarias EC, Neder JA, Cendom SP, Nery LE, Jardim JR. Heart rate at the estimated lactate threshold in patients with chronic obstructive pulmonary disease: effects on the target intensity for dynamic exercise training. Journal of Cardiopulmonary Rehabilitation. 2000;20(6):369–376. doi: 10.1097/00008483-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 75.O'Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. American Journal of Respiratory and Critical Care Medicine. 1998;157(5):1489–1497. doi: 10.1164/ajrccm.157.5.9708010. [DOI] [PubMed] [Google Scholar]
- 76.Inbar O, Oren A, Scheinowitz M, Rotstein A, Dlin R, Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20- to 70- yr-old men. Medicine and Science in Sports and Exercise. 1994;26(5):538–546. [PubMed] [Google Scholar]
- 77.Hansen JE, Sue DY, Oren A, Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. The American Journal of Cardiology. 1987;59(6):669–674. doi: 10.1016/0002-9149(87)91190-8. [DOI] [PubMed] [Google Scholar]
- 78.Solal AC, Chabernaud JM, Gourgon R. Comparison of oxygen uptake during bicycle exercise in patients with chronic heart failure and in normal subjects. Journal of the American College of Cardiology. 1990;16(1):80–85. doi: 10.1016/0735-1097(90)90460-7. [DOI] [PubMed] [Google Scholar]
- 79.Koike A, Wasserman K. Effect of acute reduction in oxygen transport on parameters of aerobic function during exercise. Annals of the Academy of Medicine Singapore. 1992;21(1):14–22. [PubMed] [Google Scholar]
- 80.Jones S, Elliott PM, Sharma S, McKenna WJ, Whipp BJ. Cardiopulmonary responses to exercise in patients with hypertrophic cardiomyopathy. Heart. 1998;80(1):60–67. doi: 10.1136/hrt.80.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gimenes AC, Neder JA, dal Corso S, et al. Relationship between work rate and oxygen uptake in mitochondrial myopathy during ramp-incremental exercise. Brazilian Journal of Medical and Biological Research. 2011;44(4):354–360. doi: 10.1590/s0100-879x2011007500023. [DOI] [PubMed] [Google Scholar]
- 82.Belardinelli R, Lacalaprice F, Carle F, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. European Heart Journal. 2003;24(14):1304–1313. doi: 10.1016/s0195-668x(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 83.Schmid JP. Detection of exercise induced ischaemia: a new role for cardiopulmonary exercise testing. European Heart Journal. 2003;24(14):1285–1286. doi: 10.1016/s0195-668x(03)00280-x. [DOI] [PubMed] [Google Scholar]
- 84.Chaudhry S, Arena R, Wasserman K, et al. Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. The American Journal of Cardiology. 2009;103(5):615–619. doi: 10.1016/j.amjcard.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bussotti M, Apostolo A, Andreini D, Palermo P, Contini M, Agostoni P. Cardiopulmonary evidence of exercise-induced silent ischaemia. European Journal of Cardiovascular Prevention and Rehabilitation. 2006;13(2):249–253. doi: 10.1097/01.hjr.0000189809.99353.76. [DOI] [PubMed] [Google Scholar]
- 86.Balady GJ, Arena R, Sietsema K, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 87.Chaudhry S, Arena RA, Hansen JE, et al. The utility of cardiopulmonary exercise testing to detect and track early-stage ischemic heart disease. Mayo Clinic Proceedings. 2010;85(10):928–932. doi: 10.4065/mcp.2010.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaudhry S, Arena R, Wasserman K, et al. The utility of cardiopulmonary exercise testing in the assessment of suspected microvascular ischemia. International Journal of Cardiology. 2011;148(1):e7–e9. doi: 10.1016/j.ijcard.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau best predicts early death in heart failure. Chest. 2012;141(5):1284–1294. doi: 10.1378/chest.11-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davies LC, Wensel R, Georgiadou P, et al. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. European Heart Journal. 2006;27(6):684–690. doi: 10.1093/eurheartj/ehi672. [DOI] [PubMed] [Google Scholar]
- 91.Arena R, Brubaker P, Moore B, Kitzman D. The oxygen uptake efficiency slope is reduced in older patients with heart failure and a normal ejection fraction. International Journal of Cardiology. 2010;144(1):101–102. doi: 10.1016/j.ijcard.2008.12.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arena R, Myers J, Abella J, et al. Prognostic significance of the oxygen uptake efficiency slope: percent-predicted versus actual value. American Journal of Cardiology. 2010;105(5):757–758. doi: 10.1016/j.amjcard.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Antoine-Jonville S, Pichon A, Vazir A, Polkey MI, Dayer MJ. Oxygen uptake efficiency slope, aerobic fitness, and V(E)-VCO2 slope in heart failure. Medicine and Science in Sports and Exercise. 2012;44(3):428–434. doi: 10.1249/MSS.0b013e31822f8427. [DOI] [PubMed] [Google Scholar]
- 94.Arena R, Guazzi M, Myers J, et al. The relationship between minute ventilation and oxygen consumption in heart failure: comparing peak VE/VO(2) and the oxygen uptake efficiency slope. International Journal of Cardiology. 2012;154(3):384–385. doi: 10.1016/j.ijcard.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fu TC, Wang CH, Lin PS, et al. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. doi: 10.1016/j.ijcard.2011.11.086. International Journal of Cardiology. In press. [DOI] [PubMed] [Google Scholar]
- 96.Myers J, Gademan M, Brunner K, Kottman W, Boesch C, Dubach P. Effects of high-intensity training on indices of ventilatory efficiency in chronic heart failure. Journal Cardiopulmonary Rehabilitation and Prevention. 2012;32:9–16. doi: 10.1097/HCR.0b013e3182343bdf. [DOI] [PubMed] [Google Scholar]
- 97.Groepenhoff H, Vonk-Noordegraaf A, Boonstra A, Spreeuwenberg MD, Postmus PE, Bogaard HJ. Exercise testing to estimate survival in pulmonary hypertension. Medicine and Science in Sports and Exercise. 2008;40(10):1725–1732. doi: 10.1249/MSS.0b013e31817c92c0. [DOI] [PubMed] [Google Scholar]
- 98.Deboeck G, Scoditti C, Huez S, et al. Exercise to predict outcome in idiopathic vs associated pulmonary arterial hypertension. European Respiratory Journal. 2012;40(6):1410–1419. doi: 10.1183/09031936.00217911. [DOI] [PubMed] [Google Scholar]
- 99.Arena RA, Guazzi M, Myers J, Abella J. The prognostic value of ventilatory efficiency with beta-blocker therapy in heart failure. Medicine and Science in Sports and Exercise. 2007;39(2):213–219. doi: 10.1249/01.mss.0000241655.45500.c7. [DOI] [PubMed] [Google Scholar]
- 100.Wolk R, Johnson BD, Somers VK, et al. Effects of β-blocker therapy on ventilatory responses to exercise in patients with heart failure. Journal of Cardiac Failure. 2005;11(5):333–339. doi: 10.1016/j.cardfail.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Agostoni P, Contini M, Magini A, et al. Carvedilol reduces exercise-induced hyperventilation: a benefit in normoxia and a problem with hypoxia. European Journal of Heart Failure. 2006;8(7):729–735. doi: 10.1016/j.ejheart.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Agostoni P, Guazzi M, Bussotti M, De Vita S, Palermo P. Carvedilol reduces the inappropriate increase of ventilation during exercise in heart failure patients. Chest. 2002;122(6):2062–2067. doi: 10.1378/chest.122.6.2062. [DOI] [PubMed] [Google Scholar]
- 103.Jaussaud J, Aimable L, Bordachar P, et al. Cardiac resynchronization therapy reduces metaboreflex contribution to the ventilatory response in heart failure population. Cardiology Research and Practice. 2012;2012:6 pages. doi: 10.1155/2012/914071.914071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ting H, Sun XG, Chuang ML, Lewis DA, Hansen JE, Wasserman K. A noninvasive assessment of pulmonary perfusion abnormality in patients with primary pulmonary hypertension. Chest. 2001;119(3):824–832. doi: 10.1378/chest.119.3.824. [DOI] [PubMed] [Google Scholar]
- 105.Oudiz RJ, Roveran G, Hansen JE, Sun XG, Wasserman K. Effect of sildenafil on ventilatory efficiency and exercise tolerance in pulmonary hypertension. European Journal of Heart Failure. 2007;9(9):917–921. doi: 10.1016/j.ejheart.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsuda H, Ogino H, Minatoya K, et al. Long-term recovery of exercise ability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. The Annals of Thoracic Surgery. 2006;82(4):1338–1343. doi: 10.1016/j.athoracsur.2006.03.105. [DOI] [PubMed] [Google Scholar]
- 107.Myers J, Dziekan G, Goebbels U, Dubach P. Influence of high-intensity exercise training on the ventilatory response to exercise in patients with reduced ventricular function. Medicine and Science in Sports and Exercise. 1999;31(7):929–937. doi: 10.1097/00005768-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Chua TP, Ponikowski P, Harrington D, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. Journal of the American College of Cardiology. 1997;29(7):1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 109.Arena R, Myers J, Abella J, et al. Development of a ventilatory classification system in patients with heart failure. Circulation. 2007;115(18):2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 110.Torchio R, Guglielmo M, Giardino R, et al. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for non-small-cell lung cancer. European Journal of Cardio-thoracic Surgery. 2010;38(1):14–19. doi: 10.1016/j.ejcts.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 111.Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K. End-tidal Pco2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127(5):1637–1646. doi: 10.1378/chest.127.5.1637. [DOI] [PubMed] [Google Scholar]
- 112.Arena R, Myers J, Abella J, et al. The partial pressure of resting end-tidal carbon dioxide predicts major cardiac events in patients with systolic heart failure. American Heart Journal. 2008;156(5):982–988. doi: 10.1016/j.ahj.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arena R, Peberdy MA, Myers J, Guazzi M, Tevald M. Prognostic value of resting end-tidal carbon dioxide in patients with heart failure. International Journal of Cardiology. 2006;109(3):351–358. doi: 10.1016/j.ijcard.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 114.Arena R, Guazzi M, Myers J, et al. Prognostic value of capnography during rest and exercise in patients with heart failure. Congestive Heart Failure. 2012;18(6):302–307. doi: 10.1111/j.1751-7133.2012.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arena R, Guazzi M, Myers J. Prognostic value of end-tidal carbon dioxide during exercise testing in heart failure. International Journal of Cardiology. 2007;117(1):103–108. doi: 10.1016/j.ijcard.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 116.Hoshimoto-Iwamoto M, Koike A, Nagayama O, et al. Prognostic value of end-tidal CO2 pressure during exercise in patients with left ventricular dysfunction. Journal of Physiological Sciences. 2009;59(1):49–55. doi: 10.1007/s12576-008-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wensel R, Opitz CF, Anker SD, et al. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106(3):319–324. doi: 10.1161/01.cir.0000022687.18568.2a. [DOI] [PubMed] [Google Scholar]
- 118.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Gas exchange detection of exercise-induced right-to-left shunt in patients with primary pulmonary hypertension. Circulation. 2002;105(1):54–60. doi: 10.1161/hc0102.101509. [DOI] [PubMed] [Google Scholar]
- 119.Oudiz RJ, Midde R, Hovenesyan A, et al. Usefulness of right-to-left shunting and poor exercise gas exchange for predicting prognosis in patients with pulmonary arterial hypertension. The American Journal of Cardiology. 2010;105(8):1186–1191. doi: 10.1016/j.amjcard.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eto Y, Koike A, Matsumoto A, et al. Early aerobic training increases end-tidal CO2 pressure during exercise in patients after acute myocardial infarction. Circulation Journal. 2004;68(8):778–783. doi: 10.1253/circj.68.778. [DOI] [PubMed] [Google Scholar]
- 121.Corrà U, Giordano A, Bosimini E, et al. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121(5):1572–1580. doi: 10.1378/chest.121.5.1572. [DOI] [PubMed] [Google Scholar]
- 122.Corrà U, Pistono M, Mezzani A, et al. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113(1):44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- 123.Murphy RM, Shah RV, Malhotra R, et al. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation. 2011;124:1442–1451. doi: 10.1161/CIRCULATIONAHA.111.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun XG, Hansen JE, Beshai JF, Wasserman K. Oscillatory breathing and exercise gas exchange abnormalities prognosticate early mortality and morbidity in heart failure. Journal of the American College of Cardiology. 2010;55(17):1814–1823. doi: 10.1016/j.jacc.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 125.Olson LJ, Arruda-Olson AM, Somers VK, Scott CG, Johnson BD. Exercise oscillatory ventilation: instability of breathing control associated with advanced heart failure. Chest. 2008;133(2):474–481. doi: 10.1378/chest.07-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Arena R, Guazzi M, Myers J, et al. Exercise oscillatory ventilation reflects diminished quality of life and perceived functional capacity in patients with heart failure. International Journal of Cardiology. 2011;153(2):213–214. doi: 10.1016/j.ijcard.2011.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nery LE, Wasserman K, French W. Contrasting cardiovascular and respiratory responses to exercise in mitral valve and chronic obstructive pulmonary diseases. Chest. 1983;83(3):446–453. doi: 10.1378/chest.83.3.446. [DOI] [PubMed] [Google Scholar]
- 128.Munhoz EC, Hollanda R, Vargas JP, et al. Flattening of oxygen pulse during exercise may detect extensive myocardial ischemia. Medicine and Science in Sports and Exercise. 2007;39(8):1221–1226. doi: 10.1249/mss.0b013e3180601136. [DOI] [PubMed] [Google Scholar]
- 129.Belardinelli R, Lacalaprice F, Carle F, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. European Heart Journal. 2003;24(14):1304–1313. doi: 10.1016/s0195-668x(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 130.Grassi B, Porcelli S, Marzorati M, et al. Metabolic myopathies: functional evaluation by analysis of oxygen uptake kinetics. Medicine and Science in Sports and Exercise. 2009;41(12):2120–2127. doi: 10.1249/MSS.0b013e3181aae96b. [DOI] [PubMed] [Google Scholar]
- 131.Whipp BJ, Higgenbotham MB, Cobb FC. Estimating exercise stroke volume from asymptotic oxygen pulse in humans. Journal of Applied Physiology. 1996;81(6):2674–2679. doi: 10.1152/jappl.1996.81.6.2674. [DOI] [PubMed] [Google Scholar]
- 132.Inbar O, Yamin C, Bar-On I, Nice S, David D. Effects of percutaneous transluminal coronary angioplasty on cardiopulmonary responses during exercise. Journal of Sports Medicine and Physical Fitness. 2008;48(2):235–245. [PubMed] [Google Scholar]
- 133.Chaudhry S, Arena R, Wasserman K, et al. Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. The American Journal of Cardiology. 2009;103(5):615–619. doi: 10.1016/j.amjcard.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. The New England Journal of Medicine. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 135.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104(16):1911–1916. [PubMed] [Google Scholar]
- 136.Chacko KM, Bauer TA, Dale RA, Dixon JA, Schrier RW, Estacio RO. Heart rate recovery predicts mortality and cardiovascular events in patients with type 2 diabetes. Medicine and Science in Sports and Exercise. 2008;40(2):288–295. doi: 10.1249/mss.0b013e31815c4844. [DOI] [PubMed] [Google Scholar]
- 137.Lacasse M, Maltais F, Poirier P, et al. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respiratory Medicine. 2005;99(7):877–886. doi: 10.1016/j.rmed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 138.Spies C, Otte C, Kanaya A, Pipkin SS, Schiller NB, Whooley MA. Association of metabolic syndrome with exercise capacity and heart rate recovery in patients with coronary heart disease in the heart and soul study. The American Journal of Cardiology. 2005;95(10):1175–1179. doi: 10.1016/j.amjcard.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nanas S, Sakellariou D, Kapsimalakou S, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clinical Cardiology. 2010;33(1):46–51. doi: 10.1002/clc.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ardic I, Kaya MG, Yarlioglues M, et al. Impaired heart rate recovery index in patients with sarcoidosis. Chest. 2011;139(1):60–68. doi: 10.1378/chest.09-3022. [DOI] [PubMed] [Google Scholar]
- 141.Dogdu O, Yarlioglues M, Kaya MG, et al. Deterioration of heart rate recovery index in patients with systemic lupus erythematosus. The Journal of Rheumatology. 2010;37(12):2511–2515. doi: 10.3899/jrheum.100163. [DOI] [PubMed] [Google Scholar]
- 142.Kaya EB, Okutucu S, Aksoy H, et al. Evaluation of cardiac autonomic functions in patients with ankylosing spondylitis via heart rate recovery and heart rate variability. Clinical Research in Cardiology. 2010;99(12):803–808. doi: 10.1007/s00392-010-0187-x. [DOI] [PubMed] [Google Scholar]
- 143.Tekin G, Tekin A, Kiliçarslan EB, et al. Altered autonomic neural control of the cardiovascular system in patients with polycystic ovary syndrome. International Journal of Cardiology. 2008;130(1):49–55. doi: 10.1016/j.ijcard.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 144.Orscelik O, Kocyigit I, Baran O, et al. Impairment of heart rate recovery index in autosomal-dominant polycystic kidney disease patients without hypertension. Blood Pressure. 2012;21(5):300–305. doi: 10.3109/08037051.2012.680691. [DOI] [PubMed] [Google Scholar]
- 145.Cade WT, Reeds DN, Lassa-Claxton S, et al. Post-exercise heart rate recovery in HIV-positive individuals on highly active antiretroviral therapy: early indicator of cardiovascular disease? HIV Medicine. 2008;9(2):96–100. doi: 10.1111/j.1468-1293.2007.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tiukinhoy S, Beohar N, Hsie M. Improvement in heart rate recovery after cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2003;23(2):84–87. doi: 10.1097/00008483-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 147.Georgiopoulou VV, Dimopoulos S, Sakellariou D, et al. Cardiopulmonary rehabilitation enhances heart rate recovery in patients with COPD. Respiratory Care. 2012;57:2095–2103. doi: 10.4187/respcare.01485. [DOI] [PubMed] [Google Scholar]
- 148.Kline CE, Crowley EP, Ewing GB, et al. Blunted heart rate recovery is improved following exercise training in overweight adults with obstructive sleep apnea. doi: 10.1016/j.ijcard.2012.04.108. International Journal of Cardiology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miossi R, Benatti FB, de Sa Pinto AL, et al. Exercise training counterbalances chronotropic incompetence and delayed heart rate recovery in systemic lupus erythematosus: a randomized trial. Arthritis Care and Research. 2012;64(8):1159–1166. doi: 10.1002/acr.21678. [DOI] [PubMed] [Google Scholar]
- 150.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. The Journal of the American Medical Association. 2000;284(11):1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 151.Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. Journal of the American College of Cardiology. 2003;42(5):831–838. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 152.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. Journal of the American College of Cardiology. 2001;38(7):1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 153.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Annals of Internal Medicine. 2000;132(7):552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 154.Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. American Heart Journal. 2006;151(4):851–e7. doi: 10.1016/j.ahj.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 155.Nanas S, Anastasiou-Nana M, Dimopoulos S, et al. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. International Journal of Cardiology. 2006;110(3):393–400. doi: 10.1016/j.ijcard.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 156.Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Heart rate recovery predicts sudden cardiac death in heart failure. International Journal of Cardiology. 2010;144(1):121–123. doi: 10.1016/j.ijcard.2008.12.149. [DOI] [PubMed] [Google Scholar]
- 157.Hansen JE, Sue DY, Oren A, Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. The American Journal of Cardiology. 1987;59(6):669–674. doi: 10.1016/0002-9149(87)91190-8. [DOI] [PubMed] [Google Scholar]
- 158.Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. Journal of Applied Physiology. 2007;103(6):1999–2004. doi: 10.1152/japplphysiol.01414.2006. [DOI] [PubMed] [Google Scholar]
- 159.Boone J, Koppo K, Barstow TJ, Bouckaert J. Aerobic fitness, muscle efficiency, and motor unit recruitment during ramp exercise. Medicine and Science in Sports and Exercise. 2010;42(2):402–408. doi: 10.1249/MSS.0b013e3181b0f2e2. [DOI] [PubMed] [Google Scholar]
- 160.Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fibre type and fitness on the oxygen uptake/power output slope during incremental exercise in humans. Experimental Physiology. 2000;85(1):109–116. [PubMed] [Google Scholar]
- 161.Myers J, Salleh A, Buchanan N, et al. Ventilatory mechanisms of exercise intolerance in chronic heart failure. American Heart Journal. 1992;124(3):710–719. doi: 10.1016/0002-8703(92)90282-z. [DOI] [PubMed] [Google Scholar]
- 162.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJS. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93(5):940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 163.Ponikowski P, Francis DP, Piepoli MF, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103(7):967–972. doi: 10.1161/01.cir.103.7.967. [DOI] [PubMed] [Google Scholar]
- 164.Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. Journal of the American College of Cardiology. 2000;36(1):194–201. doi: 10.1016/s0735-1097(00)00691-4. [DOI] [PubMed] [Google Scholar]
- 165.Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. Journal of the American College of Cardiology. 1996;28(6):1567–1572. doi: 10.1016/s0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 166.Baba R, Tsuyuki K, Kimura Y, et al. Oxygen uptake efficiency slope as a useful measure of cardiorespiratory functional reserve in adult cardiac patients. European Journal of Applied Physiology and Occupational Physiology. 1999;80(5):397–401. doi: 10.1007/s004210050610. [DOI] [PubMed] [Google Scholar]
- 167.Baba R, Nagashima M, Nagano Y, Ikoma M, Nishibata K. Role of the oxygen uptake efficiency slope in evaluating exercise tolerance. Archives of Disease in Childhood. 1999;81(1):73–75. doi: 10.1136/adc.81.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Olson TP, Joyner MJ, Johnson BD. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circulation: Heart Failure. 2010;3(2):212–219. doi: 10.1161/CIRCHEARTFAILURE.109.879684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau: physiology and reference values. European Journal of Applied Physiology. 2011:1–10. doi: 10.1007/s00421-011-2030-0. [DOI] [PubMed] [Google Scholar]
- 170.Whipp BJ, Ward SA. The coupling of ventilation to pulmonary gas exchange during exercise. In: Whipp BJ, Wasserman K, editors. Pulmonary Physiology and Pathophysiology of Exercise. New York, NY, USA: Dekker; 1991. pp. 271–307. [Google Scholar]
- 171.Van Laethem C, Bartunek J, Goethals M, Nellens P, Andries E, Vanderheyden M. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. American Heart Journal. 2005;149(1):175–180. doi: 10.1016/j.ahj.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 172.Van Laethem C, De Sutter J, Peersman W, Calders P. Intratest reliability and test-retest reproducibility of the oxygen uptake efficiency slope in healthy participants. European Journal of Cardiovascular Prevention and Rehabilitation. 2009;16(4):493–498. doi: 10.1097/HJR.0b013e32832c88a8. [DOI] [PubMed] [Google Scholar]
- 173.Williamson W, Fuld J, Westgate K, Sylvester K, Ekelund U, Brage S. Validity of reporting oxygen uptake efficiency slope from submaximal exercise using respiratory exchange ratio as secondary criterion. Pulmonary Medicine. 2012;2012:8 pages. doi: 10.1155/2012/874020.874020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gruet M, Brisswalter J, Mely L, Vallier JM. Clinical utility of the oxygen uptake efficiency slope in cystic fibrosis patients. Journal of Cystic Fibrosis. 2010;9(5):307–313. doi: 10.1016/j.jcf.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 175.Phypers BJ, Robiony-Rogers D, Pickering RM, Garden AL. Test-retest reliability of the oxygen uptake efficiency slope in surgical patients. Anaesthesia. 2011;66(8):659–666. doi: 10.1111/j.1365-2044.2011.06714.x. [DOI] [PubMed] [Google Scholar]
- 176.Baba R, Kubo N, Morotome Y, Iwagaki S. Reproducibility of the oxygen uptake efficiency slope in normal healthy subjects. Journal of Sports Medicine and Physical Fitness. 1999;39(3):202–206. [PubMed] [Google Scholar]
- 177.Akkerman M, Van Brussel M, Hulzebos E, Vanhees L, Helders PJM, Takken T. The oxygen uptake efficiency slope: what do we know? Journal of Cardiopulmonary Rehabilitation and Prevention. 2010;30(6):357–373. doi: 10.1097/HCR.0b013e3181ebf316. [DOI] [PubMed] [Google Scholar]
- 178.Arena R, Myers J, Hsu L, et al. The minute ventilation/carbon dioxide production slope is prognostically superior to the oxygen uptake efficiency slope. Journal of Cardiac Failure. 2007;13(6):462–469. doi: 10.1016/j.cardfail.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 179.Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. American Heart Journal. 2008;156(6):1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 180.Bongers BC, Hulzebos EH, Arets BG, Takken T. Validity of the oxygen uptake efficiency slope in children with cystic fibrosis and mild-to-moderate airflow obstruction. Pediatric Exercise Science. 2012;24:129–141. doi: 10.1123/pes.24.1.129. [DOI] [PubMed] [Google Scholar]
- 181.Whipp BJ, Rossiter HB, Ward SA, et al. Simultaneous determination of muscle and O2 uptake kinetics during whole body NMR spectroscopy. Journal of Applied Physiology. 1999;86(2):742–747. doi: 10.1152/jappl.1999.86.2.742. [DOI] [PubMed] [Google Scholar]
- 182.Hagberg JM, Mullin JP, Nagle FJ. Effect of work intensity and duration on recovery O2 . Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1980;48(3):540–544. doi: 10.1152/jappl.1980.48.3.540. [DOI] [PubMed] [Google Scholar]
- 183.Gaesser GA, Brooks GA. Metabolic bases of excess post-exercise oxygen consumption: a review. Medicine Science in Sports Exercise. 1984;16:29–43. [PubMed] [Google Scholar]
- 184.Yoshida T, Whipp BJ. Dynamic asymmetries of cardiac output transients in response to muscular exercise in man. Journal of Physiology. 1994;480(2):355–359. doi: 10.1113/jphysiol.1994.sp020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Groote P, Millaire A, Decoulx E, Nugue O, Guimier P, Ducloux G. Kinetics of oxygen consumption during and after exercise in patients with dilated cardiomyopathy: new markers of exercise intolerance with clinical implications. Journal of the American College of Cardiology. 1996;28(1):168–175. doi: 10.1016/0735-1097(96)00126-x. [DOI] [PubMed] [Google Scholar]
- 186.Koike A, Yajima T, Adachi H, et al. Evaluation of exercise capacity using submaximal exercise at a constant work rate in patients with cardiovascular disease. Circulation. 1995;91(6):1719–1724. doi: 10.1161/01.cir.91.6.1719. [DOI] [PubMed] [Google Scholar]
- 187.Kemps HM, Schep G, Zonderland ML, et al. Are oxygen uptake kinetics in chronic heart failure limited by oxygen delivery or oxygen utilization? International Journal of Cardiology. 2010;142(2):138–144. doi: 10.1016/j.ijcard.2008.12.088. [DOI] [PubMed] [Google Scholar]
- 188.Nanas S, Nanas J, Kassiotis C, et al. Early recovery of oxygen kinetics after submaximal exercise test predicts functional capacity in patients with chronic heart failure. European Journal of Heart Failure. 2001;3(6):685–692. doi: 10.1016/s1388-9842(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 189.Nanas S, Nanas J, Kassiotis C, et al. Respiratory muscles performance is related to oxygen kinetics during maximal exercise and early recovery in patients with congestive heart failure. Circulation. 1999;100(5):503–508. doi: 10.1161/01.cir.100.5.503. [DOI] [PubMed] [Google Scholar]
- 190.Cohen-Solal A, Laperche T, Morvan D, Geneves M, Caviezel B, Gourgon R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure: analysis with gas exchange measurements and NMR spectroscopy. Circulation. 1995;91(12):2924–2932. doi: 10.1161/01.cir.91.12.2924. [DOI] [PubMed] [Google Scholar]
- 191.Daida H, Allison TG, Johnson BD, Squires RW, Gau GT. Further increase in oxygen uptake during early active recovery following maximal exercise in chronic heart failure. Chest. 1996;109(1):47–51. doi: 10.1378/chest.109.1.47. [DOI] [PubMed] [Google Scholar]
- 192.Cohen-Solal A, Czitrom D, Geneves M, Gourgon R. Delayed attainment of peak oxygen consumption after the end of exercise in patients with chronic heart failure. International Journal of Cardiology. 1997;60(1):23–29. doi: 10.1016/s0167-5273(97)00059-4. [DOI] [PubMed] [Google Scholar]
- 193.Takaki H, Sakuragi S, Nagaya N, et al. Postexercise VO2 “Hump” phenomenon as an indicator for inducible myocardial ischemia in patients with acute anterior myocardial infarction. International Journal of Cardiology. 2006;111(1):67–74. doi: 10.1016/j.ijcard.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 194.Kriatselis CD, Nedios S, Kelle S, Helbig S, Gottwik M, von Bary C. Oxygen kinetics and heart rate response during early recovery from exercise in patients with heart failure. Cardiology Research and Practice. 2012;2012:7 pages. doi: 10.1155/2012/512857.512857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tajima A, Itoh H, Osada N, et al. Oxygen uptake kinetics during and after exercise are useful markers of coronary artery disease in patients with exercise electrocardiography suggesting myocardial ischemia. Circulation Journal. 2009;73(10):1864–1870. doi: 10.1253/circj.cj-09-0222. [DOI] [PubMed] [Google Scholar]
- 196.Chick TW, Cagle TG, Vegas FA, Poliner JK, Murata GH. Recovery of gas exchange variables and heart rate after maximal exercise in COPD. Chest. 1990;97(2):276–279. doi: 10.1378/chest.97.2.276. [DOI] [PubMed] [Google Scholar]
- 197.Pouliou E, Nanas S, Papamichalopoulos A, et al. Prolonged oxygen kinetics during early recovery from maximal exercise in adult patients with cystic fibrosis. Chest. 2001;119(4):1073–1078. doi: 10.1378/chest.119.4.1073. [DOI] [PubMed] [Google Scholar]
- 198.Levinger I, Varley M, Jerums G, Hare DL, Selig S. Oxygen (O2) kinetics during early recovery from peak exercise in patients with Type 2 diabetes. Diabetic Medicine. 2011;28(5):612–617. doi: 10.1111/j.1464-5491.2011.03229.x. [DOI] [PubMed] [Google Scholar]
- 199.Convertino VA, Goldwater DJ, Sandler H. VO2 kinetics on constant-load exercise following bed-rest-induced deconditioning. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1984;57(5):1545–1550. doi: 10.1152/jappl.1984.57.5.1545. [DOI] [PubMed] [Google Scholar]
- 200.Tanabe Y, Takahashi M, Hosaka Y, Ito M, Ito E, Suzuki K. Prolonged recovery of cardiac output after maximal exercise in patients with chronic heart failure. Journal of the American College of Cardiology. 2000;35(5):1228–1236. doi: 10.1016/s0735-1097(00)00517-9. [DOI] [PubMed] [Google Scholar]
- 201.Giardini A, Donti A, Specchia S, et al. Recovery kinetics of oxygen uptake is prolonged in adults with an atrial septal defect and improves after transcatheter closure. American Heart Journal. 2004;147(5):910–914. doi: 10.1016/j.ahj.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 202.Johnson RL. Gas exchange efficiency in congestive heart failure. Circulation. 2000;101(24):2774–2776. doi: 10.1161/01.cir.101.24.2774. [DOI] [PubMed] [Google Scholar]
- 203.Oren A, Wasserman K, Davis JA, Whipp BJ. Effect of CO2 set point on ventilatory response to exercise. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1981;51(1):185–189. doi: 10.1152/jappl.1981.51.1.185. [DOI] [PubMed] [Google Scholar]
- 204.Wasserman K, Zhang YY, Gilt A, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96(7):2221–2227. doi: 10.1161/01.cir.96.7.2221. [DOI] [PubMed] [Google Scholar]
- 205.Sue DY. Excess ventilation during exercise and prognosis in chronic heart failure. American Journal of Respiratory and Critical Care Medicine. 2011;183(10):1302–1310. doi: 10.1164/rccm.201006-0965CI. [DOI] [PubMed] [Google Scholar]
- 206.Guazzi M, Reina G, Tumminello G, Guazzi MD. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. European Heart Journal. 2005;26(5):472–480. doi: 10.1093/eurheartj/ehi060. [DOI] [PubMed] [Google Scholar]
- 207.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124(2):720–727. doi: 10.1378/chest.124.2.720. [DOI] [PubMed] [Google Scholar]
- 208.Davis JA, Whipp BJ, Wasserman K. The relation of ventilation to metabolic rate during moderate exercise in man. European Journal of Applied Physiology and Occupational Physiology. 1980;44(2):97–108. doi: 10.1007/BF00421087. [DOI] [PubMed] [Google Scholar]
- 209.Kleber FX, Vietzke G, Wernecke KD, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101(24):2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 210.Robbins M, Francis G, Pashkow FJ, et al. Ventilatory and heart rate responses to exercise better predictors of heart failure mortality than peak oxygen consumption. Circulation. 1999;100(24):2411–2417. doi: 10.1161/01.cir.100.24.2411. [DOI] [PubMed] [Google Scholar]
- 211.Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. American Heart Journal. 2008;156(6):1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 212.Myers J, Arena R, Oliveira RB, et al. The lowest VE/VCO2 ratio during exercise as a predictor of outcomes in patients with heart failure. Journal of Cardiac Failure. 2009;15(9):756–762. doi: 10.1016/j.cardfail.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schwaiblmair M, Faul C, von Scheidt W, Berghaus T. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulmonary Medicine. 2012;12:p. 23. doi: 10.1186/1471-2466-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guazzi M, Myers J, Vicenzi M, et al. Cardiopulmonary exercise testing characteristics in heart failure patients with and without concomitant chronic obstructive pulmonary disease. American Heart Journal. 2010;160(5):900–905. doi: 10.1016/j.ahj.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 215.Deboeck G, Niset G, Lamotte M, Vachiéry JL, Naeije R. Exercise testing in pulmonary arterial hypertension and in chronic heart failure. European Respiratory Journal. 2004;23(5):747–751. doi: 10.1183/09031936.04.00111904. [DOI] [PubMed] [Google Scholar]
- 216.Zhai Z, Murphy K, Tighe H, et al. Differences in ventilatory inefficiency between pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Chest. 2011;140(5):1284–1291. doi: 10.1378/chest.10-3357. [DOI] [PubMed] [Google Scholar]
- 217.Roman MA, Casaburi JD, Porszasz J, Casaburi R. Noninvasive assessment of normality of V, (D)/V (T) in clinical cardiopulmonary exercise testing utilizing incremental cycle ergometry. European Journal of Applied Physiology. 2012;113(1):33–40. doi: 10.1007/s00421-012-2407-8. [DOI] [PubMed] [Google Scholar]
- 218.Zimmerman MI, Miller A, Brown LK, Bhuptani A, Sloane MF, Teirstein AS. Estimated vs actual values for dead space/tidal volume ratios during incremental exercise in patients evaluated for dyspnea. Chest. 1994;106(1):131–136. doi: 10.1378/chest.106.1.131. [DOI] [PubMed] [Google Scholar]
- 219.Matsumoto A, Itoh H, Eto Y, et al. End-tidal CO2 pressure decreases during exercise in cardiac patients. Journal of the American College of Cardiology. 2000;36(1):242–249. doi: 10.1016/s0735-1097(00)00702-6. [DOI] [PubMed] [Google Scholar]
- 220.Myers J, Gujja P, Neelagaru S, et al. End-tidal CO2 pressure and cardiac performance during exercise in heart failure. Medicine and science in sports and exercise. 2009;41(1):19–25. doi: 10.1249/MSS.0b013e318184c945. [DOI] [PubMed] [Google Scholar]
- 221.Jones PW, French W, Weissman ML, Wasserman K. Ventilatory responses to cardiac output changes in patients with pacemakers. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1981;51(5):1103–1107. doi: 10.1152/jappl.1981.51.5.1103. [DOI] [PubMed] [Google Scholar]
- 222.Arena R, Myers J, Abella J, et al. Prognostic value of timing and duration characteristics of exercise oscillatory ventilation in patients with heart failure. The Journal of Heart and Lung Transplantation. 2008;27(3):341–347. doi: 10.1016/j.healun.2007.11.574. [DOI] [PubMed] [Google Scholar]
- 223.Olson LJ, Arruda-Olson AM, Somers VK, Scott CG, Johnson BD. Exercise oscillatory ventilation: instability of breathing control associated with advanced heart failure. Chest. 2008;133(2):474–481. doi: 10.1378/chest.07-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Koike A, Shimizu N, Tajima A, et al. Relation between oscillatory ventilation at rest before cardiopulmonary exercise testing and prognosis in patients with left ventricular dysfunction. Chest. 2003;123(2):372–379. doi: 10.1378/chest.123.2.372. [DOI] [PubMed] [Google Scholar]
- 225.Ben-Dov I, Sietsema KE, Casaburi R, Wasserman K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. The American Review of Respiratory Disease. 1992;145(4 I):776–781. doi: 10.1164/ajrccm/145.4_Pt_1.776. [DOI] [PubMed] [Google Scholar]
- 226.Ponikowski P, Anker SD, Chua TP, et al. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100(24):2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 227.Francis DP, Willson K, Davies LC, Coats AJS, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102(18):2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 228.Hanly P, Zuberi N, Gray R. Pathogenesis of cheyne-stokes respiration in patients with congestive heart failure: relationship to arterial PCO2 . Chest. 1993;104(4):1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- 229.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. The New England Journal of Medicine. 1999;341(13):949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 230.Ponikowski P, Chua TP, Anker SD, et al. Peripheral chemoreceptor hypersensitivity an ominous sign in patients with chronic heart failure. Circulation. 2001;104(5):544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 231.Sanders MH. Article reviewed: a mechanism of central sleep apnea in patients with heart failure. Sleep Medicine. 2000;1(1):63–64. doi: 10.1016/s1389-9457(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 232.Ribeiro JP. Periodic breathing in heart failure: bridging the gap between the sleep laboratory and the exercise laboratory. Circulation. 2006;113(1):9–10. doi: 10.1161/CIRCULATIONAHA.105.590265. [DOI] [PubMed] [Google Scholar]
- 233.Andreas S. Central sleep apnea and chronic heart failure. Sleep. 2000;23(4):S220–S223. [PubMed] [Google Scholar]
- 234.Piepoli MF, Kaczmarek A, Francis DP, et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114(2):126–134. doi: 10.1161/CIRCULATIONAHA.105.605980. [DOI] [PubMed] [Google Scholar]
- 235.Francis DP, Davies LC, Piepoli M, Rauchhaus M, Ponikowski P, Coats AJS. Origin of oscillatory kinetics of respiratory gas exchange in chronic heart failure. Circulation. 1999;100(10):1065–1070. doi: 10.1161/01.cir.100.10.1065. [DOI] [PubMed] [Google Scholar]
- 236.Guazzi M, Raimondo R, Vicenzi M, et al. Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. Journal of the American College of Cardiology. 2007;50(4):299–308. doi: 10.1016/j.jacc.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 237.Ribeiro JP, Knutzen A, Rocco MB, Hartley LH, Colucci WS. Periodic breathing during exercise in severe heart failure: reversal with milrinone or cardiac transplantation. Chest. 1987;92(3):555–556. doi: 10.1378/chest.92.3.555. [DOI] [PubMed] [Google Scholar]
- 238.Ingle L, Isted A, Witte KK, Cleland JGF, Clark AL. Impact of different diagnostic criteria on the prevalence and prognostic significance of exertional oscillatory ventilation in patients with chronic heart failure. European Journal of Cardiovascular Prevention and Rehabilitation. 2009;16(4):451–456. doi: 10.1097/HJR.0b013e32832a4f54. [DOI] [PubMed] [Google Scholar]
- 239.Guazzi M, Arena R, Ascione A, Piepoli M, Guazzi MD. Exercise oscillatory breathing and increased ventilation to carbon dioxide production slope in heart failure: an unfavorable combination with high prognostic value. American Heart Journal. 2007;153(5):859–867. doi: 10.1016/j.ahj.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 240.Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Exercise oscillatory breathing in diastolic heart failure: prevalence and prognostic insights. European Heart Journal. 2008;29(22):2751–2759. doi: 10.1093/eurheartj/ehn437. [DOI] [PubMed] [Google Scholar]
- 241.Kremser CB, O’Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. The American Journal of Cardiology. 1987;59(8):900–905. doi: 10.1016/0002-9149(87)91116-7. [DOI] [PubMed] [Google Scholar]
- 242.Miyagi K, Asanoi H, Ishizaka S, Kameyama T, Sasayama S. Limited value of anaerobic threshold for assessing functional capacity in patients with heart failure. Clinical Cardiology. 1993;16(2):133–137. doi: 10.1002/clc.4960160210. [DOI] [PubMed] [Google Scholar]
- 243.Dall'Ago P, Chiappa GRS, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. Journal of the American College of Cardiology. 2006;47(4):757–763. doi: 10.1016/j.jacc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 244.Zurek M, Corra U, Piepoli MF, Binder RK, Saner H, Schmid JP. Exercise training reverses exertional oscillatory ventilation in heart failure patients. European Respiratory Journal. 2012;40(5):1238–1244. doi: 10.1183/09031936.00167011. [DOI] [PubMed] [Google Scholar]
- 245.Winkelmann ER, Chiappa GR, Lima COC, Viecili PRN, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. American Heart Journal. 2009;158(5):768–e1. doi: 10.1016/j.ahj.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 246.Laughlin MH. Cardiovascular response to exercise. The American Journal of Physiology. 1999;277(6):S244–S259. doi: 10.1152/advances.1999.277.6.S244. [DOI] [PubMed] [Google Scholar]
- 247.Bodner ME, Rhodes EC. A review of the concept of the heart rate deflection point. Sports Medicine. 2000;30(1):31–46. doi: 10.2165/00007256-200030010-00004. [DOI] [PubMed] [Google Scholar]
- 248.Bevilacqua M, Savonitto S, Bosisio E, et al. Role of the frank-starling mechanism in maintaining cardiac output during increasing levels of treadmill exercise in β-blocked normal men. The American Journal of Cardiology. 1989;63(12):853–857. doi: 10.1016/0002-9149(89)90056-8. [DOI] [PubMed] [Google Scholar]
- 249.Miller TD, Gibbons RJ, Squires RW, Allison TG, Gau GT. Sinus node deceleration during exercise as a marker of significant narrowing of the right coronary artery. The American Journal of Cardiology. 1993;71(4):371–373. doi: 10.1016/0002-9149(93)90815-t. [DOI] [PubMed] [Google Scholar]
- 250.Jorde UP, Vittorio TJ, Kasper ME, et al. Chronotropic incompetence, β-blockers, and functional capacity in advanced congestive heart failure: time to pace? European Journal of Heart Failure. 2008;10(1):96–101. doi: 10.1016/j.ejheart.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 251.Pierpont GL, Voth EJ. Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. The American Journal of Cardiology. 2004;94(1):64–68. doi: 10.1016/j.amjcard.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 252.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. Journal of the American College of Cardiology. 1994;24(6):1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 253.Seshadri N, Gildea TR, McCarthy K, Pothier C, Kavuru MS, Lauer MS. Association of an abnormal exercise heart rate recovery with pulmonary function abnormalities. Chest. 2004;125(4):1286–1291. doi: 10.1378/chest.125.4.1286. [DOI] [PubMed] [Google Scholar]
- 254.Dimopoulos S, Anastasiou-Nana M, Katsaros F, et al. Impairment of autonomic nervous system activity in patients with pulmonary arterial hypertension: a case control study. Journal of Cardiac Failure. 2009;15(10):882–889. doi: 10.1016/j.cardfail.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 255.Savonen KP, Kiviniemi V, Laaksonen DE, et al. Two-minute heart rate recovery after cycle ergometer exercise and all-cause mortality in middle-aged men. Journal of Internal Medicine. 2011;270(6):589–596. doi: 10.1111/j.1365-2796.2011.02434.x. [DOI] [PubMed] [Google Scholar]
- 256.Cahalin LP, Arena R, Guazzi M. Comparison of heart rate recovery after the six-minute walk test to cardiopulmonary exercise testing in patients with heart failure and reduced and preserved ejection fraction. The American Journal of Cardiology. 2012;110(3):467–468. doi: 10.1016/j.amjcard.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 257.Minai OA, Gudavalli R, Mummadi S, Liu X, McCarthy K, Dweik RA. Heart rate recovery predicts clinical worsening in patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2012;185(4):400–408. doi: 10.1164/rccm.201105-0848OC. [DOI] [PubMed] [Google Scholar]
- 258.Aijaz B, Squires RW, Thomas RJ, Johnson BD, Allison TG. Predictive value of heart rate recovery and peak oxygen consumption for long-term mortality in patients with coronary heart disease. The American Journal of Cardiology. 2009;103(12):1641–1646. doi: 10.1016/j.amjcard.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 259.Ritt LE, Oliveira RB, Myers J, et al. Patients with heart failure in the, “intermediate range” of peak oxygen uptake: additive value of heart rate recovery and the minute ventilation/carbon dioxide output slope in predicting mortality. Journal of Cardiopulmonary Rehabilitation and Prevention. 2012;32:141–146. doi: 10.1097/HCR.0b013e31824f9ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation. 2000;102(15):1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 261.Lucini D, Milani RV, Costantino G, Lavie CJ, Porta A, Pagani M. Effects of cardiac rehabilitation and exercise training on autonomic regulation in patients with coronary artery disease. American Heart Journal. 2002;143(6):977–983. doi: 10.1067/mhj.2002.123117. [DOI] [PubMed] [Google Scholar]
- 262.Hai JJ, Siu CW, Ho HH, Li SW, Lee S, Tse HF. Relationship between changes in heart rate recovery after cardiac rehabilitation on cardiovascular mortality in patients with myocardial infarction. Heart Rhythm. 2010;7(7):929–936. doi: 10.1016/j.hrthm.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 263.Maeder MT, Ammann P, Rickli H, Brunner-La Rocca HP. Impact of the exercise mode on heart rate recovery after maximal exercise. European Journal of Applied Physiology. 2009;105(2):247–255. doi: 10.1007/s00421-008-0896-2. [DOI] [PubMed] [Google Scholar]


