Abstract
In recent years, substantial advances in T-cell immunosuppressive strategies and their translation to routine clinical practice have revolutionized management and outcomes in autoimmune disease and solid organ transplantation. More than 80 diseases have been considered to have an autoimmune etiology, such that autoimmune-associated morbidity and mortality rank as third highest in developed countries, after cardiovascular diseases and cancer. Solid organ transplantation has become the therapy of choice for many end-stage organ diseases. Short-term outcomes such as patient and allograft survival at 1 year, acute rejection rates, as well as time course of disease progression and symptom control have steadily improved. However, despite the use of newer immunosuppressive drug combinations, improvements in long-term allograft survival and complete resolution of autoimmunity remain elusive. In addition, the chronic use of nonspecifically targeted immunosuppressive drugs is associated with significant adverse effects and increased morbidity and mortality. In this article, we discuss the current clinical tools for immune suppression and attempts to induce long-term T-cell tolerance induction as well as much-needed future approaches to produce more short-acting, antigen-specific agents, which may optimize outcomes in the clinic.
Keywords: autoimmunity, chimerism, immune suppression, infection, maintenance suppression, T-cell tolerance, transplantation
T-cell-mediated pathogenesis of transplantation & autoimmune disease
Examination of current clinical practice and clinical trials [201] reveals that there is a convergence of therapeutic strategies for the treatment of autoimmune diseases and the prevention of acute transplant rejection. It appears that the immunopathologic mechanisms driving T-cell-mediated autoimmune diseases and allograft rejection are similar in that immunopathology is driven by antigen-specific T cells. In autoimmune diseases such as multiple sclerosis, disease is caused by myelin-specific T cells, while allograft rejection is caused by T cells specific for major and minor histocompatibility antigens. Thus, while the clinical outcomes in autoimmune disease and organ transplantation appear to be vastly different, the ability to specifically regulate immunopathologic T-cell responses, or induce T-cell tolerance, is the major goal in clinical treatment of both disorders.
Tolerance
T-cell activation is the result of several key steps that result in a full effector response (Box 1 & Figure 1). Targeting one or more of these stages serves as the basic strategy for tolerance induction utilizing current immunosuppressive agents. Transplantation tolerance refers to a state of sustained, specific nonresponsiveness of the recipient's immune system to donor alloantigens; while tolerance induction in autoimmune disease refers to a reinstatement of sustained, specific nonresponsiveness of the native immune system to self-antigen.
Box 1. Signal hypothesis.
Signal 1: antigen recognition
■ T cells have specific receptors that recognize allo- or self-antigens, enabling the induction of antigen-specific cellular responses. In allo-antigen recognition, T cells can initiate rejection of MHC-mismatched tissues via three distinct pathways: the direct, indirect and the recently described semidirect pathways. T-cell antigen receptors (TCRs) recognize intact allogeneic MHC molecules displayed at the surface of donor cells (direct pathway), recognize peptides derived from processed allogeneic MHC molecules presented by recipient MHC class II molecules (indirect pathway), or simultaneously recognize alloantigen presented via both the direct and indirect pathways by the same antigen-presenting (semidirect pathway). In self-antigen recognition, the first signal received is via the interaction of the CD4+ TCR with processed self-peptides on MHC class II on the antigen-presenting cell (APC) surface (indirect pathway).
Signal 2: costimulation
■ Full T-cell activation requires not only antigen recognition, but also a second distinct costimulatory signal provided by the APC. Costimulatory signals are delivered via constitutive or inducible receptors on the responding T-cell surface interacting with their ligands constitutively expressed or upregulated on the activated APC. Without this second signal, the T cell cannot be fully activated to mount an effector response. There are a growing number of characterized costimulatory receptor:ligand molecules that are key for T-cell stimulation and regulation, including the CD28:B7(CD80:CD86) and CD154(CD40L):CD40 pathways. These positive activating costimulatory signals are balanced by inhibitory inducible signals, such as CTLA4:B7, which enables downregulation of the immune response after initial T-cell activation.
Signal 3: proliferation and differentiation
■ Costimulation together with antigen recognition initiates a cascade of downstream signaling pathways and the induction of transcription factors, leading to the expression of new surface molecules such as inducible costimulatory molecules and cytokine receptors. IL-2 and other cytokines can then trigger T-cell proliferation and differentiation via their receptors, upregulated on the recently activated T cell. Hence, the immune response is activated and amplified.
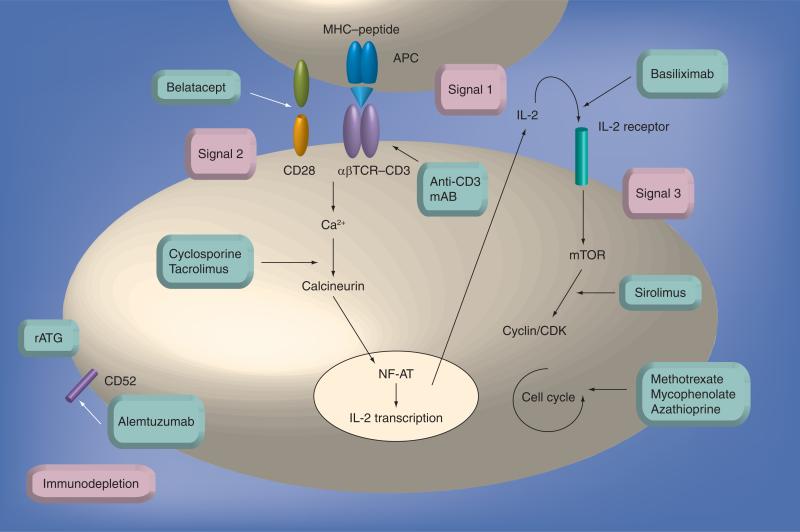
Figure 1. Current immunosuppressive drugs and their targets.
Signal 1 results from MHC–antigen recognition through the T-cell receptor–CD3 complex, a process blocked by anti-CD3 mAbs and indirectly by rituximab. Signal 2 results in costimulation, a process that can be blocked by belatacept. Costimulation activates downstream signaling pathways, resulting in calcineurin activation, a stage that can be inhibited by tacrolimus and cyclosporine A. Activated calcineurin dephosphorylates NF-AT, allowing IL-2 transcription to initiate signal 3. IL-2 receptor stimulation, a step that can be blocked by basiliximab, activates the mTOR signaling cascade, which can be inhibited by sirolimus. This pathway induces the T cell to enter the cell cycle and proliferate, which in turn can be blocked by methotrexate, mycophenolate and azathioprine. rATG exerts polyclonal effects while alemtuzumab binds to CD52, both resulting in immunodepletion. mAb: Monoclonal antibody; NF-AT: Nuclear factor of activated T cell; rATG: Rabbit antithymocyte globulin.
In the transplant setting, there are a number of patients that have developed tolerance to transplant tissues over the years. In many cases, these incidences of tolerance have been the result of patients’ noncompliance with their immune suppression regimens, weaning themselves off immune therapies over time. While immunological analysis of these patients has attempted to define the parameters through which tolerance is achieved in these patients, a tolerance fingerprint remains to be convincingly defined. Over recent years, however, further experimental models have shown that it is possible to exploit the mechanisms that normally maintain immune homeostasis and tolerance to self-antigens, to induce tolerance to alloantigen, as well as to reintroduce tolerance to self-antigen in an autoimmune setting. Based on current immunological understanding, these pathways may include the following mechanisms.
Deletion
Deletion of allo- or auto-reactive T cells can be achieved centrally in the thymus or in the periphery. Infusion of donor bone marrow into a recipient who has been conditioned by nonmyeloablative irradiation or immunotherapy enables antigen-presenting cells (APCs) to access the thymus and trigger the deletion of maturing thymocytes [1]. In the periphery, deletion can be triggered by alloantigen recognition under suboptimal conditions, including costimulation blockade as well as immunodepletion by activation of apoptotic cell death and cytolysis [2].
Anergy
This is the functional inactivation of the T-cell response to restimulation by allo- or self-antigen, and has been described both in vivo and in vitro. Some forms of T-cell anergy are also reported to result in the development of regulatory activity [3]. Costimulation blockade, as well as inhibition of downstream proliferative pathways, can trigger anergic states in T cells.
Immunoregulation
This active process results in the regulation of one cell population by the activity of another cell population. Various populations of leukocytes have been described in rodent models as having the ability to control immune responsiveness to alloantigen stimulation in both the innate and adaptive immune responses. This mechanism, although well described in experimental models, is yet to be introduced therapeutically in a sustained, clinical setting.
Clonal exhaustion
This can occur as a result of chronic antigen stimulation or antigen recognition under suboptimal conditions. The consequence is either deletion or functional inactivation of the cells that are responding to the recognized antigen. An example of such exhaustion can be seen in liver transplantation, where the large number of donor-derived APCs migrating from the liver to draining lymphoid tissues after transplantation can trigger this type of response [4].
Ignorance
This is an uncommon mechanism in the induction phase of unresponsiveness to alloantigen as it is difficult to introduce donor cells or tissue without alerting the immune system, in transplantation. This mechanism, however, does describe the natural state of some self-reactive CD4+ T-cell populations found in healthy individuals with no autoimmune pathology.
Immunosuppressive strategies in clinical practice
The ultimate goal of therapeutic intervention in transplantation and T-cell-mediated autoimmune disease is to induce immunological tolerance. Originally, the use of induction therapy in transplantation was designed to open a window whereby organs, such as the kidneys, could engraft before the administration of toxic metabolic maintenance immune suppression was required. However, work in animal studies showing the ability for short-course immune induction therapy (SCIIT) to induce long-lasting immune tolerance has raised the bar for induction therapy strategies. SCIIT may be defined as a specific, short-term immune modulation using a therapeutic agent to induce T-cell non-responsiveness, limiting the need for long-term maintenance immune suppression. Therapeutic regimens implemented in the clinic today aimed at inducing this phenomenon are focused on targeting the various steps involved in the T-cell-mediated immune response to allo- and self-antigen in an attempt to induce tolerance. As outlined earlier (Box 1), there are three main stages in this pathway: recognition of allo- or self-antigen, costimulation and proliferation/differentiation of effector T cells. The current clinical paradigm is based on blockade of at least one of these stages and/or by total immunodepletion therapy. Currently used agents appear to be more immune suppressive than tolerogenic, so successful implementation of SCIIT remains elusive. Nonetheless, current agents are capable of modulating the immune response and, when combined with maintenance immune suppression, provide a potent therapy to prevent transplant rejection (Figure 1). In addition to currently utilized agents, a number of novel therapies are in development (Table 1).
Table 1.
Maintenance immunosuppressive therapies in transplantation and autoimmune disease.
| Drugs | Mechanisms | Side effects |
|---|---|---|
| Azathioprine | Inhibits purine and DNA synthesis, inhibits cell proliferation | Bone marrow depression, opportunistic infection, macrocytosis and liver toxicity |
| Cyclosporine | Binds to cyclophilin, inhibits calcineurin phosphatase, blocks NF-AT dephosphorylation, blocks IL-2 transcription and T-cell activation | Hypertension, hyperlipidemia, nephrotoxicity, hepatotoxicity, pancreatitis, peptic ulcers, thrombotic microangiopathy, opportunistic infection, neurotoxicity, tremor, gingival hyperplasia and hirsutism |
| Methotrexate | Irreversibly inhibits DHFR, blocks folic acid synthesis, interferes with synthesis of DNA, RNA, thimidylates and proteins | Pulmonary fibrosis, hepatitis, anemia, neutropenia, hair loss, gastrointestinal symptoms and dermatitis |
| Mycophenolate mofetil | Inhibits inosine-monophosphate dehydrogenase, inhibits purine synthesis and blocks cell proliferation | Gastrointestinal symptoms, bone marrow depression, opportunistic infection in particular CMV and BK nephropathy |
| Sirolimus | Binds to FKBP12, inhibits mTOR and blocks IL-2-driven cell proliferation | Delayed graft function, delayed wound healing, mouth ulcers, pneumonitis, increased proteinuria, peripheral edema and hyperlipidemia |
| Steroids | Induces phospholipase A2 inhibitory proteins, inhibits arachidonic acid synthesis, inhibits prostaglandins and leukotrienes | Diabetes, delayed wound healing, peptic ulcers, psychosis, osteoporosis, infection, blurred vision, fluid retention, weight gain, acne and constipation |
| Tacrolimus | Binds to FKBP12, inhibits calcineurin phosphatase and blocks T-cell activation | Post-transplantation diabetes mellitus, nephrotoxicity, thrombotic microangiopathy and neurotoxicity |
| Fingolimod (FTY720) | Modulation of the spingosine-1-phosphate receptors | Adverse events reported in up to 10.7% of patients. All related to mechanism of action and include cardiovascular and infections. Isolated cases of ischemic stroke, vascular occlusion and encephalopathy have been reported [119,131] |
CMV: Cytomegalovirus; DHFR: Dihydrofolate reductase; NF-AT: Nuclear factor of activated T cell.
Immunodepletion
The more recent therapeutic strategies are based on induction therapies, which concentrate on profound immune cell depletion at the time of transplant, when immune activation is most intense.
Antithymocyte globulin
Antithymocyte (ATG) is a lymphocyte-depleting polyclonal IgG preparation with specificity towards human thymocytes. It binds primarily to peripheral blood lymphocytes, as well as to those from lymph nodes, spleen and thymus, according to data from in vivo studies in monkeys [5]. The agent's polyclonal nature enables it to display specificity towards a wide variety of antigens expressed on the surface of T cells, B cells, dendritic cells, NK cells and endothelial cells including those involved in immune response, T-cell activation, proliferation, apoptosis, signal transduction, cell adhesion and trafficking [6–8].
The precise mechanism of action underlying the immunosuppressive efficacy of rabbit (r)ATG in solid organ transplantation recipients is unknown at present, although has been primarily attributed to T-cell depletion. Data from in vitro studies suggest that rATG modulates the expression of various lymphocyte surface antigens resulting in apoptosis, antibody-dependent cytolysis or complement-dependent lysis. Lymphocyte depletion with rATG has been further demonstrated in adult renal transplant patients in several randomized, comparative clinical studies (n = 26–277) [9–12], with repopulation reported to take at least 3 months [13]. More recently, data from preclinical and clinical studies suggest that rATG therapy may induce the expansion and enrichment of certain regulatory T-cell subsets [14,15].
Clinical efficacy
Rabbit antithymocyte induction in combination with tacrolimus-based immunosuppressive therapy is more effective in preventing episodes of acute renal graft rejection than with tacrolimus-based therapy without induction, as reported by primary end point data from two randomized, open-label, multicenter trials [16,17]. Moreover, the median time to biopsy-proven acute rejection (BPAR) was >1 week longer in rATG induction than in non-induction recipients; however, it should be noted that there was no significant differences between induction and non-induction regimens in terms of patient or graft survival.
Several randomized, multicenter studies report that the efficacy of rATG induction therapy is generally no different from that of basiliximab or low-dose daclizumab (anti-IL-2R monoclonal antibodies [mAbs], described below) with regard to the incidence of BPAR, graft loss or death at 6 and 12 months post-transplantation, in the context of renal transplant recipients receiving triple immunosuppressive maintenance therapy [9,18,19]. These trials, however, were not powered to show overall superiority of one agent over the other [10], but instead were focused on safety parameters [16] at different dosage levels [19]. More robust studies are needed that are designed to specifically evaluate the efficacy of rATG relative to IL-2R mAbs, in order to definitively establish the use of rATG with respect to these monoclonal agents.
Minimized maintenance immunosuppression
The considerable morbidity concerns associated with long-term corticosteroid therapy have directed clinical studies to explore minimization of steroid use in maintenance immunosuppression. Limited data in adult and pediatric renal transplant recipients indicate that effective immunosuppression may be achieved with rATG in combination with steroid-free immunosuppressive therapy or with those using early withdrawal of steroids [20–22]. Longer-term, prospective data with larger patient numbers are needed to fully assess the efficacy of such regimens. These studies do illustrate, however, that rATG cannot be regarded as a tolerance-inducing therapy at present, but more as an immunosuppression-reducing agent; chronic maintenance therapy is still needed despite the lymphocyte-depleting effects of rATG.
Polyclonal effects
Polyclonal antibodies such as rATG have a nonspecific immunosuppressive action, which, although it results in profound lymphocyte depletion, can also lead to significant, undesirable side effects. These may include serum sickness (influenza-like symptoms due to immune complex deposition after foreign protein administration), neutralizing anti-rabbit (host response to nonhuman proteins), anti-idiotype antibody formation (antibody generation against the complementarity determining region), leukopenia, thrombocytopenia, gastrointestinal disorders and cytokine-release syndrome (due to mass release of cytokines after opsonization and destruction of mature T cells). These adverse effects present substantial comorbidity for transplant recipients, particularly as clinical data are yet to provide sound evidence of prolonged graft or patient survival with the use of rATG as an induction agent.
Susceptibility to infection & malignancy
A major concern with immunosuppressive therapy in solid organ transplantation is the risk of infections, and indeed these (bacterial, viral, fungal and protozoal) may occur in patients receiving rATG in combination with other immunosuppressive agents. Induction with rATG in combination with tacrolimus- or cyclosporine-based therapy had significantly greater overall incidence of infection compared to non-induction therapy as reported in two large clinical trials [16,17]. The incidence of cytomegalovirus and herpes simplex virus (HSV) in particular occurred in more rATG induction than non-induction recipients. In trials comparing rATG with other induction agents, the incidence of infection (bacterial, viral other than cytomegalovirus [CMV] and urinary tract infections) in high-risk patients treated with rATG was significantly greater than in those treated with basiliximab. Most studies report the use of antiviral (and in some cases antibacterial and antifungal) prophylaxis, but despite these measures, the incidence of infection with rATG remains significant. It should also be remembered that such prophylactic regimens carry their own adverse effects that can also significantly impact on patient morbidity. An increased risk of malignancies is also a concern with immunosuppressive therapy, but the incidence of malignancies such as post-transplant lymoproliferative disorder and de novo solid tumours is generally low with rATG induction and does not appear to differ from that seen with other agents [17–19,22–24].
Anti-CD52 mAb (alemtuzumab)
Anti-CD52 mAb (alemtuzumab) is a humanized rat IgG directed against the CD52 antigen, which is expressed on 95% of peripheral blood lymphocytes, NK cells, macrophages and thymocytes [25]; thus almost all mononuclear cells are affected. The profound and long-lasting lymphopenia produced after the administration of one or two doses of alemtuzumab is probably partly explained by such abundance on monocyte cell surfaces. It should be noted that examination of peripheral blood lymphocytes from human recipients post-alemtuzumab induction has identified a subset of T cells, predominantly CD4+ central memory cells, which survive despite alemtuzumab induction and appear largely resistant to depletion; these memory T cells were found to express lower CD52 levels than naive T cells [26]. CD52 is not present on granulocytes, platelets, erythrocytes or hematopoietic stem cells.
After binding to CD52, alemtuzumab causes cell death through several mechanisms: complement-mediated cytolysis, antibody-mediated cytotoxicity and apoptosis. While the plasma elimination half-life is approximately 12 days, its clinical effects are far more persistent. Lymphocyte depletion of >99% can be seen after a single dose [2], with lymph node depletion taking up to 3–5 days compared with <1 h seen in peripheral lymphocytes [27]. Different subpopulations display varying rates of recovery depending on the subpopulation of interest [2]: NK cells are almost unaffected and decrease only transiently (a population of CD52- NK cells has also been identified) [28]; monocyte and B-cell recovery can be observed at 3 and 12 months respectively; T-cell levels recover to only 50% of baseline at 36 months [29,30].
Clinical efficacy
Alemtuzumab is a powerful antilymphocyte antibody that produces profound and long-lasting lymphopenia. While originally thought to be used at increasing frequency, for induction in organ transplantation, with the aim of minimizing maintenance immunosuppression, its future availability for transplant patients is being questioned owing to rebranded exercises conducted by the manufacturer as part of their strategy to bring the drug to multiple sclerosis patients.
Alemtuzumab was first used in transplantation as an induction agent in 1998 [31], in 13 renal transplant recipients who received low-dose cyclosporine alone as maintenance therapy. At 6–12-month follow-up, patient and graft survival rates were 100% and there were two episodes of acute rejection. The 5-year results of the initial series [32], together with another 20 patients who were subsequently enrolled, reported no significant difference in graft or patient survival, or acute rejection rates when compared with retrospective, contemporaneous control group of 66 renal transplant recipients who had received no induction, but triple immunosuppression therapy alone (cyclosporine, azathioprine and prednisolone). The study did also find, however, more episodes of late rejection in alemtuzumab-treated group. Indeed, severe lymphopenia and homeostatic cytokines are known to drive the rapid homeostatic proliferation of naive and memory T cells, and lymphocytes generated under such conditions have been previously found to be potent alloreactive cells, inevitably triggering rejection in animal models [33]. Investigation of this phenomenon in human recipients by Trzonkowski et al. [26] illustrated that the recovery of the immune system post-alemtuzumab induction differed with respect to CD4+ and CD8+ T cells. While CD8+ T cells recovered to pretransplant levels by 6 months after alemtuzumab induction, the number of CD4+ T cells remained low, even at 1 year post-transplant. The precise reasons and outcomes for this remain unknown, although CD8+ T cell versus CD4+ T cell competition may play a role [28].
Despite now considerable experience with this agent, largely in renal transplantation, there are few relatively small, randomized controlled trials available and thus interpretation of evidence is limited. In two such studies widely cited in the literature [34,35], acute rejection rates, renal function and patient and graft survival in alemtuzumab recipients were all comparable with other induction or no induction regimens at 6-month [34] and 15-month [35] follow-up. In addition, 75% [34] and 80% [35] of alemtuzumab recipients remained steroid free. Subsequent randomized trials and larger retrospective studies [13,29,36] corroborate these earlier findings, supporting the use of alemtuzumab as an induction agent with corticosteroid-free/withdrawal regimens. However, similar to ATG, alemtuzumab is unable to induce complete tolerance, and requires chronic maintenance immunosuppression to prevent rejection of allograft.
In autoimmune diseases, clinical testing of alemtuzumab began in the early 1990s when it was shown to be ineffective for the treatment of rheumatoid arthritis (RA). Alemtuzumab has been explored recently as a potential treatment for relapsing–remitting multiple sclerosis (RRMS). In a Phase II trial involving 334 patients with early RRMS, alemtuzumab significantly decreased the rate of clinical relapse, reduced the risk of sustained accumulation of disability, and lessened the T2-weighted lesion burden on MRI, compared with IFN-β1a treatment [37]. These apparent clinical benefits came at a cost, however, as homeostatic peripheral T-cell expansion following lymphocyte depletion triggered autoimmunity. Immune thrombocytopenic purpura occurred in 2.8% of the alemtuzumab-treated patients, causing death in one case. A further 20% of patients receiving alemtuzumab were diagnosed with autoimmune complications of the thyroid gland.
CNI dependence
In an effort to reduce the comorbidities of maintenance regimens, several groups have looked at alemtuzumab induction and maintenance therapy without calcineurin inhibitor (CNI) agents. Unfortunately, results of pilot studies have been discouraging [29,38], with high acute rejection rates, (as much as 36% of recipients in one study). An attempt to induce donor allograft tolerance using alemtuzumab alone as an induction therapy with no maintenance immunosuppression at all resulted in 100% of patients developing acute rejection within the first month post-transplantation.
Review of rejection episodes that have occurred with alemtuzumab induction, particularly in the absence of CNIs, has highlighted some interesting findings. Several authors have reported that a number of these rejections demonstrate positive staining for C4d, indicative of acute humoral rejection [13,29,31,39]. Furthermore, examination of acute rejection biopsies taken from patients who have received both alemtuzumab induction and tacrolimus, revealed none that were humoral in origin. It has been postulated that depletion with alemtuzumab may cause dysregulation of B-cell function, with a resultant increased rate of acute humoral rejection [40]; this hypothesis needs further testing. Whatever the underlying mechanism for this phenomenon, one aspect is clear: alemtuzumab induction, despite its potency, is unable to prevent patient exposure to nephrotoxic CNI agents.
Susceptibility to infection
The profound lymphocyte depletion caused by alemtuzumab induction raises concerns with regard to susceptibility to opportunistic infection. Infective risks with the use of alemtuzumab in the treatment of hematological malignancies are well documented [41–43], although much higher doses at greater frequencies were administered to these patients than in transplantation. Studies looking at solid organ transplantation support the association of alemtuzumab use with increased frequency of unusual infections [40,44–47].
A large, recent retrospective cohort study [48] has attempted to clarify the issue. A total of 1738 patients were included, who underwent renal, liver or simultaneous pancreas kidney transplantation over a 3-year time period, having received induction with alemtuzumab, ATG or basiliximab. Strict inclusion criteria and clear definitions of viral, fungal and bacterial infections were implemented. The investigators found that although overall risk of infection was not increased with the use of alemtuzumab and was in fact lower than basiliximab, the infections that did occur were more severe and more likely to be disseminated, in particular candidal and CMV infection. Indeed, fungal infections were associated with excess mortality in the alemtuzumab group. Interestingly, another group looked at alemtuzumab induction in liver transplantation, and included in their cohort hepatitis C-positive patients. As expected, these patients did significantly worse than hepatitis C-negative patients in both induction and non-induction groups. However, in the depletional group, viral replication was frequently associated with alemtuzumab infusion; a marked increase in viral load was apparent in the 2 months following depletional therapy. This may suggest that lymphocyte depletion may somehow allow unchecked viral replication and could also lead to an earlier and more aggressive recurrence of disease [49]. Thus, the benefit of lymphocyte depletion with such potent agents as alemtuzumab should be balanced against the risk of severe infection.
Signal 1: blockade of antigen recognition
Activation of the rejection response to allograft, as well as activation of the autoimmune response to self-antigen, hinges on the recognition of antigen by the host immune system. Targeting signal 1 through the use of mAbs has been used in both transplantation and autoimmunity.
Anti-CD3 mAb
Muromonab-CD3 (OKT3), a mouse mAb binding to the CD3 component of the TCR signal-transduction complex, has been successfully used an induction agent for high-risk patients or for the treatment of corticosteroid-resistant acute rejection episodes [50]. Originally considered to act through a depletional mechanism, the return of T-cell counts within 1 week of dosing, combined with its ability to induce cytokine release, suggest receptor modulation may play a bigger role in its immunosuppressive effect [51,52]. Muronomab is no longer commercially available due to its propensity to induce severe adverse effects such as cytokine-release syndrome. OKT3 was removed from the market in 2008, due to the preference for ATG and Anti-CD52 agents. In an attempt to harness the positive aspects of anti-CD3 therapy, numerous attempts to humanize CD3 antibodies have been performed. These include teplizumab, a humanized OKT3 antibody, as well as otelxizumab and vizliximuab. Unfortunately, the biochemical engineering of these antibodies resulted in only moderate increases in the therapeutic safety. In 2011, all humanized anti-CD3 antibodies in development were terminated likely owing to toxicity and/or lack of efficacy issues.
Anti-αβTCR antibodies
Preventing signal 1 through the administration of anti-CD3 antibodies has been associated with significant drawbacks due to both cytokine release concerns and infectious complications. While attempts to humanize rat- and mouse-derived anti-CD3 antibodies have been made, this strategy has not yielded a significant decrease in these issues. This is arguably a reflection of the highly mitogenic activity of the CD3 protein, which acts as the major signaling component of the TCR, through its immunoreceptor tyrosine-based activation motifs (ITAMS; reviewed in [53]). On the other hand, the αβTCR does not contain these motifs, and as such may provide a more appropriate target for safer and more targeted T-cell modulation. Furthermore, specifically targeting the αβTCR may also have the overall benefit of preserving γδ T cells. While representing a small fraction of the total T-cell compartment, γδ T cells have been shown to be important in fighting infection. More importantly in the context of autoimmunity and transplant rejection, γδ T may play a positive role in generation of immunological tolerance [54]. In animal models, anti-αβTCR antibodies have successfully been utilized to treat EAE (an animal model of multiple sclerosis), diabetes in NOD mice (an animal model of Type 1 diabetes), as well as in numerous models of transplantation [55–59]. In addition, in the 1990s, numerous anti-αβTCR antibodies were tested in the clinic, mostly with favorable results compared with muronomab [57–68]. Today, there is one anti-αβTCR product in clinical testing, TOL101, a murine IgM that is specific for the αβTCR. No clinical data are currently available for this drug; however, in vitro studies with TOL101 suggest that this antibody is capable of modulating, in a nondepletional fashion, the αβTCR without triggering T-cell proliferation or cytokine release (Figure 2). Phase II clinical trials with TOL101 are ongoing and expected to be complete in 2012.
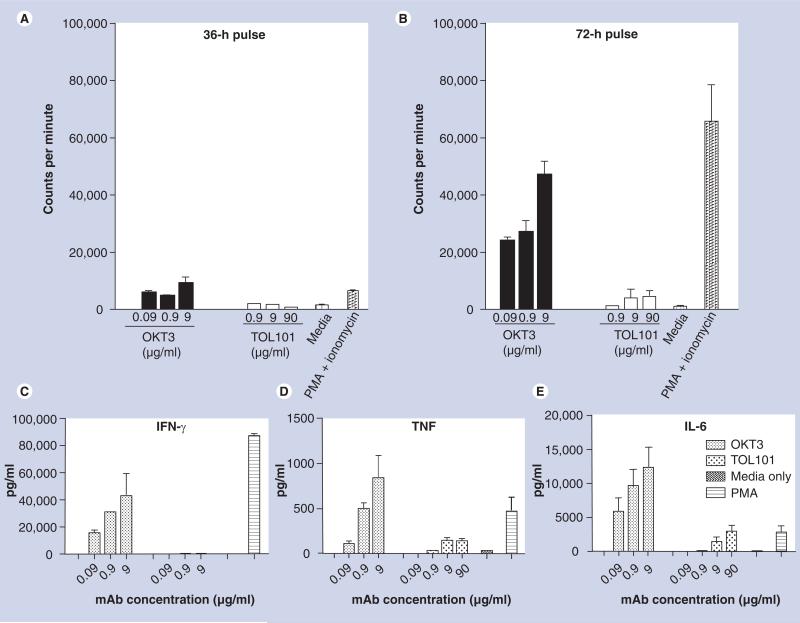
Figure 2. In vitro proliferation assays using freshly isolated human peripheral blood monocyte incubated with OKT3, TOL101, media alone (negative control) or phorbol 12-myristate 13-acetate and ionomycin (positive control).
After (A) 36 h or (B) 72 h, tritiated thymidine was added to the cultures for 12 h, after which the plates were harvested and the incorporation of thymidine measured as a reflection of the mitogenic capacity of each treatment. While OKT3 and PMA and ionomysin induced significant T-cell proliferation, TOL101 did not. The inability to induce T-cell proliferation was also reflected in a lack of proinflammatory cytokine: (C) IFN-γ, (D) TNF and (E) IL-6. Data presented are the mean and standard deviations from three individual patients’ PBMCs. These experiments have been repeated over three times with over nine independent donors.
mAb: Monoclonal antibody; PBMC: Peripheral blood monocyte; PMA: Phorbol 12-myristate 13-acetate.
Signal 2: blockade of costimulation
In a clinical setting, the inhibition of full T-cell activation by costimulatory blockade is an attempt to promote anergic tolerance induction while avoiding the many adverse effects of non-specific immunodepletion.
Cytotoxic T-lymphocyte-associated antigen 4 immunoglobulin
Cytotoxic T-lymphocyte-associated antigen (CTLA)-4 is an inducible, T-cell surface antigen that, when bound to CD80/86 receptor:ligand, delivers inhibitory signals to the activated T cell. Belatacept is a fusion protein combining the extracellular binding domain of CTLA-4 with the Fc portion of IgG1, with specificity for CD80/86 expressed on APCs. Ligation of CD80/86 by CD28 (a surface antigen constitutively expressed on T cells) usually lowers the activation threshold of these immune cells. Two CTLA-4 therapies exist, abatacept (approved for use in RA) and belatacept, with the latter representing a modified molecule that has a higher affinity and slower dissociation rate from human CD80/86 molecules, resulting in inhibition of the costimulation required for effective T-cell activation [69].
CTLA-4-Ig (abetacept) has been shown in large clinical trials to reduce the signs and symptoms of RA as well as slow radiological progression of joint damage [70–71]. However, in a randomized, placebo-controlled, Phase II trial, this agent failed to show treatment efficacy in patients with non-renal lupus on a background of oral corticosteroid therapy. This illustrates that costimulatory blockade is not a panacea for T-cell-mediated autoimmunity in general.
In the clinic, costimulatory blockade has been used more as a selective immunosuppressive agent to prevent acute rejection of allograft, or as an alternative to CNIs. Phase III clinical trials testing the ability of belatacept versus cyclosporine in renal transplantation patients has reported promising results with excellent low acute rejection rates and excellent protection of renal function. However, the incidence of post-transplant lymoproliferative disorder was reportedly higher in patients receiving higher doses of belatacept [72–74]. This observation may have provided the impetuous for the US FDA to look for longer follow-up data to confirm this drugs safety profile before it makes its final decision on whether to approve belatacept for approval or not. The approval of belatacept may represent a novel step toward CNI-free maintenance therapy.
The complexities of the human immune system present significant difficulties to the translation of such agents into clinical practice: in vivo work indicates memory and cytotoxic T cells have different costimulation requirements for complete activation, and furthermore, such blockade may also affect the function of regulatory T cells [75,76].
Signal 3: blockade of proliferation/differentiation
Activated T cells produce cytokines such as IL-2, which in turn binds to IL-2 receptors (IL-2Rs) that are expressed only on the surface of activated cells and are not present on resting T cells. IL-2R is composed of three high-affinity transmembrane protein subunits: α-(CD25), β- (CD122) and γ- (CD132) subunits, which are covalently linked. The α-subunit is specific to IL-2R only, and it is the binding of α- and β-subunits that is crucial to IL-2 signal transduction and T-cell activation, which subsequently leads to proliferation and clonal expansion of T and B cells specific to allo- or self-antigen. These cells are also stimulated to release more IL-2, further activating the immune response.
Anti-IL-2R mAb (basiliximab & daclizumab)
Basiliximab (chimeric form) and daclizumab (humanized form, currently unavailable) are commonly used in solid organ, namely renal, transplantation in low-risk recipients (usually based on transplant center specific criteria, including first allograft, living donor and low immune reactivity to a panel of antigens) [77,78]. As these drugs specifically target activated T cells, they do not cause significant lymphocyte depletion and are not associated with major adverse effects compared with lymphocyte-depleting agents. However, it is important to remember that other types of T cells, including regulatory T cells, also express CD25 and therefore the use of these agents may also impact some of the natural mechanisms of immunoregulation.
Basiliximab binds to IL-2R with similar affinity as IL-2, thereby effectively competing with IL-2 and subsequently inhibiting IL-2-driven T cell-driven proliferation. Basiliximab has a high volume of distribution, almost completely saturating IL-2R on peripheral lymphocytes within 24 h of a single dose of 2.5–25 mg in renal transplant recipients [79]. The half-life in adults is approximately 13.4 days, and IL-2R saturation and suppression can last for 4–6 weeks [80].
Clinical efficacy
Two meta-analyses evaluating the efficacy and safety of basiliximab in renal transplant recipients have been published [77,81]. Both studies showed that basiliximab was more effective than placebo in reducing acute cellular rejection 6 months after transplantation; the relative risk of acute rejection at 6 months was reported to be decreased by 35–49% in patients receiving basiliximab in both studies. However, both meta-analyses illustrated no significant differences in patient or graft survival rates between basiliximab and placebo groups at 1 year after transplantation. These findings are echoed in other randomized, double-blind, placebo-controlled trials [77,81–83].
Several randomized trials have examined the safety profile of basiliximab [82,84–86], and have reported no significant difference in type, incidence or severity of adverse events in patients who received basiliximab compared with placebo. The mAb is minimally immunogenic, and rates of adverse events, malignancy and infection are all comparable with placebo [82,87–90], and even reduced when compared with other induction agents [10,18,91].
IL-2R monoclonal antibodies have also been examined in the context of autoimmune disease. In an open-label trial involving 15 subjects with RRMS, daclizumab treatment was associated with a reduction in MRI brain activity [92], but was also associated with a reduction in the frequency and suppressive activity of circulating CD4 CD25 FoxP3 T regulatory cells [93]. However, the relevance of these seemingly paradoxical observations is uncertain in light of the absence of information about the status of T regulatory cells in the CNS.
Minimized maintenance immunosuppression
The highly selective and short-term immunosuppressive effects of basiliximab, which is confined to the highly immunogenic period immediately post-transplantation, makes this class of drug a useful substitute to steroids in early steroid withdrawal/steroid-free regimens, as illustrated in studies with liver transplantation. Several prospective clinical trials using basiliximab induction to facilitate early steroid withdrawal or complete steroid avoidance after kidney transplantation were tried and proven safe [94,95]. However, similar to alemtuzumab, the use of complete CNI avoidance protocols should be practised with extreme caution; in two studies in which CNIs were withheld for longer than a very short period, despite adequate immunosuppression with IL-2R mAbs, mycophenolate mofetil (MMF) and steroids, the acute cellular rejection rates after renal transplantation were much higher than with CNI use [96,97]. Thus, in line with ATG and alemtuzumab, basiliximab should be considered immunosuppression minimizing, rather than tolerance inducing. Patients are still exposed to chronic administration of maintenance immunosuppression and its associated co-morbidities.
High-risk patients
Brennan et al. reported an international study comparing a short course of ATG globulin (n = 141) and basiliximab (n = 137) in patients at high risk for acute rejection or DGF who received a deceased-donor renal transplantation [24]. Both groups received cyclosporine, MMF and steroids for maintenance immunosuppression. The ATG group, as compared with the basiliximab group, had lower incidences of BPAR (16 vs 26%; p = 0.02 at 1 year) and anti-body-treated BPAR (1.4 vs 8%; p = 0.005 at 1 year). The ATG group and basiliximab group had similar incidence of DGF, graft survival and patient survival. The incidences of all adverse events, serious adverse events and cancers were also similar between the two groups at 1 year. Longer-term data from a 5-year retrospective analysis [98] of this study support these findings, which suggests that the incidence of acute rejection is comparable with IL-2R mAb and ATG in kidney transplant recipients at low immunological risk, but the risk of rejection may be higher with IL-2R mAb in patients at high risk.
Small-molecule maintenance immunosuppression
The use of powerful induction therapies such as those described previously have done much to reduce the incidence of acute rejection in solid organ transplantation, as well as to alleviate symptoms and disease progression in some autoimmune conditions. Despite the introduction of such potent agents, however, clinical evidence strongly supports the use of life-long maintenance immunosuppression in the vast majority of patients to prevent acute rejection of allograft or disease relapse in conditions such as RA, systemic lupus erythematosus, psoriasis and Wegener's granulomatosis. Maintenance regimens generally consist of CNIs, which block alloantigen-dependent T-cell activation, and/or antiproliferative agents [99,100] as well as corticosteroids. These immunosuppressive drugs often target the immune response nonspecifically, and can lead to unwanted side effects including non-immunological complications, which in turn significantly contribute to patient morbidity and mortality (Table 1). In addition to approved agents, there are numerous small molecules in clinical testing. Two of the more promising agents are tofacitinib (CP 690,550) and sotrastaruin (AEB071) [101].
Tofacitinib (CP 690,550)
Cytokine signaling is key to potentiating autoimmunity and allograft rejection. Cytokines signal through two major types of cytokine receptors, referred to as type 1 and 2. These distinct membrane-bound proteins inherently lack the ability to directly trigger downstream events, and instead rely on their association with the cytoplasmic protein tyrosine kinases known as Janus kinases (JAK), named after the Roman god of doorways, Janus [102]. There are three known JAKs, which, when triggered by cytokine activation of the cytokine receptor, will mediate phosphorylation of the cytoplasmic tail of the receptor. In turn, this will attract the recruitment of signal transducers and activators of transcription (STATs), which are also phosphorylated by JAKs. Phosphorylated STATs translocate into the nucleus, where they directly regulate gene expression [102]. The combination of JAK and STAT involved in cellular signaling has a significant impact on cell function. For example, it is known that JAK3 plays an essential role in IL-2R signaling, as it is only associated with the IL-2R common γ-chain, which is used by numerous cytokines important for T-cell function. Clearly, an agent specifically capable of modulating components of the JAK–STAT pathway would have utility in transplantations and autoimmunity. In support of this notion, there are numerous JAK inhibitors in the pipelines of pharmaceutical companies. Toficitinib is in the advanced stage of the drug development process.
Recent studies in renal transplant patients showed that tofacitinib therapy, when combined with IL-2R induction, mycophenolic acid and steroids, was as effective as tacrolimus in this regimen [103–107]. In addition, toficitinib has exhibited promising results in a Phase II trial in RA, with up to 80% of patients displaying a significant reduction in disease [108,109]. However, continuing the trend for all immune suppression-based therapies, the development of infections and other adverse events remain a concern. A recent study has shown that tofacitinib inhibits both JAK1 and 3, and is capable of interfering with Th1, Th2 and Th17 differentiation and effector function [110]. These data, combined with the findings from the renal transplant study showing high doses of this agent result in increased numbers of infectious complications, support the need for further monitoring and examination of the safety profile of this and other JAK inhibitors in development.
Sotrastaurin (AEB071)
Protein kinases, through their ability to catalyze the phosphorylation of serine, threonine and tyrosine residues, play essential functions in many immunological processes including T-cell functions such as proliferation, differentiation and cytokine production. Owing to the importance of T cells in driving allograft rejection and autoimmune processes, protein kinases have long been considered an ideal therapeutic target to prevent allograft rejection and autoimmunity. However, specificity has been an issue, as there are over 500-related protein kinases (reviewed in [111], and most agents used to inhibit protein kinase activity do so through interference at a conserved ATP-binding site. However, sotrastaurin (AEB071), a low molecular weight, orally administered compound, is argued to block early T-cell activation through the specific inhibition of classical and novel protein kinase C isoforms. Preclinical in vitro testing demonstrated that sotrastaurin was capable of inhibiting T-cell activation and IL-2 production [101]. Furthermore, in rodent and monkey transplant models, sotrastaurin, alone or in combination with other agents, was capable of preventing heart and kidney allograft rejection [101,112,113].
In transplantation, the ability for sotrastaruin to suppress T-cell responses independently of the calcineurin pathway was originally thought be a significant advantage that may have paved the way for CNI-free maintenance suppression. However, early testing of sotrastaurin in combination with mycophenolic acid, without any CNIs, actually resulted in increased acute rejection rates [101]. Subsequent trials have since shown that when combined with CNIs, sotrastaurin is as effective as CNI and mycophenolic acid together, suggesting that sotrastaurin may provide an alternative to mycophenolic acid in the long term [101]. From the perspective of autoimmune disease therapy, sotrasturin has been tested in a limited protocol in psoriasis patients, where in the short term is reduced clinical severity of disease by 69% over baseline after 2 weeks of therapy [114]. Further clinical development is required to determine the long-term safety and efficacy of sotrastauin in autoimmunity and transplant patients.
Transplant tolerance through mixed chimerism & other cell-based strategies
Current therapeutic strategies to manipulate the immune response are certainly capable of reducing autoimmunity and reducing shortterm rejection rates; however, they are associated with significant adverse events, and in the case of transplantation have yielded little reduction in long-term rejection rates. Recently, in the transplant setting, there has been a shift to include concurrent cell infusions, broadly defined as hematopietic cell transplant (HCT), at the time of organ transplantation as a means to induce tolerance. In animal models of autoimmunity, infusion of apoptotic cells such as those induced through ethylene-carbodiamide cross-linking (ECDI-coupled cells), have shown promise. These strategies attempt to induce long-lasting immune tolerance, without the need maintenance immune suppression.
Hematopoietic cellular transplantation
Medawar, in 1953, originally showed that the infusion of allogenic cells into fetal or neonatal mice promoted tolerance between MHC-mismatched individuals. In his studies, Medawar proved that donor and recipient leukocytes can coexist without rejection or graft-versus-host disease development [115]. This particular phenomenon, whereby donor and recipient leukocytes coexist, is known as mixed chimerism. This popular area of tolerance research has been well described by several authors [116–118]; here, we briefly describe some of the clinical data and protocols that may allow for maintenance immune suppression-free transplantation.
The idea that mixed chimerism through bone marrow transplant could occur was supported by the observance that human bone marrow transplant patients who later received an organ transplant from the same donor accepted the organ, without the need for chronic immune suppression [119]. Since this observation, numerous studies have investigated the ability to use hematopoietic cellular transplant concurrently with solid organ transplant to induce trigger a state of allograft tolerance. However, the major limitation in broad utilization of HCT/organ transplant therapy has been the toxicity of conditioning regimens used to generate a favorable environment to allow for bone marrow engraftment. Classically, this has involved a combination of intense irradiation, immune cell depletion as well as immune suppression. Relative to currently utilized organ allograft rejection agents, these conditioning therapies carry a higher-risk profile for severe complications, and have thus hampered the development of HCT as a tool in transplantation. As a result, clinicians have spent much of the last decade pioneering less toxic conditioning regimens that may allow for safer HCT.
In one such study, Kawai and colleagues combined cylophosphamide, anti-CD2, cyclosporine and thymic irradiation prior to kidney transplantation, with donor bone marrow infusion subsequent to kidney transplant. Using this nonmyeloablative perioperative regimen, mixed chimerism was induced and stable renal allograft function without the need for maintenance immune suppression has been recorded for up to 5 years [120]. Examination of the immunological response during and after these therapies shows that the generation of chimerism is critical for long-term tolerance, but is not a guarantee. Using a similar regimen to that described by Kawai and colleagues, with the addition of anti-CD20 to the nonmyeloablation pre-transplant protocol, showed that multilineage chimerism was transient and undetectable at 14 days post-bone marrow transplant. Nonetheless, at day 24, the antidonor T-cell response, as measured by limiting dilution analysis, was described to be nondetectable, while third party response remained intact [121].
Unfortunately, the work performed by these groups used anti-CD2 antibodies that are no longer in clinical development or available commercially. Therefore, testing using agents currently available or in clinical development will be necessary. These research should include antithymocyte globulin, CTLA4-Ig as well as anti-αβTCR. Experimental studies, including those using anti-αβTCR antibodies [122–124], suggest that these may provide a useful alternative in the endeavor of combining HCT with solid organ transplant.
ECDI-coupled cell tolerance
In 1979, Miller and Claman reported that murine immune reactions against a model antigen could be prevented if the antigen was covalently linked to splenic leukocytes (using a chemical cross-linker) and administered to recipient animals [125]. In the intervening 30 years, this technique (termed ‘antigen-coupled cells’) has been successfully applied to several models of autoimmunity (including EAE and an animal model of Type 1 diabetes), viral encephalitis and transplantation of mismatched pancreatic islets and skin [126–130].
Antigen-coupled cells have been particularly useful in studies of EAE, where tolerance induction against select epitopes was used to elucidate the immunodominance hierarchy of antigens in this model [128]. Antigen-coupled cells have also been used to confer immune tolerance against several epitopes simultaneously, regardless whether the epitopes were present as separate peptides, or as part of a whole protein, or even a whole organ homogenate. Furthermore, infusion of ECDI fixation of donor leukocytes may also induce long-lasting tolerance in murine model of MHC-mismatched pancreatic islet cell transplantation. Overall, this flexibility is an important consideration for complicated diseases such as transplant rejection, multiple sclerosis and Type 1 diabetes, where several epitopes are targeted by T cells. A ongoing German clinical trial at the University of Hamburg is employing the seven identified immunodominant CNS epitopes to new-onset RRMS patients.
Future perspective
The concept of short-course immune induction therapy was pioneered in murine models, and there are now numerous ways to induce T-cell unresponsiveness in rodents. However, the translation from animal models to humans remains poor in autoimmunity and transplantation. While antigen-specific techniques for tolerance induction in humans are hopefully not far off in the future, it is clear that the current techniques for managing the allograft and autoimmune response need to be refocused. The lack of long-term graft survival in transplant patients and the clear risk of infection and cancer in any patients taking the commonly prescribed immune suppression agents suggest that new agents are required with an enhanced therapeutic index. Further investigation on large cohorts of patients that appear to have developed tolerance need to be conducted, and the stability of tolerance and the ability for inflammation to overcome tolerance all need to be addressed. Finally, the combination of novel agents with cellular transplants needs to be examined, with avenues examining how to make such therapies commercially viable in the long term.
Executive summary.
■ Current clinical tools in use for immune suppression in T-cell-mediated autoimmune disease and organ transplant have improved outcomes in these indications, but are still associated with adverse effects and would be improved by increased target specificity.
- ■ T-cell tolerance is a major goal in the clinical treatment of both T-cell-mediated autoimmune disease and organ transplant rejection, the immunopathologic mechanisms of which are similar to one another.
- – T-cell tolerance refers to a state of specific nonresponsiveness to antigen.
- – T-cell tolerance to self- or allo-antigen is the goal of tolerance-inducing therapies in the treatment of autoimmune disease or in the prevention/treatment of allograft transplant rejection, respectively.
- – Basic mechanisms of T-cell tolerance include deletion, anergy, immunoregulation, clonal exhaustion and ignorance.
- ■ Clinically, therapeutic interventions in transplantation and T-cell-mediated autoimmune disease are based either on immunodepletion or on blockade of one of the three steps of T-cell activation – antigen recognition (signal 1), co-stimulation (signal 2) and proliferation/differentiation (signal 3).
- – Immunodepletion: antithymocyte globulin (ATG; a polyclonal IgG preparation that depletes T cells, B cells, dendritic cells, NKs, endothelial cells and others) and alemtuzumab (a monoclonal anti-CD52 antibody that depletes almost all mononuclear cells) are currently widely used clinically and are effective but associated with side effects that appear to be related to the broad immune depletion.
- – Blockade of antigen recognition (T-cell receptor complex antibodies): while anti-CD3 antibodies were in use for some time in transplantation, their use has largely been replaced by ATG and alemtuzumab. Anti-αβTCR antibody is currently in clinical testing in transplantation.
- – Blockade of costimulation: CTLA-4-Ig therapies interfere with costimulation through the CD80/86-CD28 pathway.
- – Blockade of proliferation/differentiation: anti-IL-2R antibodies are currently used in transplantation (generally in low-risk patients) and have been clinically tested in autoimmune disease.
- ■ In most cases, maintenance immune suppression in the form of calcineurin inhibitors, antiproliferative agents and/or corticosteroids is also administered to transplant patients and relapsing autoimmune disease patients.
- – These maintenance immune suppression agents are nonspecific and tend to be associated with unwanted side effects.
- – Tofacitinib is a promising small-molecule maintenance immune suppression agent currently in clinical testing; this agent is a JAK inhibitor and therefore targets various aspects of immune cell activation.
- – Sotrastaurin is a small-molecule inhibior of protein kinase C and is an example of another promising small-molecule maintenance immune suppression agent currently in clinical testing.
- ■ Transplant tolerance through mixed chimerism and other cell-based strategies has recently shown promise in both transplantation and in animal models of autoimmunity.
- – The goal of cell-based tolerance strategies is to induce immune tolerance and avoid the use of maintenance immunosuppresion.
- – Hematopoietic cell transplant is conducted via transfer of bone marrow following a conditioning regimen generating a favorable environment for bone marrow engraftment. The major drawback of this strategy is the toxic nature of the conditioning regimens.
- – ECDI coupled-cell tolerance is a method of inducing specific T-cell tolerance through infusion of leukocytes covalently linked (via the chemical ECDI) to the peptides/proteins against which tolerance is desired. This method of tolerance is currently being tested clinically in multiple sclerosis patients.
Acknowledgments
S Miller and DR Getts receive research funding support from Tolera Therapeutics.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organi zation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Manilay JO, Waneck GL, Sykes M. Altered expression of Ly-49 receptors on NK cells developing in mixed allogeneic bone marrow chimeras. Int. Immunol. 1998;10(12):1943–1955. doi: 10.1093/intimm/10.12.1943. [DOI] [PubMed] [Google Scholar]
- 2.Ciancio G, Burke GW, Gaynor JJ, et al. The use of Campath-1H as induction therapy in renal transplantation: preliminary results. Transplantation. 2004;78(3):426–433. doi: 10.1097/01.tp.0000128625.29654.eb. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264(5165):1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 4.Bishop GA, Sun J, Sheil AG, Mccaughan GW. High-dose/activation-associated tolerance: a mechanism for allograft tolerance. Transplantation. 1997;64(10):1377–1382. doi: 10.1097/00007890-199711270-00001. [DOI] [PubMed] [Google Scholar]
- 5.Preville X, Flacher M, Lemauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71(3):460–468. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 6.Naujokat C, Berges C, Fuchs D, Sadeghi M, Opelz G, Daniel V. Antithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation. 2007;83(4):485–497. doi: 10.1097/01.tp.0000251975.81281.22. [DOI] [PubMed] [Google Scholar]
- 7.Dalle JH, Dardari R, Menezes J, Cordeiro P, Champagne MA, Duval M. Binding of thymoglobulin to natural killer cells leads to cell activation and interferon-γ production. Transplantation. 2009;87(4):473–481. doi: 10.1097/TP.0b013e3181949c57. [DOI] [PubMed] [Google Scholar]
- 8.Stauch D, Dernier A, Sarmiento Marchese E, et al. Targeting of natural killer cells by rabbit antithymocyte globulin and campath-1H: similar effects independent of specificity. PLoS One. 2009;4(3):e4709. doi: 10.1371/journal.pone.0004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noel C, Abramowicz D, Durand D, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J. Am. Soc. Nephrol. 2009;20(6):1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebranchu Y, Bridoux F, Buchler M, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am. J. Transplant. 2002;2(1):48–56. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 11■■.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, Phase III clinical trial of thymoglobulin versus ATGAM in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66(1):29–37. doi: 10.1097/00007890-199807150-00005. [Pivotal trial showing rATG superior to ATGAM.] [DOI] [PubMed] [Google Scholar]
- 12.Brennan DC, Flavin K, Lowell JA, et al. Leukocyte response to thymoglobulin or ATGAM for induction immunosuppression in a randomized, double-blind clinical trial in renal transplant recipients. Transplantation Proceedings. 1999;31(Suppl. 3B):S16–S18. doi: 10.1016/s0041-1345(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro R, Basu A, Tan H, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with thymoglobulin or Campath. J. Am. Coll. Surg. 2005;200(4):505–515. doi: 10.1016/j.jamcollsurg.2004.12.024. quiz A559–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J. Am. Soc. Nephrol. 2006;17(10):2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 15.Lacorcia G, Swistak M, Lawendowski C, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation. 2009;87(7):966–974. doi: 10.1097/TP.0b013e31819c84b8. [DOI] [PubMed] [Google Scholar]
- 16■■.Mourad G, Garrigue V, Squifflet JP, et al. Induction versus noninduction in renal transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2001;72(6):1050–1055. doi: 10.1097/00007890-200109270-00012. [Induction shown to reduce acute rejection.] [DOI] [PubMed] [Google Scholar]
- 17.Charpentier B, Rostaing L, Berthoux F, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation. 2003;75(6):844–851. doi: 10.1097/01.TP.0000056635.59888.EF. [DOI] [PubMed] [Google Scholar]
- 18.Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation. 2004;78(4):584–590. doi: 10.1097/01.tp.0000129812.68794.cc. [DOI] [PubMed] [Google Scholar]
- 19■■.Abou-Ayache R, Buchler M, Lepogamp P, et al. CMV infections after two doses of daclizumab versus thymoglobulin in renal transplant patients receiving mycophenolate mofetil, steroids and delayed cyclosporine A. Nephrol. Dial. Transplant. 2008;23(6):2024–2032. doi: 10.1093/ndt/gfm873. [Examination of viral complications of transplant immune suppression.] [DOI] [PubMed] [Google Scholar]
- 20.Rajab A, Pelletier RP, Henry ML, Ferguson RM. Excellent clinical outcomes in primary kidney transplant recipients treated with steroid-free maintenance immunosuppression. Clin. Transplan. 2006;20(5):537–546. doi: 10.1111/j.1399-0012.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 21.Matas AJ, Kandaswamy R, Gillingham KJ, et al. Prednisone-free maintenance immunosuppression-a 5-year experience. Am. J. Transplant. 2005;5(10):2473–2478. doi: 10.1111/j.1600-6143.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 22.Birkeland SA. Steroid-free immunosuppression in renal transplantation: a long-term follow-up of 100 consecutive patients. Transplantation. 2001;71(8):1089–1090. doi: 10.1097/00007890-200104270-00013. [DOI] [PubMed] [Google Scholar]
- 23.Hardinger KL, Rhee S, Buchanan P, et al. A prospective, randomized, double-blinded comparison of thymoglobulin versus Atgam for induction immunosuppressive therapy: 10-year results. Transplantation. 2008;86(7):947–952. doi: 10.1097/TP.0b013e318187bc67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24■.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N. Engl. J. Med. 2006;355(19):1967–1977. doi: 10.1056/NEJMoa060068. [rATG shown to be superior to basiliximab in high-risk renal transplants.] [DOI] [PubMed] [Google Scholar]
- 25.Morris PJ, Russell NK. Alemtuzumab (Campath-1H): a systematic review in organ transplantation. Transplantation. 2006;81(10):1361–1367. doi: 10.1097/01.tp.0000219235.97036.9c. [DOI] [PubMed] [Google Scholar]
- 26.Trzonkowski P, Zilvetti M, Chapman S, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am. J. Transplant. 2008;8(2):338–347. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- 27.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation. 2003;76(1):120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 28■■.Trzonkowski P, Zilvetti M, Friend P, Wood KJ. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82(10):1342–1351. doi: 10.1097/01.tp.0000239268.64408.84. [Memory T cells appear to be resistant to alemtuzumab therapy.] [DOI] [PubMed] [Google Scholar]
- 29.Knechtle SJ, Pirsch JD, H Fechner J, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am. J. Transplant. 2003;3(6):722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 30.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81(1):81–87. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 31.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351(9117):1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 32.Watson CJ, Bradley JA, Friend PJ, et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation – efficacy and safety at five years. Am. J. Transplant. 2005;5(6):1347–1353. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vathsala A, Ona ET, Tan SY, et al. Randomized trial of Alemtuzumab for prevention of graft rejection and preservation of renal function after kidney transplantation. Transplantation. 2005;80(6):765–774. doi: 10.1097/01.tp.0000166921.14670.33. [DOI] [PubMed] [Google Scholar]
- 35.Ciancio G, Burke GW, Gaynor JJ, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80(4):457–465. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman DB, Leventhal JR, Axelrod D, Gallon LG, Parker MA, Stuart FP. Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: comparison with basiliximab induction--long-term results. Am. J. Transplant. 2005;5(10):2539–2548. doi: 10.1111/j.1600-6143.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 37■■.Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 2008;359(17):1786–1801. doi: 10.1056/NEJMoa0802670. [Efficacy of alemtuzumab in multiple sclerosis patients reported.] [DOI] [PubMed] [Google Scholar]
- 38.Flechner SM, Friend PJ, Brockmann J, et al. Alemtuzumab induction and sirolimus plus mycophenolate mofetil maintenance for CNI and steroid-free kidney transplant immunosuppression. Am. J. Transplant. 2005;5(12):3009–3014. doi: 10.1111/j.1600-6143.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 39.Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80(8):1051–1059. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 40.Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl. Int. 2006;19(9):705–714. doi: 10.1111/j.1432-2277.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 41.Ravandi F, O'Brien S. Alemtuzumab. Expert Rev. Anticancer Ther. 2005;5(1):39–51. doi: 10.1586/14737140.5.1.39. [DOI] [PubMed] [Google Scholar]
- 42.Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920–2924. doi: 10.1182/blood-2003-10-3389. [DOI] [PubMed] [Google Scholar]
- 43.Dearden C. The role of alemtuzumab in the management of T-cell malignancies. Semin. Oncol. 2006;33(2 Suppl. 5):S44–S52. doi: 10.1053/j.seminoncol.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Silveira FP, Husain S, Kwak EJ, et al. Cryptococcosis in liver and kidney transplant recipients receiving anti-thymocyte globulin or alemtuzumab. Transpl. Infect. Dis. 2007;9(1):22–27. doi: 10.1111/j.1399-3062.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 45.Nath DS, Kandaswamy R, Gruessner R, Sutherland DE, Dunn DL, Humar A. Fungal infections in transplant recipients receiving alemtuzumab. Transplant. Proc. 2005;37(2):934–936. doi: 10.1016/j.transproceed.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 46.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin. Infect. Dis. 2006;43(1):16–24. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 47.Abad S, Gyan E, Moachon L, et al. Tuberculosis due to Mycobacterium bovis after alemtuzumab administration. Clin. Infect. Dis. 2003;37(2):E27–E28. doi: 10.1086/375690. [DOI] [PubMed] [Google Scholar]
- 48.Safdar N, Smith J, Knasinski V, et al. Infections after the use of alemtuzumab in solid organ transplant recipients: a comparative study. Diagn. Microbiol. Infect. Dis. 2010;66(1):7–15. doi: 10.1016/j.diagmicrobio.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Marcos A, Eghtesad B, Fung JJ, et al. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;78(7):966–971. doi: 10.1097/01.tp.0000142674.78268.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Opelz G. Efficacy of rejection prophylaxis with OKT3 in renal transplantation. Collaborative Transplant Study. Transplantation. 1995;60(11):1220–1224. [PubMed] [Google Scholar]
- 51.Midtvedt K, Fauchald P, Lien B, et al. Individualized T cell monitored administration of ATG versus OKT3 in steroid-resistant kidney graft rejection. Clin. Transplan. 2003;17(1):69–74. doi: 10.1034/j.1399-0012.2003.02105.x. [DOI] [PubMed] [Google Scholar]
- 52.Getts D, Martin A, Siemionow M, Miller S. Operational tolerance vs immune suppression, targeting the α β TCR with TOL101. Am. J. Transplant. 2010;10(Suppl. 4):210. [Google Scholar]
- 53.Getts DR, Getts MT, Mccarthy DP, Chastain EM, Miller SD. Have we overestimated the benefit of human(ized) antibodies? MAbs. 2010;2(6):682–694. doi: 10.4161/mabs.2.6.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locke NR, Stankovic S, Funda Dp, Harrison LC. TCR γ δ intraepithelial lymphocytes are required for self-tolerance. J. Immunol. 2006;176(11):6553–6559. doi: 10.4049/jimmunol.176.11.6553. [DOI] [PubMed] [Google Scholar]
- 55.Lavasani S, Dzhambazov B, Andersson M. Monoclonal antibody against T-cell receptor αβ induces self-tolerance in chronic experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2007;65(1):39–47. doi: 10.1111/j.1365-3083.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- 56.Sempe P, Bedossa P, Richard MF, Villa MC, Bach JF, Boitard C. Anti-α/β T cell receptor monoclonal antibody provides an efficient therapy for autoimmune diabetes in nonobese diabetic (NOD) mice. Eur. J. Immunol. 1991;21(5):1163–1169. doi: 10.1002/eji.1830210511. [DOI] [PubMed] [Google Scholar]
- 57.Waid TH, Lucas BA, Amlot P, et al. T10B9.1A-31 anti-T-cell monoclonal antibody: preclinical studies and clinical treatment of solid organ allograft rejection. Am. J. Kidney Dis. 1989;14(5 Suppl. 2):61–70. [PubMed] [Google Scholar]
- 58.Waid TH, Lucas BA, Thompson JS, et al. Treatment of acute cellular kidney allograft rejection with T10B9.1A-31A anti T-cell monoclonal antibody. Transplant. Proc. 1989;21(1 Pt 2):1778–1784. [PubMed] [Google Scholar]
- 59.Waid TH, Lucas BA, Thompson JS, et al. Treatment of acute cellular rejection with T10B9.1A-31 or OKT3 in renal allograft recipients. Transplantation. 1992;53(1):80–86. doi: 10.1097/00007890-199201000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Bishop MR, Henslee-Downey PJ, Anderson JR, et al. Long-term survival in advanced chronic myelogenous leukemia following bone marrow transplantation from haploidentical related donors. Bone Marrow Transplant. 1996;18(4):747–753. [PubMed] [Google Scholar]
- 61.Brown S, Lucas B, Waid T, Mckeown W, Tsuchida M, Thompson J. T10B9 action: the effect of anti-T-cell antibody mitogenicity and graft rejection on the first dose cytokine response. Transplant. Proc. 1997;29(1–2):315–316. doi: 10.1016/s0041-1345(96)00281-3. [DOI] [PubMed] [Google Scholar]
- 62.Bunin N, Aplenc R, Iannone R, et al. Unrelated donor bone marrow transplantation for children with severe aplastic anemia: minimal GVHD and durable engraftment with partial T cell depletion. Bone Marrow Transplant. 2005;35(4):369–373. doi: 10.1038/sj.bmt.1704803. [DOI] [PubMed] [Google Scholar]
- 63.Bunin N, Aplenc R, Leahey A, et al. Outcomes of transplantation with partial T-cell depletion of matched or mismatched unrelated or partially matched related donor bone marrow in children and adolescents with leukemias. Bone Marrow Transplant. 2005;35(2):151–158. doi: 10.1038/sj.bmt.1704754. [DOI] [PubMed] [Google Scholar]
- 64.Godder KT, Hazlett LJ, Abhyankar SH, et al. Partially mismatched related-donor bone marrow transplantation for pediatric patients with acute leukemia: younger donors and absence of peripheral blasts improve outcome. J. Clin. Oncol. 2000;18(9):1856–1866. doi: 10.1200/JCO.2000.18.9.1856. [DOI] [PubMed] [Google Scholar]
- 65.Waid TH, Lucas BA, Thompson JS, et al. Treatment of acute rejection with anti-T-cell antigen receptor complex alpha beta (T10B9.1A-31) or anti-CD3 (OKT3) monoclonal antibody: results of a prospective randomized double-blind trial. Transplant. Proc. 1991;23(1 Pt 2):1062–1065. [PubMed] [Google Scholar]
- 66.Beelen DW, Graeven U, Schulz G, et al. Treatment of acute graft-versus-host disease after HLA-partially matched marrow transplantation with a monoclonal antibody (BMA031) against the T cell receptor. First results of a Phase-I/II trial. Onkologie. 1988;11(1):56–58. doi: 10.1159/000216484. [DOI] [PubMed] [Google Scholar]
- 67.Beelen DW, Grosse-Wilde H, Ryschka U, et al. Initial treatment of acute graft-versus-host disease with a murine monoclonal antibody directed to the human alpha/beta T cell receptor. Cancer Immunol. Immunother. 1991;34(2):97–102. doi: 10.1007/BF01741342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker C, Pichler WJ, Koponen M, Domzig W, De Weck AL. Different effects of IL-2 addition or antibody crosslinking on T-cell subset stimulation by CD3 antibodies. Cell Immunol. 1986;101(1):195–203. doi: 10.1016/0008-8749(86)90197-8. [DOI] [PubMed] [Google Scholar]
- 69.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transplant. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 70.Vincenti F, Luggen M. T cell costimulation: a rational target in the therapeutic armamentarium for autoimmune diseases and transplantation. Annu. Rev. Med. 2007;58:347–358. doi: 10.1146/annurev.med.58.080205.154004. [DOI] [PubMed] [Google Scholar]
- 71.Ruderman EM, Pope RM. Drug insight: abatacept for the treatment of rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2006;2(12):654–660. doi: 10.1038/ncprheum0345. [DOI] [PubMed] [Google Scholar]
- 72.Larsen Cp, Grinyo J, Medina-Pestana J, et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation. 2010;90(12):1528–1535. doi: 10.1097/TP.0b013e3181ff87cd. [DOI] [PubMed] [Google Scholar]
- 73.Vincenti F, Blancho G, Durrbach A, et al. Five-year safety and efficacy of belatacept in renal transplantation. J. Am. Soc. Nephrol. 2010;21(9):1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A Phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am. J. Transplant. 2010;10(3):535–546. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 75.Koyama S, Ishii KJ, Kumar H, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 2007;179(7):4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 76.Jones ND, Van Maurik A, Hara M, et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J. Immunol. 2000;165(2):1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 77.Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for renal transplant recipients: a meta-analysis of randomized trials. Transplantation. 2004;77(2):166–176. doi: 10.1097/01.TP.0000109643.32659.C4. [DOI] [PubMed] [Google Scholar]
- 78.Vincenti F, De Andres A, Becker T, et al. Interleukin-2 receptor antagonist induction in modern immunosuppression regimens for renal transplant recipients. Transpl. Int. 2006;19(6):446–457. doi: 10.1111/j.1432-2277.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- 79.Amlot PL, Rawlings E, Fernando ON, et al. Prolonged action of a chimeric interleukin-2 receptor (CD25) monoclonal antibody used in cadaveric renal transplantation. Transplantation. 1995;60(7):748–756. doi: 10.1097/00007890-199510150-00023. [DOI] [PubMed] [Google Scholar]
- 80.Basadonna GP, Matas AJ, Gillingham KJ, et al. Early versus late acute renal allograft rejection: impact on chronic rejection. Transplantation. 1993;55(5):993–995. doi: 10.1097/00007890-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Keown P, Balshaw R, Khorasheh S, et al. Meta-analysis of basiliximab for immunoprophylaxis in renal transplantation. BioDrugs. 2003;17(4):271–279. doi: 10.2165/00063030-200317040-00006. [DOI] [PubMed] [Google Scholar]
- 82.Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet. 1997;350(9086):1193–1198. doi: 10.1016/s0140-6736(97)09278-7. [DOI] [PubMed] [Google Scholar]
- 83.Calmus Y, Scheele JR, Gonzalez-Pinto I, et al. Immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with azathioprine-containing triple therapy in liver transplant recipients. Liver Transpl. 2002;8(2):123–131. doi: 10.1053/jlts.2002.30882. [DOI] [PubMed] [Google Scholar]
- 84.Ponticelli C, Yussim A, Cambi V, et al. A randomized, double-blind trial of basiliximab immunoprophylaxis plus triple therapy in kidney transplant recipients. Transplantation. 2001;72(7):1261–1267. doi: 10.1097/00007890-200110150-00014. [DOI] [PubMed] [Google Scholar]
- 85.Lawen JG, Davies EA, Mourad G, et al. Randomized double-blind study of immunoprophylaxis with basiliximab, a chimeric anti-interleukin-2 receptor monoclonal antibody, in combination with mycophenolate mofetil-containing triple therapy in renal transplantation. Transplantation. 2003;75(1):37–43. doi: 10.1097/00007890-200301150-00007. [DOI] [PubMed] [Google Scholar]
- 86.Chen W, Bennett CF, Wang ME, et al. Perfusion of kidneys with unformulated ‘naked’ intercellular adhesion molecule-1 antisense oligodeoxynucleotides prevents ischemic/reperfusion injury. Transplantation. 1999;68(6):880–887. doi: 10.1097/00007890-199909270-00022. [DOI] [PubMed] [Google Scholar]
- 87.Kreis HA, Ponticelli C. Causes of late renal allograft loss: chronic allograft dysfunction, death, and other factors. Transplantation. 2001;71(Suppl. 11):SS5–9. [PubMed] [Google Scholar]
- 88.Kahan BD, Rajagopalan PR, Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation. 1999;67(2):276–284. doi: 10.1097/00007890-199901270-00016. [DOI] [PubMed] [Google Scholar]
- 89.Baudouin V, Crusiaux A, Haddad E, et al. Anaphylactic shock caused by immunoglobulin E sensitization after retreatment with the chimeric anti-interleukin-2 receptor monoclonal antibody basiliximab. Transplantation. 2003;76(3):459–463. doi: 10.1097/01.TP.0000073809.65502.8F. [DOI] [PubMed] [Google Scholar]
- 90.Barros VR, Rocha V, Garcia VD, Garcia CD. Anaphylactic shock after retreatment with basiliximab. Transplant. Proc. 2003;35(1):579. doi: 10.1016/s0041-1345(02)03406-1. [DOI] [PubMed] [Google Scholar]
- 91.Sollinger H, Kaplan B, Pescovitz MD, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation. 2001;72(12):1915–1919. doi: 10.1097/00007890-200112270-00008. [DOI] [PubMed] [Google Scholar]
- 92.Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch. Neurol. 2009;66(4):483–489. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oh U, Blevins G, Griffith C, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch. Neurol. 2009;66(4):471–479. doi: 10.1001/archneurol.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenberg PB, Vriesendorp AE, Drazner MH, et al. Induction therapy with basiliximab allows delayed initiation of cyclosporine and preserves renal function after cardiac transplantation. J. Heart Lung Transplant. 2005;24(9):1327–1331. doi: 10.1016/j.healun.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann. Surg. 2008;248(4):564–577. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 96.Vincenti F, Grinyo J, Ramos E, et al. Can antibody prophylaxis allow sparing of other immunosuppressives? Transplant. Proc. 1999;31(1–2):1246–1248. doi: 10.1016/s0041-1345(98)01982-4. [DOI] [PubMed] [Google Scholar]
- 97.Tran HT, Acharya MK, Mckay DB, et al. Avoidance of cyclosporine in renal transplantation: effects of daclizumab, mycophenolate mofetil, and steroids. J. Am. Soc. Nephrol. 2000;11(10):1903–1909. doi: 10.1681/ASN.V11101903. [DOI] [PubMed] [Google Scholar]
- 98.Brennan DC, Schnitzler MA. Long-term results of rabbit antithymocyte globulin and basiliximab induction. N. Engl. J. Med. 2008;359(16):1736–1738. doi: 10.1056/NEJMc0805714. [DOI] [PubMed] [Google Scholar]
- 99.Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N. Engl. J. Med. 2002;346(8):580–590. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 100.Magee CC, Pascual M. Update in renal transplantation. Arch. Intern. Med. 2004;164(13):1373–1388. doi: 10.1001/archinte.164.13.1373. [DOI] [PubMed] [Google Scholar]
- 101.Vincenti F, Kirk AD. What's next in the pipeline. Am. J. Transplant. 2008;8(10):1972–1981. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 102.O'shea JJ, Park H, Pesu M, Borie D, Changelian P. New strategies for immunosuppression: interfering with cytokines by targeting the Jak/Stat pathway. Curr. Opin. Rheumatol. 2005;17(3):305–311. doi: 10.1097/01.bor.0000160781.07174.db. [DOI] [PubMed] [Google Scholar]
- 103.Flanagan ME, Blumenkopf TA, Brissette WH, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J. Med. Chem. 2010;53(24):8468–8484. doi: 10.1021/jm1004286. [DOI] [PubMed] [Google Scholar]
- 104.Lamba M, Tafti B, Melcher M, Chan G, Krishnaswami S, Busque S. Population pharmacokinetic analysis of mycophenolic acid coadministered with either tasocitinib (CP-690,550) or tacrolimus in adult renal allograft recipients. Ther. Drug Monit. 2010;32(6):778–781. doi: 10.1097/FTD.0b013e3181f361c9. [DOI] [PubMed] [Google Scholar]
- 105.Van Gurp EA, Schoordijk-Verschoor W, Klepper M, et al. The effect of the JAK inhibitor CP-690,550 on peripheral immune parameters in stable kidney allograft patients. Transplantation. 2009;87(1):79–86. doi: 10.1097/TP.0b013e31818bbea7. [DOI] [PubMed] [Google Scholar]
- 106.Van Gurp E, Weimar W, Gaston R, et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. Am. J. Transplant. 2008;8(8):1711–1718. doi: 10.1111/j.1600-6143.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 107.Borie DC, Larson MJ, Flores MG, et al. Combined use of the JAK3 inhibitor CP-690,550 with mycophenolate mofetil to prevent kidney allograft rejection in nonhuman primates. Transplantation. 2005;80(12):1756–1764. doi: 10.1097/01.tp.0000184634.25042.ea. [DOI] [PubMed] [Google Scholar]
- 108.Cohen S, Zwillich SH, Chow V, Labadie RR, Wilkinson B. Co-administration of the JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis without need for dose adjustment. Br. J. Clin. Pharmacol. 2010;69(2):143–151. doi: 10.1111/j.1365-2125.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta P, Friberg LE, Karlsson MO, Krishnaswami S, French J. A semi-mechanistic model of CP-690,550-induced reduction in neutrophil counts in patients with rheumatoid arthritis. J. Clin. Pharmacol. 2010;50(6):679–687. doi: 10.1177/0091270009346060. [DOI] [PubMed] [Google Scholar]
- 110.Ghoreschi K, Jesson MI, Li X, et al. Modulation of Innate and Adaptive Immune Responses by Tofacitinib (CP-690,550). J. Immunol. 2011;186(7):4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roffey J, Rosse C, Linch M, Hibbert A, Mcdonald NQ, Parker PJ. Protein kinase C intervention: the state of play. Curr. Opin. Cell Biol. 2009;21(2):268–279. doi: 10.1016/j.ceb.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 112.Kovarik JM, Steiger JU, Grinyo JM, et al. Pharmacokinetics of sotrastaurin combined with tacrolimus or mycophenolic acid in de novo kidney transplant recipients. Transplantation. 2011;91(3):317–322. doi: 10.1097/TP.0b013e318203860d. [DOI] [PubMed] [Google Scholar]
- 113.Weckbecker G, Pally C, Beerli C, et al. Effects of the novel protein kinase C inhibitor AEB071 (Sotrastaurin) on rat cardiac allograft survival using single agent treatment or combination therapy with cyclosporine, everolimus or FTY720. Transpl. Int. 2010;23(5):543–552. doi: 10.1111/j.1432-2277.2009.01015.x. [DOI] [PubMed] [Google Scholar]
- 114.Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J. Clin. Invest. 2008;118(9):3151–3159. doi: 10.1172/JCI35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 116.Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat. Rev. Nephrol. 2010;6(10):594–605. doi: 10.1038/nrneph.2010.110. [DOI] [PubMed] [Google Scholar]
- 117.Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl. Int. 2008;21(12):1118–1135. doi: 10.1111/j.1432-2277.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 118■■.Siemionow M, Nasir S. Chimerism and bone marrow based therapies in transplantation. Microsurgery. 2007;27(5):510–521. doi: 10.1002/micr.20395. [Anti-αβTCR induction protocols to induce tolerance.] [DOI] [PubMed] [Google Scholar]
- 119.Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann. Intern. Med. 1991;114(11):954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 120.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Locascio SA, Morokata T, Chittenden M, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010;90(12):1607–1615. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arslan E, Klimczak A, Siemionow M. Chimerism induction in vascularized bone marrow transplants augmented with bone marrow cells. Microsurgery. 2007;27(3):190–199. doi: 10.1002/micr.20330. [DOI] [PubMed] [Google Scholar]
- 123.Siemionow M, Zielinski M, Ozmen S, Izycki D. Intraosseus transplantation of donor-derived hematopoietic stem and progenitor cells induces donor-specific chimerism and extends composite tissue allograft survival. Transplantation proceedings. 2005;37(5):2303–2308. doi: 10.1016/j.transproceed.2005.03.127. [DOI] [PubMed] [Google Scholar]
- 124.Ozer K, Izycki D, Zielinski M, Siemionow M. Development of donor-specific chimerism and tolerance in composite tissue allografts under αβ-T-cell receptor monoclonal antibody and cyclosporine a treatment protocols. Microsurgery. 2004;24(3):248–254. doi: 10.1002/micr.20034. [DOI] [PubMed] [Google Scholar]
- 125.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J. Exp. Med. 1979;149:758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo X, Pothoven KL, Mccarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc. Natl Acad. Sci. USA. 2008;105(38):14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Getts MT, Kim BS, Miller SD. Differential outcome of tolerance induction in naive versus activated Theiler's virus epitope- specific CD8+ cytotoxic T cells. J. Virol. 2007;81(12):6584–6593. doi: 10.1128/JVI.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mcrae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 1995;182(1):75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peterson JD, Karpus WJ, Clatch RJ, Miller SD. Split tolerance of Th1 and Th2 cells in tolerance to Theiler's murine encephalomyelitis virus. Eur. J. Immunol. 1993;23:46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- 130.Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 2006;203(12):2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brinkmann V, Billich A, Baumruker T, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9(11):883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 201.Clinical trials homepage. http://clinicaltrials.gov.