Abstract
Two major influences on how the brain processes music are maturational development and active musical training. Previous functional neuroimaging studies investigating music processing have typically focused on either categorical differences between “musicians versus nonmusicians” or “children versus adults.” In the present study, we explored a cross-sectional data set (n=84) using multiple linear regression to isolate the performance-independent effects of age (5 to 33 years) and cumulative duration of musical training (0 to 21,000 practice hours) on fMRI activation similarities and differences between melodic discrimination (MD) and rhythmic discrimination (RD). Age-related effects common to MD and RD were present in three left hemisphere regions: temporofrontal junction, ventral premotor cortex, and the inferior part of the intraparietal sulcus, regions involved in active attending to auditory rhythms, sensorimotor integration, and working memory transformations of pitch and rhythmic patterns. By contrast, training-related effects common to MD and RD were localized to the posterior portion of the left superior temporal gyrus/planum temporale, an area implicated in spectrotemporal pattern matching and auditory–motor coordinate transformations. A single cluster in right superior temporal gyrus showed significantly greater activation during MD than RD. This is the first fMRI which has distinguished maturational from training effects during music processing.
Keywords: Auditory discrimination, Developmental, Musical training
Introduction
The brains of musicians are considered an ideal lens through which functional and structural plasticity may be examined (for reviews, see Münte et al., 2002; Schlaug, 2001; Wan and Schlaug, 2010; Zatorre et al., 2007). During performance, a musician must rapidly integrate sensory cues (auditory, visual, proprioceptive) and motor commands (articulatory, respiratory, limb coordination) within his or her own person, as well as with other musicians engaging in the same activities. The learning of this rich and dynamic process is often begun at an early age and sustained over the course of many years. Thus, two important factors that influence brain function and structure in musicians are the duration/intensity of musical training (and the concomitant explicit learning of perceptual–musical skills), and normal maturational development (and the concomitant implicit learning of perceptual–musical skills).
Intensive training and practice on an instrument (including the voice) is nearly always a prerequisite for musicianship, and has been investigated extensively. Apart from differences in task (e.g., perception, working memory, or production), studies may also be distinguished by their statistical designs: specifically, how musician status was analyzed. Most cross-sectional functional magnetic resonance imaging (fMRI) investigations have used the categorical distinction (and subsequent statistical contrast) “musicians vs. nonmusicians,” in both perception tasks (e.g., Gaab et al., 2003; Koelsch et al., 2005; Ohnishi et al., 2001) and production tasks (e.g., Bangert et al., 2006; Hund-Georgiadis and von Cramon, 1999; Meister et al., 2005). Artificial dichotomizations, however, result in well-known costs to statistical power (e.g., MacCallum et al., 2002), reducing the likelihood that true effects will be detected. Given the level of conservativeness with which statistical parametric maps are already thresholded (e.g., Lieberman and Cunningham, 2009), this additional loss of power is problematic. Nevertheless, only a handful of studies have parameterized some aspect of musical training—for example, years since commencement of training or intensity of musical practice—and used regression techniques to examine the association between that parameter and task-related functional activations (e.g., Kleber et al., 2010; Ohnishi et al., 2001).
Compared to the large fMRI literature exploring musical training effects, there have been few cross-sectional investigations exploring developmental aspects of brain activation during music processing. Early explorations of functional activation across the lifespan were hindered by methodological concerns, particularly the fidelity of normalizing the scans of children and adults to a common template (e.g., Gaillard et al., 2001). Subsequent empirical work, however, demonstrated that systematic changes in brain anatomy are below the effective resolution of fMRI once standard spatial smoothing algorithms have been applied (Burgund et al., 2002; Kang et al., 2003). With respect to brain anatomy, Burgund et al. (2002) measured the locations of 45 sulcus coordinates and 66 outer-boundary coordinates (in all three planes) in the brains of 20 children (aged 7–8) and 20 adults (aged 18–30) after all brains had been transformed to the same adult-derived template. A difference score between children and adults was then computed at each coordinate. Of these total 111 difference scores, only 5 were greater than 4 mm, and none was greater than 7 mm. With respect to fMRI activations, Kang et al. (2003) examined eight reliable activations elicited by a visuomotor task, and reported highly consistent results between children and adults with respect to activation time courses and the mean and variability of activation foci locations. A number of subsequent investigations have cited these empirical findings in choosing to use regression designs and a common normalization template to explore differences in fMRI activation across wide age ranges; for example, during visual working memory (Ofen et al., 2007 [ages 8–24]), word reading (Turkeltaub et al., 2003 [ages 6–22]) or word generation (Brown et al., 2005 [ages 7–32]).
Only one previous cross-sectional study has investigated maturational differences in music processing. Koelsch et al. (2005) compared activations elicited during a harmonic discrimination task (regular versus irregular chord progressions) in children aged 9.5–11 and adults aged 20–36. Because children and adults were analyzed separately rather than in a single design, however, no direct contrasts between the age groups were performed, and thus no inferences could be made about whether (and where) patterns of fMRI activation were associated, positively or negatively, with age.
In sum, no previous fMRI study has simultaneously explored the influence of maturational development and musical training on brain function. In the present study, we used multiple linear regression to analyze the contributions of age and training (while controlling for task performance; cf. Brown et al., 2005) on fMRI activation during a same/different two-choice musical phrase discrimination task.
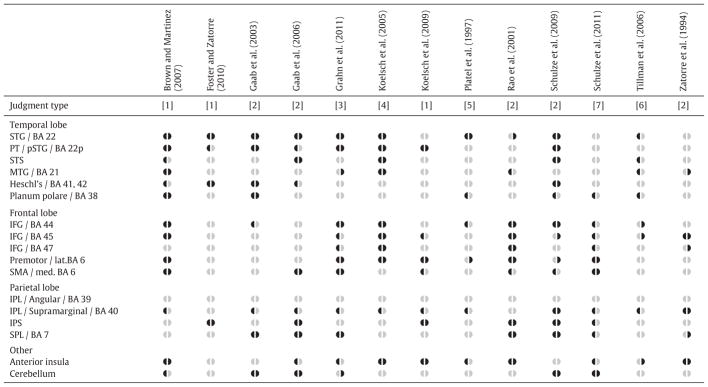
As summarized in Table 1, two-choice discrimination tasks are common among fMRI investigations of music processing, and elicit wide, bilateral activations across the cortex (frontal, temporal, and parietal lobes), the anterior insula, and the cerebellum. This wide pattern of activation is favorable for the present design, as it yields the potential to explore differences associated with both age and training throughout the brain. Specifically, we will explore whether any of the regions identified in Table 1 show activation that correlates with age and training (as well as task performance), using behavioral and imaging data from 84 subjects participating in a large study on the effects of music training across the lifespan. Previous in-depth reports on this data set have focused on behavioral (Forgeard et al., 2008; Norton et al., 2005) and morphological (Hyde et al., 2009; Schlaug, 2001; Schlaug et al., 2009) changes associated with musical training in young subjects. Understanding how age and training influences patterns of fMRI activation in a cross-sectional sample may inform future longitudinal work exploring maturation- and training-mediated changes in auditory processing (for reviews, see Besson et al., 2007; Jäncke, 2009; Kraus and Chandrasekaran, 2010).
Table 1.
Regions of interest in the present study, derived from reported or clearly visualized significant fMRI activations in 13 two-choice musical discrimination tasks. Anatomical and/or Brodmann area (BA) labels are provided together, as different authors use different terminology.
|
Dark symbols (◖ and ◗) indicate significant left or right hemisphere activation, respectively; pale gray symbols indicate no significant activation. Judgment type: [1] same/different pairs of melodies or rhythms (Brown and Martinez, 2007; Foster and Zatorre, 2010a, 2010b; Koelsch et al., 2009); [2] same/different tones within a melody (Gaab et al., 2003, 2006; Rao et al., 2001; Schulze et al., 2009; Zatorre et al., 1994); [3] speeding up/slowing down rhythms (Grahn et al., 2011); [4] regular/irregular harmonic progressions (Koelsch et al., 2005); [5] yes/no change in pitch or change in rhythm in a sequence (Platel et al., 1997); [6] timbre A/timbre B of a target chord (Tillman et al., 2006); [7] similar to [2], but here referring to significantly greater activity during explicit rehearsal of pitches versus syllables (Schulze et al., 2011).
Abbreviations: IFG: inferior frontal gyrus; IPL: inferior parietal lobule; IPS: intraparietal sulcus; lat.: lateral; med.: medial; MTG: middle temporal gyrus; PT: planum temporale; SPL: superior parietal lobule; SMA: supplementary motor area; STG: superior temporal gyrus; pSTG: posterior STG.
Methods
Subjects
Behavioral and imaging data were obtained from 84 individuals participating in a large study on the effects of music training on brain structure and function: 28 adults (aged 21–33) and 28 children (aged 9–11) participating in a cross-sectional arm, and 28 children (aged 5–7) participating in a longitudinal arm. Handedness was classified in adults per the Annett handedness questionnaire (Annett, 1970), and the same questionnaire was adapted for children as described in Norton et al. (2005). All subjects were classified as consistently right-handed. For the 42 subjects with musical training, the primary instrument (tallied for 5-to-7 s/9-to-11 s/Adults) was from the keyboard (12/4/8), string (1/8/6), or woodwind (1/2/0) family. All subjects (as well as the parents of the children) gave informed, written consent prior to taking part in the study, which was approved by the Internal Review Board of Beth Israel Deaconess Medical Center.
In each of the three age groups (5-to-7, 9-to-11, Adult), half of the subjects (n=14) had received musical training, and half (n=14) had not. No subject reported having absolute pitch. Demographic data from these six cells are presented in Table 1.
Musical stimuli and tasks
The scanner task comprised a same/different melodic discrimination (MD) or rhythmic discrimination (RD) judgment of pairs of five-note musical phrases (Fig. 1) via a button press with the index finger of the left (“same”) or right (“different”) hand. All phrases were recorded using a marimba-like sound (Cubase Universal Sound Module no. 13) to minimize any potential experience bias with a practiced instrument. These stimuli and paradigm have been used successfully in a previous study with young children (Overy et al., 2004). A single run was three minutes in duration and consisted of 12 trials: eight phrase pairs (either MD or RD) and four silence (motor control) trials (S), during which subjects heard no auditory stimulus, but made a bimanual button press after an auditory cue. Within each run, 3 same and 5 different phrase pairs were presented. All subjects completed four runs (2 MD and 2 RD, in alternation). Subjects were familiarized with the discrimination task during a behavioral testing session prior to scanning.
Fig. 1.
Sample stimuli for the MD and RD task. Asterisks indicate a change in Phrase 2.
In designing the study, the primary aim with respect to the scanner task was to make it feasible for children to perform. For this reason, we chose not to manipulate melodic or rhythmic parameters (e.g., Schönwiesner et al., 2005; Zatorre and Belin, 2001), and did not include a separate “listen only” baseline condition (e.g., Zatorre et al., 1994).
Regressor specification
Three regressors were used in all analyses. Two were task-independent (Age and Training), and one was task-dependent (Performance). We did not model the age at onset of training in our regression because the range of onset ages in our sample was substantially smaller than in previous investigations which did model age at onset. Age was quantified in years. Training was quantified as the “cumulative dose” of instrumental instruction and practice hours since the onset of musical training (including private lesson and ensemble time), as in previous studies (e.g., Bengtsson et al., 2005b; Kleber et al., 2010). Training hours were derived via retrospective questionnaires given to the adult subjects and the parents of children from the cross-sectional arm (9-to-11 year olds); and via weekly practice journals kept by parents of children in the longitudinal arm (5-to-7 year olds).
To guard against mistakenly attributing performance-mediated patterns of activation to either of our task-independent factors (cf. Brown et al., 2005), Performance was quantified as the sensitivity index d′ Z(hit rate) − Z(false alarm rate), with appropriate correction for rates equal to 0 or 1 (Macmillan and Creelman, 2005, chap. 2). A unique d′ was calculated for each subject across all four runs (2 MD and 2 RD) and used as the regressor in all analyses. (There was no significant difference in d′ between MD and RD, as will be discussed in the Results.)
As both Age and Training were positively skewed, they were natural log transformed: ln(value+1). Under this transformation, the variance inflation factor for all regressors was acceptable (Age: 2.01; Training: 1.34; Performance: 2.40), indicating a valid regression model without multicollinearity (Kutner et al., 2004).
Image acquisition
Functional images were acquired via a sparse sampling design (e.g., Gaab et al., 2003, 2006) on a 3T General Electric magnetic resonance imaging scanner using a gradient-echo EPI-sequence with an echo time of 25 ms and a 64×64 mm matrix. Using a mid-sagittal scout image, 26 slices were acquired over 1.75 s with a voxel size of 3.8×3.8×4 mm. Scanning repetition time (TR) was kept constant at 15 s; stimuli were jittered between three time points such that the onset of the first axial slice occurred 1.25, 2.25, or 3.25 s after the end of the second phrase in each pair. The data from these three time points were combined during statistical analysis to allow for individual differences in hemodynamic response time across brain regions.
Image processing, first-level analysis, and contrast specification
All first-level image processing steps (movement correction, normalization to the MNI EPI template, smoothing with an isotropic 8 mm FWHM kernel, and resampling to 2 mm cubic voxels) were performed using the SPM5 software suite (www.fil.ion.ucl.ac.uk/spm/).
First-level analysis used a finite-impulse response basis function (window length=1 s, order=1) with scaling set to global normalization. Low frequency drifts were removed using a temporal high-pass filter with a cutoff of 200 s. Temporal autocorrelation was modeled as a first-order autoregressive [AR(1)] process. A box-car function was applied with an epoch length of 1 to the fMRI time series (12 acquisitions within each run: 8 MD or RD, 4 S), and no temporal derivatives were applied.
In designing the first-level contrasts, several interrelated issues were at play. Because we did not parametrically manipulate melodic or rhythmic properties and did not have a “listen only” baseline condition, we anticipated (and wished to accurately capture) similar patterns of activation during MD and RD. However, if such differences were present, they too should be accurately captured. To satisfy all these requirements, a set of three contrasts was constructed for each subject.
The first two contrasts directly captured relative differences in activation between MD and RD: MD>RD (i.e., [MD>S]>[RD>S]); and RD>MD (i.e., [RD>S]>[MD>S]). To capture relative similarities in activation between MD and RD, the third contrast used of the “minimum statistic” (Nichols et al., 2005) at the first level (Rudert and Lohmann, 2008): MD∧RD (i.e., min([MD>S],[RD>S])). The minimum statistic map simply takes the more negative parameter estimate (β-value) between the two constituent maps. Statistically, it is both more accurate and more conservative than the average of the two conditions (as would be calculated in a repeated-measures or flexible factorial design). For example, a given voxel with a high β-value in MD>S a null β-value in RD>S will take the null value rather than the (possibly supra-threshold) average value. Thus, a positive β-value in MD∧RD indicates at least some activation across both task conditions. Defined in this way, all contrasts may be analyzed and thresholded identically at the second level, described below.
Second-level analysis
Second-level (random-effects) analyses using the Multiple Regression module in SPM5 were performed separately on the three first-level contrasts from all subjects, with Age, Training, and Performance as (mean-centered) regressors. For each contrast, five SPM t-maps were generated: four “partial correlation SPMs” (that is, separate voxel-wise positive and negative correlations with Age, Training, and Performance), and one “average subject SPM” interpreted as the expected response for a subject of average age, training, and performance after removing error associated with these three linear effects (Nichols, 2008).
Anatomical regions of interest
Given the already ambitious scope of this aim, we restrict our analyses to regions strongly associated with task-induced increases in activation (i.e., Table 1), rather than task-induced decreases in activation (i.e., within the default mode network; Buckner et al., 2008; Raichle et al., 2001). A single anatomical mask was created a priori as the union of bilateral masks from the Automated Anatomical Labeling (AAL) toolbox (Tzourio-Mazoyer et al., 2002) and queried using the WFU Pick Atlas (Maldjian et al., 2003). The full mask covered six broad regions: (1) superior temporal gyrus (plus pole), middle temporal gyrus (plus pole), Heschl’s gyrus, and temporal (Rolandic) operculum; (2) opercular, orbital, and triangular inferior frontal gyrus; (3) precentral gyrus and supplementary motor area; (4) superior parietal lobule and inferior parietal lobule/supramarginal gyrus; (5) insula; and (6) cerebellum (lobules I–X, crus I, crus II). This image was used to define a small volume correction (“small” being relative in this case, as the mask retained 46,776 voxels of the total 156,984 in-brain voxels), which was then thresholded at voxel-level p=.001 and cluster-level p=.05 (FWE corrected). (SPM8 was used to correctly visualize significant activations within the anatomical mask in Figs. 3 and 4).
Fig. 3.
Thresholded activation in the average subject SPMs. (a) Activation common to both MD and RD (scatter plot inserts visualize the simple correlations between Age and the mean β-value from each active cluster). (b) A different scatter plot illustrating the similarity of voxel values between second-level MD>S and RD>S average subject SPMs. (c) Significantly increased activation in MD relative to RD. Abbreviations: pre-SMA: presupplementary motor area; STG: superior temporal gyrus. r-values are partial correlations with p<.05 (*) or p<.001 (***). The scatter plot was made using a custom-built data visualization toolbox (http://tools.robjellis.net).
Fig. 4.
Thresholded activation in the MD∧RD positive partial correlation with Age. Scatter plots visualize the simple correlations between Age and the mean β-value from each active cluster in temporofrontal junction (a), premotor cortex (b), and intraparietal sulcus (c). r-values are partial correlations with p<.001 (***).
Scatter plots
Finally, to confirm that any observed linear correlations were not spuriously driven by subjects at either end of the spectrum, MarsBaR v0.43 (http://marsbar.sourceforge.net/) was used to extract each subject’s mean β-value from each significant cluster. To aid interpretation, simple correlation scatter plots were created.
Results
Behavioral data
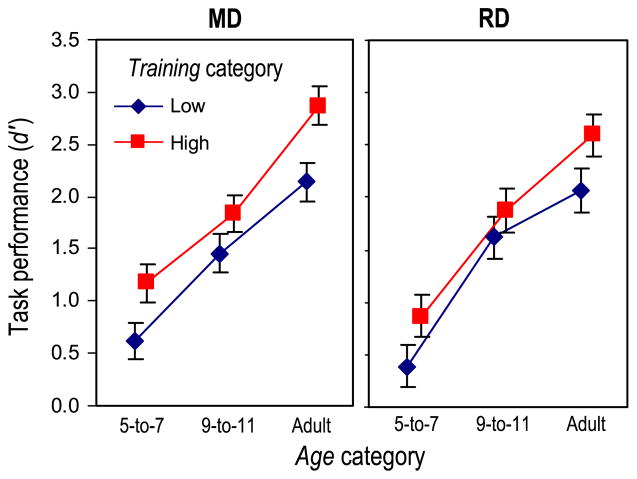
Task performance (d′) was expected to increase both with age and with level of musical training. To test this, a 3 (Age group)×2 (Training group)×2 (Task: MD vs. RD) analysis of variance (ANOVA) was performed. As seen in Fig. 2, a significant main effect was present for Age (p<.0001, η2=.453) and Training (p<.0001, η2=.054), but not Task (p=.259, η2=.002). (η2 is the proportion of total variance in the ANOVA uniquely explained by a given effect; Keppel and Wickens, 2004). The Age×Training interaction was not significant (p=.525, η2=.006), nor was this interaction further modulated by Task (p=.930, η2<.001). Furthermore, MD and RD d′ values were highly correlated across the sample (r=.697, p<.0001). This analysis reveals that Age and Training had statistically independent influences on d′ (together explaining 50% of the total variance); indeed, even small amounts of training in the 5-to-7 year old group were reflected in increased d′. The overall similarity of performance in the MD and RD conditions motivated our decision to use the same d′ regressor across all multiple regression SPMs, as noted in the Section 2.3.
Fig. 2.
Task performance (d′) as a function of Age, Training, and Task (MD vs. RD). r-values are partial correlations.
Imaging data
Average subject effects
First, we examined the MD∧RD average subject SPMs. Fig. 3a presents surface renderings and sections for supra-threshold activations, and Table 3 presents the associated SPM statistics. The statistically average subject in our paradigm (age=[e2.57 − 1]=12.07 years; cumulative training dose=[e3.15 − 1]=22.35 hours; d′=1.90; cf. Nichols, 2008) exhibited activation in bilateral superior temporal gyrus (STG), middle temporal gyrus (MTG), and presupplementary motor area (Pre-SMA; cf. Johansen-Berg et al., 2004), consistent with previous musical discrimination paradigms in adult subjects (cf. Table 1).
Table 3.
SPM statistics for all significant activations in the MD∧RD average subject SPMs and positive partial correlation SPMs for Age and Training. Where present, probabilistic labels (“Assigned to”) are given for individual peaks, as defined by the SPM5 Anatomy toolbox (version 1.8; Eickhoff et al., 2005).
| Effect | Extent (voxels) | p-value (cluster) | Peak location a | Assigned to | Peak t | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|
| Avg. sub. | 2178 | <.0001 | L. Sup. temporal | Te 1.0 [30%] b | 11.92 | −50 | −26 | 4 |
| L. Mid. temporal | Te 3 [40%] c | 10.55 | −60 | −32 | 8 | |||
| L. Sup. temporal | — | 7.28 | −54 | 0 | −6 | |||
| 2109 | <.0001 | R. Sup. temporal | Te 3 [40%] c | 10.97 | 66 | −22 | 8 | |
| R. Sup. temporal | — | 10.74 | 58 | −20 | 2 | |||
| R. Sup. temporal | Te 1.0 [50%] b | 9.87 | 60 | −8 | 2 | |||
| 507 | .001 | Pre-SMA d | Area 6 [50%] d | 7.48 | 2 | 10 | 54 | |
| Pre-SMA d | Area 6 [40%] d | 6.26 | 2 | 6 | 64 | |||
| Age | 500 | .001 | L. Premotore | Area 6 [ 70%] | 5.15 | −56 | 2 | 36 |
| L. Inf. frontal oper. | Area 44 [60%] | 4.21 | −58 | 14 | 24 | |||
| 370 | .006 | L. Inf. frontal orb. | — | 5.51 | −40 | 18 | −14 | |
| L. Sup. temporal pole | — | 4.06 | −48 | 8 | −18 | |||
| 281 | .014 | L. Inf. parietal | hIP 2 [30%] f | 5.04 | −40 | −48 | 52 | |
| L. Inf. parietal | hIP 3 [50%] f | 4.73 | −34 | −52 | 44 | |||
| Training | 248 | .029 | L. Sup. temporal | — | 4.36 | −60 | −48 | 12 |
Abbreviations: Inf.: inferior; L.: left; Mid.: middle; oper.: opercular; orb.: orbital; Post.: posterior; R.: right; Pre-SMA: presupplementary motor area; Sup.: superior.
Notes:
AAL locations implicitly refer to gyri;
subregion of BA 41;
subregion of BA 22;
neither the AAL or the Anatomy toolbox differentiates pre-SMA from SMA-proper;
the AAL does not differentiate BA 4 from BA 6;
subregion of the intraparietal sulcus.
Second, we examined the MD>RD and RD>MD average subject SPMs. At pvoxel < .001, there were here were no significant clusters in either contrast. This null result is consistent with our prediction that the present stimuli and paradigm would elicit largely similar patterns of activation during MD and RD. As a further illustration of this point, Fig. 3b presents a “voxel-wise scatter plot” of all 156,984 t-values in the MD>S and RD>S average subject SPMs (estimated at the second level using the same three-factor multiple regression design). The strong voxel-wise correlation (r=.900) further reflects the overall similarity between MD and RD at the group level.
However, we hypothesized that MD>RD might reveal some activation in right STG, based on a previous report using these same stimuli (Overy et al., 2004). Applying a new small volume correction consisting solely of the AAL masks for right STG plus its pole (3082 in-brain voxels), a single cluster emerged at pvoxel < .001 (Fig. 3c; 157 voxels; peak at {64, −10, −4}; pcluster =.007). (At pvoxel < .001, there were no supra-threshold voxels in right MTG or its pole.)
These average subject effects, however, were not the focus of the present study. Statistically, activation in an average subject SPM is statistically independent from activation in a partial correlation SPM, as the two utilize distinct calculations for their t-values (i.e., a one-sample t-test versus a regression slope t-test). Thus, the activations (or lack thereof) in Fig. 3 should not be seen as definitive. Rather, Fig. 3 serves simply as a manipulation check prior to exploring the primary question of interest: how Age, Training, or Performance might modulate patterns of fMRI activation during musical discrimination.
Age effects
Significant positive (but not negative) partial correlations with Age were present in MD∧RD, visualized in Fig. 4. Neither positive nor negative correlations with Age were present in the MD>RD or RD>MD partial correlation SPMs. Table 3 presents the associated statistics. Three distinct left hemisphere clusters were significant at pvoxel < .001: (1) temporofrontal junction (Fig. 4a; i.e., planum polare [BA 38; 51% of the cluster within this AAL mask], orbital IFG [BA 47; 31%], and anterior insula [BA 13; 18%]); (2) premotor cortex (lateral BA 6; 77%) extending into opercular (BA 44; 12%) and triangular (BA 45; 11%) inferior frontal gyrus; and (3) intraparietal sulcus (with 70% and 30% in inferior and superior parietal lobules, respectively). No clusters were significant in the right hemisphere.
Training effect
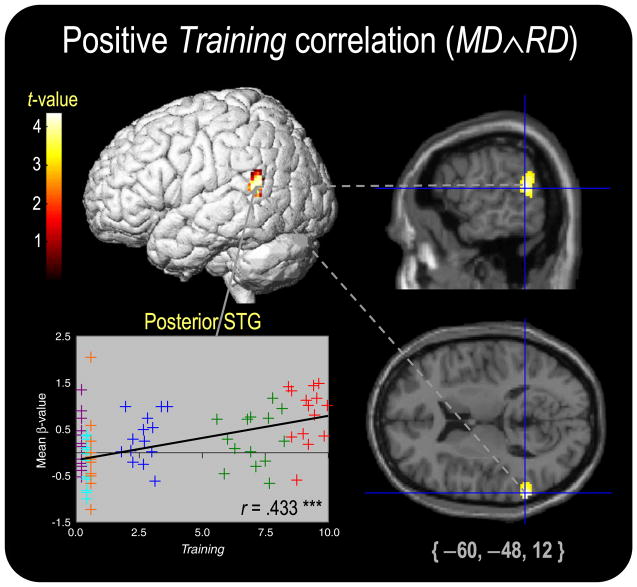
A positive partial correlation with Training was present in MD∧RD (shown in Fig. 5), but not in MD>RD or RD>MD. A single cluster was significant in left posterior STG (BA 22p), with a peak located within the probabilistic limits of the planum temporale defined by Westbury et al. (1999) after conversion to Talairach space using GingerALE (www.brainmap.org/ale). (The entire cluster is inferior to the probabilistic limits of inferior parietal cortex defined by the Anatomy toolbox.) This cluster possessed no spatial overlap with clusters in the positive Age correlation. No significant negative correlations with Training were present in the MD∧RD, MD>RD, and RD>MD partial correlation SPMs.
Fig. 5.
Thresholded activation in the MD∧RD positive partial correlation with Training. Scatter plots visualize the simple correlations between Training and the mean β-value from the active cluster. r-values are partial correlations with p<.001 (***).
Performance effects
No significant positive or negative partial correlations with Performance were present in the partial correlation SPMs for MD∧RD, MD>RD, or RD>MD at either pvoxel < .001 or pvoxel < .005: the largest observed cluster had 2 voxels (pcluster =.978) and 24 voxels (pcluster = .999) at these respective thresholds.
Discussion
The present study used multiple linear regression to statistically isolate the contributions of maturational development (quantified as chronological age) and musical training (quantified as cumulative hours) to fMRI activation associated with performing melodic discrimination (MD) and rhythmic discrimination (RD) tasks. MD/RD was deemed a useful task with which to investigate maturational and training-mediated differences during music processing, for two reasons. First, MD/RD tasks have been frequently explored in both healthy individuals (cf. Table 1) and neuropsychological patients (e.g., Milner, 1962; Samson and Zatorre, 1988). Second, MD/RD elicits wide activation across the brain (cf. Table 2), yielding the potential for significant correlations with the regressors of interest. A major statistical advantage of our design is the increase in power (or more accurately, the prevented loss of power) achieved by using continuous predictor variables (Age, Training, Performance) rather than artificially categorized variables (“musicians vs. nonmusicians”; “children vs. adults”).
Table 2.
Demographics for the 84 subjects, grouped into six “cells” as a function of age and training.
| Cell | Males/females | Mean (SD)/range
|
|||
|---|---|---|---|---|---|
| Age at scan (years) | Age of commencement (years) | Practice (years) | Cumulative dose (h) | ||
| 5-to-7 U | 7/7 | 6.23 (.56)/5.10–7.11 | — | — | — |
| 5-to-7 T | 5/9 | 6.46 (.80)/5.04–7.38 | 6.03 (.77)/4.84–6.91 | .38 (.17)/.16–.82 | 15.57 (8.86)/4.94–35.91 |
| 9-to-11 U | 8/6 | 10.07 (.67)/9.10–11.16 | — | — | — |
| 9-to-11 T | 5/9 | 10.28 (.73)/9.08–11.12 | 5.74 (1.35)/4.01–8.46 | 4.61 (1.61)/1.24–6.63 | 1535 (1144)/268–3792 |
| Adult U | 6/8 | 27.17 (3.50)/20.96–33.00 | — | — | — |
| Adult T | 8/6 | 25.87 (2.85)/21.51–31.33 | 5.21 (1.05)/4.00–8.00 | 19.07 (4.51)/10.00–26.00 | 10,888 (5053)/4473–20,849 |
T: trained; U: untrained. “Cumulative dose” is the total number of hours since the beginning of training; for details, see Section Regression specification.
We intentionally designed our task to be successfully performed by both children and adults, and thus did not parametrically manipulate melodic or rhythmic properties in the stimuli (e.g., Schönwiesner et al., 2005; Zatorre and Belin, 2001). We thus expected largely similar patterns of activation during MD and RD conditions. This hypothesis received support via a direct comparison of t-values in the MD>S and RD>S average subject SPMs (Fig. 3b). Additionally, only one small cluster in right STG was significant in MD>RD after applying a very small volume correction. Furthermore, although MD∧RD revealed significant partial correlations with the regressors of interest, MD>RD and RD>MD did not. For all these reasons, we focus the scope of our discussion towards exploring brain regions and cognitive processes common to both melodic and rhythmic discrimination, and how they are modulated by age and training.
Age-related effects
Strong positive partial correlations with age (Fig. 3; Table 3) were found in three spatially distinct left hemisphere regions: temporofrontal junction (TFJ; planum polare, orbital IFG, and anterior insula), ventral premotor cortex (vPMC), and intraparietal sulcus (IPS). Activation in each of these regions is frequent in two-choice musical discrimination paradigms (cf. Table 1), as well as other paradigms which require attention or working memory operations on rhythmic auditory stimuli (discussed below). Two detailed reviews (Janata and Grafton, 2003; Lewis and Miall, 2003) have independently highlighted the involvement of all three regions in attention and working memory, particularly with respect to stimulus sequencing, timing, and temporal tracking. Here, we will propose that the observed pattern of results reflects a maturational component to the recruitment of regions which together support (1) dynamic attending to an unfolding musical event in both pitch space and time (TFJ and vPMC) and (2) working memory operations in pitch space and time (IPS). (We here use “attending” to refer to a temporally-guided continuous mental action, so as to avoid confusion with “attentional set” or “attentional demands”; cf. Janata and Grafton, 2003; Jones and Boltz, 1989). We first review evidence that supports this idea before relating it to the observed maturation effects.
Left TFJ
Recent fMRI meta-analyses of orbital IFG (Vigneau et al., 2006), planum polare (Olson et al., 2007), and anterior insula (Mutschler et al., 2009) each discuss aspects of processing of complex auditory signals such as speech and music, implicating the TFJ as a region of higher-order auditory association cortex. One aspect of this processing machinery particularly relevant to the current paradigm is the temporal sequencing and tracking of auditory stimuli that evolve over time.
A number of previous auditory perception fMRI studies highlight the role of left TFJ in temporal sequencing and tracking would be consistent with this notion. For example, Levitin and Menon (2003) reported focal activation in left orbital IFG/anterior insula during passive listening to temporally coherent (versus temporally scrambled) excerpts of music. Noesselt et al. (2003) reported activation in left insula and left planum polare during a passive word listening task that increased with word presentation rate (i.e., a stimulus tempo effect). Grahn et al. (2011) reported increased activation in left anterior insula/orbital IFG during an auditory (versus a visual) tempo discrimination task, and attribute this modality difference as reflecting the enhanced sense of beat in the auditory modality (cf. Patel et al., 2005). Motor production studies also suggest a role for TFJ in sequencing and tracking.
Left vPMC
In addition to its well-known involvement in both rhythmic motor production tasks (both complex rhythms and simple isochronous rhythms; e.g., Bengtsson et al., 2005a; Jäncke et al., 2000), left vPMC is also active during non-motor tasks that require accurate attending in time. Schubotz and von Cramon (2002) reported bilateral vPMC activation that increased with the complexity of a pitch deviant detection task within isochronous sequences of 12 isochronous tones. Grahn and McAuley (2009) found correlations between individual differences in listeners’ ability to “hear” an implied isochronous beat during a silence between two to-be-judged rhythms and activation in both left premotor cortex and left insula. Chen et al. (2008) reported bilateral vPMC activation while subjects passively listened to or anticipated subsequent synchronized tapping with auditory rhythms (as well as expected activation during actual tapping). Schulze et al. (2011) also reported left vPMC activation during non-motor covert rehearsal of a previously presented sequence of pitches or syllables.
It is interesting to note that vPMC activation during music perception and production tasks frequently extends into opercular IFG/BA 44 (e.g., Chen et al., 2008; Jäncke et al., 2000; Schubotz and von Cramon, 2002; Schulze et al., 2011), as was also the case with our findings. This pattern of activation might suggest that an articulatory rehearsal component of working memory (cf. Baddeley, 2003; Hickok and Poeppel, 2007) is at work, as has been proposed previously (Schubotz and von Cramon, 2002; Schulze et al., 2011).
Taken together, these results suggest a role for vPMC in tracking a dynamic, temporally predictable sequence (cf. Schubotz and von Cramon, 2002), an activity that may be relevant to the motor system (cf. Chen et al., 2008) particularly when motor responses (e.g., go/no-go button presses, synchronization or continuation tapping, rhythm reproductions) are required, independent of a particular effector system (cf. Bengtsson et al., 2005a).
IPS
As reviewed in Foster and Zatorre (2010a), the IPS is a multisensory integration region, receiving input from visual, auditory, and tactile sensory cortices anatomical inputs from visual, auditory, and tactile sensory cortices. As such, IPS serves as an ideal location in which abstract transformations of sensory information (such as visual or auditory objects) are performed in working memory to prepare and guide future decisions or actions (for a discussion, see Grefkes and Fink, 2005). Within the auditory domain, previous studies have shown bilateral IPS activation during two-choice discrimination tasks in which a musical phrase must be compared to a pitch-shifted version (Foster and Zatorre, 2010a) or temporally reversed version (Zatorre et al., 2010).
It is clear from Table 1 that explicit transformations in working memory are not the only types of operations that elicit IPS activity. Consistent with the reviews of Janata and Grafton (2003) and Lewis and Miall (2003), IPS may also play a role in attending. For example, Coull and Nobre (1998) reported strong left IPS activation in a task which required attending to both spatial and temporal properties of a visual cue. Left IPS activation was also present in the “listen with anticipation to tap” condition (Chen et al., 2006) that also elicited vPMC activation.
Maturation and entrainment
We have suggested that the TFJ, vPMC, and IPS jointly support rhythmic attending and working memory operations on auditory sequences. In addition to the studies discussed above, support for this hypothesis can also be found from the developmental literature on the perception and production of temporal intervals. Specifically, as individuals move from childhood into adulthood, they show increased flexibility in synchronizing and attending to different levels within a metrical hierarchy (eighth-note level, quarter-note level, etc.; e.g., Drake et al., 2000; McAuley et al., 2006). The improved ability to entrain or “lock in” to a temporal sequence at multiple timescales facilitates dynamic attending via the phase locking of attentional (neural) oscillators at those different timescales (Jones, 2009; Large and Jones, 1999). The substantial age-related improvements in performance on the MD/RD task (Fig. 2) might thus be explained by improved entrainment to (and subsequent working memory operations on) the to-be-discriminated stimuli (all of which corresponded to a 4/4 metrical structure) at multiple timescales, driven by increased recruitment of the TFJ, vPMC, and IPS.
General maturational effects
The possibility that general physiologic factors related to maturation (rather than specific to music processing) contributed to age-related effects cannot be excluded. Changes to blood oxygenation hemodynamic response (e.g., Richter and Richter, 2003) and baseline blood flow (e.g., Biagi et al., 2007) could alter the relationship between measured fMRI signal and neuronal activity. More fundamentally, higher synapse and neuronal density in children (e.g., Huttenlocher, 1979) may lead to an altered neuronal response during many tasks. However, the spatially distinct pattern of age-correlated differences (Figs. 4a and b) compared to the average subject response (Fig. 3a) suggests a maturational response specific to particular aspects of a network involved in music processing rather than a more generic maturational effect on fMRI responses.
Partial correlations with training
A single region was found to show a partial correlation with Training during both MD and RD: left posterior STG/planum temporale (PT; e.g., Shapleske et al., 1999). As reviewed by Griffiths and Warren (2002) and Warren et al. (2005), bilateral PT activation is found in response to a wide range of auditory stimuli (simple sound patterns, pitch sequences, objects in auditory space, environmental sounds, voices, and speech) and experimental paradigms (perception, auditory object spatial rotation, working memory, and covert rehearsal). One interpretation of these diverse findings is that PT serves as “computational engine” involved in sequencing spectrotemporal patterns and comparing them to stored templates (Griffiths and Warren, 2002), facilitating an auditory input/motor output coordinate transformation which may also involve ventral premotor cortex (Warren et al., 2005). Such processing machinery would certainly benefit the discrimination task used in our paradigm, and indeed, the MD∧RD average subject activation (Fig. 3a) shows strong pSTG activation (as do other auditory discrimination paradigms; cf. Table 1). The present results suggest that left pSTG is selectively modulated by musical training.
This asymmetry in training-mediated pSTG activation dovetails nicely with previous findings. The PT has a well-known, leftward hemispheric asymmetry (Geschwind and Levitsky, 1968) that is associated with the hemispheric lateralization of language (e.g., Moffat et al., 1998; Steinmetz et al., 1991). This leftward asymmetry is further exaggerated in musicians with absolute pitch (e.g., Keenan et al., 2001; Schlaug et al., 1995), and performance on a pitch-naming test is positively correlated both with left PT volume (Zatorre et al., 1998) and left PT fMRI activation during passive listening to music (Ohnishi et al., 2001). Our results add to these findings, showing that left posterior pSTG activation during an active discrimination task (rather than passive listening) is modulated by the extent of musical training in non-AP possessors.
Caveats
The results of any study must be viewed through the window framed jointly by its subjects, stimuli, paradigm, analysis, and inferential logic. With respect to our subjects (as is the case with any cross-sectional design based on a convenience sample), inferences can only be made about differences associated with a predictor variable, and not changes associated with a predictor variable. With respect to our stimuli, we did not systematically manipulate melodic or rhythmic properties in a parametric fashion, preventing a potentially more sensitive analysis (in both the conceptual and the statistical sense) of the differences in neural mechanism behind melodic versus rhythmic discrimination, as in Zatorre and Belin (2001). With respect to our paradigm, ours precluded us from isolating listening/attending processes from working memory/rehearsal processes, as in Schulze et al. (2011).
With respect to our analysis, although multiple regression has many advantages, inferences can only be made about factors included in the model. We modeled two task-independent variables (age and hours of training), but did not include another important factor (age of onset of training) due to a very limited range of values (4.00–8.46). The age at which musical training commenced influences structural (e.g., Schlaug et al., 1995) and functional (e.g., Ohnishi et al., 2001) differences within musicians’ brains. The amount of training itself also has pronounced effects on musicians’ brain morphometry (e.g., Bengtsson et al., 2005b; Schlaug, 2001). Structural differences associated with either age or training can mediate observed patterns of fMRI activation (cf. Foster and Zatorre, 2010b). The lack of performance-related effects (quantified here as here, d′) at the voxel level is also noteworthy; further analyses (perhaps exploring signal change within specific ROIs) may reveal more subtle effects of performance on activation patterns.
Finally, with respect to making inferences, we have constrained ourselves to discussing how age and training modulate patterns of activation in fMRI. This is an accurate statement, but is moot with respect to questions concerning developmental neuroanatomy and neurophysiology (cf. Brown et al., 2005; Poldrack, 2010).
Longitudinal implications
Although the present study was cross-sectional in nature, its results will inform subsequent analyses of the longitudinal arm of our investigation of the effects of training on music processing in children (e.g., 2009; Hyde et al., 2009; Schlaug et al., 2005). Other groups using a shorter-term longitudinal design (i.e., training over weeks or months rather than years) have already reported evidence of functional plasticity in children (e.g., Fujioka et al., 2006 [11–14 months]; Moreno and Besson, 2006 [8 weeks]; Moreno et al., 2009 [24 weeks]; 2011 [4 weeks]).
Conclusion
We interpret the distinct cortical regions associated with age and the duration musical training to be related to specific cognitive operations at work during a musical discrimination task. Age effects were localized to regions implicated in attending to and performing working memory operations on dynamic auditory stimuli; training effects were localized to a region known both for its role in spectrotemporal pattern matching and auditory–motor coordinate transformations. These findings improve our understanding of how maturational development and musical training shape brain function.
Acknowledgments
We thank our previous research assistants (C. Alexander, L. Blake, K. Brumm, K. Cronin, L. Forbes, U. Iyengar, M. Rosam, and L. Zhu) for test preparation, behavioral testing, and imaging data collection; the participating children and their families for their cooperation in taking part in our experiments; and Joyce Chen, Jonathan Freeman, and two anonymous reviewers for helpful suggestions. This work was supported by grants from the National Science Foundation (BCS-0132508 and BCS-0518837) and supplemental funds from the Dana Foundation and the Grammy Foundation. Dr. Schlaug also acknowledges support from the National Institutes of Health (DC009823 and DC008796).
References
- Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61 (3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4 (10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, Heinze HJ, et al. Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. NeuroImage. 2006;30 (3):917–926. doi: 10.1016/j.neuroimage.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Ehrsson HH, Forssberg H, Ullén F. Effector-independent voluntary timing: behavioural and neuroimaging evidence. Eur J Neurosci. 2005a;22 (12):3255–3265. doi: 10.1111/j.1460-9568.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson S, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005b;8 (9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Besson M, Schön D, Moreno S, Santos A, Magne C. Influence of musical expertise and musical training on pitch processing in music and language. Restor Neurol Neurosci. 2007;25:399–410. [PubMed] [Google Scholar]
- Biagi L, Abbruzzese A, Bianchi MC, Alsop DC, Del Guerra A, Tosetti M. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging. 2007;25 (4):696–702. doi: 10.1002/jmri.20839. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ. Activation of premotor vocal areas during musical discrimination. Brain Cogn. 2007;63 (1):59–69. doi: 10.1016/j.bandc.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15 (3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17 (1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Chen JL, Zatorre RJ, Penhune VB. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage. 2006;32 (4):1771–1781. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, Zatorre Robert J. Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex. 2008;18 (12):2844–2854. doi: 10.1093/cercor/bhn042. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18 (18):7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C, Jones MR, Baruch C. The development of rhythmic attending in auditory sequences: attunement, referent period, focal attending. Cognition. 2000;77 (3):251–288. doi: 10.1016/s0010-0277(00)00106-2. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25 (4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Forgeard Marie, Winner E, Norton A, Schlaug G. Practicing a musical instrument in childhood is associated with enhanced verbal ability and nonverbal reasoning. PLoS One. 2008;3 (10):e3566. doi: 10.1371/journal.pone.0003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NEV, Zatorre RJ. A role for the intraparietal sulcus in transforming musical pitch information. Cereb Cortex. 2010a;20 (6):1350–1359. doi: 10.1093/cercor/bhp199. [DOI] [PubMed] [Google Scholar]
- Foster NEV, Zatorre RJ. Cortical structure predicts success in performing musical transformation judgments. NeuroImage. 2010b;53 (1):26–36. doi: 10.1016/j.neuroimage.2010.06.042. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Ross B, Kakigi R, Pantev C, Trainor L. One year of musical training affects development of auditory cortical-evoked fields in young children. Brain. 2006;129:2593–2608. doi: 10.1093/brain/awl247. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gaser C, Zaehle T, Jäncke L, Schlaug G. Functional anatomy of pitch memory—an fMRI study with sparse temporal sampling. NeuroImage. 2003;19 (4):1417–1426. doi: 10.1016/s1053-8119(03)00224-6. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gaser C, Schlaug G. Improvement-related functional plasticity following pitch memory training. NeuroImage. 2006;31 (1):255–263. doi: 10.1016/j.neuroimage.2005.11.046. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB, Xu B. Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. NeuroImage. 2001;13 (2):239–249. doi: 10.1006/nimg.2000.0681. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161 (3837):186. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Grahn J, McAuley J. Neural bases of individual differences in beat perception. NeuroImage. 2009;47 (4):1894–1903. doi: 10.1016/j.neuroimage.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Henry MJ, McAuley JD. FMRI investigation of cross-modal interactions in beat perception: audition primes vision, but not vice versa. NeuroImage. 2011;54 (2):1231–1243. doi: 10.1016/j.neuroimage.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207 (1):3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25 (7):348–353. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8 (5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, von Cramon DY. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res. 1999;125 (4):417–425. doi: 10.1007/s002210050698. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163 (2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. Musical training shapes structural brain development. J Neurosci. 2009;29 (10):3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Grafton ST. Swinging in the brain: shared neural substrates for behaviors related to sequencing and music. Nat Neurosci. 2003;6 (7):682–687. doi: 10.1038/nn1081. [DOI] [PubMed] [Google Scholar]
- Jäncke L. Music drives brain plasticity. F1000 Biology Reports. 2009;1:78. doi: 10.3410/B1-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Loose R, Lutz K, Specht K, Shah N. Cortical activations during paced finger-tapping applying visual and auditory pacing stimuli. Cogn Brain Res. 2000;10 (1–2):51–66. doi: 10.1016/s0926-6410(00)00022-7. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, Smith SM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR. Musical time. In: Hallam S, Cross I, Thaut M, editors. Oxford Handbook of Music Psychology. Oxford; New York: 2009. pp. 81–92. [Google Scholar]
- Jones MR, Boltz M. Dynamic attending and responses to time. Psychol Rev. 1989;96 (3):459–491. doi: 10.1037/0033-295x.96.3.459. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. NeuroImage. 2003;19 (1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Thangaraj V, Halpern AR, Schlaug G. Absolute pitch and planum temporale. NeuroImage. 2001;14 (6):1402–1408. doi: 10.1006/nimg.2001.0925. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. 4. Prentice Hall; Upper Saddle River, NJ: 2004. [Google Scholar]
- Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M. The brain of opera singers: experience-dependent changes in functional activation. Cereb Cortex. 2010;20 (5):1144–1152. doi: 10.1093/cercor/bhp177. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Schulze K, Alsop D, Schlaug G. Adults and children processing music: an fMRI study. NeuroImage. 2005;25 (4):1068–1076. doi: 10.1016/j.neuroimage.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Schulze K, Sammler D, Fritz T, Müller K, Gruber O. Functional architecture of verbal and tonal working memory: an fMRI study. Hum Brain Mapp. 2009;30 (3):859–873. doi: 10.1002/hbm.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nat Rev Neurosci. 2010;11 (8):599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Kutner M, Nachtsheim C, Neter J. Applied Linear Regression Models. 4. McGraw-Hill; 2004. [Google Scholar]
- Large EW, Jones MR. The dynamics of attending: how people track time-varying events. Psychol Rev. 1999;106 (1):119–159. [Google Scholar]
- Levitin DJ, Menon V. Musical structure is processed in “language” areas of the brain: a possible role for Brodmann Area 47 in temporal coherence. NeuroImage. 2003;20 (4):2142–2152. doi: 10.1016/j.neuroimage.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13 (2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4 (4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7 (1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. Lawrence Erlbaum; 2005. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McAuley JD, Jones MR, Holub S, Johnston HM, Miller NS. The time of our lives: life span development of timing and event tracking. J Exp Psychol Gen. 2006;135 (3):348–367. doi: 10.1037/0096-3445.135.3.348. [DOI] [PubMed] [Google Scholar]
- Meister I, Krings T, Foltys H, Boroojerdi B, Müller M, Töpper R, Thron A. Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: implications for cortical motor organization. Hum Brain Mapp. 2005;25 (3):345–352. doi: 10.1002/hbm.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner BA. Laterality effects in audition. In: Mountcastle V, editor. Interhemispheric Relations and Cerebral Dominance. Johns Hopkins Press; Baltimore, MD: 1962. pp. 177–195. [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right hemisphere speech representation. Brain. 1998;121 (Pt 12):2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- Moreno S, Besson M. Musical training and language-related brain electrical activity in children. Psychophysiology. 2006;43:287–291. doi: 10.1111/j.1469-8986.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Moreno S, Marques C, Santos A, Santos M, Castro SL, Besson M. Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cereb Cortex. 2009;19:712–723. doi: 10.1093/cercor/bhn120. [DOI] [PubMed] [Google Scholar]
- Moreno S, Bialystok E, Barac R, Schellenberg EG, Cepeda NJ, Chau T. Short-term music training enhances verbal intelligence and executive function. Psychol Sci. 2011;22:1425–1433. doi: 10.1177/0956797611416999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münte TF, Altenmuller E, Jäncke L. The musician’s brain as a model of neuroplasticity. Nat Rev Neurosci. 2002;3 (6):473–478. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457 (2):66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Nichols TE. Re: multiple regression: testing an interaction at the second level. SPM email discussion list. 2008 Retrieved a from https://www.jiscmail.ac.uk/cgi-bin/wa.exe?A2=SPM;PPOtEw;20080318094435%2B0000.
- Nichols TE, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25 (3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Shah NJ, Jäncke L. Top-down and bottom-up modulation of language related areas—an fMRI study. BMC Neurosci. 2003;4 (1):13. doi: 10.1186/1471-2202-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton Andrea, Winner E, Cronin K, Overy K, Lee DJ, Schlaug G. Are there pre-existing neural, cognitive, or motoric markers for musical ability? Brain Cogn. 2005;59 (2):124–134. doi: 10.1016/j.bandc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nat Neurosci. 2007;10 (9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Asada T, Aruga M, Hirakata M, Nishikawa M, Katoh A, et al. Functional anatomy of musical perception in musicians. Cereb Cortex. 2001;11 (8):754–760. doi: 10.1093/cercor/11.8.754. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Overy K, Norton AC, Cronin KT, Gaab N, Alsop DC, Winner E, Schlaug G. Imaging melody and rhythm processing in young children. Neuroreport. 2004;15 (11):1723–1726. doi: 10.1097/01.wnr.0000136055.77095.f1. [DOI] [PubMed] [Google Scholar]
- Patel AD, Iversen JR, Chen Y, Repp BH. The influence of metricality and modality on synchronization with a beat. Exp Brain Res. 2005;163 (2):226–238. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- Platel H, Price C, Baron J, Wise R, Lambert J, Frackowiak R, Lechevalier B, et al. The structural components of music perception. A functional anatomical study. Brain. 1997;120 (2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Interpreting developmental changes in neuroimaging signals. Hum Brain Mapp. 2010;31 (6):872–878. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci. 2001;98 (2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4 (3):317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Richter W, Richter M. The shape of the fMRI BOLD response in children and adults changes systematically with age. NeuroImage. 2003;20 (2):1122–1131. doi: 10.1016/S1053-8119(03)00347-1. [DOI] [PubMed] [Google Scholar]
- Rudert T, Lohmann G. Conjunction analysis and propositional logic in fMRI data analysis using Bayesian statistics. J Magn Reson Imaging. 2008;28 (6):1533–1539. doi: 10.1002/jmri.21518. [DOI] [PubMed] [Google Scholar]
- Samson S, Zatorre RJ. Melodic and harmonic discrimination following unilateral cerebral excision. Brain Cogn. 1988;7 (3):348–360. doi: 10.1016/0278-2626(88)90008-5. [DOI] [PubMed] [Google Scholar]
- Schlaug G. The brain of musicians. A model for functional and structural adaptation. Ann N Y Acad Sci. 2001;930:281–299. [PubMed] [Google Scholar]
- Schlaug G, Jäncke L, Huang Y, Steinmetz H. In vivo evidence of structural brain asymmetry in musicians. Science (New York, NY) 1995;267 (5198):699–701. doi: 10.1126/science.7839149. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Overy K, Winner E. Effects of music training on the child’s brain and cognitive development. Ann N Y Acad Sci. 2005;1060:219–230. doi: 10.1196/annals.1360.015. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Forgeard M, Zhu L, Norton Andrea, Norton Andrew, Winner E. Training-induced neuroplasticity in young children. Ann N Y Acad Sci. 2009;1169:205–208. doi: 10.1111/j.1749-6632.2009.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönwiesner M, Rübsamen R, von Cramon DY. Hemispheric asymmetry for spectral and temporal processing in the human anterolateral auditory belt cortex. Eur J Neurosci. 2005;22 (6):1521–1528. doi: 10.1111/j.1460-9568.2005.04315.x. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Predicting perceptual events activates corresponding motor schemes in lateral premotor cortex: an fMRI study. NeuroImage. 2002;15 (4):787–796. doi: 10.1006/nimg.2001.1043. [DOI] [PubMed] [Google Scholar]
- Schulze K, Gaab N, Schlaug G. Perceiving pitch absolutely: comparing absolute and relative pitch possessors in a pitch memory task. BMC Neurosci. 2009;10:106. doi: 10.1186/1471-2202-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K, Zysset S, Mueller K, Friederici AD, Koelsch S. Neuroarchitecture of verbal and tonal working memory in nonmusicians and musicians. Hum Brain Mapp. 2011;32 (5):771–783. doi: 10.1002/hbm.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 1999;29 (1):26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Volkmann J, Jäncke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left-and right-handers. Annals of Neurology. 1991;29 (3):315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- Tillmann B, Koelsch S, Escoffier N, Bigand E, Lalitte P, Friederici AD, von Cramon DY. Cognitive priming in sung and instrumental music: activation of inferior frontal cortex. NeuroImage. 2006;31 (4):1771–1782. doi: 10.1016/j.neuroimage.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P, Gareau L, Flowers D, Zeffiro T, Eden G. Development of neural mechanisms for reading. Nat Neurosci. 2003;6 (7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15 (1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30 (4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist. 2010;16 (5):566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Wise RJS, Warren JD. Sounds do-able: auditory–motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28 (12):636–643. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Westbury CF, Zatorre RJ, Evans AC. Quantifying variability in the planum temporale: a probability map. Cereb Cortex. 1999;9 (4):392–405. doi: 10.1093/cercor/9.4.392. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11 (10):946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E. Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci. 1994;14 (4):1908–1919. doi: 10.1523/JNEUROSCI.14-04-01908.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre Robert J, Perry DW, Beckett CA, Westbury CF, Evans AC. Functional anatomy of musical processing in listeners with absolute pitch and relative pitch. Proc Natl Acad Sci U S A. 1998;95 (6):3172–3177. doi: 10.1073/pnas.95.6.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory–motor interactions in music perception and production. Nat Rev Neurosci. 2007;8 (7):547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Halpern AR, Bouffard M. Mental reversal of imagined melodies: a role for the posterior parietal cortex. J Cogn Neurosci. 2010;22 (4):775–789. doi: 10.1162/jocn.2009.21239. [DOI] [PubMed] [Google Scholar]