Abstract
Event-related oscillations (EROs) represent highly heritable neuroelectric correlates of cognitive processes that manifest deficits in alcoholics and in offspring at high risk to develop alcoholism. Theta ERO to targets in the visual oddball task has been shown to be an endophenotype for alcoholism. A family-based genome-wide association study was performed for the frontal theta ERO phenotype using 634583 autosomal single nucleotide polymorphisms (SNPs) genotyped in 1560 family members from 117 families densely affected by alcohol use disorders, recruited in the Collaborative Study on the Genetics of Alcoholism. Genome-wide significant association was found with several SNPs on chromosome 21 in KCNJ6 (a potassium inward rectifier channel; KIR3.2/GIRK2), with the most significant SNP at P = 4.7 × 10-10). The same SNPs were also associated with EROs from central and parietal electrodes, but with less significance, suggesting that the association is frontally focused. One imputed synonymous SNP in exon 4, highly correlated with our top three SNPs, was significantly associated with the frontal theta ERO phenotype. These results suggest KCNJ6 or its product GIRK2 account for some of the variations in frontal theta band oscillations. GIRK2 receptor activation contributes to slow inhibitory postsynaptic potentials that modulate neuronal excitability, and therefore influence neuronal networks.
Introduction
The electroencephalogram (EEG) recorded from the scalp during cognitive tasks contains oscillation patterns in specific frequency bands associated with task processing. These event-related oscillations (EROs) provide a versatile framework to generate coordination and communication during complex brain operations (Buzsaki 2010; Buzsaki & Draguhn 2004); they bind neural ensembles by providing windows of opportunity for neurons to fire enabling functional integration of networks (Fries 2005). EROs in specific frequency bands [delta (1.0-2.5 Hz); theta (3.0-7.0 Hz); alpha (7.5-12.0 Hz); beta (12.5-29.0 Hz); and gamma (>29.0 Hz)] have been attributed to specific cognitive processes during normal and pathological brain function (Babiloni et al. 2011; Basar et al. 2001; Klimesch et al. 2001; Rothenberger 2009). Delta EROs are associated with signal evaluation and decision-making (Basar et al. 1999; Schurmann et al. 2001), while theta EROs are important for processes underlying frontal inhibitory control, conscious awareness, recognition memory and episodic retrieval (Gevins et al. 1998; Klimesch et al. 2001; Klimesch et al. 1994). Theta oscillations have been implicated in sensorimotor integration (Bland & Oddie 2001; O'Keefe & Recce 1993), evaluating loss and gain (Kamarajan et al. 2008) and several processes associated with memory (Jacobs et al. 2006; Klimesch et al. 2008; Vertes 2005). Theta rhythm plays a role in information processing using an attentional double-gating mechanism, “filtering-in” signals for effective registration and encoding of selected information and “filtering-out” interfering inputs (Vinogradova 1995). Brain oscillations have been shown to be stable, highly heritable (van Beijsterveldt & Boomsma 1994; van Beijsterveldt et al. 1996), and to be reliable endophenotypes that reflect a shared liability between alcoholism and related disorders (Porjesz et al. 2005). Power estimates of oscillations are more heritable than event-related potential (ERP) components, giving them a slight edge as endophenotypes (de Geus 2010).
Alcoholism is part of a spectrum of disinhibitory disorders, which include externalizing and substance use disorders; a shared set of genetic factors influencing impulse control are postulated to underlie these co-occurring disinhibitory disorders (Kendler et al. 2003; Lahey et al. 2011). Hence, examining neuroelectric phenotypes that reflect shared liabilities provides a powerful strategy to investigate underlying risk for alcoholism and related disorders (Porjesz & Rangaswamy 2007; Rangaswamy & Porjesz 2008). Our earlier studies have shown that both delta and theta power are significantly reduced in alcoholics and adolescent offspring of alcoholics when compared with normal controls during target processing in a visual oddball paradigm (Jones et al. 2006b; Rangaswamy et al. 2007). Frontal theta ERO has successfully served as an endophenotype in family and case-control genetic studies in the Collaborative Study on the Genetics of Alcoholism (COGA) (Chen et al. 2009;Jones et al. 2006a; Jones et al. 2004; Zlojutro et al. 2011). This study examines the theta ERO endophenotype recorded at the midline frontal (Fz) electrode in response to targets in a visual oddball paradigm in a family-based genome-wide association study (GWAS). This study also evaluates the scalp topography of associations for theta ERO and single nucleotide polymorphisms (SNPs) in the most significant gene. The advantage of a family-based study design is robustness against population substructure and the availability of the genotypes of both parents, which enables a more correct evaluation of genotype errors. This is the first family-based GWAS of EROs.
Materials and Methods
Participants
Alcoholic and community probands and their families were recruited and tested as part of the national multi-site COGA. Alcoholic probands were recruited from inpatient and outpatient treatment facilities; all participants were administered the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al. 1994; Hesselbrock et al. 1999), and alcoholic subjects met criteria for alcohol dependence (DSM-IV). Data from the six COGA collection sites were included in the analysis: SUNY Downstate Medical Center at Brooklyn, New York; University of Connecticut Health Science Center; Washington University School of Medicine in St. Louis; University of California at San Diego; University of Iowa, and Indiana University School of Medicine. Recruitment and assessment procedures have been outlined previously (Begleiter et al. 1995; Foroud et al. 2000; Nurnberger et al. 2004; Reich et al. 1998) and are available at zork.wustl.edu/niaaa/coga_instruments/resources.html. Institutional review boards at each site approved the research protocols in the COGA study and written consent was obtained from each individual before participation.
Prior to neurophysiological assessments, alcoholic subjects were required to abstain from alcohol for a minimum of 3 weeks and not exhibit withdrawal symptoms. Subjects were excluded from neurophysiological assessment if they had any of the following: (1) recent substance or alcohol use (i.e., positive breath-analyzer test and/or urine screen), (2) hepatic encephalopathy/cirrhosis of the liver, (3) significant history of head injury, seizures or neurosurgery, (4) uncorrected sensory deficits, (5) taking medication known to influence brain functioning, (6) history/symptoms of psychoses, (7) positive test for human immunodeficiency virus, (8) other acute/chronic medical illnesses that affects brain function, and (9) a score of less than 25 on the Mini Mental State Examination (MMSE) (Folstein et al. 1975).
Prioritization of families for the COGA family-based GWAS was based on the number of alcohol dependent family members, the number of individuals who supplied DNA and the number of family members with electrophysiological measurements. The family-based GWAS of the theta ERO phenotype only included families of primarily Caucasian descent (to reduce heterogeneity of the sample) and those with measurements of the theta ERO phenotype at the frontal (Fz) lead. Thus, the dataset for the family-based GWAS of theta ERO comprised 1,560 individuals (male=738, female=822) ranging in age from 7 to 74 years (male: 7-74 years, average = 30.7 years; female: 7-72 years, average = 31.6 years) from 117 multi-generational families affected with alcoholism. Family sizes ranged from 4 to 39 individuals and had an average of 13.4 subjects per family (Figure S1).
Visual Oddball Task
A three stimulus visual oddball task was employed with 280 visual stimuli of three different types: targets (rarely occurring letter X to which the subjects responded quickly and accurately with a button press, non-targets (frequently occurring white squares) and novels (rarely occurring random colored geometric figures). Stimuli subtended a visual angle of 2.5° with stimulus durations of 60 ms and inter-stimulus intervals of 1.625 ms. The task comprised 35 target, 35 novel and 210 non-target stimuli with a probability of occurrence of 0.125, 0.125 and 0.750, respectively. The stimuli were presented pseudo-randomly with the only constraint that the targets and novels always followed non-targets.
Event-Related Potential Recording
All six collaborating sites used identical experimental procedures and EEG acquisition hardware and software programs. Subjects were seated comfortably 1 m from a monitor in a dimly lit sound-attenuated RF-shielded booth (Industrial Acoustics Company, Bronx, NY, USA), and wore a 19-channel electrode cap (Electro-Cap International, Inc., Eaton, OH, USA) as specified by the International 10-20 System for Electrode Placement (Figure S2). The nose served as reference and the forehead served as ground. Electrode impedances were maintained below 5 kΩ. Electrical activity was amplified 10,000 times using Sensorium EPA-2 Electrophysiology amplifiers (Charlotte, VT, USA) was recorded continuously over a bandwidth of 0.02 - 100.0 Hz on a Neuroscan system (Version 4.1 to 4.5; Neurosoft, Inc., El Paso, TX, USA) at sampling rates of 256 Hz, 500 Hz and 512 Hz, and stored for further analysis. During analysis all signals were re-sampled to 256 Hz and bandpass filtered between 0.05 and 55.0 Hz. Artifact rejection threshold was set at 100 μV. A minimum of 20 epochs of 100 milliseconds pre-stimulus to 750 milliseconds post-stimulus artifact free trials for each stimulus was required for analysis.
ERO Energy Estimation
Estimates of localized power of non-stationary evoked potential time series were obtained using the S-transform, a time-frequency representation method developed by Stockwell et al. (Stockwell 2007; Stockwell et al. 1996). This method has been previously described and implemented in our laboratory to evaluate event-related signals in the time-frequency domain (Jones et al. 2006b; Kamarajan et al. 2006; Rangaswamy et al. 2007).
Phenotype
Event-related electrophysiological data for the target stimulus from the visual oddball task were analyzed. The amplitude envelope of the S-transform time-frequency region was averaged across single trials, per individual, to obtain estimates of event-related total power. Mean power was calculated for each electrode within time-frequency regions of interest that were defined by frequency band ranges and time intervals (Lachaux et al. 2003). The primary phenotype used for the family GWAS was the total power in the theta (3.0-7.0 Hz) oscillation at the frontal midline channel (Fz) extracted from the 300-700 ms time window, which corresponds to the window of the P300 component in the event-related waveforms. The total theta power measure was also extracted for the central (Cz) and parietal (Pz) channels for secondary analyses. As the theta ERO phenotype showed significant age and gender effects with amplitude, age and gender were included as covariates in the genetic analyses.
Genotyping and Quality Control
Samples from the COGA DNA and Cell Repository at Rutgers University were genotyped at the Genome Technology Access Center at Washington University School of Medicine in St. Louis using the Illumina Human OmniExpress array 12.VI (731,444 SNPs; Illumina, San Diego, CA, USA) on 2098 subjects selected from 118 densely affected families. An additional 224 subjects from these 118 families had been genotyped in a previous case-control GWAS by the Center for Inherited Disease Research (CIDR) at John Hopkins University using the Illumina Human 1M-Duo BeadChip technology (1,041,465 SNPs; Illumina, San Diego, CA, USA; Edenberg et al. 2010). To insure quality control, 51 subjects previously genotyped at CIDR were included among the 2098 subjects genotyped at Washington University.
The genotypic data set of 731 444 SNPs was carefully examined to ensure high quality standards (Turner et al. 2011). In this QC, each SNP was first examined for genotypic completeness. A genotype call score threshold of 0.15 was used, as recommended by Illumina Technical Support, which led to the removal of 1.7% of SNPs from further analysis. For those cases (n = 162, 0.33%) in which duplication or deletion was observed, the entire chromosome harboring this copy number variant was removed from further analysis. Inconsistencies (a non-missing, but different, genotype on the two arrays) of the 544 276 overlapping SNPs on the Illumina Human 1M-Duo and the Illumina Human OmniExpress arrays were tested using the 51 twice-genotyped subjects. We found 571 SNPs with more than one discrepancy and removed those SNPs in all 2322 subjects. Deviations from Hardy-Weinberg equilibrium (HWE) were evaluated using 442 genotyped founders, and SNPs that failed the HWE test at p < 10−6 were removed. SNPs with minor allele frequency less than 0.01 were removed. In total, 634 627 autosomal SNPs were analyzed for association.
The software package PLINK v1.07 (Purcell et al. 2007) was used to calculate pairwise identity-by-descent (IBD) estimates of 100 000 SNPs, which were not in linkage disequilibrium with each other, to verify the reported pedigree structure of the 118 families. Based on these IBDs, some family structures were modified. Using the software PEDCHECK v1.1 (O'Connell & Weeks 1998) on the modified pedigrees, 2899 SNPs with 2 or more inconsistencies with Mendelian inheritance were identified and removed. Ethnic stratification was assessed with EIGENSTRAT (Price et al. 2006) using the 100 000 SNPs and the HapMap Caucasian reference samples, and no individual was excluded based on the EIGENSTRAT v3.0 results.
Statistical Genetic Analyses
Genome-wide association tests with the frontal theta ERO phenotype to target stimuli were performed on 1560 samples from 117 of the 118 families genotyped; one of the 118 families was excluded because there were no theta ERO measurements available. Association testing was carried out assuming an additive model using the generalized disequilibrium test. Phenotype data was derived from multivariate linear regression models which were constructed from log-transformed theta power recorded at Fz, controlling for log-transformed age and stratified by gender and the residual values were fit to a standard normal distribution to create z-scores. Secondary analysis examined the topographic distribution of association signals in the KCNJ6 region using total theta power at Cz and Pz scalp locations.
Imputation of SNPs in the KCNJ6 region was performed using the program BEAGLE version 3.3.1 (Browning & Browning 2009) (http://www.sph.umich.edu/csg/abecasis/MaCH/). We used reference data from the European popultaion in the August 2010 release of the 1000 Genomes Project provided with the Beagle release for our European American sample. SNPs with a final r2, the estimated squared correlation between the estimated allele dosage and the true allele dosage, >0.30 were used. For individual-level genotype data, we retained genotypes having a probability ≥80% (from the gprob metric in Beagle); otherwise that genotype was set to missing. To account for uncertainty, we used the mean of the distribution of imputed genotypes, which corresponds to an expected allelic or genotypic count (dosage) for each individual. Subsequently, association tests of the Fz theta ERO phenotype were performed.
Results
Event-related electrophysiological data for the target stimulus from the visual oddball task yielded measures of total power in the theta (3.0-7.0 Hz) oscillation at the frontal midline channel (Fz). This measure was estimated for 1560 individuals from the signals in the 300-700 milliseconds time window, which corresponded to the time window of the P300 component in the event-related waveforms. The mean power in the theta band for the frontal midline channel was 30.2 ± 18.7 μV2 (males = 28.2 μV2; females = 32.0 μV2).
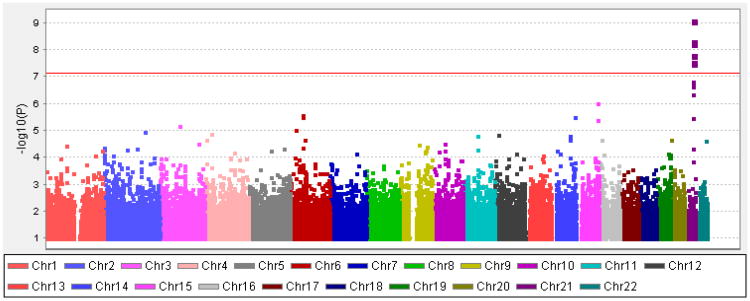
The quantile-quantile (QQ) plot of predicted and observed association results is presented in Fig. S3. The genomic inflation factor λ is 1.00, indicating that there is no bias of the test statistic. Figure 1 displays the P-values of association tests for the theta Fz phenotype in a Manhattan plot. The most significant results were located on chromosome 21, with seven SNPs reaching genome-wide significant P-values (see Table 1 for SNPs with p < 1 × 10−5). The SNP with the lowest P-value (4.7 × 10−10) was rs2835872 in KCNJ6 on chromosome 21. The G allele of rs2835872 was associated with lower theta power at Fz. This marker, along with 10 other SNPs listed in Table 1, were located within the introns of KCNJ6.
Figure 1. Manhattan plot of genome-wide association results for theta ERO at Fz.
Negative log-transformed p- values for SNPs are plotted against position on each chromosome. One genomic region on chromosome 21 contains SNPs that exceed the genome-wide significance threshold of 7.88 × 10−8 (indicated by red line).
Table 1.
Top SNPs with p < 1 × 10−5 associated with theta power at Fz in family GWAS.
| chr | gene | function | SNP | BP | A1 a | A2 | Freq. b | Effect c | p |
|---|---|---|---|---|---|---|---|---|---|
| 21 | KCNJ6 | intron | rs2835872 | 39,027,272 | G | A | 0.681 | -0.145 | 4.70×10−10 |
| 21 | KCNJ6 | intron | rs702860 | 39,008,629 | A | G | 0.699 | -0.139 | 3.10×10−9 |
| 21 | KCNJ6 | intron | rs2835850 | 39,012,977 | T | C | 0.700 | -0.139 | 3.50×10−9 |
| 21 | KCNJ6 | intron | rs857975 | 39,001,613 | C | A | 0.699 | -0.135 | 9.20×10−9 |
| 21 | KCNJ6 | intron | rs857978 | 38,998,126 | C | T | 0.678 | -0.129 | 1.90×10−8 |
| 21 | rs2835831 | 38,987,233 | T | C | 0.684 | -0.129 | 2.00×10−8 | ||
| 21 | rs2835837 | 38,990,443 | G | A | 0.681 | -0.129 | 2.10×10−8 | ||
| 21 | KCNJ6 | intron | rs2154553 | 39,004,501 | A | G | 0.696 | -0.130 | 1.00×10−7 |
| 21 | KCNJ6 | intron | rs12482570 | 39,077,777 | A | G | 0.671 | -0.123 | 1.10×10−7 |
| 21 | KCNJ6 | intron | rs2835893 | 39,060,893 | G | A | 0.672 | -0.126 | 1.50×10−7 |
| 21 | KCNJ6 | intron | rs1787422 | 39,076,961 | T | C | 0.610 | -0.115 | 1.90×10−7 |
| 21 | rs2835833 | 38,987,897 | G | A | 0.683 | -0.123 | 2.90×10−7 | ||
| 14 | rs2766692 | 100,684,192 | G | A | 0.691 | -0.110 | 2.10×10−6 | ||
| 22 | PRR5-ARHGAP8 | intron | rs16992796 | 45,183,014 | G | A | 0.050 | -0.236 | 3.00×10−6 |
| 15 | rs7181753 | 96,844,727 | T | C | 0.199 | 0.128 | 3.00×10−6 | ||
| 3 | rs9860340 | 87,783,976 | A | G | 0.749 | -0.122 | 3.60×10−6 | ||
| 21 | KCNJ6 | intron | rs858008 | 39,065,630 | C | T | 0.512 | 0.099 | 4.30×10−6 |
| 21 | KCNJ6 | intron | rs2835886 | 39,040,342 | C | T | 0.586 | 0.101 | 4.60×10−6 |
| 6 | rs9395865 | 53,307,694 | T | C | 0.279 | 0.106 | 6.10×10−6 | ||
| 11 | C11orf84 | intron | rs10897449 | 63,592,621 | T | C | 0.453 | -0.099 | 7.20×10−6 |
| 6 | FAM65B | intron | rs4256430 | 24,863,075 | A | G | 0.465 | -0.099 | 8.40×10−6 |
| 6 | rs4712029 | 53,317,012 | G | A | 0.238 | 0.114 | 8.40×10−6 |
A1 is the reference allele.
Freq. is the allele frequency of the reference allele A1.
Effect is the effect of the reference allele A1 on the phenotype theta power recorded at Fz.
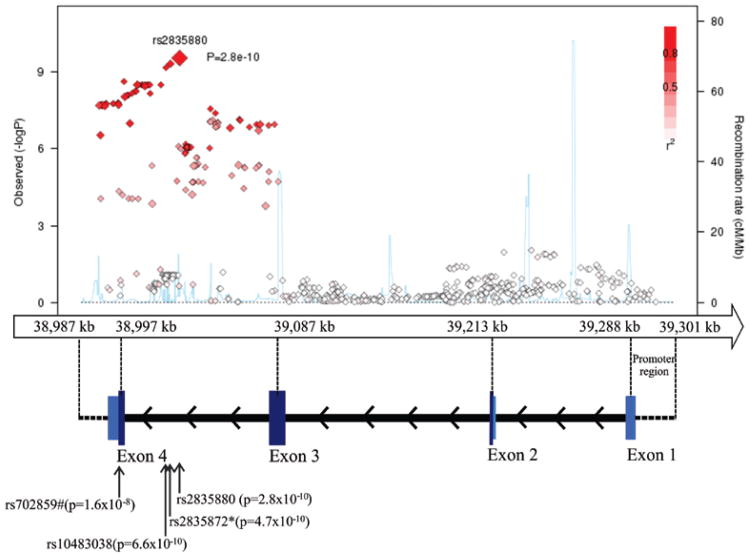
Given the significant findings of KCNJ6 SNPs, we imputed additional SNPs in this region. Our imputed data show that the top three highly correlated SNPs (r2 > 0.99) in introns of KCNJ6 are associated with theta Fz at a genome-wide significance level (rs2835880, imputed, P = 2.80 × 10−10; rs2835872, genotyped, P = 4.70 × 10−10; rs10483038, imputed, P = 6.60 × 10−10). A synonymous SNP, rs702859 (imputed) in KCNJ6 that is highly correlated with the top three SNPs, rs2835880, rs2835872, and rs10483038 (r2 > 0.8) is associated with theta Fz at P-value 1.60 ×10−8 (Table S1). Figure 2 displays KCNJ6 in the chromosome 21q22 association with theta Fz for both genotyped and imputed SNPs.
Figure 2. Plot of chromosome 21q22 association with theta power at Fz. Negative log-transformed p- values for SNPs are plotted against position on chromosome 21q22.
* indicates genotyped SNP
# indicates synonymous coding SNP
To evaluate the topographic specificity of the significant findings of KCNJ6 SNPs with the theta ERO phenotype at Fz, we investigated the association of SNPs in KCNJ6 with theta ERO power at the central midline (Cz) and parietal midline (Pz) channels. The association analysis results for Cz and Pz for KCNJ6 SNPs did not reach genome-wide significance levels, nor yield as many P-values < 1 × 10−4 as Fz. There are 15 SNPs with P-values < 1 × 10−4 for Fz while there is only one for Cz and none for Pz. The most significant SNP for Fz, rs2835872, was also most significant for Cz (6.00 × 10-5). The SNP with the lowest P-value for Pz, rs2835850, (P = 1.10 × 10−4), reached genome-wide significance for Fz. This suggests that the theta oscillations in all three regions shared a genetic determinant in KCNJ6, and that the frontal channel shows the strongest signal.
In addition to KCNJ6, some evidence of association (P < 1.0 × 10-5) was found with SNPs in or near other genes (Table 1). These other findings were in PRR5-ARHGAP8 on chromosome 22, C11orf84 on chromosome 11, and FAM65B on chromosome 6.
Discussion
The results from this family GWAS have provided the first genome-wide significant association of theta oscillations with three SNPs in KCNJ6. These SNPs were associated with theta oscillations across the scalp; however, the strongest associations were frontal (Fz). One imputed synonymous SNP in exon four, highly correlated with our top three SNPs, was significantly associated with the frontal theta ERO phenotype. These results suggest a role for KCNJ6 or its product GIRK2 in variations of frontal theta band oscillations.
The protein encoded by KCNJ6 is known as GIRK2, GIRK 2a and KIR 3.2, and is part of a superfamily of inward rectifier channels. GIRK2 is widely distributed in the brain and is an important functional element in dopaminergic, cholinergic, GABAergic and glutamatergic synapses (Saenz del Burgo et al. 2008). Studies have suggested that three different splice variants of GIRK2 (GIRK2a, GIRK2b and GIRK2c) channels may exist in neurons (Isomoto et al. 1997). Of the four GIRK channels (GIRK 1-4) expressed in mammals, GIRK 1-3 are abundantly expressed in the brain (Wickman et al. 2000). Of these, only GIRK2 is capable of forming both heterotetrameric (with GIRK1/3) and homotetrameric functional channels (Liao et al. 1996). By virtue of its channel properties, GIRK2 contributes to the slow inhibitory postsynaptic potentials (IPSCs) as a result of GABAB action (Luscher et al. 1997; Nicoll 2004).
Activity of GIRK receptors results in hyperpolarization that decreases neuronal excitability and this in turn directly influences activity levels in neurons. There are several lines of evidence that highlight the role of inhibition in tuning responses and pacing oscillations and establishing synchrony during cognitive processing in the brain (Isaacson & Scanziani 2011; review). A simulation study examining decision time and theta rhythm suggests that a mixture of slow and fast inhibition can affect the power in the theta band and speed up the reaction times in a decision-making network (Smerieri et al. 2010).
Very few studies have examined electrophysiology and genetics in humans. Small effects from several genes associated with neurotransmitters may contribute to the variation in P300 and theta and delta oscillations during cognitive tasks. The neurochemical basis of the target stimulus response and P300 component has been suggested to be triggered by glutamatergic activity and modulated by influences from both cholinergic and GABAergic sources (Frodl-Bauch et al. 1999; Kenemans & Kahkonen 2011). Previous studies conducted in COGA examining the genetics of EROs have employed whole genome linkage followed by candidate gene association methods on family and case control data. Based on the regions identified in whole genome linkage scans with theta EROs, we identified candidate genes, namely, GRM8 (metabotropic glutamate receptor) and CHRM2 (muscarinic cholinergic receptor), and subsequently reported that SNPs in both candidate genes are associated with theta EROs to targets (Chen et al. 2009; Jones et al. 2006a; Jones et al. 2004). In this study, we also found several SNPs in both CHRM2 and GRM8 genes were significantly associated with frontal theta EROs; however, not quite at the level of genome-wide significance (Table S2). Another study endorsing a role for the thalamus in cognitively relevant brain rhythms has suggested both metabotropic glutamate receptor and muscarinic cholinergic receptor activation can cause rhythmic bursting at alpha/theta frequencies (Hughes et al. 2008). Since the GIRK2 is an important functional element in dopaminergic, cholinergic, GABAergic and glutamatergic synapses (Saenz del Burgo et al. 2008), a functional involvement of the GIRK2 channels in the oscillatory dynamics of theta band can be speculated.
The activation of GIRK channels can influence neuronal networks at different levels in the brain via different mechanisms: (1) neuronal self-inhibition – where the transmitter released from the dendrites activates the GIRK channels on the same cell (Bacci et al. 2004), (2) neuron-to-neuron inhibition – a result of activation of post synaptic receptors such as GABAB (Newberry & Nicoll 1985), D2 (Beckstead & Williams 2007) and group II metabotropic glutamate receptors (Dutar et al. 1999) by the relevant transmitters released from presynaptic neurons and (3) network level inhibition – a result of ambient levels of neuromodulators like adenosine and somatostatin, which are endogenous G protein coupled receptor agonists. Somatostatin may alter the oscillatory behavior of thalamic networks through postsynaptic activation of GIRK channels together with presynaptic inhibition (Sun et al. 2002); in addition, endogenous adenosine may suppress gamma oscillations in the hippocampus, which also activate GIRK channels (Pietersen et al. 2009).
Animal models have shown GIRK channels are important effectors in both opioid- and ethanol-induced analgesia (Ikeda et al. 2002), as well as being directly activated by ethanol with the involvement of a discrete alcohol pocket (Aryal et al. 2009). Another study on rat midbrain dopaminergic neurons suggests that the action of ethanol occurs on activated GIRK channels downstream of the GABAB receptors (Federici et al. 2009). The authors suggest that the enhancing effects of ethanol on GABAB responses could modulate alcohol intake and the mental and motor performance in an acute intoxicative phase. These receptors are the focus for developing new analgesics (Lotsch & Geisslinger 2011) or therapeutic compounds that could mitigate the effects of alcohol. Hence, this may also be a pathway by which alcohol can modulate oscillatory brain activity.
A candidate gene study looking for genetic variants of risk for nicotine dependence identified a marker in KCNJ6 gene as one of their top signals (Saccone et al. 2007). Recently, another study examined a few SNPs in the promoter region of KCNJ6 and found some association with these SNPs for alcohol dependence in adults and for hazardous drinking behavior in adolescents who were exposed to early life stress (Clarke et al. 2011). Another recent study has suggested epistatic interaction of KCNJ6 with CREB1 (cyclic adenosine 5′-phosphate (adenosine monophosphate)-response element binding protein) may influence rumination, which is a core cognitive feature of depression (Lazary et al. 2011).
A polymorphism in this gene has been associated with opioid effects on analgesia and methadone replacement dose (Lotsch et al. 2010). A study examining dopamine-dependent phenotypes in GIRK2 knockout mice noted the presence of dopamine-dependent hyperactivity and enhanced responses to drugs that stimulate dopamine neurotransmission (Arora et al. 2010). However, these phenotypes were not solely attributable to the loss of GIRK (1/2) signaling in dopamine neurons, but could be due to adaptations in the mesolimbic dopamine system that facilitated excitatory glutamatergic neurotransmission. Therefore the authors suggest that drugs of abuse may evoke adaptations to promote chronic use through a regulation of GIRK signaling strength in dopaminergic neurons or their input neurons.
In conclusion, the results of the present study suggesting a role for the KCNJ6 gene in variations of frontal theta band oscillations are very compelling. Although all the neurotransmitter interactions and the functional role of GIRK2 channels on brain electrophysiology and behavior is yet to be completely defined, there is robust evidence (as reviewed above) for an important role of this channel in regulating the excitability of neuronal networks. Our findings underscore the potential for identifying meaningful genetic correlates of brain oscillations associated with pathophysiology of neuropsychiatric conditions.
Despite these strong findings, there are some limitations and caveats to bear in mind. As this method is designed for common variants, studies are planned to examine rare variants (through sequencing). Furthermore, increasing the sample size and meta-analysis with other data sets will improve power to identify associations. Future studies using an independent data sample are needed to confirm this association and examine the functional importance of these associations.
Supplementary Material
Acknowledgments
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, H. Edenberg, L. Bierut, includes ten different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, J. Nurnberger Jr., T. Foroud); University of Iowa (S. Kuperman, J. Kramer); SUNY Downstate (B. Porjesz); Washington University in St. Louis (L. Bierut, A. Goate, J. Rice, K. Bucholz); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield); Southwest Foundation (L. Almasy), Howard University (R. Taylor) and Virginia Commonwealth University (D. Dick). Other COGA collaborators include: L. Bauer (University of Connecticut); D. Koller, S. O’Connor, L. Wetherill, X. Xuei (Indiana University); Grace Chan (University of Iowa); N. Manz, M. Rangaswamy (SUNY Downstate); A. Hinrichs, J. Rohrbaugh, J-C Wang (Washington University in St. Louis); A. Brooks (Rutgers University); and F. Aliev (Virginia Commonwealth University). A. Parsian and M. Reilly are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, currently a consultant with COGA, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Funding support for GWAS genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the National Institute on Alcohol Abuse and Alcoholism, the NIH GEI (U01HG004438), and the NIH contract “High throughput genotyping for studying the genetic contributions to human disease” (HHSN268200782096C).
References
- Arora D, Haluk DM, Kourrich S, Pravetoni M, Fernandez-Alacid L, Nicolau JC, Lujan R, Wickman K. Altered neurotransmission in the mesolimbic reward system of Girk mice. Journal of neurochemistry. 2010;114:1487–1497. doi: 10.1111/j.1471-4159.2010.06864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nature neuroscience. 2009;12:988–995. doi: 10.1038/nn.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Lizio R, Ferri R, Rodriguez G, Marzano N, Frisoni GB, Rossini PM. Resting state cortical rhythms in mild cognitive impairment and Alzheimer's disease: electroencephalographic evidence. Journal of Alzheimer's disease: JAD. 2011;26(3):201–214. doi: 10.3233/JAD-2011-0051. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience letters. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Reich T, Hesselbrock VM, Porjesz B, Li TK, Schuckit MA, Edenberg HJ, Rice JP. The collaborative study on the genetics of alcoholism. Alcohol Health Res World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Human genetics. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. American journal of human genetics. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behavioural brain research. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. American journal of human genetics. 2009;84:210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of studies on alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, Edenberg HJ, Hesselbrock V, Nurnberger J, Jr, Kuperman S, O'Connor SJ, Schuckit MA, Bauer LO, Tischfield J, Rice JP, Bierut L, Goate A, Porjesz B. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B:359–368. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Laucht M, Ridinger M, Wodarz N, Rietschel M, Maier W, Lathrop M, Lourdusamy A, Zimmermann US, Desrivieres S, Schumann G. KCNJ6 is associated with adult alcohol dependence and involved in gene × early life stress interactions in adolescent alcohol drinking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1142–1148. doi: 10.1038/npp.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Vu HM, Perkel DJ. Pharmacological characterization of an unusual mGluR-evoked neuronal hyperpolarization mediated by activation of GIRK channels. Neuropharmacology. 1999;38:467–475. doi: 10.1016/s0028-3908(98)00206-8. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev F, Dick D, Hesselbrock V, Hinrichs A, Kramer J, Kuperman S, Nurnberger JI, Jr, Rice JP, Schuckit MA, Taylor R, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcoholism, clinical and experimental research. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Nistico R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. The European journal of neuroscience. 2009;29:1369–1377. doi: 10.1111/j.1460-9568.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, Goate A, Rice J, Flury L, Koller DL, Bierut LJ, Conneally PM, Nurnberger JI, Bucholz KK, Li TK, Hesselbrock V, Crowe R, Schuckit M, Porjesz B, Begleiter H, Reich T. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcoholism, clinical and experimental research. 2000;24:933–945. [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in cognitive sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Human factors. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- de Geus EJ. From genotype to EEG endophenotype: a route for post-genomic understanding of complex psychiatric disease? Genome medicine. 2010;2:63. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Errington A, Lorincz ML, Kekesi KA, Juhasz G, Orban G, Cope DW, Crunelli V. Novel modes of rhythmic burst firing at cognitively-relevant frequencies in thalamocortical neurons. Brain research. 2008;1235:12–20. doi: 10.1016/j.brainres.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi T, Kumanishi T, Yano R, Sora I, Niki H. Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys? Neuroscience research. 2002;44:121–131. doi: 10.1016/s0168-0102(02)00094-9. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. The Japanese journal of physiology. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. NeuroImage. 2006;32:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, et al. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behavior genetics. 2006a;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Stimus A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2006b;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biological psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, Pandey AK, Roopesh BN, Stimus AT, Porjesz B. Theta oscillations during the processing of monetary loss and gain: a perspective on gender and impulsivity. Brain research. 2008;1235:45–62. doi: 10.1016/j.brainres.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of general psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kahkonen S. How human electrophysiology informs psychopharmacology: from bottom-up driven processing to top-down control. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:26–51. doi: 10.1038/npp.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Rohm D, Gruber W. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain research Cognitive brain research. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain research. 2008;1235:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalography and clinical neurophysiology. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Chavez M, Lutz A. A simple measure of correlation across time, frequency and space between continuous brain signals. J Neurosci Meth. 2003;123:175–188. doi: 10.1016/s0165-0270(02)00358-8. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of general psychiatry. 2011;68:181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazary J, Juhasz G, Anderson IM, Jacob CP, Nguyen TT, Lesch KP, Reif A, Deakin JF, Bagdy G. Epistatic interaction of CREB1 and KCNJ6 on rumination and negative emotionality. European Neuropsychopharmacology. 2011;21:63–70. doi: 10.1016/j.euroneuro.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotsch J, Geisslinger G. Pharmacogenetics of new analgesics. British journal of pharmacology. 2011;163:447–460. doi: 10.1111/j.1476-5381.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotsch J, Pruss H, Veh RW, Doehring A. A KCNJ6 (Kir3.2, GIRK2) gene polymorphism modulates opioid effects on analgesia and addiction but not on pupil size. Pharmacogenetics and genomics. 2010;20:291–297. doi: 10.1097/FPC.0b013e3283386bda. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. The Journal of physiology. 1985;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA. My close encounter with GABA(B) receptors. Biochemical pharmacology. 2004;68:1667–1674. doi: 10.1016/j.bcp.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O'Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Archives of general psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. American journal of human genetics. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Pietersen AN, Lancaster DM, Patel N, Hamilton JB, Vreugdenhil M. Modulation of gamma oscillations by endogenous adenosine through A1 and A2A receptors in the mouse hippocampus. Neuropharmacology. 2009;56:481–492. doi: 10.1016/j.neuropharm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. TheScientificWorldJournal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain research. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, Foroud T, Hesselbrock V, Schuckit MA, Bucholz K, Porjesz B, Li TK, Conneally PM, Nurnberger JI, Jr, Tischfield JA, Crowe RR, Cloninger CR, Wu W, Shears S, et al. Genome-wide search for genes affecting the risk for alcohol dependence. American journal of medical genetics. 1998;81:207–215. [PubMed] [Google Scholar]
- Rothenberger A. Brain oscillations forever--neurophysiology in future research of child psychiatric problems. Journal of child psychology and psychiatry, and allied disciplines. 2009;50:79–86. doi: 10.1111/j.1469-7610.2008.01994.x. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Human molecular genetics. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz del Burgo L, Cortes R, Mengod G, Zarate J, Echevarria E, Salles J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. The Journal of comparative neurology. 2008;510:581–606. doi: 10.1002/cne.21810. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2001;39:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Smerieri A, Rolls ET, Feng J. Decision time, slow inhibition, and theta rhythm. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14173–14181. doi: 10.1523/JNEUROSCI.0945-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell RG. Why use the S-transform? Fields Inst Commun. 2007;52:279–309. 414. [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S transform. Ieee T Signal Proces. 1996;44:998–1001. [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Somatostatin inhibits thalamic network oscillations in vitro: actions on the GABAergic neurons of the reticular nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:5374–5386. doi: 10.1523/JNEUROSCI.22-13-05374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Armstrong LL, Bradford Y, Carlson CS, Crawford DC, Crenshaw AT, de Andrade M, Doheny KF, Haines JL, Hayes G, Jarvik G, Jiang L, Kullo IJ, Li R, Ling H, Manolio TA, Matsumoto M, McCarty CA, McDavid AN, et al. Quality control procedures for genomewide association studies. In: Haines Jonathan L, et al., editors. Current protocols in human genetics. Chapter 1. 2011. p. 19. Unit1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. Expression, control, and probable functional significance of the neuronal theta-rhythm. Progress in neurobiology. 1995;45:523–583. doi: 10.1016/0301-0082(94)00051-i. [DOI] [PubMed] [Google Scholar]
- Wickman K, Karschin C, Karschin A, Picciotto MR, Clapham DE. Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:5608–5615. doi: 10.1523/JNEUROSCI.20-15-05608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, Bierut LJ, Goate A, Hesselbrock V, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit MA, Foroud T, Edenberg HJ, Porjesz B, Almasy L. Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156B:44–58. doi: 10.1002/ajmg.b.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.