Abstract
Organelles in homogenates from autotrophic cells of Chlorogonium elongatum were separated on linear sucrose gradients. The distribution of enzymes typical of leaf peroxisomes was determined.
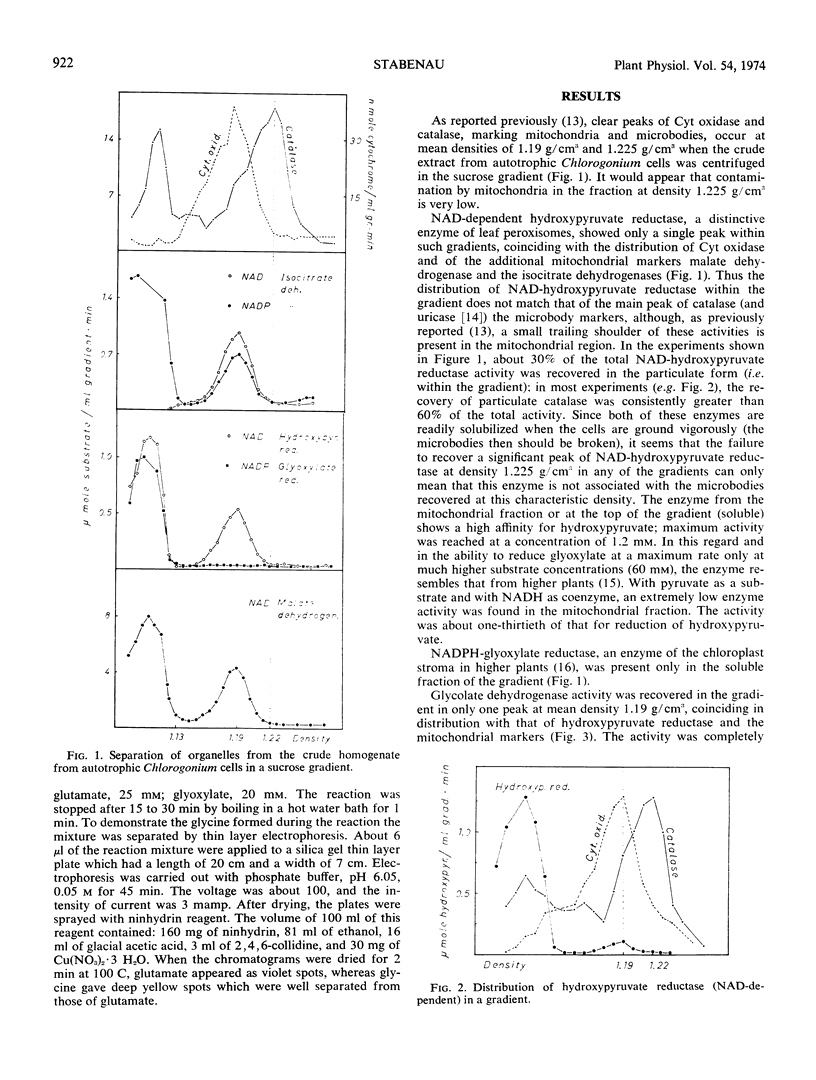
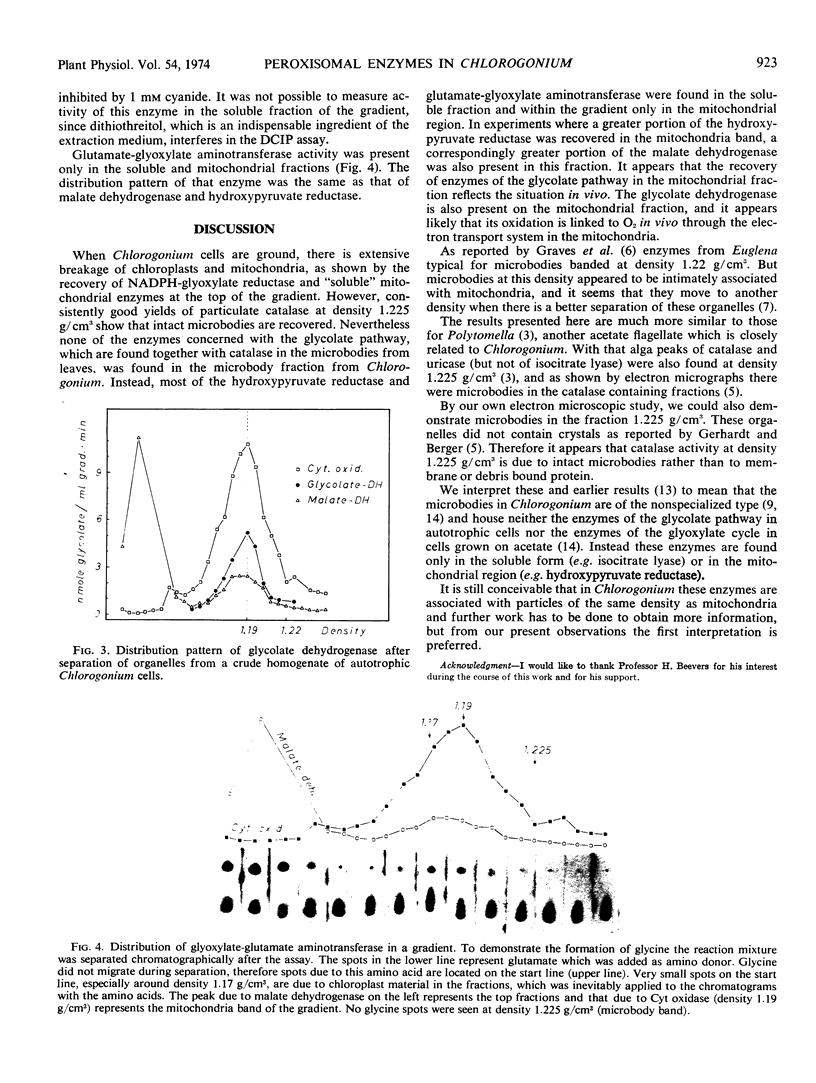
Whereas more than 60% of the catalase activity was particulate and recovered in microbodies at a mean density of 1.225 g/cm3 within the gradient, in most experiments only 5 to 10% (as a maximum 30%) of the NAD-dependent hydroxypyruvate reductase was particulate, and this was recovered principally at density 1.19 g/cm3. This distribution coincides with that of cytochrome oxidase, malate dehydrogenase, and isocitrate dehydrogenase, the mitochondrial markers. Glyoxylate-glutamate aminotransferase and glycolate dehydrogenase showed a similar distribution pattern to that of NAD-dependent hydroxypyruvate reductase. Thus in Chlorogonium the enzymes of the glycolate pathway are not associated with the microbodies that are recovered at density 1.225 g/cm3.
The single large chloroplasts of the Chlorogonium cells are broken during grinding, and this probably accounts for the finding that NADP-glyoxylate reductase was recovered only in the soluble fractions of the gradient.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca. Arch Mikrobiol. 1971;80(3):205–218. [PubMed] [Google Scholar]
- Graves L. B., Jr, Trelease R. N., Grill A., Becker W. M. Localization of glyoxylate cycle enzymes in glyoxysomes in Euglena. J Protozool. 1972 Aug;19(3):527–532. doi: 10.1111/j.1550-7408.1972.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Graves L. B., Trelease R. N., Becker W. M. Particulate nature of glycolate dehydrogenase in euglena: possible localization in microbodies. Biochem Biophys Res Commun. 1971 Jul 16;44(2):280–286. doi: 10.1016/0006-291x(71)90596-1. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Zelitch I., Day P. R. Glycolate oxidase activity in algae. Plant Physiol. 1968 Feb;43(2):289–291. doi: 10.1104/pp.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]



