Abstract
Previous studies demonstrated effectiveness of selective sigma-receptor (σR) agonists (DTG, PRE-084) as reinforcers in rats trained to self-administer cocaine. Like cocaine, these drugs increased nucleus accumbens shell dopamine levels, and effects of DTG, but not PRE-084, on dopamine appeared to be mediated by σRs. Additionally, σR antagonists blocked self-administration of σR agonists, but were inactive against reinforcing and neurochemical effects of cocaine. Thus pharmacologically distinct mechanisms likely underlie the reinforcing and neurochemical effects of σR agonists and cocaine. The present study further examined the cocaine-like effects of σR agonists in rats trained to discriminate injections of cocaine from saline to assess the similarity of their subjective effects. Standard dopamine-uptake inhibitors (WIN 35,428, methylphenidate), but neither σR agonist (PRE-084, DTG) produced full cocaine-like discriminative-stimulus effects. The lack of effects of σR agonists was obtained regardless of route of administration (i.p., s.c. or i.v.) or pretreatment time (5- or 30-min before sessions). The present results demonstrate differences in the discriminative-stimulus effects of cocaine and selective σR agonists, indicating that an overlap of subjective effects is not necessary for σR agonist self-administration. The previously found differences in neurochemical effects of cocaine and σR agonists may contribute to their different subjective effects.
Keywords: cocaine; discriminative-stimulus effects; σ receptor; dopamine uptake inhibitor; DTG; PRE-084; WIN 35,428; methylphenidate
Introduction
Previous studies have suggested a role for the σ receptor (σR) in several of the behavioral effects of cocaine. For example, an early study showed that the σR antagonists, BMY 14802 and rimcazole, blocked the locomotor stimulant effects of cocaine at doses that were inactive when administered alone (Menkel et al., 1991). This antagonist action was more selective than that obtained with dopamine receptor antagonists, which only blocked the locomotor-stimulant effects of cocaine at doses that also decreased activity when administered alone (see also Chausmer and Katz, 2001). Other studies showed that more selective σR antagonists, such as 1-[2-(3,4-dichlorophenyl)-ethyl]-4-methylpiperazine (BD 1063), also blocked cocaine-induced locomotor stimulant effects, as well as convulsions and lethality in mice (Matsumoto et al., 2001). In further studies, several σR antagonists attenuated the development of cocaine-induced locomotor sensitization (Ujike et al., 1992; Witkin et al., 1993). These studies prompted others described below on the role of σRs in various behavioral effects related to the abuse liability of stimulant drugs.
Several studies have indicated that, despite the above cited antagonism of cocaine by several σR antagonists, σR agonists were devoid of cocaine-like behavioral effects. For example, the σR agonist (+)-pentazocine did not affect locomotor activity in naïve mice (Okuyama et al., 1996). Other σR agonists, 1,3-di-o-tolylguanidine (DTG) and 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride (SA 4503), only decreased locomotor activity in rats across the range of behaviorally active doses (Maj et al., 1996; Rodvelt et al., 2011a; Rodvelt et al., 2011b) . On the other hand, the σR agonist DTG potentiated cocaine induced locomotor stimulant effects in rats (Skuza, 1999). In rats trained to discriminate injections of 10 mg/kg cocaine from saline in a shock avoidance procedure, DTG (1 and 10 mg/kg, 30 minutes before sessions) failed to produce effects different from vehicle (Ukai et al., 1997). Further, a recent study on substitution of the σR agonist, SA 4503, for cocaine (5 mg/kg) demonstrated that SA 4503 produced at most partial, but not full, substitution in rats trained with food to discriminate injections of cocaine from saline under a fixed-ratio ten-response schedule of reinforcement (Rodvelt et al., 2011a).
Consistent with the lack of stimulant-like effects of σR agonists, (+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethylbut-3-en-1-ylamine hydrochloride (igmesine) and 2-(4-morpholinethyl) 1-phenylcyclohexanecarboxylate hydrochloride (PRE-084), both selective σR agonists, failed to produce place conditioning under conditions in which cocaine was effective (Romieu et al., 2002). In contrast, σR antagonists given in combination with cocaine can block the development of a place preference (Romieu et al., 2000). The place conditioning studies together with those focused on locomotor activity with σR agonists and antagonists suggest that stimulation of σRs is necessary but insufficient for these two effects of cocaine.
In other studies, the σR agonist, igmesine, reinstated a previously extinguished cocaine place-conditioned response in mice (Romieu et al., 2004). In addition, a recent study from our laboratory indicated the σR agonists, DTG and PRE-084, were reinforcing in rats with a history of cocaine self administration. Further, both σR agonists increased dopamine concentrations in the shell of the nucleus accumbens and those effects of DTG, but not PRE-084 were antagonized by the σR antagonist, BD 1008 (Garcés-Ramírez et al., 2011). In contrast, σR antagonists had no effect on cocaine self-administration (Hiranita et al., 2010; see also Martin-Fardon et al., 2007) nor on cocaine-induced increases in nucleus accumbens dopamine (Garcés-Ramírez et al., 2011). In addition, pretreatment with i.p. injections of PRE-084 or DTG before sessions produced leftward shifts in the cocaine self-administration dose-effect curve, suggesting an additive effect or possibly a potentiation of the reinforcing effects of cocaine (Hiranita et al., 2010).
The present studies were prompted by the findings by Romieu et al., 2004, and Hiranita et al., 2010, and re-examined the potential of σR agonists to produce cocaine-like discriminative-stimulus effects. A substantial overlap with cocaine in the discriminative (subjective) effects of these drugs would hypothetically explain the σR agonist-induced reinstatement of place conditioning or the substitution of σR agonists in subjects trained to self-administer cocaine. Thus, the present study examined the substitution of the σR agonists, PRE-084 or DTG, for cocaine in rats trained with food to discriminate injections of cocaine from saline under a fixed-ratio twenty-response schedule of reinforcement. We compared cocaine-like discriminative-stimulus effects of the σR agonists with those of standard dopamine uptake inhibitors, WIN 35,428 and methylphenidate. In the previous study, both σR agonists potentiated the reinforcing effects of cocaine, when pretreated i.p. 30 minutes before sessions (Hiranita et al., 2010). In addition, i.v. injections of DTG maintained responding, when substituted for those of cocaine, On the other hand, s.c. injections of DTG 30 minutes before sessions have been reported to produce their own discriminative-stimulus effects in naïve rats (Holtzman, 1989). Therefore, both agonists were injected either i.p., s.c. or i.v‥
Methods
Subjects
Male Sprague-Dawley rats (weighing approximately 300 g at the start of the study), obtained from Charles River Laboratories (Wilmington, MA) served as subjects after acclimation to the laboratory for at least one week. Food (Scored Bacon Lover Treats, BIOSERV, Frenchtown, NJ) and tap water were available in their home cages. After acclimation, weights of rats were maintained at approximately 320 g by adjusting their daily food ration. The animal housing room was temperature- and humidity-controlled and maintained on a 12:12-h light:dark cycle with lights on at 07:00 h. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by AAALAC International.
Apparatus
Experimental sessions were conducted with rats placed in operant-conditioning chambers (modified ENV-203, Med Associates, St. Albans, VT) having inside measurements of 23.0 cm L ×29.0 cm W×21.0 cm H. These chambers were enclosed within sound-attenuating cubicles equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two response levers, 5.0 cm from the midline and 4.0 cm above the grid floor. A downward displacement of a lever with a force approximating 20 g defined a response, which always activated a relay mounted behind the front wall of the chamber producing an audible “feedback” click. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of food pellets was mounted behind a 5.0×5.0 cm opening in the front wall midline between the two levers and 2.0 cm above the floor.
Procedures
Rats were initially trained with food reinforcement to press both levers, and were eventually trained to press one after cocaine (10 mg/kg, i.p.), and the other after saline (i.p.) injection. Each response produced an audible click. The ratio of responses to food pellets (fixed ratio or FR) was gradually increased until, under the final conditions, the completion of 20 consecutive responses on the cocaine- or saline-appropriate lever produced a food pellet delivery. Incorrect responses reset the FR response requirement. The right- vs. left-assignment of cocaine- and saline-associated levers was counterbalanced among subjects.
Subjects were placed in chambers immediately after injection. There was a five min timeout period during which lights were off and responses produced an audible click, but had no other scheduled consequences. Following the timeout, the house light was turned on until the completion of the FR 20 response requirement. Food presentation was followed by a 20-sec timeout during which all lights were off and responses had no scheduled consequences other than the feedback click. Sessions ended after 20 food presentations or 15 min, whichever occurred first. Cocaine or saline training sessions were scheduled in a double-alternation sequence (i.e., cocaine-saline-saline-cocaine). Training continued until subjects met the criteria on four consecutive sessions of ≥ 85% cocaine- or saline-appropriate responding over the entire session, as well as during the first FR of the session.
Once these criteria were met, testing began. Test sessions were conducted with the administration of different doses of cocaine, standard dopamine uptake inhibitors (WIN 35,428 and methylphenidate) or selective σR agonists (PRE-084 and DTG) before sessions. Test sessions were identical to training sessions with the exception that completion of the FR requirement on either lever was reinforced. All data collection and programming of behavioral contingencies was accomplished with software from Med Associates, Inc (St. Albans, VT).
For habituation to i.v. injections, subjects were placed daily before sessions for several minutes into a flat bottom restrainer (model FB-LG, Braintree Scientific, Inc., MA) which measured 8.57 cm diameters×21.59 cm. Subjects were intravenously injected with saline (1.0 ml/kg) inside the restrainer, removed from the restraint, and then given an i.p. injection of saline or cocaine (10 mg/kg) until the above training criteria were met. Subjects were tested as described above with i.v. injection of different doses of cocaine or DTG in a volume of 1.0 mg/ml administered in the restrainer, followed by an i.p. injection of saline (1.0 ml/kg) 5 min before sessions.
Drugs
The following drugs were used: (-)-cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO, Molecular Weight: 339.8), WIN 35,428 ((−)-3β-(4-Fluorophenyl)tropan-2β-carboxylic acid methyl ester tartrate, NIDA, Drug Supply Program, Molecular Weight: 443.9), methylphenidate hydrochloride (NIDA, Molecular Weight: 269.8), PRE-084 hydrochloride (Tocris, Ballwin, MO, Molecular Weight: 353.9), and DTG (Sigma-Aldrich, Molecular Weight: 239.3). Drug pretreatments were administered by the i.p., s.c. or i.v. routes. Cocaine, WIN 35,428, and methylphenidate were administered i.p. five min before sessions. In addition, PRE-084 and DTG were administered i.p. or i.v. at five or 30 min before sessions or s.c. at 30 min before sessions. All drug solutions were prepared fresh daily in 0.9% NaCl, with the exception of DTG (initially dissolved in 1 N HCl and neutralized with 1 N NaOH). Pretreatment times and doses of drugs used in the present study were chosen based on published (Hiranita et al., 2010; Garcés-Ramírez et al., 2011) or preliminary data obtained in this laboratory.
Data analysis
The percentage of cocaine lever responding was calculated by dividing the total number of responses on the cocaine-associated lever by the total number of responses. Rate of responding was calculated by dividing the total number of responses by the session time (excluding timeout periods) and is expressed as percentage of saline (control) rate of responding. These data are shown as mean values (±SEM) for groups of subjects at each drug dose. Response rate exclusion criteria were applied to the % cocaine lever selection data such that any dose at which more than half of the sample size did not respond was considered a potentially unreliable indication of lever selection and was removed from the analysis. Dose-effect curves for percent cocaine lever responding were analyzed using standard linear regression techniques, from which ED50 values with 95% confidence limits (Snedecor and Cochran, 1967) were calculated. Only points on the linear part of the ascending portions of the dose-effect curves were used. For compounds that did not fully substitute for cocaine standard analysis of variance was conducted to determine if drug substitution differed from saline vehicle controls. Comparisons were considered significant when p<0.05.
Results
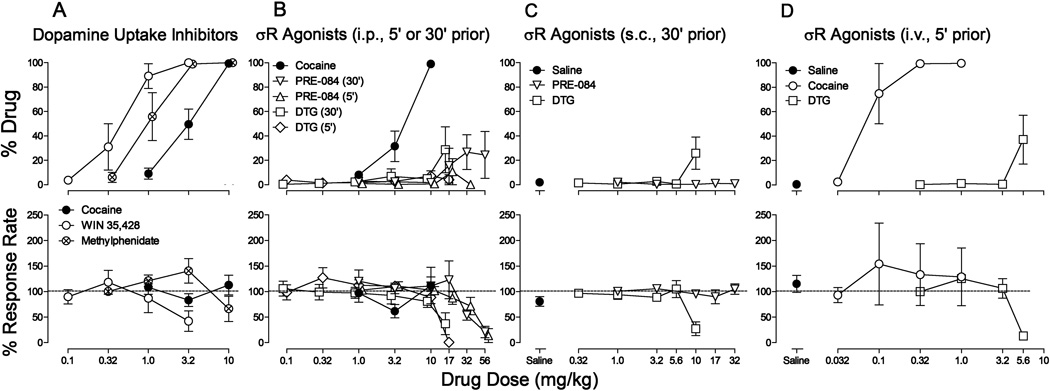
Cocaine produced a dose-dependent increase in the percentage of responses on the cocaine-appropriate lever (Fig. 1A and B, top panels, filled circles), with an ED50 value of 9.62 µmol/kg (Table 1). Similar dose-dependent effects of the dopamine uptake inhibitors, WIN 35,428 and methylphenidate, were obtained (Fig. 1A, top panel, open circles and circles with cross hatch, respectively). WIN 35,428 was approximately 10-fold and methylphenidate 3-fold more potent than cocaine on a molar basis (Table 1). Cocaine did not affect response rates across the range of doses tested whereas both WIN 35,428 and methylphenidate decreased rates of responding at the highest doses tested (Fig. 1A, bottom panel).
Figure 1.
Substitution of the standard dopamine uptake inhibitors (WIN 35,428 and methylphenidate), and the standard σR agonists (PRE-084 and DTG) in rats trained to discriminate injections of cocaine from saline. Horizontal axes, drug dose in mg/kg (log scale). Vertical axes: upper panels, percentage of responses on the cocaine-appropriate lever; lower panels, percentage of response rate after saline administration. Each point represents the mean ± SEM (N=5–18). The percentage of responses emitted on the cocaine-appropriate lever was considered unreliable, and not plotted, if less than half of the subjects responded at that dose. Panel A: Cocaine (filled circles), WIN 35,428 (open circles), and methylphenidate (circles with cross hatch). All three drugs were administered i.p. at 5 min before sessions. Panel B: Cocaine (filled circles), PRE-084 (open triangles down and up), and DTG (open squares and diamonds). Cocaine was administered i.p. at 5 min before sessions. PRE-084 and DTG were administered i.p. at either 5 or 30 min before sessions. Panel C: Saline (filled circles), PRE-084 (open triangles down), and DTG (open squares). All test compounds were administered s.c. at 30 min before sessions. Panel D: Saline (filled circles), cocaine (open circles), and DTG (open squares). All test compounds were administered i.v. at 5 min before sessions. Note that the dopamine uptake inhibitors dose-dependently produced cocaine-like responding and fully substituted for cocaine and that neither σR agonist produced cocaine-like responding that different from vehicle.
TABLE 1.
Comparisons of potencies and maximal effects of the dopamine uptake inhibitors and σR agonists in substituting for cocaine and affecting rates of responding
| Drugs | % Cocaine Response | Response Rates | |
|---|---|---|---|
| ED50 Value (µmol/kg) and 95% CLs |
Maximum Effect (% Cocaine Responding) |
ED50 Value (µmol/kg) and 95% CLs |
|
| Cocaine (i.p., 5’ prior) | 9.62 (7.96–11.7) |
99.4 ± 0.220 @ 10 mg/kg |
Nonsignificant linear regression |
| WIN 35,428 (i.p., 5’ prior) | 1.04 (0.678–1.55) |
100 ± 0.00 @ 3.2 mg/kg |
6.05 (2.51–4.26×106) |
| Methylphenidate (i.p., 5’ prior) |
3.42 (2.33–4.89) |
100 ± 0.00 @ 10 mg/kg |
Nonsignificant linear regression |
| PRE-084 (i.p., 30’ prior) | No substitution | 26.7 ± 14.3 @ 32 mg/kg |
106 (72.1–242) |
| DTG (i.p., 30’ prior) | No substitution | 28.6 ± 18.8 @ 17 mg/kg |
60.6 (48.1–108) |
| PRE-084 (i.p., 5’ prior) | No substitution | 10.7 ± 10.7 @ 17 mg/kg |
100 (78.8–136) |
| DTG (i.p., 5’ prior) | No substitution | 7.27 ± 4.11 @ 10 mg/kg |
52.6 (47.2–58.1) |
| PRE-084 (s.c., 30’ prior) | No substitution | 2.02 ± 1.84 @ 1.0 mg/kg |
Nonsignificant linear regression |
| DTG (s.c., 30’ prior) | No substitution | 25.8 ± 13.2 @ 10 mg/kg |
35.3 (27.0–76.9) |
| Cocaine (i.v., 5’ prior) | 0.241 (0.126–0.400) |
99.6 ± 0.205 @ 1.0 mg/kg |
Nonsignificant linear regression |
| DTG (i.v., 5’ prior) | No substitution | 37.1 ± 20.0 @ 5.6 mg/kg |
18.7 (15.0–25.7) |
In contrast to the dopamine uptake inhibitors, neither of the σR agonists, PRE-084 nor DTG, substituted for cocaine when administered i.p. at either 5 or 30 min before testing (Fig. 1B, top panel, open symbols). The % cocaine-appropriate responding was not statistically different from that obtained with vehicle (all F values < 1.43; NS). Both PRE-084 and DTG were studied at doses from those that had no effects on the rates of responding to those that decreased response rates to less than 50% of control response rates (Fig. 1B, bottom panel, open symbols). When tested 30 min after s.c. administration (Fig. 1C), the overall ANOVA for DTG was significant (F4,20 = 3.78; p = < 0.02), though post-hoc comparisons indicated that effects at no dose were significantly different from vehicle. The effects of PRE-084 tested 30 min after s.c. administration were not significant (F5,20 = 1.05; NS). The highest dose of DTG decreased response rates to less than 50% of control, whereas PRE-084 administered s.c. had no effects on response rates up to 32 mg/kg (Fig. 1C, lower panel).
Intravenous injections of cocaine produced a dose-dependent increase in the percentage of responses on the cocaine-appropriate lever (Fig. 1D, top panels, open circles), with an ED50 value of 0.241 µmol/kg (Table 1). The potency of i.v. cocaine was approximately forty-fold greater than that for the i.p. route. Across the range of doses of cocaine tested cocaine did not significantly affect response rates (Fig. 1D, bottom panels, open circles, F4,12=0.41; NS). In contrast, DTG did not substitute for cocaine when administered i.v. at 5 min before testing (Fig. 1D, top panel, open squares). The % cocaine-appropriate responding was statistically different from that obtained with vehicle (F4,12=3.31; p < 0.05). However, the subsequent post-hoc Bonferroni t-test indicated no significant differences in DTG dose (all t values ≤ 2.90; all p values ≥ 0.053). The highest dose of DTG decreased response rates to less than 50% of control rates (Fig. 1D, lower panel, open squares).
Discussion
The present study was initiated following studies showing that σR agonists were reinforcing in rats trained to self-administer cocaine (Hiranita et al., 2010) suggesting that the drugs might possess cocaine-like subjective effects. In contrast to the self-administration results, neither of the σR agonists tested, PRE-084 or DTG, produced cocaine-like discriminative effects different from vehicle across the range of behaviorally active doses in rats trained to discriminate cocaine (10 mg/kg) from saline injections. As expected based on previous reports (e.g., Colpaert et al., 1979; Witkin et al., 1991; Li et al., 2006), the standard dopamine uptake inhibitors, WIN 35,428 and methylphenidate, both fully substituted for cocaine.
While the previous self-administration results suggested that σR agonists would produce cocaine-like discriminative effects, other studies have suggested otherwise. For example, in rats trained to discriminate injections of cocaine (10 mg/kg) from saline in a shock avoidance procedure (Ukai et al., 1997) s.c. injections of DTG (1 and 10 mg/kg, 30 minutes before sessions) were no different from vehicle (see also Cohen and Sanger, 1994). Additionally, two recent studies on substitution of another σR agonist, SA 4503, in rats trained with food reinforcement to discriminate either cocaine (5.0 mg/kg; Rodvelt et al., 2011a) or methamphetamine (0.5 mg/kg; Rodvelt et al., 2011b) from saline also indicated a failure of that σR agonist to fully reproduce the discriminative-stimulus effects of those psychomotor stimulant drugs.
The absence of cocaine-like discriminative-stimulus effects of both PRE-084 and DTG was also obtained in the present study with different pretreatment times (5 or 30 minutes before sessions). In addition, because the self administration of σR agonists was obtained with i.v. injections different routes of administration (i.p., s.c., or i.v.) were studied. The absence of cocaine-like discriminative-stimulus effects at different pretreatment times and different routes of administration further substantiated the distinctiveness of the discriminative-stimulus or subjective effects of σR agonists and those of psychomotor stimulants. Consistent with this suggestion are findings that rats trained to discriminate selective σR agonists (Steinfels et al., 1988; Holtzman, 1989) did not show substitution with cocaine or other psychostimulants. Other studies using drug discrimination procedures have demonstrated that the σR agonist, (+)-pentazocine, fully substituted in rats trained to discriminate drugs other than stimulants, ethanol (Hundt et al., 1998) or buprenorphine (Holtzman, 1997). Together these previous results are consistent with the suggestion of little, if any, overlap of cocaine-like and σR-agonist discriminative-stimulus effects. Thus, the reinforcing effectiveness of the σR agonists in rats with a history of cocaine self-administration in the previous study (Hiranita et al., 2010) probably did not result from subjective effects of σR agonists that were similar to those of cocaine.
The standard dopamine uptake inhibitors, WIN 35,428 and methylphenidate, both fully reproduced cocaine-like discriminative-stimulus effects, as has been shown previously (Colpaert et al., 1979; Witkin et al., 1991; Li et al., 2006). Further, indirect agonists that release dopamine, such as d-amphetamine and methamphetamine, have been reported to produce full cocaine-like discriminative-stimulus effects and dopamine receptor antagonists block the discriminative-stimulus effects of cocaine (Barrett and Appel, 1989; Witkin et al., 1991; Terry et al., 1994). A previous study (Garcés-Ramírez et al., 2011) using in vivo microdialysis showed that both PRE-084 and DTG increase concentrations of dopamine in the nucleus accumbens shell, a brain region important for the actions of abused drugs (Pontieri et al., 1995; Tanda et al., 1997). However, the dose of PRE-084 that increased dopamine levels was approximately thirty-fold higher than the dose that maintained responding in rats trained to self-administer cocaine (Hiranita et al., 2010). Importantly, none of the selective σR antagonists BD 1008 or BD 1063 antagonized the neurochemical effects of PRE-084 (Garcés-Ramírez et al., 2011) indicating that those effects were not σR mediated. In contrast, the reinforcing effects of PRE-084 in self-administration studies were sensitive to the σR antagonists (Hiranita et al., 2010). Thus it appears that there is a separation in the pharmacological mechanisms underlying the reinforcing effects of cocaine and σR agonists, and it is likely that the separation of pharmacological mechanisms contributes to the differences in discriminative-stimulus effects.
In summary, the present study demonstrated dissimilarities in the discriminative-stimulus effects of cocaine and selective σR agonists in rats. Those differences suggest that the reinforcing effects of the σR agonists (Hiranita et al., 2010) are not based on substantial overlap of the subjective effects of the two classes of drugs. Previous studies suggest a distinction between the neurochemical effects of cocaine and σR agonists that may contribute to the differences in subjective effects of the two classes of drugs.
Acknowledgments
We thank Patty Ballerstadt for administrative assistance and Bettye Campbell for a technical assistance.
The work reported herein was supported by the Intramural Research Program of the National Institute on Drug Abuse. The animal housing facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and all procedures were conducted in accordance with the guidelines of the Institutional Care and Use Committee of the NIDA Intramural Research Program and the 1996 National Research Council and Guide for Care and Use of Laboratory Animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett RL, Appel JB. Effects of stimulation and blockade of dopamine receptor subtypes on the discriminative stimulus properties of cocaine. Psychopharmacology (Berl) 1989;99:13–16. doi: 10.1007/BF00634445. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J Exp Anal Behav. 1994;61:213–221. doi: 10.1901/jeab.1994.61-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, De La Garza R, 2nd, Cunningham KA. Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav. 1997;57:601–607. doi: 10.1016/s0091-3057(96)00434-0. [DOI] [PubMed] [Google Scholar]
- Cohen C, Sanger DJ. Effects of sigma ligands on the cocaine discriminative stimulus in rats. In: Harris LS, editor. Problems of Drug Dependence 1994. NIDA Research Monograph 153. Rockville, Maryland: U.S. Department of Health and Human Services; 1995. p. 380. [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. Discriminative stimulus properties of cocaine: neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav. 1979;10:535–546. doi: 10.1016/0091-3057(79)90229-6. [DOI] [PubMed] [Google Scholar]
- Garcés-Ramírez L, Green J, Hiranita T, Kopajtic T, Mereu M, Thomas A, Mesangeau C, Narayanan S, McCurdy C, Katz J, Tanda G. Sigma receptor agonists: Receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis in rats. Biol Psychiatry. 2011;69:208–217. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman SG. Opioid- and phencyclidine-like discriminative effects of ditolylguanidine, a selective sigma ligand. J Pharmacol Exp Ther. 1989;248:1054–1062. [PubMed] [Google Scholar]
- Holtzman SG. Discriminative stimulus effects of buprenorphine in the rat. Psychopharmacology (Berl) 1997;130:292–299. doi: 10.1007/s002130050242. [DOI] [PubMed] [Google Scholar]
- Hundt W, Danysz W, Holter SM, Spanagel R. Ethanol and N-methyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998;135:44–51. doi: 10.1007/s002130050484. [DOI] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Maj J, Rogoz Z, Skuza G. Some behavioral effects of 1,3-di-o-tolylguanidine, opipramol and sertraline, the sigma site ligands. Pol J Pharmacol. 1996;48:379–395. [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, Katz JL, Witkin JM. Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Imagawa Y, Tomisawa K. Behavioral evidence for modulation by sigma ligands of (+)MK-801-induced hyperlocomotion in monoamine-depleted mice. Neuropharmacology. 1996;35:467–474. doi: 10.1016/0028-3908(95)00193-x. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Rodvelt KR, Lever SZ, Lever JR, Blount LR, Fan KH, Miller DK. SA 4503 attenuates cocaine-induced hyperactivity and enhances methamphetamine substitution for a cocaine discriminative stimulus. Pharmacol Biochem Behav. 2011a;97:676–682. doi: 10.1016/j.pbb.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodvelt KR, Oelrichs CE, Blount LR, Fan KH, Lever SZ, Lever JR, Miller DK. The sigma receptor agonist SA4503 both attenuates and enhances the effects of methamphetamine. Drug Alcohol Depend. 2011b doi: 10.1016/j.drugalcdep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. The sigma1 (sigma1) receptor activation is a key step for the reactivation of cocaine conditioned place preference by drug priming. Psychopharmacology (Berl) 2004;175:154–162. doi: 10.1007/s00213-004-1814-x. [DOI] [PubMed] [Google Scholar]
- Skuza G. Effect of sigma ligands on the cocaine-induced convulsions in mice. Pol J Pharmacol. 1999;51:477–483. [PubMed] [Google Scholar]
- Snedecor G, Cochran W, editors. Statistical Methods. 6th ed. Ames, IA: Iowa State College; 1967. [Google Scholar]
- Steinfels GF, Alberici GP, Tam SW, Cook L. Biochemical, behavioral, and electrophysiologic actions of the selective sigma receptor ligand (+)-pentazocine. Neuropsychopharmacology. 1988;1:321–327. [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. Sigma (sigma) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci. 1992;50:PL129–PL134. doi: 10.1016/0024-3205(92)90466-3. [DOI] [PubMed] [Google Scholar]
- Ukai M, Mori E, Kameyama T. Modulatory effects of morphine, U-50488H and 1,3- di-(2-tolyl)guanidine on cocaine-like discriminative stimulus in the rat using two-choice discretetrial avoidance paradigm. Methods Find Exp Clin Pharmacol. 1997;19:541–546. [PubMed] [Google Scholar]
- Witkin JM, Nichols DE, Terry P, Katz JL. Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther. 1991;257:706–713. [PubMed] [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–482. [PubMed] [Google Scholar]