Abstract
The toxicity of environmental chemicals such as nitrates, thiocynates, and perchlorates, some therapeutics, and dietary goitrogens can lower thyroidal iodine uptake and result in hypothyroidism and goiter. Iodine sufficiency, essential for normal thyroid hormone synthesis, is critical during gestation to assure that sufficient thyroxine (T4) and iodine reach the developing fetus. Spot urinary iodide (UI) measurements are used globally to indicate and monitor iodine sufficiency of populations. In individuals, however, UI are not routinely measured; instead, normal serum thyroid-stimulating hormone (TSH) and T4 concentrations serve as surrogate indicators of iodine sufficiency as well as thyroidal health. Our objective was to examine the relationship between UI concentrations and serum T4 and TSH concentrations in individuals in an ‘‘iodine-sufficient population.’’ Using a cross-sectional sample of the US population (n = 7628) from the National Health and Nutrition Examination Survey (NHANES III; 1988–1994) database, we examined the relationship among UI, T4, and TSH in pregnant and nonpregnant women and in men (15–44 years). There was a lack of relationship between UI (or UI/Cr) concentrations and serum T4 or TSH concentrations. Therefore, TSH and T4 are not appropriate markers of UI concentrations in this population. Monitoring the status of iodine nutrition of individuals in the United States may be important because serum TSH and T4 concentrations do not indicate low iodine status.
Keywords: urinary iodine measurements, maternal thyroxine T4 monitoring, TSH, pregnancy, iodine deficiency, prevention of neurological damage, NHANES
Iodine deficiency is the world’s leading cause of preventable mental impairments.1 In accordance with WHO/UNICEF/ICCIDD recommendations,2 UI concentration is the major indicator of iodine nutrition. Deficiency is defined as either severe (median UI ,< 20 µg/L), moderate (20–49 µg/L), or mild (50– 99 µg/L); sufficiency is 100 µg/L or higher. Generally, UI concentrations correlate with goiter surveys. It is thought that all degrees of iodine deficiency affect maternal and neonatal thyroid function as well as the mental development of the child—the damage increases with the extent of the deficiency.1 Severe iodine deficiency during gestation can result in cretinism, hearing loss, and severe fetal neurologic damage.3 To provide optimal guidelines, the Daily Reference Intakes (DRI) established for iodine by the Food and Nutrition Board of the Institute of Medicine are age and gender specific.4 The optimal level of iodine intake to prevent thyroid disease may be a relatively narrow range around the recommended daily iodine intake.5
Iodine plays a central role in thyroid physiology, being both a major constituent of thyroid hormones and a regulator of thyroid gland function. Goitrogens are substances that can decrease iodine availability or interfere with its tissue utilization. This can lead to inadequate thyroid hormone production, resulting in thyroid enlargement (goiter) and hypothyroidism. Two general categories of foods that have been associated with disrupted thyroid hormone production in humans: soybeanrelated foods containing isoflavons and cruciferous vegetables containing isothiocyanates. Environmental toxins such as nitrates, thiocynates, and perchlorates may affect the amount of iodine availability for thyroid hormone synthesis. Among the drugs that interfere with iodine uptake and hormone production are lithium carbonate, methimazole (MMI), propyl-thiouracil (PTU), β blockers such as propranolol (Inderal), metoprolol (Lopressor), phenylbutazone, calcium, and fluorides in the water supply. These may be accentuated by low dietary iodine intake.
Iodine intake and excretion are in a steady state with renal excretion, approximating the amount of iodine ingested and absorbed. Therefore, UI concentration is the prime indicator of nutritional iodine status. Monitoring of UI excretion is useful in determining the status of iodine nutrition of a population.2,6 Daily iodine intake can be estimated by measuring 24-hour iodine excretion or by random spot urine sampling calculated either in relation to urinary creatinine excretion or as UI concentration per liter. Spot UI measurements can identify excess as well as deficiency in iodine intake and are acceptable for population health purposes.1 But, the spot urine test is a less reliable indicator of iodine status in individuals (compared to populations) because it reflects recent iodine intake.7 The UI to creatinine ratio (UI/Cr) corrects for urine dilution. The UI/Cr in random single voided urine specimens is considered a reliable method to quantify iodide in individuals,8 more reliable than random spot UI measurement because of day-to-day variability in iodine intake, variability in water consumption for any individual, and in the amount of time it takes for iodine exposure to equilibrate. Optimally, for the most reliable assessment of iodine status of an individual, more than one 24-hour UI specimen should be collected. During pregnancy creatinine concentrations are lower, UI excretion is higher (because of the higher glomerular filtration rate), there is higher demand for iodine by the fetus, and pregnancy-specific thyroid hormone changes occur.9
Iodine deficiency can result in lower than normal serum T4 (hypothyroxinemia) or in hypothyroidism reflected in higher than normal serum TSH concentrations (with or without subnormal T4). When iodine supplies are low, however, the thyroid is capable of up-regulation, which results in a free thyroxine (FT4) surge. During pregnancy low iodine supply and a first-trimester FT4 surge may not result in increased maternal TSH but may be crucial for the prevention of learning disabilities in a significant number of unborn children.10 It is therefore important to maintain sufficient iodine intake as well as normal levels of T4.
To test the hypothesis that low UI would be associated with lower serum T4 and higher TSH concentrations and that within-range TSH concentrations are predictive of ‘‘normal’’ UI, we sought to reconcile the population and individual approaches to assessing iodine sufficiency.
METHODS
Using the NHANES III database, we analyzed the relationship between iodine status reflected in UI concentrations or UI/Cr (µg/g) and the standard biologic markers serum TSH and total T4. Due to the sensitivity of the developing fetal brain to sufficient thyroid hormone and iodine concentrations, of particular interest were the relationships between low UI excretion and TSH as well as T4 concentrations during pregnancy.
The National Health and Nutrition Examination Survey (NHANES) is a stratified, multistage probability study designed to give national normative estimates of the health and nutritional status of the US civilian, noninstitutionalized population. NHANES III was conducted from 1988 through 1994.11 The ongoing cross-sectional survey represents, but does not include, all 50 states and the District of Columbia. Biologic samples were collected from participants for a large number of biochemical indicators of health status. Based on iodine excretion data measured in NHANES III it was determined that the US population is iodine sufficient according to WHO criteria.12 The 2.5th to 97.5th percentiles of UI concentrations for women of childbearing age (14 to 44 years) were 1.8–65 µg/dL (36–539 µg/g creatinine).13 According to the same NHANES III database, 6.9% of pregnant women surveyed were moderately to severely iodine deficient (UI levels < 5 µg/dL).12
The NHANES III files (EXAMINATION, LAB, and LAB2) were downloaded from the CDC web site. Information on gender, age at examination, pregnancy status, urinary iodine (UI), creatinine (Cr), thyroxine (T4), thyroid-stimulating hormone (TSH), anti–thyroid peroxidase antibody (TPOAb) and antithyroglobulin antibody (TgAb) were extracted from these files and merged into a single analytic file on the basis of subject ID number.
T4 was measured using an immunoassay for T4 (Roche Molecular Biochemicals, Indianapolis, IN) that had a ‘‘normal’’ reference range of 57.9 nmol/L to 169.9 nmol/L (4.5 µg/dL to 13.2 µg/dL).
TSH was measured with a chemiluminescence immunometric assay (Nichols Institute Diagnostics, San JuanCapistrano, CA).14 The working range for this method is 0.01 mIU/L to 50 mIU/L. The manufacturer’s reference interval for the test was 0.39–4.6 mIU/L.
Thyroglobulin antibodies (TgAb) and thyroid peroxidase Ab (TPOAb) were measured by a highly sensitive direct RIA system (Kronus, San Clemente, CA).15,16 The normal range for TPOAb in humans is <0.5 IU/mL, and that for TgAb in humans is <1.0 IU/mL. NHANES III subjects who tested positive for antithyroid antibodies (anti-TPOAb and anti-TgAb) or who were not disease-free were removed from the analysis.
Urinary iodine was determined by the Iodine Research Laboratory, University of Massachusetts Medical Center (Worcester, MA). Spot urine samples were collected because collection of 24-hour urine samples was not feasible for survey purposes. Subjects were instructed to fast for 10–16 hours before the morning examination or for 6 hours before the afternoon or evening examination. Fasting urine samples are known to give a reasonable estimate of UI on a population basis. The duration of the fast was recorded. UI concentrations were determined using the Sandell-Koltoff reaction as modified by Benotti et al.17,18 UI LOD was 0.2 µg/dL. Iodine standards were prepared from analytic potassium iodate (KIO3), covering the range 0.0–0.3 µg/mL iodine, and were analyzed in duplicate with every 10 urine samples. Urine concentrations were calculated from the slope and y-intercept of the standard curve. Samples above the higher standard were diluted, and values below 0.1 µg/mL were repeated. A quality control sample was digested and analyzed every 10 urine samples. The coefficient of variance for UI determination ranged from 2.7% to 7.0%. For the conversion of units to equivalent SI units: 1.0 µg/dL = 0.07874 µmol/L; 1.0 pmol/L = 12.7 pg/dL. The criteria for iodine deficiency in a population has been established by the World Health Organization (WHO), which stated that the median UI concentration in a population should be >10 µg/dL, and <20% of the population should have UI concentrations of <5 µg/dL.6
Urinary Creatinine. Daily iodine intake is most closely estimated by the amount of iodine excreted in the urine in 24 hours.To compensate for the lack of 24-hour urine collection, creatinine has been used to adjust for urine dilution and for comparison with other databases. NHANES III creatinine was measured by the Jaffe´ alkaline picrate method. The LOD was 1 mg/dL. Creatinine concentration standards (50–300 mg/dL) were analyzed in duplicates with every 60 urine samples. Urinary creatinine concentrations were calculated from the slope and y-intercept of the standard curve. A quality control sample was analyzed with every 20 urine samples. Samples were repeated for values <10 and >300 mg/dL. The coefficient of variance for urinary creatinine determination ranged from 1.5% to 7.7%. According to WHO criteria, if the UI/Cr is used for iodine evaluation, the ratio should be >50 µg I/g Cr.2
Statistical Analysis
Statistical analyses were performed only on subjects 15–44 years (referred to as of ‘‘child-bearing age’’). Exclusion criteria included NHANES III participants who reported thyroid disease, goiter, or use of thyroid medications. Subjects who were positive for anti-TgAb or TPOAb (or both) were excluded. Missing SSN values and out-of-range UI (>150 µg/dL), T4 (>15 µg/dL), or TSH (>4.5 mIU/L) values were omitted from primary analyses because they were clearly outside the normal range. In the secondary analyses, however, the relationship between UI concentrations and TSH was further examined by including persons with TSH concentrations above 4.49 µg/dL but without antibodies, thyroid disease, or thyroid medications. Values are based on the nonparametric Mann-Whitney U test (for comparing TSH and T4 concentrations across 2 groups), Kruskal-Wallis test (for comparing TSH and T4 concentrations across 3 groups), and χ2 analyses (for comparing 2-way classification tables).
RESULTS
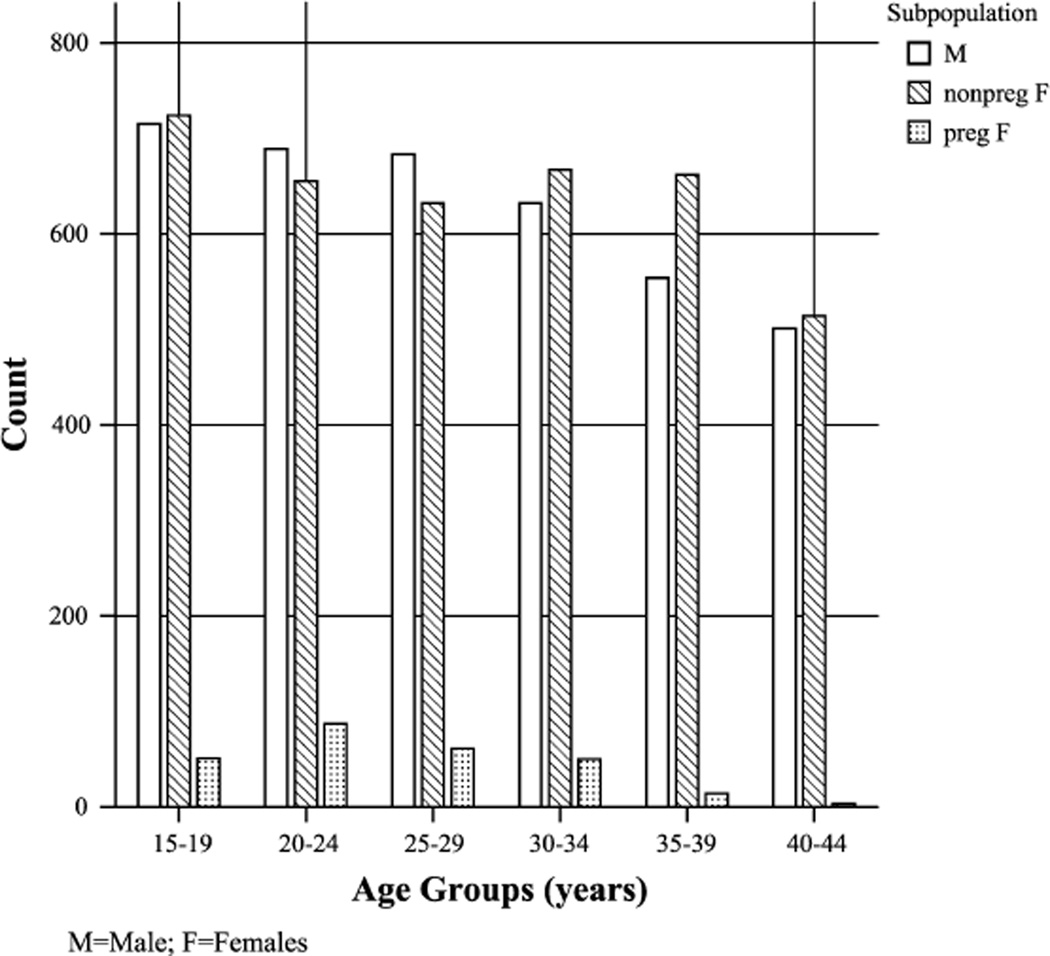
The distribution of the population by age is presented in Figure 1. For the population of childbearing age (15–44 years) and among those with normal-range TSH and T4 values, we compared UI concentrations, T4 and TSH levels across 3 groupings of the population: men and nonpregnant and pregnant women (Table 1).
FIGURE 1.
Distribution of ages (5-year increments) in sub-populations.
TABLE 1.
Comparison of UI, T4, and TSH Concentrations in NHANES III (1988–1994): Men vs. Nonpregnant Women (age 15–44 years)
| Variable | Male (n = 3774) |
Female (n=3854) |
P |
|---|---|---|---|
| Urinary iodine (µg/dL) | GM 15.80 ± 1.01 | GM 13.0 6 ±.01 | |
| Med 16.3 | Med 13.8 | 0.000 | |
| Range 1.01–146 | Range 0.5–140 | ||
| Thyroxine (T4 (µg/dL) | 8.50 ± 0.03 | 9.30 ± 0.03 | |
| Med 8.5 | Med 9.2 | 0.000 | |
| Range 0.4–14.7 | Range 0.4–14.9 | ||
| T4 (nmol/L) | 110.10 ± 0.40 | 119.2 ± 0.41 | |
| Med 109.4 | Med 117.1 | 0.000 | |
| Range 5.1–189.2 | Range 5.1–191.8 | ||
| TSH (µU/mL = mU/L) | GM 1.70 ± 0.04 | GM 1.50 ± 0.03 | |
| Med 1.3 | Med 1.3 | 0.000 | |
| Range 0.01–4.4 | Range 0.01–4.5 |
UI and TSH are presented as geometric means ± SE, and T4 as arithmetic mean ± SE.
UI concentrations are significantly lower (z = − 210.4, P < 0.001) in nonpregnant women of childbearing age (GM = 13.0 µg/dL) than in men in the same age group (GM = 15.8 µg/dL). T4 levels are significantly higher in nonpregnant women than in men (z = −15.7, P < 0.001).
TSH concentrations have differently shaped distributions in men and women. Women have the lower geometric mean (P < 0.001) but an almost identical median (z = −5.0, P < 0.001). The normal reference intervals for T4 in women of reproductive age are 4.5 to 13.2 µg/dL,19 and 5.8 to 14.4 µg/dL during pregnancy.9 The reference intervals for TSH in women of reproductive age are 0.39 to 4.6 mIU/L,19 and 0.24 to 3.0 mIU/L during pregnancy.9
UI and T4 medians and means were significantly higher in pregnant than in nonpregnant women (UI [z = 22.2, P < 0.05]; T4 [z = − 13.6, P < 0.001]) (Table 2), whereas TSH levels were significantly lower in pregnant than in nonpregnant women (z = − 2.7, P < 0.01). Because of these differences in UI, T4, and TSH values, we examined the relationships between UI and T4 and UI and TSH separately for each group. The relationships were analyzed by comparing the median ranks of the TSH and T4 levels across individuals within each group classified into 1 of 3 UI ranges:20 ‘‘moderate to severe iodine deficiency’’ (UI < 5 µg/dL), ‘‘mild deficiency to optimal’’ (UI 5–20 µg/dL), and ‘‘adequate and more than adequate’’ (UI > 20 µg/dL). Additionally, to adjust for dilution, these analyses were replicated using UI/Cr, rather than UI itself, to group individuals into the same 3 groups but with a fourth group, individuals with UI/Cr concentrations higher than 200.01 µg/g. The fourth group allowed us to examine creatinine-adjusted UI in the highest group. Because creatinine values were not available for all individuals, the number of participants is slightly different in the comparison tables (Tables 3–8).
TABLE 2.
Comparison of UI, T4, and TSH Concentrations in NHANES III (1988–1994): Pregnant vs Nonpregnant Women (age 15–44 years)
| Variable | Not Pregnant (n = 3854) |
Pregnant (n=266) |
P |
|---|---|---|---|
| Urinary iodine (µg/dL) | 13.0 ± 1.01 | 14.9 ± 1.04 | |
| Med 13.8 | Med 14.8 | 0.031 | |
| Range 0.5–146 | Range 0.9–94 | ||
| Thyroxine (T4) (µg/dL) | 9.3 ± 0.01 | 11.1 ± 1.2 | |
| Med 9.1 | Med 11.2 | 0.000 | |
| Range 0.4–14.9 | Range 3.5–14.9 | ||
| T4 (nmol/L) | 119.2 ± 0.40 | 143.3 ± 1.6 | |
| Med 117.1 | Med 144.1 | 0.000 | |
| Range 5.1–191.8 | Range 45–191.8 | ||
| TSH (µU/mL = mU/L) | 1.5 ± 0.03 | 1.1 ± 0.10 | |
| Med 1.2 | Med 1.1 | 0.007 | |
| Range 0.0–4.5 | Range 0.0–4.0 |
UI and TSH are presented as the geometric means ± SE, and T4 as arithmetic means ± SE.
TABLE 3.
Nonpregnant Women (age 15–44 years): Comparisons of T4 and TSH values Across UI Ranges
| Variable | UI ≤ 5 mg/dL (n = 483) |
UI 5 to <20 µg/dL (n = 2214) |
UI ≥ 20 µg/dL (n = 1157) |
P |
|---|---|---|---|---|
| Thyroxine (T4) (µg/dL) | 9.22 ± 0.09 | 9.22 ± 0.04 | 9.35 ± 0.06 | |
| Med 9.2 | Med 9.0 | Med 9.2 | 0.101 | |
| Range 0.4–14.5 | Range 0.4–14.9 | Range 0.4–14.9 | ||
| T4 (nmol/L) | 118.62 ± 1.18 | 118.65 ± 0.54 | 120.35 ± 0.75 | |
| Med 118.4 | Med 115.8 | Med 118.4 | 0.101 | |
| Range 5.1–186.6 | Range 5.1–191.8 | Range 5.1–191.8 | ||
| TSH (µU/mL = mU/L) | GM 1.6 ± 0.10 | GM 1.4 ± 0.04 | GM 1.5 ± 0.06 | |
| Med 1.3 | Med 1.20 | Med 1.2 | 0.157 | |
| Range: 0.0–4.3 | Range 0.0–4.3 | Range 0.0–4.5 |
Descriptive data are summarized in the format: mean ± SE (T4) or geometric mean ± SE (TSH) & median, range (minimum–maximum). Total n = 3854.
TABLE 8.
Men (age 15–44 years): Comparisons of T4 and TSH Values Across 4-level UI/Cr Ranges
| Variable | UI/Cr 0–50 µg/g (n = 480) |
UI/Cr 50.01–100 µg/g (n = 1354) |
UI/Cr 100.01–200 µg/g (n = 1317) |
UI/Cr 200.01+ µg/g (n=620) |
P |
|---|---|---|---|---|---|
| Thyroxine (T4) (µg/dL) | 8.49 ± 0.09 | 8.55 ± 0.05 | 8.52 ± 0.05 | 8.65 ± 0.07 | |
| Med 8.4 | Med 8.5 | Med 8.5 | Med 8.6 | 0.685 | |
| Range 2.7–14.6 | Range 0.4–14.3 | Range 0.4–14.6 | Range 0.4–14.7 | ||
| T4 (nmol/L) | 109.3 ± 1.1 | 110.1 ± 0.61 | 109.7 ± 0.63 | 111.3 ± 0.90 | |
| Med 108.1 | Med 109.4 | Med 109.4 | Med 110.7 | 0.685 | |
| Range 5.1–191.8 | Range 5.1–184.0 | Range 5.1–187.9 | Range 5.1–189.2 | ||
| TSH (µU/mL = mU/L) | GM 1.1 ± 0.03 | GM 1.2 ± 0.01 | GM 1.33 ± 0.02 | GM 1.39 ± 0.02 | |
| Med 1.2 | Med 1.3 | Med 1.3 | Med 1.3 | <0.001 | |
| Range 0.1–4.4 | Range 0.0–4.4 | Range 0.0–4.4 | Range 0.0–4.2 |
Descriptive data are summarized in the format mean ± SE or geometric mean ± SE and median, range (minimum–maximum). Total n = 3771.
In nonpregnant women, there was no significant relationship between UI concentrations and T4 concentrations (, P > 0:1) (Table 3). Similarly, the TSH values were not significantly different across UI ranges (, P > 0:1) in these women.
For Table 4, the nonparametric comparisons were performed across the 4 UI/Cr level groupings, and the P values are given in these tables. When the UI/Cr ratio is used instead of UI alone, TSH concentrations are in fact associated with UI/Cr level in nonpregnant women. However, this relationship is in the opposite direction to that predicted: TSH is lower in individuals with lower UI/Cr ratios and tends to increase as UI/Cr increases.
TABLE 4.
Nonpregnant Women (age 15–44 years): Comparisons of T4 and TSH Values Across 4-level UI/Cr Ranges
| Variable | UI/Cr 0–50 µg/g (n = 398) |
UI/Cr 50.01–100 µg/g (n = 1358) |
UI/Cr 100.01–200 µg/g (n = 1392) |
UI/Cr 200.01+µg/g (n = 704) |
P |
|---|---|---|---|---|---|
| Thyroxine (T4) (µg/dL) | 9.32 ± 0.10 | 9.26 ± 0.05 | 9.29 ± 0.05 | 9.18 ± 0.07 | |
| Med 9.2 | Med 9.0 | Med 9.1 | Med 9.0 | 0.611 | |
| Range 0.4–14.9 | Range 0.4–14.9 | Range 0.4–14.9 | Range 0.4–14.9 | ||
| T4 nmol/L | 120.0 ± 1.30 | 119.1 ± 0.70 | 119.5 ± 0.68 | 118.2 ± 0.95 | |
| Med 118.4 | Med 115.8 | Med 117.1 | Med 117.1 | 0.611 | |
| Range 5.1–191.8 | Range 5.1–191.8 | Range 5.1–191.8 | Range 5.1–191.8 | ||
| TSH (µU/mL = mU/L) | GM 1.1 ± 0.03 | GM 1.1 ± 0.02 | GM 1.2 ± 0.02 | GM 1.3 ± 0.02 | |
| Med 1.3 | Med 1.1 | Med 1.3 | Med 1.3 | <0.001 | |
| Range 0.1–4.2 | Range 0.0–4.3 | Range 0.0–4.5 | Range 0.0–4.4 |
Descriptive data are summarized in the format mean ± SE or geometric mean ± SE and median, range (minimum–maximum). Total n = 3852.
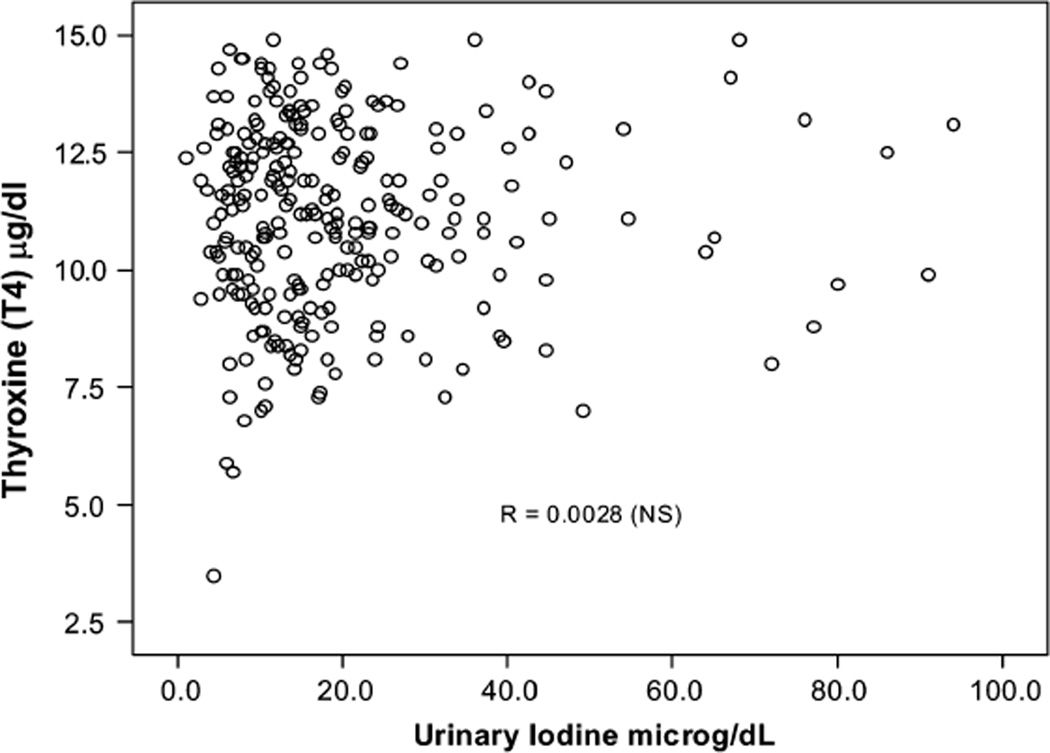
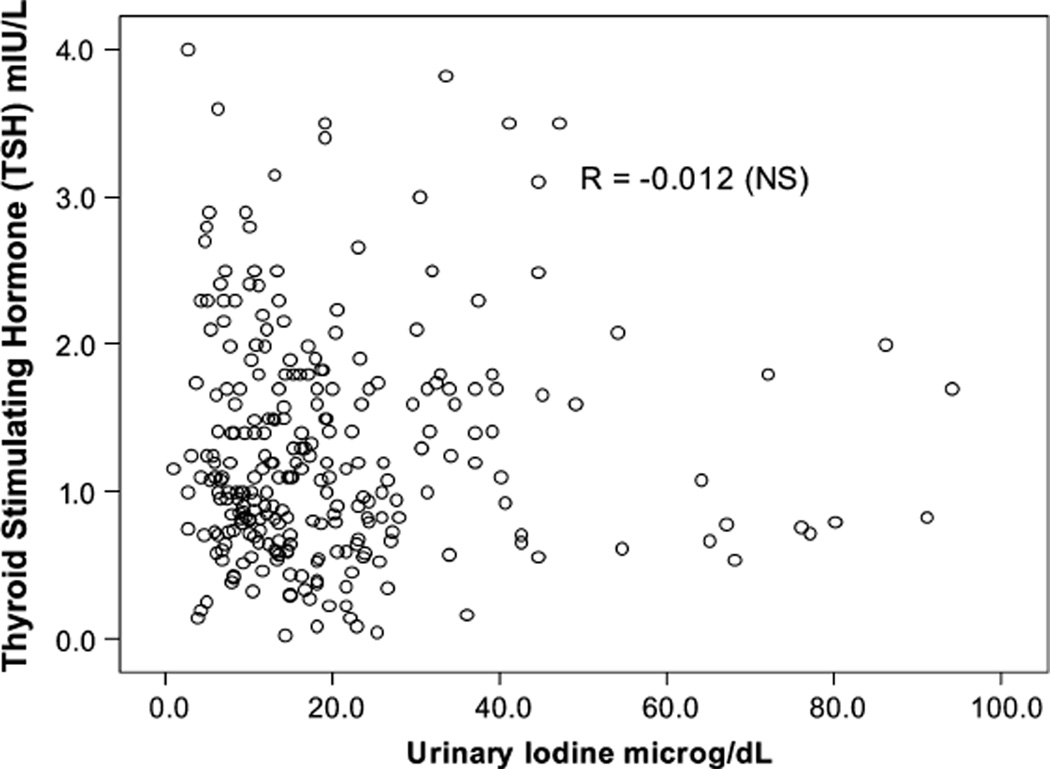
Iodine deficiency was defined by the UI < 5 µg/dL cutoff during pregnancy, similar to the nonpregnant women and men in the sample, although it has been illustrated that UI excretion is higher during pregnancy as a result of a pregnancy-related increase in glomerular filtration rate, (thus representing higher UI excretion and not higher iodine intake). In this group of pregnant women there was no association between UI or UI/Cr and T4 and TSH concentrations (all P > 0.10) (Tables 5 and 6). There were no significant differences in mean T4 and TSH concentrations across the 3 intervals, as illustrated by scattergrams (Figs. 2,3).
TABLE 5.
Pregnant Women (age 15–44 years): Comparisons Across UI Ranges
| Variable | UI ≤ 5 µg/dL (n = 16) |
UI 5 to <20 µg/dL (n = 163) |
UI ≥ 20 µg/dL (n = 87) |
P |
|---|---|---|---|---|
| Thyroxine (T4) (µg/dL) | 11.03 ± 0.63 | 11.1 ± 0.16 | 11.21 ± 0.2 | |
| Med 11.35 | Med 11.3 | Med 11.1 | 0.966 | |
| Range 3.5–14.3 | Range 5.7–14.9 | Range 7.0–14.9 | ||
| T4 (nmol/L) | 141.97 ± 8.18 | 142.87 ± 2.11 | 144.33 ± 2.54 | |
| Med 146.1 | Med 145.4 | Med 142.9 | 0.966 | |
| Range 45.0–184.0 | Range 73.4–191.8 | Range 90.1–191.8 | ||
| TSH (µmU/mL = mU/L) | GM 1.10 ± 0.60 | GM 1.10 ± 0.10 | GM 1.10 ± 0.10 | |
| Med 1.4 | Med 1.1 | Med 1.1 | 0.807 | |
| Range 0.2–4.0 | Range 0.0–3.6 | Range 0.1–4.0 |
Descriptive data are summarized in the format mean ± SE or geometric mean ± SE and median, or range (minimum– maximum). n = 266.
The recommended iodine intake for adults is 150 µg/day; for pregnant women 220 µg/day (Corresponding to UI of 150 µg/day), and during lactation 290 µg/day.10
TABLE 6.
Pregnant Women (age 15–44 years): Comparisons of T4 and TSH Values Across 4-level UI/Cr Ranges
| Variable | UI/Cr 0–50 µg/g (n = 13) |
UI/Cr 50.01–100 µg/g (n = 77) |
UI/Cr 100.01–200 µg/g (n = 101) |
UI/Cr 200.01+µg/g (n = 75) |
P |
|---|---|---|---|---|---|
| Thyroxine T4 (µg/dL) | 10.93 ± 0.62 | 11.03 ± 0.24 | 11.14 ± 0.21 | 11.26 ± 0.22 | |
| Med 10.6 | Med 11.4 | Med 11.1 | Med 11.4 | 0.893 | |
| Range 7.1–14.7 | Range 5.7–14.5 | Range 3.5–14.9 | Range 5.9–14.9 | ||
| T4 (nmol/L) | 140.7 ± 8.01 | 142.0 ± 3.04 | 143.4 ± 2.69 | 144.94 ± 2.81 | |
| Med 136.4 | Med 146.7 | Med 142.9 | Med 146.7 | 0.893 | |
| Range 91.4–198.2 | Range 73.4–186.6 | Range 45.0–191.8 | Range 75.9–191.8 | ||
| TSH (µU/mL = mU/L) | GM 1.2 ± 0.26 | GM 1.1 ± 0.07 | GM 1.0 ± 0.08 | GM 1.1 ± 0.08 | |
| Med 1.2 | Med 1.0 | Med 1.2 | Med 1.1 | 0.392 | |
| Range 0.3–3.6 | Range 0.2 ± 4.0 | Range 0.0–3.8 | Range 0.1–3.5 |
Descriptive data are summarized in the format mean ± SE or geometric mean ± SE and median, range (minimum–maximum). Total n = 266.
FIGURE 2.
Relationship between urinary iodine (UI) and thyroxine (T4) levels in pregnant women.
FIGURE 3.
Relationship between urinary iodine (UI) and thyroid-stimulating hormone (TSH) levels in pregnant women.
In men, there was no apparent consistent relationship between UI and T4, similar to pregnant and nonpregnant women in this age group (Table 7). There was a significant difference in TSH concentrations across the 3 UI range intervals (, P < 0:05). Similar to the findings in nonpregnant women, TSH concentrations increased with higher UI/Cr concentrations.
TABLE 7.
Men (Age 15–44 years): Comparisons Across UI Ranges
| Variable | UI ≤ 5 µg/dL (n = 263) |
UI 5 –<20 µg/dL (n = 2093) |
UI ≥ 20 µg/dL (n = 1445) |
P |
|---|---|---|---|---|
| Thyroxine (T4) (µg/dL) | 8.52 ± 0.13 | 8.56 ± 0.04 | 8.54 ± 0.05 | |
| Med 8.4 | Med 8.5 | Med 8.5 | 0.824 | |
| Range 0.4–14.5 | Range 0.4–14.6 | Range 0.4–14.7 | ||
| T4 (nmol/L) | 109.64 ± 1.62 | 110.22 ± 0.50 | 109.91 ± 0.59 | |
| Med 146.1 | Med 109.4 | Med 109.4 | 0.824 | |
| Range 5.1–108.1 | Range 5.1–187.9 | Range 5.1–189.2 | ||
| TSH (µU/mL = mU/L) | GM 1.6 ± 0.104 | GM 1.70 ± 0.10 | GM 1.81 ± 0.10 | |
| Med 1.3 | Med 1.3 | Med 1.33 | 0.044 | |
| Range 0.2 ± 3.8 | Range 0.0–4.4 | Range 0.0–4.4 |
Descriptive data are summarized in the format mean ± SE (T4) or geometric mean ± SE (TSH) and median, range (minimum–maximum).
Secondary analyses were carried out to further examine the inconsistency of our results with common thought (ie, that TSH can indicate iodine sufficiency). We classified individuals in the age range with TSH levels between 0.4 µg/dL and 4.5 µg/dL (but without antibodies, thyroid disease, or thyroid medications) as ‘‘normal’’ and those with TSH values greater than 4.5 as ‘‘hypothyroid’’ and used χ2 analyses to examine the relationship between TSH level and the 3-level UI range variable in Table 3, Table 5, and Table 7. We found no relationship between UI and TSH levels for either group of women (both P > 0.13, data not shown), and the association for men was toward increasing proportions of normal men in groups with increasing UI ranges (, P = 0:048). These results (data not shown) confirmed our original findings, suggesting that, in iodine-sufficient populations such as in the United States, any relationship between iodine levels (as reflected by UI) and TSH is a positive linear one; ie, low UI levels were associated with lower, not higher, TSH levels.
DISCUSSION AND CONCLUSIONS
Iodine plays a central role in thyroid physiology, being both a major constituent of thyroid hormones and a regulator of thyroid gland function. Iodine deficiency and excess interfere with thyroid gland function and are expected to result in decreased concentrations of T4 and elevated TSH.21 Iodine deficiency occurs when iodine intake falls below recommended levels. We analyzed a large population database and examined the validity of thyroid function tests as predictors of individual iodine status. Low UI did not correspond to out-of-range TSH or T4 in this iodine-sufficient population.
The limitations of this study are centered on measurements. We did not include smoking, dietary goitrogens, and other environmental factors in our analysis. Because of the nature of the NHANES III survey, serumT4 and not FT4 was measured: Tg, triiodothyronine (T3), and thyroid gland size were not assessed; spot fasting urine samples and not 24-hour urine specimens were collected. The study is cross sectional and therefore does not include individual changes over time; the study is based on self-reported and not clinically verified medical history; and pregnancy data are not trimester-specific. It is likely that without these measurement issues a stronger positive linear relationship between UI and T4 or TSH would have been observed.
The supply of iodine regulates thyroid hormonogenesis and alters thyroid sensitivity to TSH (‘‘thyroid autoregulation’’). When we looked at those subjects with specifically higher or lower than normal T4 or TSH concentrations, we still found no association between these thyroid analytes and UI levels. In fact, it seems that the most efficient balance between UI and T4 (or TSH) in men and in nonpregnant women is at these low UI concentrations and that TSH concentration increases slightly with the increase in UI concentrations. While in pregnant women the range is higher (100–200 µg/g), similar to ranges reported in iodine deficient areas (100–200 µg/g and 200–300 µg/g).22,23 It is possible that thyroid autoregulation and a compensatory increase in serum T3 concentrations in subjects with low iodine intake (reflected in low UI) leads to normalizing of thyroid functions in this population. Other factors (eg, selenium and vitamin deficiencies and other dietary and environmental differences from this NHANES population) might explain previous results. Thus, serum T4 and TSH concentration do not reflect iodine deficiency in this population and cannot be reliably interpreted to reflect iodine sufficiency.
The median UI in NHANES III, was 145 µg/dL (UI/Cr 124.6 µg/g).12 As a median this UI concentration is considered normal, but overall it indicates that the spread is great enough that there are a substantial number of people who are below 100 µg/dL, indicating a deficiency in iodine intake. During pregnancy this deficiency is likely to create increased stress. Adequate iodine intake before and during gestation is critical to support the pregnancy and for normal fetal neurodevelopment.24–26 Iodine requirements are normally increased during pregnancy, the postpartum period, and lactation,4 and restricted iodine availability during gestation presents an additional challenge to the maternal thyroid gland. The physiologic adaptation of the thyroid associated with a normal pregnancy9,27 is frequently replaced by pathologic changes in conditions of iodine deficiency or even during mild iodine restriction.3,10 Several markers have been identified for enhanced thyroidal stimulation associated with iodine restriction during an otherwise normal pregnancy, such as relative hypothyroxinemia and increased serum TSH and Tg concentrations.27 Prompt treatment of maternal hypothyroidism, identified by increased serum TSH, is being advocated to mitigate a negative effect. A series of studies indicate, however, that even a moderate transient period of maternal hypothyroxinemia at the beginning of neurogenesis disrupts neuronal migration into cortical layers in the rat.10,25,26 In addition to thyroid hormone synthesis, iodine is independently involved in regulatory processes such as iodine-dependent autoregulation of thyroid function, iodine may block the conversion of T4 to T3, in regulation of cell proliferation, apoptosis, and thyroid autoimmunity, and it may affect IL-2R production as well as influence the antigenicity of thyroglobulin.
Although serum TSH concentration is the biomarker for neonatal thyroid screening as a monitoring tool for the control of iodine deficiency, a 1994 WHO report raised doubts about the specificity of serum TSH in older children, adults, and pregnant women in assessing hypothyroidism induced by iodine deficiency.24 Serum TSH, T4, or T3 and radioactive iodine uptake are considered less useful than UI measurements as indicators for the assessment of iodine nutrition of a population, although the variations caused by daily fluctuations in iodine intake, even during gestation, are expected to be subtle and likely undetectable.20
UI measurements are used for estimation of iodine status of populations and are arguably not as reliable in determining the iodine status of an individual. This is because UI concentrations are not a direct measure of sufficiency; a single measurement of UI can reflect recent iodine intake and thyroid hormone catabolism.8 The human thyroid provides large stores of Tg and T4 to supply iodine for several weeks. Between 5000 and 10,000 µg of hormonal iodine is stored within the thyroid gland, acting as a protective pool and providing an iodine source in the absence of dietary iodine intake.28 Therefore, it is plausible that low UI levels reliably reflect low iodine intake, at least in the weeks preceding UI measurements. Such a period of iodine inadequacy may have serious consequences, especially during gestation.10 Although higher UI concentrations may represent recent iodine intake, low UI concentrations (even in the United States) probably reflect a prolonged low-iodine status in an individual. This is also illustrated in patients who had undergone thyroidectomy; in preparation for radioiodide ablation therapy, even in the absence of a thyroid gland (and therefore no iodine reserves), these patients are required to follow a strict low-iodine diet for 2 weeks to decrease their UI levels to <5 µg/dL.29,30
The thyroid gland concentrates iodide (I−) against an electrochemical gradient by a carrier-mediated mechanism, the sodium iodide symporter (NIS), driven by ATP.28 A similar I− uptake mechanism is found in other organs, including salivary glands, stomach, choroid plexus, and mammary glands, but only in the thyroid does TSH regulate the process. Low plasma iodide concentrations are thought to increase the expression of NIS as well as extend the half-life of plasma iodide. Thyroid hormone production is affected negatively by persistant lack of iodine, triggering a temporary increase in serum TSH concentration to induce an increase in T4 production. This leads to an appropriate decrease in serum TSH concentrations, resulting in mean serum concentrations of FT4 and TSH within the normal range. Thus, the thyroid would have compensated for the deficiency in iodine. Although a narrower reference interval for TSH and for FT4 may have assisted in detecting mild to moderate degrees of iodine deficiency, TSH, FT4, or T4 are not the appropriate markers to provide reliable conclusions relating to a patient’s iodine status, especially in the case of women during pregnancy.
The clinical guidelines of the US Preventive Task Force recognize that the positive predictive value of TSH in detecting thyroid disease is low when it is used for screening primary care populations.31 Moreover, the interpretation of a positive test result can often be complicated by an underlying illness, and other clinical issues such as autoimmunity, thyroid disease, drug-related effects, exposure to environmental and dietary goitrogens, and other physiologic stressors.
We conclude that low UI did not correspond to out-of-range TSH or T4 in this iodine-sufficient population. Iodine deficiency occurs when iodine intake falls below recommended levels.24 The physiological significance of low iodine excretion, and the consequences of subclinical effects of low iodine intake need further examination. We illustrate that thyroid function tests cannot serve as indirect measures of iodine sufficiency in this population. In order to evaluate subtle iodine deficiencies at the individual level, in the absence of UI testing, a new biologic measure needs to be identified. Without such a new standard, we are not likely to be able to prevent the consequences of low iodine and fetal neurodevelopmental risk due to subtle thyroid hormone deficiency during pregnancy.
ACKNOWLEDGMENTS
We are grateful to Dr. John Dunn, a true inspiration, who was passionate about the fight for the eradication of iodine deficiency worldwide and supported us continuously in all of our studies of iodine and its epidemiology in the United States. We would like to thank Dr. Del A. Fisher, Dr. John H. Lazarus, and Dr. J. Jonklaas for reading the manuscript and making helpful comments. This study was supported by NIH GCRC grant number 5-MO1-RR-13297-S1.
REFERENCES
- 1.Delange F. Iodine deficiency as a cause of brain damage. Postgrad Med J. 2001;77:217–220. doi: 10.1136/pmj.77.906.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ICCIDD, UNICEF, WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A guide for programme managers, 2nd ed. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 3.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine, Food and Nutrition Board, National Academy of Sciences. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington DC: National Academy Press; 2003. pp. 1–28. [Google Scholar]
- 5.Pedersen IB, Knudsen N, Jorgesnsen T, et al. Large differences in incidences of overt hyper- and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey. J Clin Endocrinol Metab. 2002;87:4462–4469. doi: 10.1210/jc.2002-020750. [DOI] [PubMed] [Google Scholar]
- 6.Dunn JT. What‘s happening to our iodine? J Clin Endocrinol Metab. 1998;83:3398–3400. doi: 10.1210/jcem.83.10.5209. [DOI] [PubMed] [Google Scholar]
- 7.Soldin OP. Controversies in urinary iodine determinations. Clin Biochem. 2002;35:575–579. doi: 10.1016/s0009-9120(02)00406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab SJ, Christensen RL, Dougherty K, et al. Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Med. 1987;147:943–944. [PubMed] [Google Scholar]
- 9.Soldin OP, Tractenberg RE, Hollowell JG, et al. Trends and associations across trimesters in iodine sufficiency: trimester-specific changes in maternal thyroid hormone, TSH and thyroglobulin concentrations during gestation. Thyroid. 2004;12:1084–1090. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morreale de Escobar G, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.National Center of Health Statistics. Plan and operation of the health and nutrition examination survey, 1982–84. Vital Health Stat. 1985;1 [PubMed] [Google Scholar]
- 12.Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994) J Clin Endocrinol Metab. 1988;83:3401–3408. doi: 10.1210/jcem.83.10.5168. [DOI] [PubMed] [Google Scholar]
- 13.Soldin OP, Soldin SJ, Pezzullo JC. Urinary iodine percentile ranges in the United States. Clin Chim Acta. 2003;328:185–190. doi: 10.1016/s0009-8981(02)00429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols Institute Diagnostics. TSH Product insert, TSH-Third Generation. San Juan Capistrano, CA: Nichols Institute; 1992. [Google Scholar]
- 15.Gunter EW, Lewis BL, Koncikowski SM. Kronus. Laboratory Methods Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Hyattsville, MD: CDC; 1996. Thyroid Peroxidase Antibody (TPO Antibody), K.P.I., RIA Kit Insert. [Google Scholar]
- 16.Gunter EW, Lewis BL, Koncikowski SM. Kronus. Laboratory Methods Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Hyattsville, MD: CDC; 1996. Thyroglobulin Antibody (TgAb), K.P.I., RIA kit insert P/N 114J. [Google Scholar]
- 17.Benotti J, Benolti N, Pino S, et al. Determination of total iodine in urine, stool, diets, and tissue. Clin Chem. 1965;11:932–936. [PubMed] [Google Scholar]
- 18.Public Health Service, US Department of Health, Education, and Welfare. DHEW Publication (HSM) 73–1310, ser. 1, no. 10a and 10b. Vol. 16. Washington, DC: US Government Printing Office; 1973. Plan and operation of the health and nutrition examination survey; pp. 63–71. [Google Scholar]
- 19.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 20.Delange F, Burgi H, Chen ZP, et al. World status of monitoring of iodine deficiency disorders control programs. Thyroid. 2002;12:915–924. doi: 10.1089/105072502761016557. [DOI] [PubMed] [Google Scholar]
- 21.Braverman LE, Utiger RD. Werner and Ingbar’s The Thyroid. A Fundamental and Clinical Text. Philadelphia: Lippincott-Raven; 2000. pp. 103–120. [Google Scholar]
- 22.Buchinger W, Lorenz-wawschinek O, Semlitsch G, et al. Thyrotropin and thyroglobulin as an index of optimal iodine intake: correlation with iodine excretion of 39,913 euthyroid patients. Thyroid. 1997:593–7. doi: 10.1089/thy.1997.7.593. [DOI] [PubMed] [Google Scholar]
- 23.Moulopoulos DS, Koutras DA, Mantzos J, et al. The relation of serum T4 and TSH with the urinary iodine excretion. J. Endocrinol Invest. 1988;11:437–9. doi: 10.1007/BF03349078. [DOI] [PubMed] [Google Scholar]
- 24.WHO/UNICEF/ICCIDD. Indicators for Assessing Iodine Deficiency Disorders and Their Control Through Salt Iodization. Geneva: WHO; 1994. pp. 1–55. [Google Scholar]
- 25.Lavado-Autric Lavado-Autric R, Auso E, Garcia-Velasco JV, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111:1073–1082. doi: 10.1172/JCI16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auso E, Lavado-Autric R, Cuevas E, et al. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–4047. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- 27.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 28.Cavalieri RR. Iodine metabolism and thyroid physiology: current concepts. Thyroid. 1997;7:177–181. doi: 10.1089/thy.1997.7.177. [DOI] [PubMed] [Google Scholar]
- 29.Pluijmen MJHM, Eustatia-Rutten C, Goslings BM, et al. Effect of lowiodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol. 2003;58:428–435. doi: 10.1046/j.1365-2265.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 30.Park JT, Hennessey JV. Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid. 2004;14:57–63. doi: 10.1089/105072504322783858. [DOI] [PubMed] [Google Scholar]
- 31.US Preventive Task Force. Clinical guidelines. Screening for thyroid disease: recommendation statement. Ann Intern Med. 2004;140:125–127. doi: 10.7326/0003-4819-140-2-200401200-00014. [DOI] [PubMed] [Google Scholar]