Abstract
In the mammalian brain, kynurenine aminotransferase II (KAT II) and kynurenine 3-monooxygenase (KMO), key enzymes of the kynurenine pathway of tryptophan degradation, form the neuroactive metabolites kynurenic acid (KYNA) and 3-hydroxykynurenine (3-HK), respectively. Although physically segregated, both enzymes use the pivotal kynurenine pathway metabolite L-kynurenine as a substrate. We studied the functional consequences of this cellular compartmentalization in vivo using two specific tools, the KAT II inhibitor BFF 122 and the KMO inhibitor UPF 648. The acute effects of selective KAT II or KMO inhibition were studied using a radiotracing method in which the de novo synthesis of KYNA, and of 3-HK and its downstream metabolite quinolinic acid (QUIN), is monitored following an intrastriatal injection of 3H-kynurenine. In naïve rats, intrastriatal BFF 122 decreased newly formed KYNA by 66%, without influencing 3-HK or QUIN production. Conversely, UPF 648 reduced 3-HK synthesis (by 64%) without affecting KYNA formation. Similar, selective effects of KAT II and KMO inhibition were observed when the inhibitors were applied acutely together with the excitotoxin QUIN, which impairs local KP metabolism. Somewhat different effects of KMO (but not KAT II) inhibition were obtained in rats that had received an intrastriatal QUIN injection 7 days earlier. In these neuron-depleted striata, UPF 648 not only decreased both 3-HK and QUIN production (by 77% and 66%, respectively) but also moderately raised KYNA synthesis (by 27%). These results indicate a remarkable functional segregation of the two pathway branches in the brain, boding well for the development of selective KAT II or KMO inhibitors for cognitive enhancement and neuroprotection, respectively.
Keywords: Excitotoxicity, 3-Hydroxykynurenine, Kynurenic acid, Quinolinic acid
INTRODUCTION
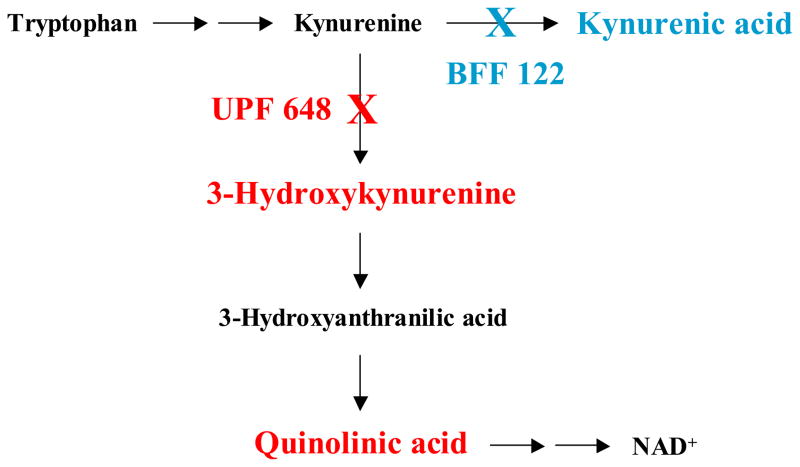
The kynurenine pathway (KP) of tryptophan degradation contains at least three neuroactive metabolites (“kynurenines”), namely kynurenic acid (KYNA), 3-hydroxykynurenine (3-HK) and quinolinic acid (QUIN) (Fig. 1). These compounds, which occur in the mammalian brain in nanomolar concentrations, may be involved in physiological processes but have so far gained most notoriety for their possible role in the pathophysiology of neurological and psychiatric disorders such as Huntington’s Disease, Alzheimer’s Disease and schizophrenia (Schwarcz and Pellicciari, 2002; Stone and Darlington, 2002; Reinhard, 2004; Erhardt et al., 2007). In particular, increases in brain KYNA levels, by virtue of the compound’s ability to antagonize two receptors with close functional links to learning and memory [the α7 nicotinic acetylcholine (α7nACh) and the NMDA receptor], may cause cognitive impairment (Chess and Bucci, 2006; Chess et al., 2007) but may also enhance resistance to excitotoxic insults (Schwarcz et al., 1984). On the other hand, the enhanced formation of the free radical generator 3-HK and the NMDA receptor agonist QUIN, possibly acting jointly (Guidetti and Schwarcz, 1999; Chiarugi et al., 2001b), may lead to neurodegeneration. These and related hypotheses have not only stimulated investigations into the biology of brain kynurenines but also prompted efforts to selectively manipulate their fate and function in the brain.
Figure 1.
The kynurenine pathway of tryptophan metabolism in mammalian cells. “X” indicates the selective inhibition of kynurenine aminotransferase II (KAT II; by BFF 122) and kynurenine 3-monooxygenase (KMO; by UPF 648), respectively.
All kynurenines derive from a pivotal parent compound, L-kynurenine, which gains ready access to the brain once it is formed peripherally through the oxidative opening of tryptophan’s indole ring (Gàl and Sherman, 1980; Fukui et al., 1991). L-kynurenine is then actively taken up by various brain cells, which, interestingly, have evolved distinct mechanisms for its further metabolism (Guillemin et al., 2001, 2007). Thus, L-kynurenine in astrocytes is irreversibly transaminated to KYNA (the “KYNA branch” of the KP), which cannot be metabolized further intracellularly but is promptly released from the cell (Turski et al., 1989; Guillemin et al., 1999, 2001). In contrast, initiated by kynurenine 3-monooxygenase (KMO), 3-HK and its downstream product QUIN are preferentially synthesized in microglial cells and then further degraded enzymatically (the “QUIN branch” of the KP; Heyes et al., 1996). KYNA production can be catalyzed by three aminotransferases [kynurenine aminotransferases (KATs) I and II and mitochondrial aspartate aminotransferase (mitAAT)], of which KAT II appears to be the most prominent in the rat and human brain (Guidetti et al., 1997, 2007a).
Although the development of brain-penetrable inhibitors of KYNA, 3-HK and QUIN synthesis is still in its infancy, selective KAT II and KMO inhibitors are available and have been successfully used in experimental proof-of-concept studies in animals. Thus, blockade of KAT II, causing a decrease in brain KYNA, may have cognition-enhancing effects (Pellicciari et al., 2006), whereas KMO inhibition, resulting in the reduction of brain 3-HK and QUIN, may provide benefits in neurodegenerative diseases (Carpenedo et al., 1994; Miranda et al., 1997; Moroni et al., 1999; Richter and Hamann, 2003; Clark et al., 2005; Ceresoli-Borroni et al., 2007; Gregoire et al., 2008). These pharmacological experiments, together with related studies using genetic approaches (Alkondon et al., 2004; Giorgini et al., 2005, 2008; Potter et al., 2006; Bergeron et al., 2007), support the existence of functionally significant links between cerebral KP metabolism and brain physiology and pathology.
With the impending development of KAT II and KMO inhibitors for possible clinical use, it became relevant to examine possible interactions between the two physically segregated branches of the brain’s KP. In other words, it became important to assess whether KAT II inhibition may lead to elevated 3-HK and/or QUIN levels and possibly heightened risk of neurotoxicity and whether, conversely, KMO inhibition may raise KYNA levels and adversely affect cognitive processes. For lack of specific experimental tools, these questions had not been addressed so far. We now infused two potent and specific inhibitors of rat KMO and KAT II, respectively, into the rat striatum to monitor their acute effect on overall KP metabolism initiated by the intrastriatal co-infusion of 3H-kynurenine (Guidetti et al., 1995) (see Fig. 1). Using this experimental approach, we documented a remarkable degree of functional segregation between the two branches of the KP in the brain.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (200–250 g; Charles River Laboratories, Kingston, NY, USA) were used in all experiments. All animals were housed in a temperature-controlled, AAALAC (American Association for Accreditation of Laboratory Animal Care)-approved animal facility on a 12hr/12hr-light/dark cycle with free access to food and water. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland.
Chemicals
L-kynurenine (sulfate), KYNA, DL-3-HK, QUIN, pyruvate (sodium salt), pyridoxal-5′-phosphate, Trizma (Tris) base, glucose-6-phosphate, and glucose-6-phosphate dehydrogenase were purchased from Sigma-Aldrich (St. Louis, MO). NADPH (tetrasodium) was obtained from Alexis Biochemicals (San Diego, CA).
The KMO inhibitor UPF 648 [(1S,2S)-2-(3,4-dichlorobenzoyl)-cyclopropane-1-carboxylic acid] was synthesized as described (Amori, 2003). First, (1R)-(−)-dimenthyl succinate was prepared by reacting succinyl anhydride and L-menthol in the presence of p-toluenesulfonic acid (toluene, reflux, 24 h). After recrystallization from methanol (89% yield), the product was reacted with lithium 2,2,6,6-tetramethylpiperidine amide and bromochloromethane (tetrahydrofurane, −78°C, 4 h) to generate dimenthyl (1S,2S)-cyclopropane-1,2-dicarboxylate after flash chromatography on silica gel (petroleum ether:ethyl acetate = 98:2) (45% yield). This diastereoisomeric diester was submitted to basic hydrolysis (KOH, water/methanol, 60°C, 4 h) to give (1S,2S)-cyclopropane-1,2-dicarboxylic acid after sublimation (84% yield). The resulting dicarboxylic acid, after conversion into the corresponding diacyl chloride (oxalyl chloride, rt, 12 h), was transformed into dimethyl (1S,2S)-cyclopropane-1,2-dicarboxylate [methanol, 23°C, 12 h; flash chromatography on silica gel (petroleum ether:ethyl acetate = 85:15)] (93% yield). The dimethyl derivative was then submitted to monohydrolysis (KOH, methanol, 60°C, 5 h), yielding (1S,2S)-cyclopropane-1,2-dicarboxylic acid monomethyl ester (80% yield). The product was then transformed (N-methoxy-N-methylamine hydrochloride, carbon tetrabromide, pyridine, triphenylphosphine, dichloromethane, 23°C, 2 h) into the corresponding N-methyl-N-methoxyamide (Weinreb amide), after flash chromatography on silica gel (petroleum ether:ethyl acetate = 7:3) (61% yield). Reaction of the Weinreb amide with a Grignard reagent, prepared from 1-bromo-3,4-dichlorobenzene (Mg, tetrahydrofurane, 60°C) in tetrahydrofurane at rt led to methyl (1S,2S)-2-(3,4-dichlorobenzoyl)-cyclopropane-1-carboxylate, after flash chromatography on silica gel (petroleum ether:ethyl acetate = 98:2) (60% yield). Final hydrolysis of the resulting methyl ester produced UPF 648 after trituration of the crude product from n-hexane (90% yield).
The KAT II inhibitor BFF 122 [(S)-(−)-9-(4-aminopiperazine-1-yl)-8-fluoro-3-methyl-6-oxo-2,3,5,6-tetrahydro-4H-1-oxa-3a-aza-phenalene-5-carboxylic acid] was prepared as follows: a solution of (S)-(−)-9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid (Sigma-Aldrich; 10.0 g) and piperazine (24.3 g) in dimethylsulfoxide (DMSO) (100 ml) was stirred for 8 h at 100°C and was then cooled to 23°C and concentrated in vacuo. The resulting yellow solid (5.4 g) was taken up in 50% glacial acetic acid (140 ml), and stirred for 4.5 h with 40 ml of an aqueous solution of 5.4 g NaNO2. The mixture was filtered, washed with water, and dried. 5.9 g of the resulting intermediate solid were taken up in 240 ml 50% glacial acetic acid. The mixture was then heated with Zn (5.9 g) for 45 min at 70°C, cooled to 23°C, and concentrated in vacuo. The resulting residue was purified by HPLC using a CAPCELL PAK C18 column (4.6 mm × 25 cm; Analis, Gent, Belgium), yielding 3.3 g of BFF 122 as a white trifluoroacetate salt.
All other chemicals were of the highest available purity and were purchased from various commercial suppliers. 5-[3H]-Kynurenine (specific activity 12.0 Ci/mmol) was custom-synthesized by Amersham Corp. (Arlington Heights, IL). Before use, 3H-kynurenine was routinely purified by HPLC as described by Guidetti et al. (1995).
Partial enzyme purification
KAT I and KAT II were partially purified from rat liver according to the procedures described by Guidetti et al. (1997). mitAAT was partially purified from rat brain (Guidetti et al., 2007a).
Enzyme assays
KAT activity
Total KAT activity was measured using minor modifications of the procedure developed by Okuno at al. (1991). The reaction mixture contained the enzyme preparation (80 μl), 20 μl aliquots of the test compounds (or water for controls), 3H-kynurenine (50 nCi; diluted with unlabelled kynurenine to reach a final concentration of 2 μM), 1 mM pyruvate, 70 μM pyridoxal-5′-phosphate and 150 mM Tris-acetate (pH 7.4) in a total volume of 200 μl. The enzyme preparation consisted of brain homogenate, diluted 1:10 with a buffer containing 500 mM Tris-acetate, 50 nM pyridoxal 5′-phosphate and 1.1 mM 2-mercaptoethanol (pH 7.4). Where indicated, tissue homogenate was substituted by partially purified KAT appropriately diluted with the same buffer. Blanks were prepared by boiling the homogenate for 10 min or by omitting the enzyme preparation. Active samples and blanks were incubated for 2 h at 37°C, and the reaction was terminated by adding 20 μl of 50 % (w/v) trichloroacetic acid and 1 ml of 0.1 M HCl. The denatured proteins were removed by centrifugation, and newly synthesized 3H-KYNA was recovered from Dowex 50W cation exchange columns and quantified by liquid scintillation spectrometry.
KMO activity
For the determination of KMO activity, rat brain tissue was diluted (1:10, w/v) with Krebs buffer (KB) containing 100 mM Tris-HCl (pH 8.1), 10 mM KCl and 1 mM EDTA, and sonicated. Eighty μl of the resulting tissue homogenate were incubated for 40 min at 37°C with 20 μl aliquots of aqueous solutions of the test compounds (or water in controls), 10 μM kynurenine, 1 mM NADPH, 3 mM glucose-6-phosphate and 1U/ml glucose-6-phosphate dehydrogenase in a total volume of 200 μl. The reaction was terminated with 50 μl of 6% perchloric acid, the denatured proteins were removed by centrifugation, and newly synthesized 3-HK was quantified by HPLC with electrochemical detection (Coulochem 5100A; ESA, Chelmsford, MA) at an oxidation potential of +0.2 mV (Heyes and Quearry, 1988).
QUIN-induced striatal lesions
Striatal lesions were induced by a focal infusion of 300 nmoles QUIN, administered over 10 min in 2.5 μl of phosphate buffered saline (PBS), pH 7.4, as described previously (Schwarcz et al., 1983). The injection coordinates were: AP: 1 mm anterior to bregma, L: ± 2.7 mm from the midline, V: 4.7 mm below the dura. The contralateral striatum received an infusion of 2.5 μl PBS.
Intrastriatal injection of 3H-kynurenine
The radioactive tracer method described by Guidetti et al. (1995) was used to study acute KP metabolism in the brain in vivo. To this end, rats were anesthetized with chloral hydrate (360 mg/kg, i.p.), and 6 μl of 3H-kynurenine (2.5 μCi; 35 μM) were infused intrastriatally over 10 min, either alone or in combination with test compounds, using the same injection coordinates listed above.
In experiments designed to study the effects of KMO or KAT II inhibition during the early stages of excitotoxic striatal neurodegeneration, 360 nmoles of QUIN were co-infused with 3H-kynurenine (alone or with test compounds).
To assess the effect of KAT II or KMO inhibition in the excitotoxically lesioned striatum, experiments were carried out 7 days after an intrastriatal injection of 300 nmoles QUIN or PBS.
All animals were euthanized 2 h after the start of the 3H-kynurenine infusion, and their brain was quickly removed. The striata were rapidly dissected out on ice, frozen on dry ice and stored at −80°C until processed. After thawing, tritiated KP metabolites were separated, detected and analyzed as described previously (Guidetti et al., 1995). The production of newly formed 3H-KYNA, 3H-3-HK and 3H-QUIN was expressed as a percentage of the total radioactivity recovered per striatum.
RESULTS
Inhibition of KAT and KMO in rat brain homogenate
Initial in vitro experiments were designed to characterize the potency and specificity of BFF 122 and UPF 648 as inhibitors of KAT and KMO, respectively, in whole forebrain homogenate (Table 1). BFF 122 inhibited KAT activity almost completely at both 1 and 0.1 mM. The effect was still remarkable at 0.01 mM (70 ± 1 % inhibition). At the same three concentrations, BFF 122 did not affect KMO activity significantly. In contrast, UPF 648 totally blocked KMO at 0.1 and 0.01 mM and was still highly active at 0.001 mM (81 ± 10 % inhibition), but the compound was essentially ineffective at blocking KAT activity (Table 1).
Table 1.
Inhibition of rat brain KAT and KMO activity by BFF 122 and UPF 648 in vitro
| % Inhibition | |||
|---|---|---|---|
| KMO | KAT | ||
| BFF 122 (mM) | 1 | 17 ± 8@ | 93 ± 1 |
| 0.1 | 7 ± 10 | 90 ± 1 | |
| 0.01 | 7 ± 6 | 70 ± 1 | |
| UPF 648 (mM) | 0.1 | 100 ± 9 | 5 ± 6 |
| 0.01 | 92 ± 7 | 4 ± 0 | |
| 0.001 | 81 ± 10 | 4 ± 1 | |
Data are the mean ± SEM of three separate experiments.
n = 4.
Inhibition of KAT I, II and mitAAT by BFF 122
To evaluate the specificity of BFF 122 as a KAT II inhibitor, the compound was tested using partially purified preparations of liver KAT I and KAT II, and brain mitAAT. As shown in Table 2, KAT II activity was totally blocked by as little as 0.01 mM BFF 122, whereas the compound was far less active as an inhibitor of KAT I and mitAAT. At 0.001 mM, BFF 122 still inhibited KAT II activity by 43 ± 6 %.
Table 2.
Inhibition of partially purified KAT I, KAT II and mitAAT by BFF 122 in vitro
| % Inhibition | ||||
|---|---|---|---|---|
| KAT I | KAT II | mitAAT | ||
| BFF 122 (mM) | 1 | 26 ± 1 | 100 ± 16 | 33 ± 5 |
| 0.1 | 8 ± 1 | 100 ± 9 | 11 ± 3 | |
| 0.01 | 0 ± 2 | 100 ± 9 | 9 ± 2 | |
| 0.001 | 0 ± 2 | 43 ± 6 | 0 ± 4 | |
Enzyme preparations were obtained as described in the text. Data are the mean ± SEM of three separate experiments.
Effect of BFF 122 and UPF 648 on striatal KP metabolism in vivo
In line with the results reported by Guidetti et al. (1995), tritiated KYNA, 3-HK and QUIN were recovered from the striatum 2 h following an intrastriatal injection of 3H-kynurenine. In naïve animals, the yield of 3H-KYNA, 3H-3-HK and 3H-QUIN, expressed as a percentage of total recovered radioactivity, was 1.4 ± 0.1, 1.9 ± 0.4 and 0.2 ± 0.0, respectively.
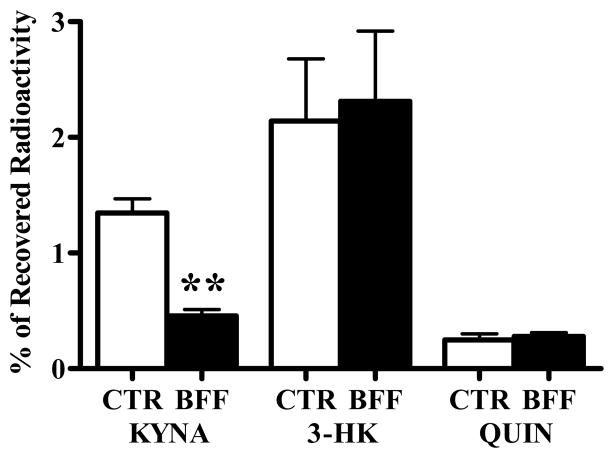
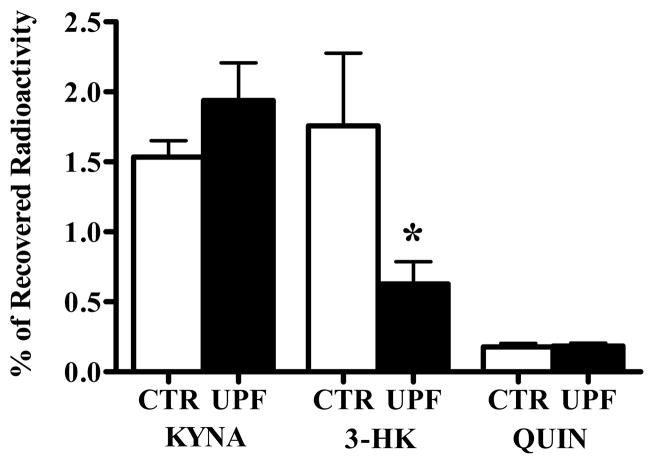
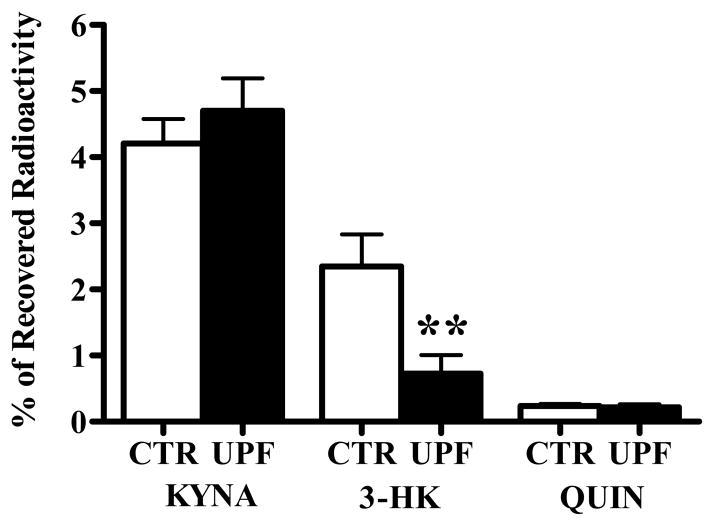
Next, we examined the in vivo effects of BFF 122 and UPF 648 on the metabolism of 3H-kynurenine. In these experiments, the formation of 3H-KYNA, 3H-3-HK and 3H-QUIN in the striatum receiving 3H-kynurenine and the enzyme inhibitors was compared to synthesis in the contralateral striatum, which received 3H-kynurenine alone. 1 mM BFF 122 (i.e. 6 nmoles in 6 μl) reduced newly formed KYNA by 66%, but did not affect either 3-HK or QUIN neosynthesis (Fig. 2). On the other hand, 0.1 mM UPF 648 (i.e. 0.6 nmoles in 6 μl) caused a significant decrease in the de novo synthesis of 3-HK (by 64%) without influencing the neosynthesis of QUIN or KYNA (Fig. 3).
Figure 2.
Effect of BFF 122 (1 mM) on the de novo synthesis of tritiated KP metabolites in the naive rat striatum. 3H-Kynurenine was injected in the absence (CTR) or presence (BFF) of the KAT II inhibitor, and the data were analyzed as described in the text. Data are the mean + SEM (n = 6). ** p < 0.01 vs. the contralateral control (paired t-test).
Figure 3.
Effect of UPF 648 (0.1 mM) on the de novo synthesis of tritiated KP metabolites in the naïve rat striatum. 3H-Kynurenine was injected in the absence (CTR) or presence (UPF) of the KMO inhibitor, and the data were analyzed as described in the text. Data are the mean + SEM (n = 6). * p < 0.05 vs. the contralateral control (paired t-test).
Effect of BFF 122 and UPF 648 on KP metabolism in the presence of QUIN
In order to analyze the effect of a selective block of either KAT II or KMO under acute excitotoxic conditions, BFF 122 or UPF 648 were co-injected with 360 nmoles QUIN and 3H-kynurenine. In these experiments, too, de novo KYNA, 3-HK and QUIN production in the presence of the enzyme inhibitors was compared to the contralateral striatum, which received only 360 nmoles QUIN and 3H-kynurenine.
In agreement with the previously described acute increase in KYNA formation in the very early stages of excitotoxic neurodegeneration (Ceresoli-Borroni et al., 1999), addition of QUIN to the perfusion solution resulted in an approximately 2.5-fold increase in the proportion of metabolized 3H-kynurenine that was recovered as 3H-KYNA. In spite of a trend towards reduced 3-HK production, the neosynthesis of 3-HK or QUIN was not significantly influenced in these animals (Fig. 4).
Figure 4.
Effect of BFF 122 (1 mM) on the de novo synthesis of tritiated KP metabolites in the presence of 360 nmoles QUIN. 3H-Kynurenine and QUIN were injected in the absence (CTR) or presence (BFF) of the KAT II inhibitor, as described in the text. Data are the mean + SEM (n = 4). * p < 0.05 vs. the contralateral control (paired t-test).
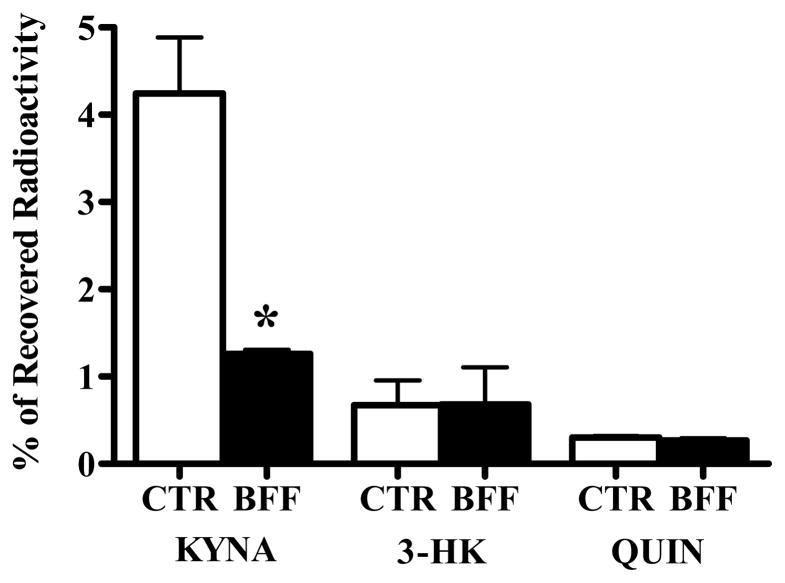
BFF 122, at 1 mM, inhibited the de novo production by 70% without affecting the formation of 3-HK and QUIN (Fig. 4). Co-administration of 0.1 mM UPF 648 and 360 nmoles QUIN caused a 68% reduction in 3-HK formation but had no effect on KYNA or QUIN neosynthesis (Fig. 5).
Figure 5.
Effect of UPF 648 (0.1 mM) on the de novo synthesis of tritiated KP metabolites in the presence of 360 nmoles QUIN. 3H-Kynurenine and QUIN were injected in the absence (CTR) or presence (UPF) of the KMO inhibitor, as described in the text. Data are the mean + SEM (n = 7). ** p < 0.01 vs. the contralateral control (paired t-test).
Effect of BFF 122 and UPF 648 in neuron-depleted tissue in vitro
In preparation of subsequent in vivo experiments, we next studied the respective potencies of BFF 122 and UPF 648 in striatal tissue homogenate obtained 7 days after a focal injection of 300 nmoles of QUIN. The in vitro potency of both enzyme inhibitors in lesioned tissue was very similar to that seen in tissue homogenate from the contralateral striatum (which had received PBS 7 days earlier). Thus, 1 mM BFF 122 completely inhibited KAT activity in tissue obtained from either lesioned or contralateral striatum without affecting KMO activity. On the other hand, 0.1 mM UPF 648 blocked KMO in both lesioned and contralateral striatum, but did not interfere with KAT activity in either tissue (Table 3).
Table 3.
Inhibition of striatal KAT and KMO activity by BFF 122 and UPF 648 in vitro
| % Inhibition | |||||
|---|---|---|---|---|---|
| KMO | KAT | ||||
| Lesioned | Contralateral | Lesioned | Contralateral | ||
| BFF 122 | 1 mM | 10 ± 14 | 1 ± 5 | 100 ± 12 | 90 ± 1 |
| UPF 648 | 0.1 mM | 94 ± 4 | 80 ± 11 | 0 ± 10 | 12 ± 8 |
Tissue homogenates were prepared from chronically QUIN-lesioned or contralateral striata as described in the text. Data are the mean ± SEM of three separate experiments.
Effect of BFF 122 and UPF 648 on KP metabolism in the excitotoxically lesioned striatum in vivo
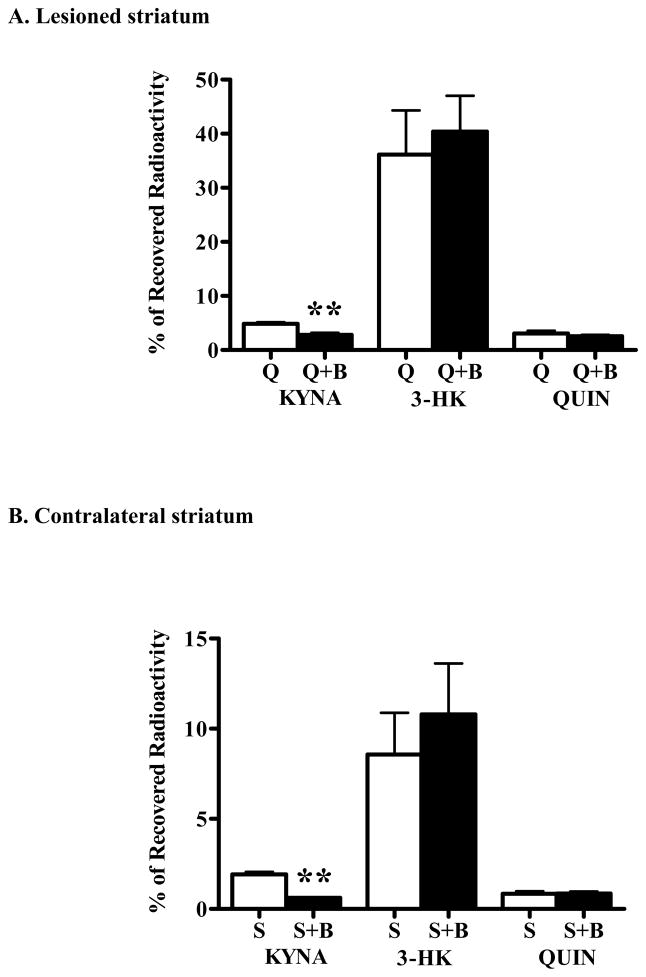
We next examined the effects of BFF 122 and UPF 648 on 3H-kynurenine metabolism in the QUIN-lesioned striatum in vivo. In these experiments, 1 mM BFF 122 and 3H-kynurenine were co-injected into both lesioned and contralateral striatum. A separate group of unilaterally lesioned rats received bilateral injections of 3H-kynurenine alone.
As shown previously (Guidetti et al., 1995; Ceresoli et al., 1997), striatal KP metabolism was abnormal in the neuron-depleted tissue. Thus, in the chronically lesioned striata, the de novo formation of 3H-KYNA, 3H-3-HK and 3H-QUIN was 2.5-, 4.2- and 3.6-fold higher, respectively, than in the contralateral, PBS-injected tissues (cf. open bars in Fig. 6A with open bars in Fig. 6B). Notably, however, the production of all three KP metabolites in the contralateral striatum also tended to be higher than in the acute experiments (cf. open bars in Fig. 6B with open bars in Figs. 2 and 3).
Figure 6.
Effect of BFF 122 (1 mM; “B”) on the de novo synthesis of tritiated KP metabolites in unilaterally QUIN-lesioned animals. As detailed in the text, separate groups of animals, unilaterally lesioned with QUIN in the striatum, received bilateral infusions of either 3H-kynurenine alone (n = 11; open bars) or 3H-kynurenine and the KAT II inhibitor (n = 9; solid bars). Bargraphs illustrate between-group comparisons of the effects of BFF 122 (A) in the lesioned (“Q”) and (B) the contralateral, PBS-treated (“S”) striatum, respectively. Data are the mean + SEM. ** p < 0.01 vs. the striatum infused with 3H-kynurenine alone (unpaired t-test).
In the excitotoxically lesioned as well as in the contralateral striatum, which had received PBS 7 days earlier, BFF 122 reduced newly formed KYNA but did not affect the de novo production of 3-HK or QUIN (p < 0.01 each) (Fig. 6A,B).
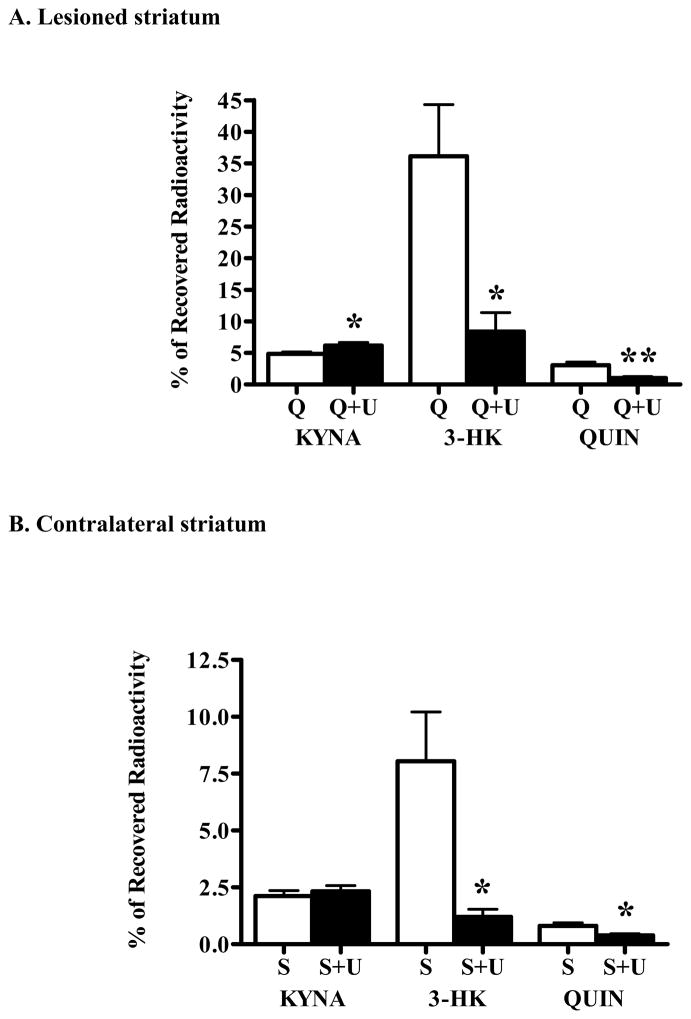
Applying an identical experimental design, separate rats were used to study the effect of KMO inhibition on the de novo synthesis of KP metabolites in the lesioned striatum. These animals were bilaterally injected with 0.1 mM UPF 648 and 3H-kynurenine in PBS. 0.1 mM UPF 648 significantly reduced the neosynthesis of 3-HK and QUIN in the lesioned striatum (by 77 % and 66%, respectively) and moderately (27%) but significantly increased the de novo formation of KYNA (Fig. 7A). An analogous trend was seen in the PBS-injected, contralateral striatum, though the effect of enhanced KYNA production did not reach statistical significance (Fig. 7B).
Figure 7.
Effect of UPF 648 (0.1 mM; “U”) on the de novo synthesis of tritiated KP metabolites in unilaterally QUIN-lesioned animals. As detailed in the text, separate groups of animals, unilaterally lesioned with QUIN in the striatum, received bilateral infusions of either 3H-kynurenine alone (n = 11; open bars) or 3H-kynurenine and the KMO inhibitor (n = 7; solid bars). Bargraphs illustrate between-group comparisons of the effects of UPF 648 in (A) the lesioned (“Q”) and (B) the contralateral, PBS-treated (“S”) striatum, respectively. Data are the mean + SEM. ** p < 0.01 and * p < 0.05 vs. the striatum infused with 3H-kynurenine alone (unpaired t-test).
Combined effect of BFF 122 and UPF 648 on KP metabolism in the excitotoxically lesioned striatum in vivo
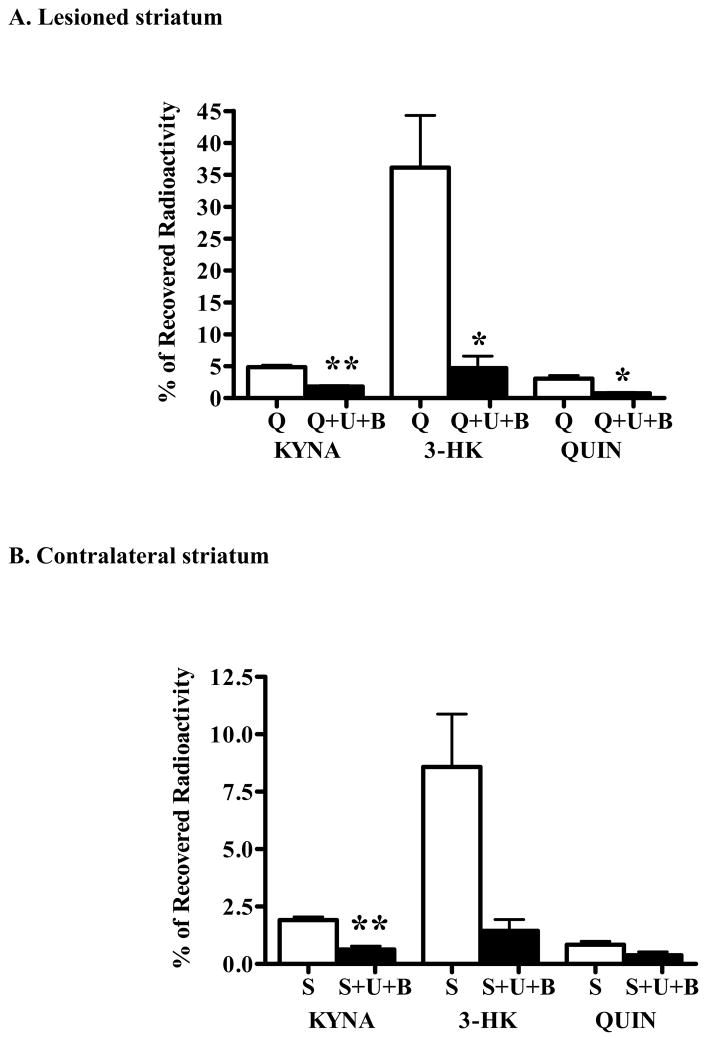
In a final experiment, we assessed the effects of the simultaneous inhibition of KAT II and KMO on KP metabolism in the chronically lesioned striatum. To this end, animals were bilaterally co-injected with a cocktail containing 1 mM BFF 122, 0.1 mM UPF 648 and 3H-kynurenine. Compared to controls (i.e. lesioned rats receiving 3H-kynurenine only; see above), KYNA, 3-HK and QUIN neosynthesis in the lesioned striatum was reduced by 63%, 87% and 75%, respectively (Fig. 8A). A similar profile was found in the contralateral striatum, where the formation of the three neuroactive KP metabolites was reduced by 67%, 83% and 55%, respectively (Fig. 8B).
Figure 8.
Combined effect of BFF 122 (1 mM) and 0.1 mM UPF 648 (0.1 mM) on the de novo synthesis of tritiated KP metabolites in unilaterally QUIN-lesioned animals. As detailed in the text, separate groups of animals, unilaterally lesioned with QUIN in the striatum, received bilateral infusions of either 3H-kynurenine alone (n = 11; open bars) or 3H-kynurenine and the two enzyme inhibitors (“U+B”; n = 4; solid bars). Bargraphs illustrate between-group comparisons of the effects of BFF 122 + UPF 648 in (A) the lesioned (“Q”) and (B) the contralateral, PBS-treated (“S”) striatum, respectively. Data are the mean + SEM. ** p <0.01 and * p < 0.05 vs. the striatum infused with 3H-kynurenine alone (unpaired t-test).
DISCUSSION
The present study addressed the question whether and to what extent the two segments of the KP, leading to the neuroactive compounds KYNA, and 3-HK and QUIN, respectively, are functionally related in the rat brain in vivo. Because of the purported role of these KP metabolites in brain physiology and pathology, this issue has been widely annotated in the recent literature, and it is frequently presumed that the two metabolic routes are closely associated and interdependent. However, for lack of appropriate tools, this assumption had so far not been validated experimentally. Our results demonstrate a remarkable functional segregation of the two KP branches when pathway metabolism is stimulated by a single, acute surge in cerebral L-kynurenine levels.
To investigate the possible link between the KYNA and the QUIN branch of the KP in the rat brain, we took advantage of the availability of two pharmacological agents, the newly synthesized KAT II inhibitor BFF 122, whose specificity for KAT II was ascertained in vitro prior to its use in the living animal, and the specific KMO inhibitor UPF 648 (Amori, 2003; Pellicciari et al., 2003). The acute effects of these compounds were studied in the rat striatum using an in vivo radiotracing method, in which the de novo synthesis of KYNA and 3-HK and its downstream metabolite, the excitotoxin QUIN, were determined 2 h following an intrastriatal injection of 3H-kynurenine. This methodology permits the simultaneous, sensitive quantification of all three neuroactive KP metabolites and therefore reveals the relative efficacies of the two alternative pathways of kynurenine degradation.
The acute synthesis rate of 3H-KYNA, 3H-3-HK and 3H-QUIN in the striatum of naïve rats was essentially identical to that reported by Guidetti et al. (1995), with an approximate ratio of 1 to 1.7 to 0.2 for the formation of the three metabolites. Co-infusion of 3H-kynurenine with BFF 122 or UPF 648, at concentrations shown to inhibit KAT II or KMO selectively, caused attenuations of the synthesis of KYNA and 3-HK, respectively. The effects were specific for the KP branch containing the compounds’ target enzyme, i.e. KAT II blockade did not influence 3-HK and, similarly, KMO inhibition did not affect KYNA formation. These data provided the first evidence for a functional segregation of the two enzymatic cascades that are initiated by the pivotal metabolite kynurenine (Fig. 1).
Similar effects were observed when the enzyme inhibitors were applied together with an excitotoxic amount of QUIN (360 nmoles). These experiments were designed to address the question whether an acute excitotoxic insult, which rapidly causes an enhanced production of KYNA (Wu and Schwarcz, 1996; Ceresoli-Borroni et al., 1999), alters the normal equilibrium between the two KP branches. Only a trend toward reduced 3H-3-HK formation was seen during this early stage of excitotoxic degeneration, but the results did not attain statistical significance. The use of BFF 122 or UPF 648 then demonstrated that KAT II and KMO maintain their functional segregation in the face of a harmful neuronal insult.
The situation was again similar, but not identical, in the chronically neuron-depleted striatum, using rats that had received an intrastriatal QUIN (300 nmoles) injection 7 days earlier. In these animals, which had lost the vast majority of intrinsic neurons and efferents (Schwarcz et al., 1983), the incorporation of kynurenine into all three of its neuroactive downstream metabolites was substantially enhanced. This was in agreement with previous findings and further confirmed the preferential, though not exclusive (Guillemin et al., 2007), non-neuronal localization of the KP in the rat brain (Guidetti et al., 1995). In the lesioned striatum, BFF 122 – which was separately shown to maintain its potency and selectivity in neuron-depleted tissue (Table 3) –, still reduced the de novo synthesis of KYNA substantially without affecting 3-HK and QUIN formation. The use of UPF 648, which is also equipotent in normal and lesioned tissue, illustrated that the neosynthesis of 3-HK can be successfully attenuated pharmacologically under conditions of extensive neuron loss and pronounced astro- and microglial activation (Björklund et al., 1986; Calka et al., 1996). Interestingly, these latter experiments were the only ones indicating a functional cross-talk between the two KP branches, showing a moderate elevation in newly synthesized KYNA in UPF 648-treated, lesioned striata. Interpreted teleologically, this increase – like the rapid elevation of KYNA formation seen during on acute excitotoxic insult (cf. controls in Fig. 2 and 4) – may be an effort of the tissue to enhance its neuroprotective capacity. Not unexpectedly, simultaneous inhibition of both KAT II and KMO resulted in a dramatic reduction in the formation of all KP metabolites studied.
Our results have interesting implications, both for the understanding of the basic neurobiology of kynurenines and for the suspected role of these metabolites in diseases affecting the brain. First, the availability of the selective inhibitor BFF 122 provided direct evidence that KAT II, rather than KAT I or mitAAT, is responsible for most of the rapid KYNA formation in the rat brain in vivo. While species differences exist (Guidetti et al., 2007a), these results effectively counter the argument that KAT II might not be critical or sufficiently specific to catalyze the transamination of kynurenine to KYNA (Han et al., 2008). Second, our data show that the two branches of the KP are segregated in the normal brain, supporting a proposition based on anatomical, lesion and cell culture studies, namely that KYNA formation takes place preferentially in astrocytes whereas the degradation of kynurenine to 3-HK and further to QUIN occurs mainly in microglial cells (Heyes et al., 1996; Guillemin et al., 1999, 2001). Thus, it appears that blood-borne kynurenine, after entering the brain from the periphery (Gàl and Sherman, 1980; Fukui et al., 1991), is transported into either astrocytes or microglia for further sequestration. Whereas the former process has been characterized in detail (Speciale et al., 1989), the latter is inferred from the present data and the microglia-specific expression of KMO (Guillemin et al., 2001). This cellular compartmentalization probably accounts for the fact that we observed no functional interactions between the two KP arms when we acutely inhibited either KAT II, an almost exclusively astrocytic enzyme (Guidetti et al., 2007b), or KMO.
The independence of the two branches of the KP was maintained during the early stages of excitotoxic neuronal degeneration, during which the brain produces and releases enhanced levels of the neuroprotective agent KYNA (Wu and Schwarcz, 1996; Ceresoli-Borroni et al., 1999; see above). In other words, acute blockade of KYNA synthesis in a situation of developing neuron damage does not lead to a compensatory up-regulation of the neurotoxic QUIN branch of the KP. This, as well as the failure of BFF 122 to influence 3-HK and QUIN neosynthesis in chronically neuron-depleted tissue, i.e. in a situation where cellular kynurenine uptake and the activities of KMO and other microglial KP enzymes are disproportionately activated (Speciale et al., 1989; Saito et al., 1993a,b; Guidetti et al., 1995), bodes well for the development of KYNA synthesis inhibitors as cognition enhancers in patients with neurodegenerative disorders (see below).
The modest but significant increase in KYNA neosynthesis caused by selective KMO inhibition in the lesioned striatum also has clinical ramifications and therefore deserves special consideration. Based on our demonstration that UPF 648 appeared to be especially potent in lesioned tissue (Figure 7), we favor the possibility that this effect is related to the substantial differences in the Km values of KMO (18 μM; Bender and McCreanor, 1982) and KAT II (0.7–0.9 mM depending on the co-substrate; Guidetti et al., 1997) with respect to their common substrate, kynurenine. Thus, the greatly increased flux through the QUIN branch of the KP in the chronically neuron-depleted, gliotic tissue may have made KMO more susceptible to inhibition by UPF 648, causing an overflow of kynurenine via reverse transport out of microglial cells (Kaper et al., 2007). Extracellular kynurenine would then be avidly taken up by activated astrocytes and readily converted to KYNA. This and alternative hypotheses are currently being evaluated in our laboratories.
Selective manipulations of the two branches of the KP in the brain have been repeatedly suggested to hold promise in the treatment of a wide variety of brain diseases, ranging from neurodegenerative and other neurological diseases to psychiatric disorders including schizophrenia and depression (Lapin, 1981; Schwarcz et al., 1984; Stone, 1993; Moroni, 1999; Németh et al., 2006; Erhardt et al., 2007; Oxenkrug, 2007; Müller and Schwarz, 2007; Dantzer et al., 2008). Based on an impressive catalogue of supportive pre-clinical studies, it is widely assumed that an up-regulation of KYNA function, or 3-HK- and QUIN synthesis inhibitors, might enhance the brain’s neuroprotective potential (see Schwarcz and Pellicciari, 2002, and Stone and Darlington, 2002, for reviews). Selective blockade of KAT II leading to reduced KYNA formation, on the other hand, is increasingly viewed as an innovative approach to enhance cognitive function by disinhibiting α7nACh and NMDA receptors (Chess and Bucci, 2006; Pellicciari et al., 2006; Potter et al., 2006; Bergeron et al., 2007; Chess et al., 2007). In addition, since several pathway metabolites serve distinct and diverse roles as immunomodulators, pharmacological interventions targeting KP enzymes have attracted the interest of basic and clinical (neuro)immunologists and (neuro)virologists (Christen et al., 1990; Kerr et al., 1997; Chiarugi et al., 2001a; Belladonna et al., 2007; Schroecksnadel et al., 2008).
Although the results of the present study, indicating a surprisingly clearcut functional segregation between the two KP arms in the brain, are reassuring for drug development efforts targeting KAT II or KMO, our data only speak to the effects of acute enzyme inhibition, albeit under both physiological and pathological conditions. Future studies will need to address the clinically more relevant question whether prolonged manipulations of either or both KP arms have similarly distinct consequences.
Acknowledgments
We dedicate this publication to the memory of our dear friend Paolo Guidetti, who passed away on December 28, 2007. We are grateful to Dr. S. Ono for the synthesis of BFF 122 and Dr. M. Okuyama for valuable comments on the manuscript. This work was supported by USPHS grants NS57715 and AG22074.
References
- Amori L. PhD Dissertation. University of Perugia; 2003. Disegno e sintesi di nuovi potenziali inibitori della chinurenina 3-idrossilasi, enzima della via metabolica delle chinurenine. [Google Scholar]
- Alkondon M, Pereira EF, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belladonna ML, Puccetti P, Orabona C, Fallarino F, Vacca C, Volpi C, Gizzi S, Pallotta MT, Fioretti MC, Grohmann U. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007;84:S17–S20. doi: 10.1097/01.tp.0000269199.16209.22. [DOI] [PubMed] [Google Scholar]
- Bender DA, McCreanor GM. The preferred route of kynurenine metabolism in the rat. Biochim Biophys Acta. 1982;717:56–60. doi: 10.1016/0304-4165(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Wu H-Q, Potter MC, Guidetti P, Schwarcz R. Mice with reduced brain kynurenic acid levels have high extracellular glutamate and show enhanced LTP in the hippocampus. Soc Neurosci Abstr. 2007;32:807.15. [Google Scholar]
- Björklund H, Olson L, Dahl D, Schwarcz R. Short-and long-term consequences of intracranial injections of the excitotoxin quinolinic acid as evidenced by GFA immunohistochemistry of astrocytes. Brain Res. 1986;371:267–277. doi: 10.1016/0006-8993(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Calka J, Wolf G, Schmidt W. Induction of cytosolic NADPH-diaphorase/nitric oxide synthase in reactive microglia/macrophages after quinolinic acid lesions in the rat striatum: an electron and light microscopical study. Histochem Cell Biol. 1996;105:81–89. doi: 10.1007/BF01450881. [DOI] [PubMed] [Google Scholar]
- Carpenedo R, Chiarugi A, Russi P, Lombardi G, Carlà V, Pellicciari R, Mattoli L, Moroni F. Inhibitors of kynurenine hydroxylase and kynureninase increase cerebral formation of kynurenate and have sedative and anticonvulsant activities. Neuroscience. 1994;61:237–243. doi: 10.1016/0306-4522(94)90227-5. [DOI] [PubMed] [Google Scholar]
- Ceresoli G, Guidetti P, Schwarcz R. Metabolism of [5-3H]-kynurenine in the developing rat brain in vivo: effect of intrastriatal ibotenate injections. Dev Brain Res. 1997;100:73–81. doi: 10.1016/s0165-3806(97)00029-1. [DOI] [PubMed] [Google Scholar]
- Ceresoli-Borroni G, Guidetti P, Schwarcz R. Acute and chronic changes in kynurenate formation following an intrastriatal quinolinate injection in rats. J Neural Transm. 1999;106:229–242. doi: 10.1007/s007020050153. [DOI] [PubMed] [Google Scholar]
- Ceresoli-Borroni G, Guidetti P, Amori L, Pellicciari R, Schwarcz R. Perinatal kynurenine 3-hydroxylase inhibition in rodents: pathophysiological implications. J Neurosci Res. 2007;85:845–854. doi: 10.1002/jnr.21183. [DOI] [PubMed] [Google Scholar]
- Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326–332. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarugi A, Cozzi A, Ballerini C, Massacesi L, Moroni F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience. 2001a;102:687–695. doi: 10.1016/s0306-4522(00)00504-2. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Meli E, Moroni F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J Neurochem. 2001b;77:1310–1318. doi: 10.1046/j.1471-4159.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect Immun. 2005;73:5249–5251. doi: 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92:203–209. doi: 10.1016/j.physbeh.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Gàl EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res. 1980;5:223–239. doi: 10.1007/BF00964611. [DOI] [PubMed] [Google Scholar]
- Giorgini F, Guidetti P, Nguyen Q, Bennett SC, Muchowski PJ. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat Genet. 2005;37:526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini F, Möller T, Kwan W, Zwilling D, Wacker JL, Hong S, Tsai LC, Cheah CS, Schwarcz R, Guidetti P, Muchowski PJ. Histone deacetylase inhibition modulates kynurenine pathway activation in yeast, microglia, and mice expressing a mutant huntingtin fragment. J Biol Chem. 2008;283:7390–7400. doi: 10.1074/jbc.M708192200. [DOI] [PubMed] [Google Scholar]
- Grégoire L, Rassoulpour A, Guidetti P, Samadi P, Bédard PJ, Izzo E, Schwarcz R, Di Paolo T. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav Brain Res. 2008;186:161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5–3H]kynurenine in the rat +brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem. 1995;65:2621–2632. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Schwarcz R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur J Neurosci. 1999;11:3857–3863. doi: 10.1046/j.1460-9568.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007a;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007b;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Armati PJ, Brew BJ. Kynurenine pathway metabolism in human astrocytes. Adv Exp Med Biol. 1999;467:125–131. doi: 10.1007/978-1-4615-4709-9_18. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Cai T, Tagle DA, Robinson H, Li J. Substrate specificity and structure of human aminoadipate aminotransferase/kynurenine aminotransferase II. Biosci Rep. 2008;28:205–215. doi: 10.1042/BSR20080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Quearry BJ. Quantification of 3-hydroxykynurenine in brain by high-performance liquid chromatography and electrochemical detection. J Chromatogr. 1988;428:340–344. doi: 10.1016/s0378-4347(00)83925-0. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320:595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper T, Looger LL, Takanaga H, Platten M, Steinman L, Frommer WB. Nanosensor detection of an immunoregulatory tryptophan influx/kynurenine efflux cycle. PLoS Biol. 2007;5:e257. doi: 10.1371/journal.pbio.0050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SJ, Armati PJ, Pemberton LA, Smythe G, Tattam B, Brew BJ. Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages. Neurology. 1997;49:1671–1681. doi: 10.1212/wnl.49.6.1671. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Kynurenines and seizures. Epilepsia. 1981;22:257–265. doi: 10.1111/j.1528-1157.1981.tb04108.x. [DOI] [PubMed] [Google Scholar]
- Miranda AF, Boegman RJ, Beninger RJ, Jhamandas K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience. 1997;78:967–975. doi: 10.1016/s0306-4522(96)00655-0. [DOI] [PubMed] [Google Scholar]
- Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Peruginelli F, Carpenedo R, Pellegrini-Giampietro DE. Neuroprotective effects of kynurenine-3-hydroxylase inhibitors in models of brain ischemia. Adv Exp Med Biol. 1999;467:199–206. doi: 10.1007/978-1-4615-4709-9_26. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Németh H, Toldi J, Vécsei L. Kynurenines, Parkinson’s disease and other neurodegenerative disorders: preclinical and clinical studies. J Neural Transm Suppl. 2006;70:285–304. doi: 10.1007/978-3-211-45295-0_45. [DOI] [PubMed] [Google Scholar]
- Okuno E, Schmidt W, Parks DA, Nakamura M, Schwarcz R. Measurement of rat brain kynurenine aminotransferase at physiological kynurenine concentrations. J Neurochem. 1991;57:533–540. doi: 10.1111/j.1471-4159.1991.tb03783.x. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Ann N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Amori L, Costantino G, Giordani A, Macchiarulo A, Mattoli L, Pevarello P, Speciale C, Varasi M. Modulation of the kynurine pathway of tryptophan metabolism in search for neuroprotective agents. Focus on kynurenine-3-hydroxylase. Adv Exp Med Biol. 2003;527:621–628. doi: 10.1007/978-1-4615-0135-0_71. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Rizzo R, Costantino G, Marinozzi M, Amori L, Guidetti P, Wu HQ, Schwarcz R. Modulators of the kynurenine pathway of tryptophan metabolism. Synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. Chem Med Chem. 2006;1:528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Guidetti P, Calu DJ, Schwarcz R. Improved behavioral performance of kynurenine aminotransferase II-deficient mice in hippocampus-based learning and memory paradigms. Soc Neurosci Abstr. 2006;32:265.9. [Google Scholar]
- Reinhard JF., Jr Pharmacological manipulation of brain kynurenine metabolism. Ann N Y Acad Sci. 2004;1035:335–349. doi: 10.1196/annals.1332.020. [DOI] [PubMed] [Google Scholar]
- Richter A, Hamann M. The kynurenine 3-hydroxylase inhibitor Ro 61-8048 improves dystonia in a genetic model of paroxysmal dyskinesia. Eur J Pharmacol. 2003;478:47–52. doi: 10.1016/j.ejphar.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Saito K, Nowak TS, Jr, Markey SP, Heyes MP. Mechanism of delayed increases in kynurenine pathway metabolism in damaged brain regions following transient cerebral ischemia. J Neurochem. 1993a;60:180–192. doi: 10.1111/j.1471-4159.1993.tb05836.x. [DOI] [PubMed] [Google Scholar]
- Saito K, Quearry BJ, Saito M, Nowak TS, Jr, Markey SP, Heyes MP. Kynurenine 3-hydroxylase in brain: species activity differences and effect of gerbil cerebral ischemia. Arch Biochem Biophys. 1993b;307:104–109. doi: 10.1006/abbi.1993.1567. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel K, Sarcletti M, Winkler C, Mumelter B, Weiss G, Fuchs D, Kemmler G, Zangerle R. Quality of life and immune activation in patients with HIV-infection. Brain Behav Immun. 2008;22:881–889. doi: 10.1016/j.bbi.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Foster AC, French ED, Whetsell WO, Jr, Köhler C. Excitotoxic models for neurodegenerative disorders. Life Sci. 1984;35:19–32. doi: 10.1016/0024-3205(84)90148-6. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Speciale C, Hares K, Schwarcz R, Brookes N. High-affinity uptake of L-kynurenine by a Na+-independent transporter of neutral amino acids in astrocytes. J Neurosci. 1989;9:2066–2072. doi: 10.1523/JNEUROSCI.09-06-02066.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Turski WA, Gramsbergen JB, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J Neurochem. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Schwarcz R. Seizure activity causes elevation of endogenous extracellular kynurenic acid in the rat brain. Brain Res Bull. 1996;39:155–162. doi: 10.1016/0361-9230(95)02087-x. [DOI] [PubMed] [Google Scholar]