Abstract
Functional networks in the human brain give rise to complex cognitive and perceptual abilities. While the decrease of functional connectivity is linked to neurological and psychiatric disorders, less is known about the consequences of increased functional connectivity. One population that has exceptionally enhanced perceptual abilities is people with absolute pitch (AP) — an ability to categorize tones into pitch classes without reference. AP has been linked to exceptional talent as well as to psychiatric and neurological conditions. Here we show that AP possessors have increased functional activation during music listening, as well as increased degrees, clustering, and local efficiency of functional correlations, with the difference being highest around the left superior temporal gyrus. Our results provide the first evidence that increased functional connectivity in a small-world brain network is related to exceptional perceptual abilities in a healthy population.
Keywords: Pitch, Emotion, Music, fMRI, Small-world network
Introduction
The concept of the human connectome as a comprehensive description of structural and functional brain networks is a focus of recent interest in neuroscience (Achard et al., 2006; Bullmore and Bassett, 2011; Sporns, 2011). Studies have identified networks of brain regions that are intrinsically connected or are synchronously activated by certain tasks (Fox et al., 2005; Reijneveld et al., 2007). These networks of functional connectivity mediate behavioral performance on complex behaviors such as perception, memory, and emotional processing (Buchsbaum et al., 2005; Caclin and Fonlupt, 2006; Ginestet and Simmons, 2011), and are impaired in neurological and/or psychiatric disorders and conditions such as autism, Alzheimer's disease, obsessive-compulsive disorder, schizophrenia, and synesthesia (Bassett et al., 2008; Hanggi et al., 2011; Just et al., 2004; Liu et al., 2008; Supekar et al., 2008; Whitfield-Gabrieli et al., 2009; Zhang et al., 2011). Understanding the relationship between functional connectivity and behavior will inform a comprehensive description of the human brain.
While impairments of functional connectivity in neurological or psychiatric disorders are informative for understanding the human brain network, an equally informative approach is to relate exceptional, above-normal behaviors to functional brain networks. One model group of healthy individuals known to have an above-normal behavioral phenotype as well as increased structural connectivity is people with absolute pitch. Absolute pitch (AP) is the ability to name musical pitches without a reference. It has traditionally been viewed as a sign of talent or giftedness partly due to its possession throughout history by exceptional musicians such as Mozart (Ward, 1999). More recently, however, AP has also been associated with conditions such as autism and Williams syndrome, due to the increased incidence of AP in these populations (Bonnel et al., 2003; Brown et al., 2003; Heaton et al., 2008; Lenhoff et al., 2001). AP is linked to both genetic and environmental factors (Athos et al., 2007; Baharloo et al., 2000; Deutsch et al., 2006; Gregersen et al., 2001), which possibly interact at the level of brain structure and function. Due to its uniqueness at the levels of behavioral characteristics, brain structure and function, and population distributions, AP has been posited as a unique model for understanding the influence of genes and development on neural and cognitive function (Zatorre, 2003). Furthermore, the increased incidence of AP in neurological and/or psychiatric disorders such as autism, combined with autism-like performance on various tasks in people with AP (Brown et al., 2003), suggests that AP may be an optimal model for understanding these conditions in a healthy population that is free from comorbid disorders.
The AP brain has known characteristics in both structure and function. Structural neuroimaging revealed increased leftward asymmetry of the posterior superior temporal lobe (planum temporale) in AP musicians (Keenan et al., 2001; Schlaug et al., 1995). Diffusion Tensor Imaging (DTI) of white matter showed increased structural connectivity between the superior temporal gyrus (STG) and middle temporal gyrus (MTG) in AP possessors (Loui et al., 2011), especially in the left hemisphere, which correlated with behavioral assessments of AP acuity (cf.Bermudez and Zatorre, 2009). Recent results from graph theory as an approach to compare cortical thickness between AP subjects and controls showed a decrease in overall degrees of correlations in AP subjects, but an increase in degrees in superior temporal regions (Jancke et al., 2012). Functionally, AP musicians elicit increased activations in the left superior temporal sulcus in a pitch memory task compared to non-AP musicians (Schulze et al., 2009) and increased perceptual processing at the primary cortical level as well as decreased working memory demands as evidenced by ERP studies (Itoh et al., 2005; Klein et al., 1984). Given that AP is linked to increases in structural asymmetry, structural connectivity, and functional activity in superior temporal regions, we expected that functional connectivity would be increased in AP subjects, especially in superior temporal regions during music listening.
In this study we asked how AP and non-AP brains differ in functional activations and functional connectivity in a music-related task. Task fMRI, specifically the task of emotional arousal judgment, was chosen because it requires music listening, thus stimulating brain regions that are sensitive to musical stimuli. At the same time, the emotional judgment task drives the direction of attention towards features that are unrelated to absolute pitch. This minimizes behavioral differences between the groups that might confound the functional differences in brain activity. Graph theory was used in this sparse-sampled task fMRI study to test whether AP subjects recruit enhanced functional networks compared to controls during music listening, and whether results were dependent on task-driven activations.
Materials and methods
For the present fMRI study we asked how the functional network of the AP brain might differ from the non-AP brain during music listening. To design a functional MRI task that requires music listening, but does not bias the results towards AP possessors (e.g. using a pitch-labeling task would bias results towards AP possessors), we employed a musical task that can reliably be performed regardless of AP possession: the task of emotional arousal judgment. Emotions can be construed as being a two-dimensional space between valence and arousal (Russell, 1980), which applies to various stimuli including music (Schubert, 2004). Previous results on music and emotion (Bachorik et al., 2009) had shown that arousal in music can be parametrically manipulated to elicit reliable and consistent behavioral ratings of arousal. Thus, for the present music listening fMRI task we chose music that was parametrically varied in arousal, and performed an fMRI study on AP possessors and matched controls while they listened to music compared to a silent rest condition. The functional activations elicited from this task were then compared between AP subjects and controls who were matched in age, sex, ethnicity, IQ, and number of years and age of onset of musical training (see Materials and methods for details). Graph theory analysis (Rubinov and Sporns, 2010) was used to compare the small-world properties of functional brain networks between AP and control groups.
Subjects
Thirty healthy volunteers (15 AP musicians and 15 non-AP musician controls) were recruited via advertisements online and at local music schools and conservatories. Subjects were matched for age, sex, ethnicity, and number of years and age of onset of musical training. All subjects in both groups were right-handed, as determined using the Edinburgh Handedness Inventory (Oldfield, 1971). Average age was 25 (SD=5) for AP possessors and 26 (SD=5) for non-AP possessors. Average age of onset of musical training was 6 years for both groups (SD=2.8 for APs; 1.6 for non-APs). Average number of years of musical training was 16 years (SD=6 years) in the AP group and 17 (SD=6.75 years) in the non-AP group. The ethnic distribution was also matched between the groups, with 10 Caucasians and 5 East Asians in the AP group and 9 Caucasians and 6 East Asians in the non-AP group. Five of the East Asian subjects in each group reported speaking a tonal language fluently (Mandarin and Cantonese Chinese). IQ as assessed using the Shipley's verbal and abstract tests (see Behavioral procedure) was 120 (SD=5.2) in the AP group and 118 (SD=3.6) in the non-AP group. T-tests confirmed that there were no significant between-group differences in any of these variables (all p's>.3).
Behavioral procedures
A survey was administered to all subjects to assess their linguistic and musical background. To control for possible between-group differences in IQ, we conducted Shipley's verbal and abstract tests (Shipley, 1940), which have been shown to be a predictor of IQ (Paulson and Lin, 1970).
AP was confirmed using an established pitch labeling test (Keenan et al., 2001; Loui et al., 2011) in which 52 trials were presented. Each trial contained one computer-generated sine wave tone (500-ms duration with a 50-ms rise and decay time) with a fundamental frequency ranging from 370 Hz (F#3) to 739.97 Hz (F#4) in the equal-tempered Western scale. The subject's task was to label each pitch by writing down the letter name (including any accidentals) on an answer sheet upon hearing each tone. The inter-tone interval was 2 s. In accordance to previous studies (Bermudez and Zatorre, 2009), subjects were classified as AP possessors if they scored a mean deviation of 1.0 semitone or less.
As a follow-up analysis, subjects were categorized as AP1 if they scored a mean deviation of less than 0.5 semitones, AP2 if their mean deviation score was between 0.5 and 1.5 semitones, and non-AP if their mean deviation score was above above 1.5 semitones.
Stimuli
Musical clips that were presented in the fMRI were chosen from a larger battery of musical stimuli that had been previously rated for emotional valence and arousal (Bachorik et al., 2009) and were shown to elicit consistent and reliable arousal ratings. Audio stimuli consisted of 12-s clips of music from different genres, with rise and fall times of 500 ms respectively. All audio stimuli were loudness-normalized to avoid arousal effects being due to differences in loudness alone.
fMRI data acquisition
All images were acquired in a 3 T General Electric scanner. A T1-weighted anatomical image with a voxel resolution of 0.93×0.93×1.5 mm was acquired in addition to three runs (with 26 acquisitions each) of gradient echo echo-planar imaging (EPI) using a sparse temporal sampling paradigm (Gaab et al., 2003; Ozdemir et al., 2006). The T2*-weighted EPI sequence had an effective repetition time (TR) of 15 s, an echo time (TE) of 30 ms, an acquisition time (TA) of 1.8 s for 26 axial slices with an acquisition matrix of 64×64 resulting in a voxel size of 3.8×3.8×4 mm3. Twenty-six whole brain volumes were acquired in each of three functional runs, each of which included 2 dummy volumes to allow time for steady state magnetization resulting in a total of 72 acquisitions (3 runs×24 acquisitions) across the music and rest conditions. Order of music and rest trials was counterbalanced. In the “Music” condition, subjects listened to 12-s musical sound clips, followed by a 500 ms burst of white noise. Subjects’ task was to make judgments on the level of emotional arousal in each sound clip after the short noise burst via a button-press. In a control condition (“Rest”), subjects heard silence followed by the 500 ms noise burst, which was monaurally presented in a counterbalanced order. Upon hearing the noise burst, subjects’ task was to indicate via button-press whether the noise came from the left ear or the right. The purpose of the fMRI task was to identify a network of regions related to listening to music with different levels of emotional arousal. This network would then be compared between AP and control groups.
fMRI data analysis
FMRI data analysis was done in MATLAB and the SPM5 toolbox (Friston et al., 1994). Images were realigned, normalized using SPM5's EPI template, and smoothed using an 8 mm Gaussian kernel. Each trial was modeled using a Finite Impulse Response (FIR) at the first level. Music and rest trials were modeled separately at the first level. Each first-level contrast image was then entered into a second-level analysis comparing AP and non-AP subjects’ responses to music compared to rest conditions.
Graph theory analysis of functional connectivity
To compare the functional networks between two groups, small-world brain networks provide a useful approach to investigations of functional connectivity, both in resting-state fMRI data and in task-related fMRI data (Bassett and Bullmore, 2006; Ginestet and Simmons, 2011; Hagmann et al., 2008; Reijneveld et al., 2007) in normal populations as well as in special populations such as individuals with schizophrenia, temporal lobe epilepsy, high-functioning autism, obsessive compulsive disorder, and grapheme-color synesthesia (Bassett et al., 2008; Hanggi et al., 2011; Koshino et al., 2005; Liu et al., 2008; Zhang et al., 2011). The network statistics that can be gleaned from graph theory analysis yield powerful information about the community structure of brain regions in different groups of subjects, that cannot be accomplished using conventional measures of functional connectivity (e.g. bivariate or partial correlation). To conduct graph theory analysis, fMRI time-series data were extracted from the 90 cerebral regions defined by the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002), an anatomical parcellation that interfaces with SPM (Friston et al., 1994) and has been used for automated labeling of functional activations in several previous graph theory analyses (Achard et al., 2006; Liu et al., 2008). ROIs from the AAL atlas were reduced in size using the fslmaths -ero function in FSL (Smith et al., 2004); this was to ensure that each ROI covered only gray matter, was similar in size between the two hemispheres (particularly important if the two groups differ in hemispheric asymmetry), and was limited to the same region for each subject. After its size was reduced, each ROI was then masked with the gray matter mask that was segmented using SPM from each individual subject's anatomical (T1) scan using SPM's VBM toolbox and then thresholded to include only voxels above the 90th percentile in gray matter signal. The resulting ROIs were not significantly different in volume (mm3) between AP and non-AP groups (t((178)=–0.11, n.s.), nor were they different in volume between the left and right hemispheres for either group (This was confirmed by a 2-way ANOVA with factors of Group and Hemisphere: Group: F(1,176)=0.01, n.s. Hemisphere: F(1,176)=0.02, n.s. Group×Hemisphere interaction: F(1,176)<0.001, n.s.). The time-series were extracted for each ROI using MarsBar (Brett et al., 2002) and normalized by the mean of each run to remove global effects of each run. The time-series for each ROI was then averaged separately across all AP and non-AP subjects to obtain a mean time-series for each ROI for each group. Bivariate correlations were performed between each pair of ROIs to obtain a 90×90 correlation matrix for each group. This correlation matrix was used for small-world network analysis using the Brain Connectivity Toolbox in MATLAB (Rubinov and Sporns, 2010). A series of correlation values from 0.05 to 0.55 were tested as the cutoff threshold for significant correlation. For each threshold level, we computed the network characteristics of degree, connection strength, clustering coefficient, and local efficiency. The degree is the most basic network measure that indicates the number of connections to each node. The clustering coefficient is a useful measure of functional segregation, indicating the fraction of neighboring nodes of each node that are also neighbors of each other — thus, the cliquishness of a node (Watts and Strogatz, 1998). Local efficiency is another measure of segregation; it is the inverse of the average shortest distance between each node in a subgraph and reveals the efficiency of each node within the network in transporting information. Strength is the sum of weights of links connected to each node (Latora and Marchiori, 2001). Significance for network statistics was evaluated at a correlation threshold level of r= 0.5. Finally, network statistics were visualized separately for the AP and non-AP groups for a visual comparison of network statistics within and between groups: each network is shown with 90 nodes where degree is represented by size of each node and clustering coefficient is represented by color of each node.
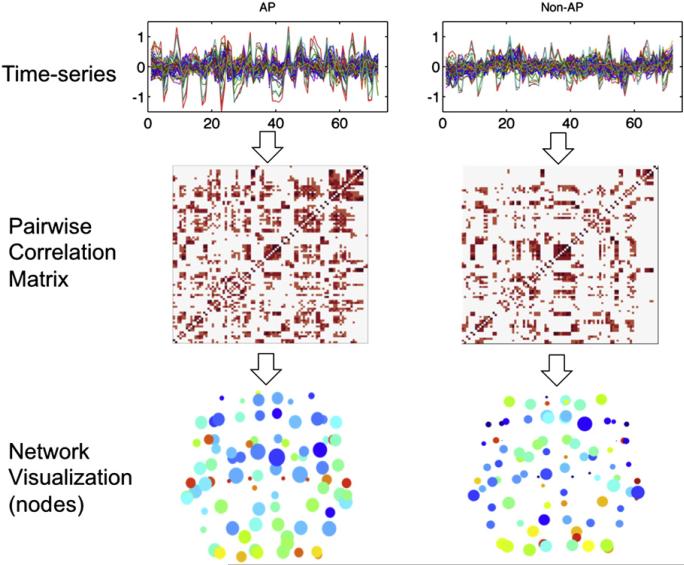
In follow-up analyses to determine whether differences in network statistics were driven by the music-listening trials or the rest trials, graph theory analysis was conducted separately for data collected from Music condition scans (48 acquisitions) and Rest condition scans (24 acquisitions). As the sparse-temporal sampling paradigm used a long TR of 15 s, we expected that relatively little of the BOLD signal from each TR would result from carryover effects of the previous TR. The normalized BOLD signal from each ROI of each subject was extracted separately for scans following Music trials and following Rest trials. Bivariate correlations were performed for each pair of ROIs to obtain a 90×90 correlation matrix for each subject's Music and Rest conditions. These correlation matrices were averaged between subjects in each group to obtain a mean correlation matrix for each group. Network statistics (degree, strength, clustering, local efficiency) were computed from the mean correlation matrix after thresholding was applied at correlation coefficients ranging from 0.05 to 0.55. Significance for differences in network statistics was evaluated again at the correlation threshold of r=0.5. An overview of the small world network analysis pipeline is shown in Fig. 4.
Fig. 4.
Network analysis pipeline for functional connectivity. Top: mean time-series data were obtained from fMRI scans of both AP and non-AP groups. Middle: Pairwise correlation matrices were obtained between every pair of regions from the modified AAL atlas. Bottom: Network statistics were calculated and visualized in brain space. Axial views of graphs obtained from both subject groups are shown here, with size of each node corresponding to degrees and color of each node corresponding to clustering coefficient.
Results
Behavioral ratings
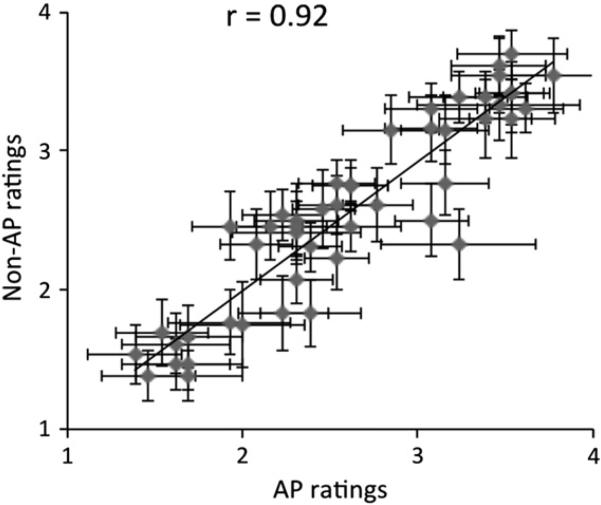
All subjects were consistently able to make arousal ratings for musical clips in the scanner. Fig. 1 shows a correlation between AP and non-AP subjects’ average ratings for each song. Arousal ratings of AP and non-AP subjects were highly correlated (r=0.92, p<0.0001) and not significantly different from each other (t(28)=0.39, p=0.70). These behavioral data confirm that the task engaged both groups similarly, and that neuroimaging results are not explained by behavioral confounds.
Fig. 1.
Behavioral results from arousal ratings for music. Each point represents mean ratings for AP group (X-axis) and the non-AP group (Y-axis) for a single trial. Error bars represent between-subject standard errors. Behavioral responses from the two groups are highly correlated.
Higher activations in AP: whole brain fMRI
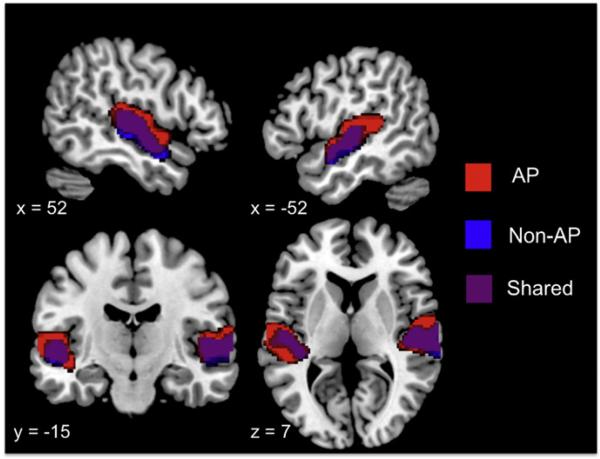
In response to music, both groups of subjects showed significant activations in the bilateral Heschl's gyrus (HG), superior temporal gyrus (STG), and middle temporal gyrus (MTG), with the extent of the activations being larger in the AP group. Fig. 2 shows activations in each group of subjects in the music vs. rest contrast at the p<0.05 FWE level.
Fig. 2.
Second-level activations for all music vs. rest for the AP group (red) and the non-AP group (blue), and the overlap between the groups (purple). Results are at the p<0.05 (FWE-corrected) level, showing activations in the bilateral Heschl's gyri, superior temporal gyri and middle temporal gyri, with a wider spread of activations in the AP group, especially in the left superior temporal gyrus. (For interpretation of the references to color in this figure legend, the reader is referred to the web of this article.)
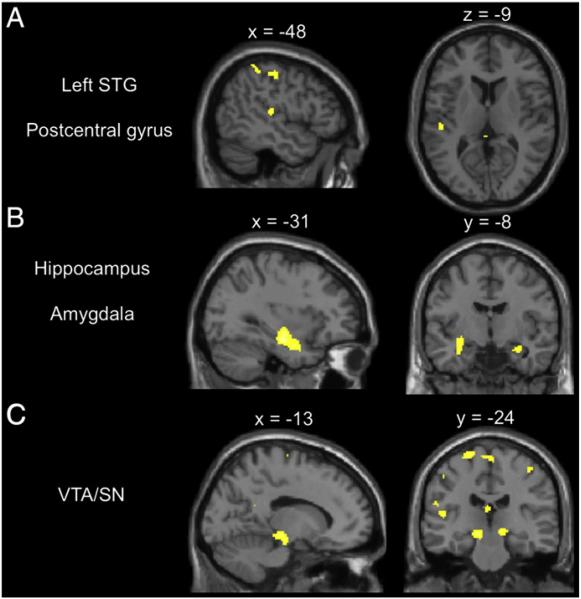
In a direct contrast between AP and non-AP groups, the AP group showed higher activations in the left STG, a region known to be important in sound perception. AP subjects also showed higher activations in the postcentral gyrus and superior parietal lobule, regions known to be involved in multisensory integration. In addition, AP subjects also showed higher activations in the left and right amygdala, hippocampus, and ventral tegmental area or substantia nigra (VTA/SN in the midbrain) in the limbic and dopaminergic reward-processing systems. Fig. 3 shows the direct contrast of AP vs. non-AP subjects at the p<0.05 (cluster-corrected) level. The finding of simultaneously higher functional activations in the left STG and sensory-integration regions in the parietal lobe as well as emotion and reward processing regions in the hippocampus, amygdala, and VTA/SN suggest that the AP group may have increased functional connectivity between auditory regions and other regions in the brain.
Fig. 3.
Interactions between group (AP vs. non-AP) and task (music vs. rest), showing increased activations in the AP group during music listening. Results are significant at the p<0.05 (cluster-corrected) level. A) Activations in the left superior temporal gyrus (x=–48, y=–24, z=–9) and postcentral gyrus (x=–48, y=–24, z=50). B) Additional activations in the hippocampus and amygdala (x=–31, y=–8, z=–21). C) Additional activations in the ventral tegmental area/substantia nigra of the midbrain (x=–13, y=–24, z=–12).
Increased small-world network properties in AP: graph theory
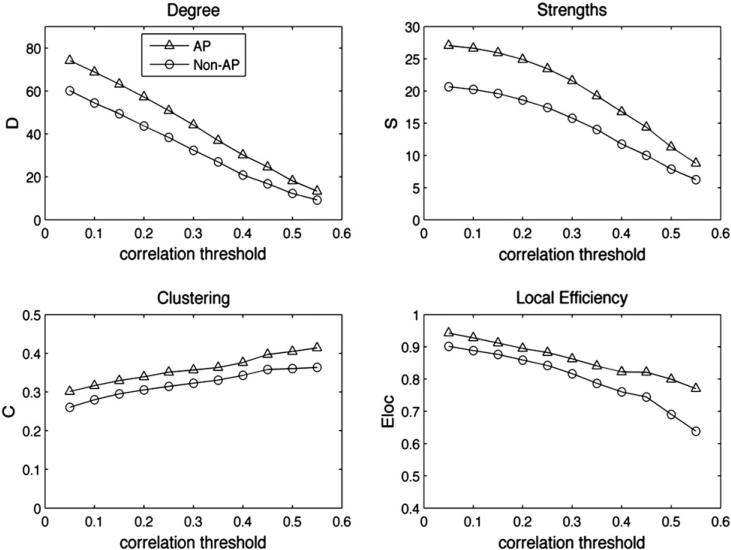
To investigate the hypothesis of increased functional connectivity in AP subjects, we conducted a graph theory analysis to compare the small-world network properties of the two groups (see Materials and methods and Fig. 4 for the analysis procedures). Compared to non-AP subjects, the AP subjects’ network showed significantly more degrees (F(1,178)=21.1, p<0.001) and increased strengths (F(1,178)=18.1, p<0.001), as well as increased local efficiency (F(1,178)=3.79, p=0.05) and increased clustering (F(1,178)=13.0, p<0.001). The differences were robust to different levels of thresholding of the connection matrix from cutoff values of r=0.05 through 0.55: for all different correlation coefficients at which thresholding was applied to the connection matrix, AP subjects showed consistently higher degrees, higher connection strengths and local efficiency, and higher clustering coefficients compared to the non-AP group (Fig. 5). To assess whether results might change with different ROIs, time-series data from the original unmodified (unreduced) AAL atlas were also extracted and used as a second dataset for graph theory analysis. Results from time-series derived from the original AAL atlas were similar to the reduced atlas: AP subjects’ network showed significantly more degrees (F(178)=41.6, p<0.001) and higher strengths (F(178)=38.8, p<0.001), as well as increased local efficiency (F(178)=34.8, p<0.001) and higher clustering (F(178)=15.6, p<0.001). Since the unmodified AAL atlas yielded the same pattern of results as the reduced atlas, in the following analyses we used the reduced set of ROIs, as these are verified as well-matched in size and covering only gray matter in both groups of subjects.
Fig. 5.
Small-world network statistics for the whole brain comparing AP and non-AP groups in degree, strength, clustering, and local efficiency for networks thresholded at correlation strengths of r=0.05 through 0.55.
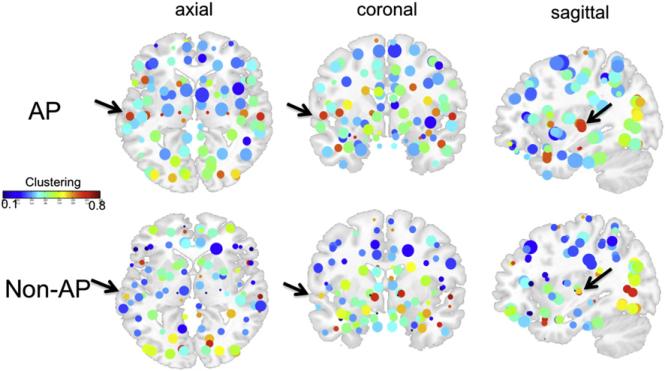
Fig. 6 shows the functional network of AP and non-AP groups, where the size of each node represents the degree of the corresponding ROI, whereas the color of each node represents the clustering coefficient of the corresponding ROI. Nodes have increased degrees and clustering in the AP group, as reflected by nodes that are larger and have warmer colors in the AP network. Superior temporal regions show the warmest colors in the AP group, indicating that AP subjects have increased clustering in the superior temporal regions. In the left STG, increased clustering was verified by a z-test (Z=1.4, p<0.05) comparing the clustering of LSTG (0.68) against clustering scores in all 89 regions in the rest of the brain (mean=0.40, standard deviation=0.13). This increased clustering was not observed in the LSTG in non-AP subjects (clustering=0.56, Z=1.15, n.s.). For the right STG, increased clustering relative to the 89 remaining regions in the brain (including the LSTG) was observed in both AP subjects (clustering=0.68, Z=2.1, p<0.05) and non-AP subjects (clustering=0.74, Z=2.18, p<0.05). Taken together, the visualized network of clustering and degree statistics in the AP and non-AP brains (Fig. 6), the threshold-independent increase in network statistics in the AP brain (Fig. 5), and the z-tests comparing the left and right STG against other brain regions in the AP and non-AP groups confirm that the AP group has a network with higher degrees, strengths, clustering, and local efficiency of functional connectivity, with differences in clustering between the AP and non-AP groups being strongest in the LSTG. These findings are consistent with electro-physiological and neuroimaging results that find structural and functional differences in AP, with effects centered on the LSTG (Itoh et al., 2005; Loui et al., 2011; Schlaug et al., 1995; Schulze et al., 2009).
Fig. 6.
Functional networks from correlation matrices in AP and non-AP data overlaid on a T1 template brain. Each node is a single region of interest. Color of each node corresponds to clustering coefficients. Size of each node corresponds to degrees. Note that nodes are generally larger in the AP than in the non-AP brain, suggesting that degrees of connectivity are higher in the AP group. Furthermore, the nodes in superior temporal regions (indicated by black arrows) are brighter red in the AP group, representing increased clustering in the superior temporal lobe in the AP brain. (For interpretation of the references to color in this figure legend, the reader is referred to the web of this article.)
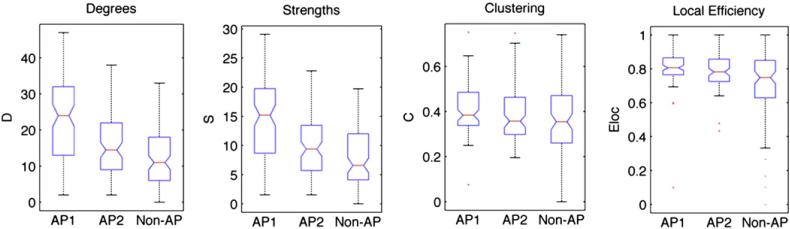
Network properties reflect behavioral acuity of AP
If small-world networks are accurate measures of the functional connectivity that is enhanced in AP subjects, then properties of the small-world networks should reflect individual subjects’ performance on pitch-categorization tests. To assess the relationship between AP acuity and small-world network properties of degree, strengths, clustering, and local efficiency, subjects were divided into AP1 (highly accurate), AP2 (mostly accurate), and non-AP (less accurate) groups based on their performance on the pitch labeling test (see Materials and methods: Behavioral procedure). This post-hoc behavioral distinction resulted in 10 AP1 subjects, 6 AP2 subjects, and 14 non-AP subjects. A comparison of the same small-world network properties revealed a consistent pattern: AP1 subjects showed highest degrees, strengths, clustering, and local efficiency, followed by AP2 subjects and then by non-AP subjects (Fig. 7). These differences in network statistics were highly significant in all cases (one-way ANOVAs comparing three groups: Degree: F(2,267)=35.4, p<0.001. Strengths: F(2,267)=33.6, p<0.001. Clustering: F(2,267)=3.05, p<0.05. Local efficiency: F(2,267)=11.8, p<0.001), surviving Bonferroni correction for post-hoc comparisons between the three groups. This link between behavior and network measures provides further support for the relationship between pitch categorization ability and the small-world network properties of the brain.
Fig. 7.
Small-world network statistics of the whole brain comparing AP1, AP2, and non-AP groups as defined post-hoc using the pitch labeling test.
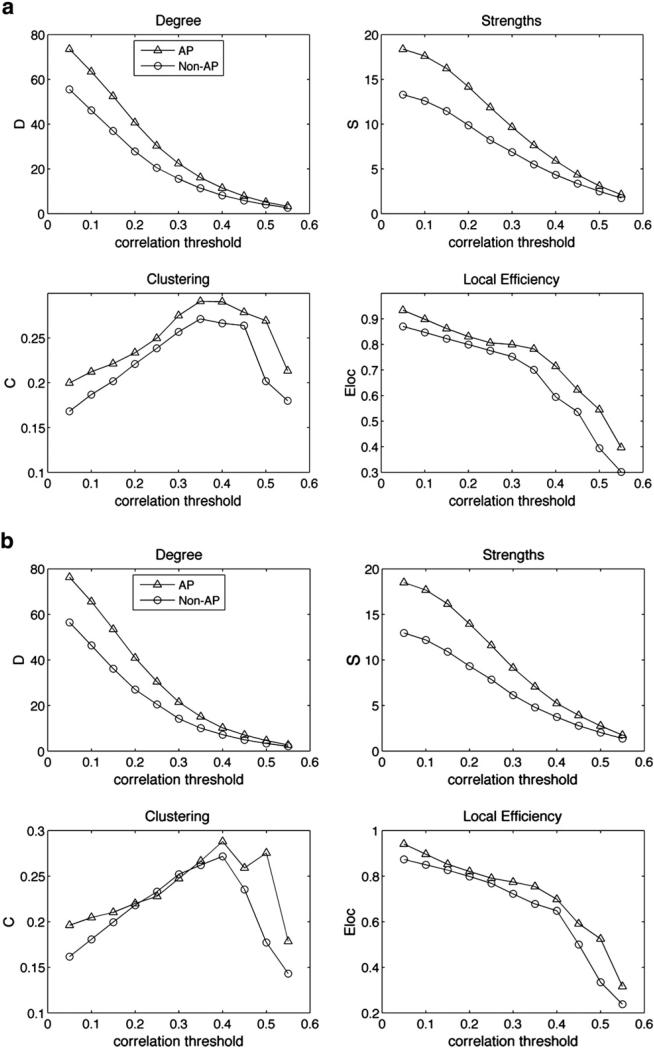
Differences in network statistics replicate for Rest-only and Music-only scans
One important question emerging from the graph theory analysis concerns whether the observed differences in network statistics, which are based on correlations between pairs of ROIs, may be completely driven by the task, or whether they would be observed even without task manipulations. To tease apart the contributions of task-driven and task-free data in these network differences between AP and control groups, we separated the sparse-sampled data into Music-only trials and Rest-only trials. Correlation matrices were obtained by pairwise correlation between time-series for each ROI obtained from the normalized sequence of scans corresponding to Music trials and Rest trials separately (see Materials and methods for details). Network statistics were obtained from each mean correlation matrix for Rest and Music trials and were again compared between groups. Results showed the same pattern of increased degrees (F(1,178)=6.4, p=0.01), strengths (F(1,178)=5.5, p=0.02), clustering (F(1,178)=4.8, p=0.03), and local efficiency (F(1,178)=7.5, p=0.006) in the AP brain even during rest trials (Fig. 8a). Similar increases in network statistics for the AP group were also observed during music trials (degree: F(1,178)=12.2, p<0.001; strengths: F(1,178)=11.5, p<0.001; clustering: F(1,178)=10.1, p=0.001; local efficiency: F(1,178)=12.3, p<0.001; Fig. 8b). These results from selected scans from the entire time-series replicate the original result of increased network statistics in the AP group and demonstrate that the differences are not explained by task manipulations.
Fig. 8.
Small-world networks statistics comparing AP and non-AP groups for correlation thresholds of 0.05 to 0.55 similar to Fig. 5, but separately for Rest condition trials (a) and Music condition trials (b).
Discussion
Results showed increased degrees and strengths of functional connections, as well as increased clustering and local efficiency in the AP brain, with the difference highest around the left superior temporal gyrus. These results provide the first evidence that increased functional connectivity in a small world network is related to exceptional perceptual abilities in a healthy population. In addition to increased functional activations in superior temporal regions that are important in sound perception and categorization, AP subjects further showed increased activations in multisensory-integration regions as well as emotion processing and reward systems during music listening. This was observed despite similar task demands and behavioral output in emotional ratings between the AP and non-AP groups. Results are consistent with fMRI studies that show increased superior temporal activations in AP subjects during the processing of speech (Oechslin et al., 2010) and leftward dominance during music processing in AP musicians in superior temporal regions (Ohnishi et al., 2001; Schulze et al., 2009). As this fMRI study adopted a sparse-temporal sampling design (Gaab et al., 2003), we ensured that brain activations were not confounded by noise from the MR scanner; thus these differences could not have been influenced by scanner noise. While the current methods cannot distinguish between VTA and SN activations in the midbrain, both of these regions are involved in reward prediction in the dopaminergic pathway (D'Ardenne et al., 2008), which codes for pleasurable responses to music (Salimpoor et al., 2011). These reward signals affect long-term memory formation in the hippocampus and emotional processing in the amygdala (Schott et al., 2008; Wittmann et al., 2005), which enhance auditory processing especially for musicians and for highly pleasurable music (Blood and Zatorre, 2001; Herdener et al., 2010; Watanabe et al., 2008). The findings of higher activations in the postcentral gyrus and hippocampus, amygdala, and VTA/SN regions in the AP group may reflect additional engagement of multisensory-integration and emotional memory and reward-processing during music listening in AP subjects. However, the high correlation between behavioral ratings for AP and non-AP groups suggests that rather than the AP possessors using an additional set of regions specifically to perform the task of emotional arousal judgment, increased activations in the AP group may be due to differences in intrinsic connectivity between superior temporal regions and distal regions in the AP brain, rather than task-specific differences in AP possessors.
Since the relationship between structural and functional connectivity is complex, care must be taken to ensure that reports of between-group functional differences are not biased methodologically by structural differences. ROI-based analyses of functional connectivity, such as the graph theory analyses shown here, cannot be biased by anatomical differences in the choice of ROIs between the groups. Here we constrained our ROIs in the functional connectivity analyses so that they show no differences in size between the two groups, and between the left and right hemispheres. Using graph theory analysis with functional correlations obtained from this refined set of atlas-defined ROIs, we report the first evidence for increased functional connectivity in AP possessors. Graph theory analysis showed increased connectivity in the AP possessors’ small world brain network, with higher degrees of functional connectivity, increased connection strengths, higher local efficiency, and higher clustering in the AP brain. Increased clustering was centered around the superior temporal regions, areas known to be important in sound perception. Network statistics also reflect categories of performance obtained from behavioral scores on the AP test, suggesting a relationship between increased functional connectivity in the small-world network and pitch perception and categorization ability. Furthermore, the heightened network statistics in the AP group was still observed even when scans in response to music listening and scans in response to rest conditions were analyzed separately, suggesting that the increased functional connectivity in the AP brain was not a simple result of our task manipulations, but may reflect a generally heightened functional network within the AP brain. Results converge with recent graph theory analyses comparing AP and control subjects in cortical thickness data in showing enhanced connectivity in perisylvian (superior temporal) regions, but the present results differ in showing a global increase in functional connectivity whereas the cortical thickness data showed a global decrease in the brain overall but a local increase in clustering specific to the peri-sylvian regions (Jancke et al., 2012). These differences may reflect a dichotomy between structural and functional hyperconnectivity in AP, where structure is locally hyperconnected but function is globally hyperconnected. Future studies will need to assess both global and local connectivity in structure as well as function for a comprehensive characterization of the AP brain network.
The present study established functional differences in the AP brain by combining several approaches. Firstly, we observed functional fluctuations during music perception by applying an emotional rating task that does not rely on AP ability, thus avoiding behavioral confounds while ensuring that all auditory stimuli were similar across subjects and were appropriately attended to and processed. By comparing behavioral output of the two groups, we could ensure that the task was not biased for one group of subjects. Secondly and perhaps more generally, to our knowledge this is the first use of network theory analysis on sparse-sampled data. Although any high-frequency components of brain activations cannot be captured with the long TR of 15 seconds, the current design ensures that subjects heard all auditory stimuli in silence, rather than having results on the auditory cortex be confounded by noise from the scanner. The use of graph theory and small-world network statistics allows us to glean network information from fMRI data such as efficiency and clustering, so that the small-world network properties of AP and control groups can be compared for the first time; in this regard the small world network analysis goes beyond other functional connectivity analysis methods for fMRI data. This is a new application of graph theory to a relatively normal population; however, results are consistent with other populations that have been hypothesized to be associated with AP, such as autism, OCD, and synesthesia (Hanggi et al., 2011; Noonan et al., 2009; Zhang et al., 2011).
The present results of increased degrees, strengths, clustering and efficiency in AP possessors are independent of the correlation coefficient that we adopt to threshold the pairwise connectivity matrix. This confirms that the differences in functional connectivity between AP possessors and controls are robust and independent of threshold differences. Previous results from comparing resting state and task-related networks (Mennes et al., 2010) have suggested that neural activity during resting state and task performance are characterized by common patterns of functional connectivity. Thus we expect that the differences between AP and non-AP brain networks, although extracted from task-related data on music listening, may apply more generally to intrinsic functional networks subserving sound processing that differentiate AP possessors from controls. This was confirmed by a follow-up analysis in which we calculated network statistics separately for subsets of data corresponding to Music trials and Rest trials. While the Rest trials that are extracted from task fMRI data cannot be taken to reflect true resting state activity (Waites et al., 2005), separating the task data from the rest data in this sparse-sampled design, which uses the long TR of 15 s thereby assuming minimal to no influence of BOLD signal between successive TRs, is effective in dissociating the effects of the task manipulations (i.e. fluctuations in the time-series due to task onset and offset) from calculations of network statistics. Compared to the network statistics obtained from the full dataset, there was more variability as a function of threshold selection in network statistics obtained from Music-only and Rest-only data, possibly because selecting subsets of data resulted in fewer acquisitions for each comparison, thus resulting in more noise. Despite this increased dependence on correlational threshold, small-world network results from the Rest-only and Music-only data are similar to results from the full time-series in showing significantly increased network statistics in AP possessors, suggesting that the enhanced functional connectivity is not task-dependent, but may reflect intrinsic differences in connectivity among AP possessors.
These findings converge with anatomical results (Schlaug et al., 1995) that highlight the role of superior temporal regions, specifically planum temporale, in AP. Results also converge with diffusion tensor imaging data (Loui et al., 2011), which showed increased structural connectivity in AP subjects between superior temporal and middle temporal regions (STG and MTG). Enhancements in functional connectivity as seen in the network analysis in this study are also found in superior and middle temporal regions, but are more global in the whole brain generally, with effects centering around STG. The differences between the present fMRI results and previous DTI results may arise from differences between anatomical structure and task-related fMRI. The MTG was not significantly activated in the general linear model, possibly because the task of emotional arousal judgment did not require subjects to access their stored templates of pitch categories, as retrieving categories of pitch classes involves MTG for AP subjects as seen from anatomy-behavior correlations (Loui et al., 2011) and from function-behavior correlations (Oechslin et al., 2010; Schulze et al., 2009). Together these results provide support for intrinsic structural and functional differences in the AP brain.
The present results extend anatomical studies by demonstrating that functional networks, which are enhanced in a musical task in AP subjects, are also observable from correlating sparse-sampled time-series data. Previous studies have found that the human brain is organized intrinsically into default mode and task-related networks (Fox et al., 2005). These slow, spontaneous fluctuations may be present and detectable in sparse-sampled fMRI data. The present results suggest that functional fluctuations in distinct brain regions are more highly correlated in the AP brain, with increased efficiency and clustering especially in superior temporal regions known to be important in sound processing and perception.
The current findings of increased functional activation and small-world connectivity in the AP brain network provide a link between heightened functional networks and heightened structural networks that may enable superior perceptual categorization ability in the behavior of AP possessors. These findings suggest that the absolute pitch population may be a valid model to help understand special populations such as autism and synesthesia (Bonnel et al., 2003; Heaton et al., 2008; Rouw and Scholte, 2007) — conditions that are also thought to be characterized by local hyperconnectivity.
Acknowledgments
We thank Robert Rowe and Sourcetone, LLC for providing us with sound samples used in this study. We thank Robert J. Ellis for useful discussions and two anonymous reviewers for helpful suggestions on a previous version of the manuscript. This research was made possible by support from NIH R01 DC 009823 and the Templeton Foundation for Positive Neuroscience.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26(1):63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athos EA, Levinson B, Kistler A, Zemansky J, Bostrom A, Freimer N, Gitschier J. Dichotomy and perceptual distortions in absolute pitch ability. Proc. Natl. Acad. Sci. U. S. A. 2007;104(37):14795–14800. doi: 10.1073/pnas.0703868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachorik J, Bangert M, Loui P, Larke K, Berger J, Rowe R, Schlaug G. Emotion in motion: investigating the time-course of emotional judgments of musical stimuli. Music Percept. 2009;26(4):355–364. [Google Scholar]
- Baharloo S, Service SK, Risch N, Gitschier J, Freimer NB. Familial aggregation of absolute pitch. Am. J. Hum. Genet. 2000;67(3):755–758. doi: 10.1086/303057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28(37):9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez P, Zatorre RJ. A distribution of absolute pitch ability as revealed by computerized testing. Music Percept. 2009;27(2):89–101. [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U. S. A. 2001;98(20):11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J. Cogn. Neurosci. 2003;15(2):226–235. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox.. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brown WA, Cammuso K, Sachs H, Winklosky B, Mullane J, Bernier R, Folstein SE. Autism-related language, personality, and cognition in people with absolute pitch: results of a preliminary study. J. Autism Dev. Disord. 2003;33(2):163–167. doi: 10.1023/a:1022987309913. discussion 169. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48(4):687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Caclin A, Fonlupt P. Functional and effective connectivity in an fMRI study of an auditory-related task. Eur. J. Neurosci. 2006;23(9):2531–2537. doi: 10.1111/j.1460-9568.2006.04773.x. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Deutsch D, Henthorn T, Marvin EW, Xu H. Absolute pitch among American and Chinese conservatory students: prevalence differences, and evidence for a speech-related critical period. J. Acoust. Soc. Am. 2006;119(2):719–722. doi: 10.1121/1.2151799. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Gaab N, Gaser C, Zaehle T, Jancke L, Schlaug G. Functional anatomy of pitch memory — an fMRI study with sparse temporal sampling. Neuroimage. 2003;19(4):1417–1426. doi: 10.1016/s1053-8119(03)00224-6. [DOI] [PubMed] [Google Scholar]
- Ginestet CE, Simmons A. Statistical parametric network analysis of functional connectivity dynamics during a working memory task. Neuroimage. 2011;55(2):688–704. doi: 10.1016/j.neuroimage.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Gregersen PK, Kowalsky E, Kohn N, Marvin EW. Early childhood music education and predisposition to absolute pitch: teasing apart genes and environment. Am. J. Med. Genet. 2001;98(3):280–282. doi: 10.1002/1096-8628(20010122)98:3<280::aid-ajmg1083>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanggi J, Wotruba D, Jancke L. Globally altered structural brain network topology in grapheme-color synesthesia. J. Neurosci. 2011;31(15):5816–5828. doi: 10.1523/JNEUROSCI.0964-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton P, Davis RE, Happe FG. Research note: exceptional absolute pitch perception for spoken words in an able adult with autism. Neuropsychologia. 2008;46(7):2095–2098. doi: 10.1016/j.neuropsychologia.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Herdener M, Esposito F, di Salle F, Boller C, Hilti CC, Habermeyer B, Cattapan-Ludewig K. Musical training induces functional plasticity in human hippocampus. J. Neurosci. 2010;30(4):1377–1384. doi: 10.1523/JNEUROSCI.4513-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Suwazono S, Arao H, Miyazaki K, Nakada T. Electrophysiological correlates of absolute pitch and relative pitch. Cereb. Cortex. 2005;15(6):760–769. doi: 10.1093/cercor/bhh177. [DOI] [PubMed] [Google Scholar]
- Jancke L, Langer N, Hanggi J. Diminished whole-brain but enhanced peri-sylvian connectivity in absolute pitch musicians. J. Cogn. Neurosci. 2012;24(6):1447–1461. doi: 10.1162/jocn_a_00227. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Thangaraj V, Halpern AR, Schlaug G. Absolute pitch and planum temporale. Neuroimage. 2001;14(6):1402–1408. doi: 10.1006/nimg.2001.0925. [DOI] [PubMed] [Google Scholar]
- Klein M, Coles MG, Donchin E. People with absolute pitch process tones without producing a P300. Science. 1984;223(4642):1306–1309. doi: 10.1126/science.223.4642.1306. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lenhoff HM, Perales O, Hickok G. Absolute pitch in Williams syndrome. Music Percept. 2001;18(4):491–503. [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131(4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Loui P, Li HC, Hohmann A, Schlaug G. Enhanced connectivity in absolute pitch musicians: a model of hyperconnectivity. J. Cogn. Neurosci. 2011;23(4):1015–1026. doi: 10.1162/jocn.2010.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50(4):1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Muller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin MS, Meyer M, Jancke L. Absolute pitch-functional evidence of speech-relevant auditory acuity. Cereb. Cortex. 2010;20(2):447–455. doi: 10.1093/cercor/bhp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Asada T, Aruga M, Hirakata M, Nishikawa M, Imabayashi E. Functional anatomy of musical perception in musicians. Cereb. Cortex. 2001;11(8):754–760. doi: 10.1093/cercor/11.8.754. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assesment and analysis of handedness: the edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. Neuroimage. 2006;33(2):628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Paulson MJ, Lin T. Predicting WAIS IQ from Shipley-Hartford scores. J. Clin. Psychol. 1970;26(4):453–461. doi: 10.1002/1097-4679(197010)26:4<453::aid-jclp2270260415>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Reijneveld JC, Ponten SC, Berendse HW, Stam CJ. The application of graph theoretical analysis to complex networks in the brain. Clin. Neurophysiol. 2007;118(11):2317–2331. doi: 10.1016/j.clinph.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Rouw R, Scholte HS. Increased structural connectivity in grapheme-color synesthesia. Nat. Neurosci. 2007;10(6):792–797. doi: 10.1038/nn1906. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. J. Pers. Soc. Psychol. 1980;39(6):1161–1178. [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011;14(2):257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Steinmetz H. In vivo evidence of structural brain asymmetry in musicians. Science. 1995;267(5198):699–701. doi: 10.1126/science.7839149. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 2008;28(52):14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert E. Modeling perceived emotion with continuous musical features. Music Percept. 2004;21(4):561–585. [Google Scholar]
- Schulze K, Gaab N, Schlaug G. Perceiving pitch absolutely: comparing absolute and relative pitch possessors in a pitch memory task. BMC Neurosci. 2009;10(1):106. doi: 10.1186/1471-2202-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. J. Psychol. 1940;9:371–377. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Ann. N. Y. Acad. Sci. 2011;1224(1):109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput. Biol. 2008;4(6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum. Brain Mapp. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WD. Absolute pitch. In: Deutsch D, editor. The Psychology of Music. Academic Press; 1999. pp. 265–298. [Google Scholar]
- Watanabe T, Yagishita S, Kikyo H. Memory of music: roles of right hippocampus and left inferior frontal gyrus. Neuroimage. 2008;39(1):483–491. doi: 10.1016/j.neuroimage.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of /‘small-world/’ networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Duzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45(3):459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Absolute pitch: a model for understanding the influence of genes and development on neural and cognitive function. Nat. Neurosci. 2003;6(7):692–695. doi: 10.1038/nn1085. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, Gong Q. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J. Psychiatry Neurosci. 2011;36(1):23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]