Abstract
Stromal cell-derived factor-1 (SDF-1) and its membrane receptor C-X-C chemokine receptor type 4 (CXCR4) are involved in the homing and migration of multiple stem cell types, neovascularization, and cell proliferation. This study investigated the hypothesis that bone marrow–derived mesenchymal stem cells (BMSCs) accelerate skin wound healing in the mouse model by overexpression of CXCR4 in BMSCs. We compared SDF-1 expression and skin wound healing times of BALB/c mice, severe combined immunodeficiency (SCID) mice, and immune system–deficient nude mice after 60Co radiation–induced injury of their bone marrow. The occurrence of transplanted adenovirus-transfected CXCR4-overexpressing male BMSCs in the wound area was compared with the occurrence of untransfected male BALB/c BMSCs in 60Co-irradiated female mice skin wound healing areas by Y chromosome marker analyses. The wound healing time of BALB/c mice was 14.00±1.41 days, whereas for the nude and SCID mice it was 17.16±1.17 days and 19.83±0.76 days, respectively. Male BMSCs could be detected in the surrounding areas of 60Co-irradiated female BALB/c mice wounds, and CXCR4-overexpressing BMSCs accelerated the wound healing time. CXCR4-overexpressing BMSCs migrate in an enhanced manner to skin wounds in a SDF-1–expression-dependent manner, thereby reducing the skin wound healing time.
Introduction
Located on the surface of the human body, the skin is the most vulnerable tissue. In addition, as the organ with the most coverage of the human body, the skin plays an important role in maintaining internal environment stability and preventing dehydration and infection. As the first line of defense for protecting against invasion of microorganisms and chemical substances, keeping the skin intact and functioning is a major clinical consideration. It is important to facilitate the healing of skin wounds to revive the skin's barrier function. Currently, within conventional treatment methods, the skin autografting method as the source of new skin not only causes new wound defects but is also constrained by limited skin tissue availability. Allogenic skin grafting, or dermatoheteroplasty, tends to cause immunological rejection or communication illness. Artificial skin, generated by inoculating fibrocytes or epidermal cells, has the tendency to decay and is subject to apoptosis; it is also expensive and is thus not suitable for use in clinical practice (Attinger et al., 2006; Gottrup, 2008, Schierle et al., 2009). Therefore, how to boost wounded skin's repair and reconstruction of its lost functions remains a focus of research.
Bone marrow–derived mesenchymal stem cells (BMSCs) are one kind of cells with the potential of multidirectional differentiation within adult stem cells (ASCs). The advantages of BMSCs in regenerative medicine are that they are convenient to obtain, easy to be cultured and amplified in vitro, have relative low immunogenicity, and are free from being involved in ethical controversies like embryonic stem cell transplantation (Kruase et al., 2001; Sanchez-Ramos et al., 2000; Shiota et al., 2007). Moreover, BMSCs are more accessible for the import of exogenous genes, thus making them a supporting shuttle that is ideal for exogenous gene therapy (Minguell et al., 2001). Therefore, we chose BMSCs as the appropriate carrier for seeded cells and gene therapy for our research of skin wound repair. In addition, we studied BMSCs because they are able to secrete a number of growth factors and cytokines (Besse et al., 2000), particularly stromal cell-derived factor-1 (SDF-1) and its receptor C-X-C chemokine receptor type 4 (CXCR4), which are crucial for homing and migration of multiple stem cell types and contribute to recovery of neurological functions (Shichinohe et al., 2007) as well as neovascularization and cell proliferation (Sharma et al., 2010).
In this study, we investigated whether BMSCs gather during the skin wound repair process into the area surrounding the healing wound via exogenous application. Furthermore, on the basis of known chemotaxis principles, we modified the BMSCs by gene transfection to promote their transfer to the wound surfaces and studied whether enhanced BMSC migration into skin wounds might affect the skin wound repair.
Materials and Methods
Cells collection and detection of CXCR4 expression
Isolation and culture of BMSCs
Male mice [BALB/c, nude, and severe combined immunodeficient (SCID)] were anesthetized, respectively, with 0.75% pentobarbital sodium (45 mg/kg) injected into the enterocoelia. Then a cavity was cut into the tibia under aseptic conditions and washed several times with Dulbecco's modified Eagle medium (DMEM)/F12 culture medium. The collected washing fluid was centrifuged and the supernatant discarded. The pellets containing the cells were suspended in DMEM/F12 complete culture medium, containing 100 IU/mL penicillin and 100 μg/mL streptomycin with 10% fetal bovine serum (FBS), at a density of 4×105/cm2 and cultured under a 5%60Co and saturated humidity conditions at 37°C. When the cells reached 90% confluence, they were passaged in the same growth medium. BMSCs were identified by their ability for multidirectional differentiation (Fig. 1). Gradually the extracted BMSCs were purified and amplified to the third passage, before being further treated and injected. The experiments were performed in adherence with the Guide for the Care and Use of Laboratory Animals and were approved by the Ethics Committee of the Third Military Medical University, China.)
FIG. 1.
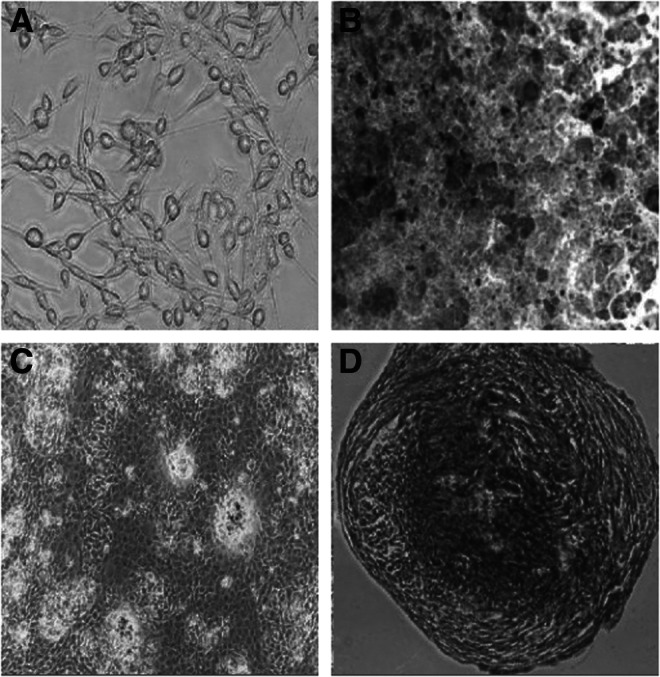
The cultivation and identification of BMSCs. (A) Cultivated in DMEM/F12 complete culture medium, BMSCs adhered on to the surface of the flask and most of them were fibroblast-like and oval in shape. (B) Fourteen days of lipoblast induction. (C) Twenty days of osteogenic induction. (D) Twenty-eight days of chondrogenic induction.
CXCR4 overexpression of BMSCs
The CXCR4-coding DNA was amplified from mouse back skin cDNA and integrated into an adenovirus shuttle vector carrying a green fluorescent protein (GFP) gene (AdTrack vector). Packaging and amplification was done using 293 cells with subsequent virus titer measurement (Adv-CXCR4). The third-passage BMSCs (BALB/c, nude, and SCID) were moved to 25-cm2 culture bottles, and the virions were added to the cells in a concentration of multiplicity of infection (MOI)=1 plaque-forming unit (PFU) per cell. After the completion of the transfection, the cells were washed with 5 mL of DMEM/F12 with 10% FBS, and the transfected BMSCs culture was incubated at 5%CO2, with saturated humidity and at 37°C for another 3 days in DMEM/F12 with 10% FBS.
CXCR4 suppression of BMSCs
The third-passage BMSCs (BALB/c, nude, and SCID mice) were placed in six-well plates until reaching 70% confluence on the day of infection. Cells were infected by adding the CXCR4 shRNA lentiviral particles (sc-35422-V, Santa Cruz, CA, USA) to 2 mL of Polybrene medium mixture and incubated overnight. Incubation continued for 3 days in DMEM/F12 with 10% FBS. Select stable clones expressing the CXCR4 shRNA via puromycin dihydrochloride selection (sc-108071, Santa Cruz, CA, USA).
CXCR4 expression on relevant BMSCs
The relevant BMSCs, were collected and analyzed for CXCR4 transcription genes via semiquantitative reverse transcription PCR with CXCR4 primers (CXCR4-P1, 5′-GGCCGTCTATGTG GGTGTCTGG-3′; CXCR4-P2, 5′-TGGCCCTTGGAGTGTGA CAG-3′) and protein expression via western blotting (ab2074, Abcam, Cambridge, UK).
Back skin wound model construction and detection of SDF-1 expression in wounds
Mice wound model
Female mice, whose weight varied from 18 to 22 grams (20.0±1.5 grams), were irradiated with 60Co (radiation dose 3.5 Gy), causing radiation injury to their bone marrow. A 1.5-cm-diameter wound was cut into the shaved back skin on the center line of the spinal column of the above-mentioned mice with a formed perforator, and the wound surface was covered with sterile gauze after hemostasis.
SDF-1 expression in wounds
The tissues surrounding the wounds (BALB/c, nude, and SCID mice, respectively, n=8) were cut out 1, 3, 5, 7, and 14 days after the initial wounding, and total RNA was extracted for constructing cDNA. The resulting cDNA was analyzed for SDF-1 gene transcription by fluorescent quantitative RT-PCR. Primers used were: SDF-1-P1, 5′-CCAAGGTCGTCGCCGTGCT-3′; SDF-1-P2, 5′-TGACGTTGGCTCTGGCGATGT-3′; GAPDH-P1, 5′-ACCCATCACCATCTTCCAGGAG-3′; GAPDH-P2, 5′-GAA GGGGCGGAGATGATGAC-3′). The control groups received only 60Co treatment.
Effect of CXCR4 expression on BMSC migration toward the concentration gradient of SDF-1
Migration in vivo
Female mice wound models (BALB/c, nude, and SCID mice, respectively, n=6) were constructed as described above and randomly divided into four groups with 6 mice per group. Each animal was injected with 0.4 mL of carboxyfluorescein diacetate N-succinimidyl ester (CFDA-SE)–marked cells (1×107/mL) by the caudal vein as follows: Group A, normal male BMSCs; group B, CXCR4-overexpressing male BMSCs; group C, CXCR4-knockdown male BMSCs; and group D, treated only with normal saline. At 1, 3, 5, 7, and 14 days after treatment, the surface skin surrounding the wound was collected. One sample was embedded in frozen sections and detected by fluorescence microscope/laser confocal microscope; the other was for quantifying the number of male BMSCs in the tissues surrounding the skin wound in the female mice. We detected expression of the Y chromosome by real-time quantitative PCR analysis using the SYBR Green system. Specific primers were: Y-P1, 5′-CTGCAGTTGCCTCAAC AAAACT-3′; Y-P2, 5′-GGTGTGCAGCTCTACTCCAGTCT-3′. Melting curves were generated to ensure the purity of the amplified product. Data were analyzed according to the comparative cycle threshold (Ct) method and were normalized by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression (Table 1).
Table 1.
Drawing of the Standard Curve of Y Chromosome FQ-PCR Detection
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| ΔCt | 10.837 | 13.198 | 16.896 | 20.156 | 23.946 | 27.475 |
| Genome mass (ng) | 20 | 2.0 | 0.2 | 0.02 | 0.002 | 0.0002 |
| ΔCt=−3.398Log(genome mass)+14.954 | ||||||
Slope factor K=−3.398; intercept b=14.954, R2=0.995.
Migration in vitro
For assessment of the motility of BMSCs with different levels of CXCR4 expression treated with SDF-1 (PeproTech, NJ, USA), the migration assay was performed in a 12-well transwell plate with 8-μm pore size filter inserts (Corning Costar, NY, USA). First, each type of BMSC (BALB/c, nude, and SCID mice, n=3) was divided into three groups as follows: Group A, male BMSCs; group B, CXCR4-overexpressing male BMSCs; and group C, CXCR4 knockdown male BMSCs. Then, 1 mL of experimental cells (5×104/mL) were seeded into upper wells. After 1 h of cell attachment, 0.5 mL of medium with SDF-1 (0, 5, 10, 15 ng/mL) was added to the lower wells and incubated for 8 h at 5%CO2, saturated humidity, and 37°C. At the end point, the cells on the upper side of the inserts were removed completely by swabbing. The transmigrated cells on both the underside of the membrane and the well bottom were measured quantitatively using MTS reagent (Promega, Madison, WI, USA). The optical densities in experimental groups were normalized as a percentage.
Observations for wound healing
Wound models of female mice with different immunity states (BALB/c, nude, and SCID, n=6) were constructed and treated as described above. Every day after injury, the healing time and general condition of the wounds were observed. Skin wound healing was defined as the wound being closed completely and no inflammatory exudation present. At the same time, the wound was photographed with a scale plate using a digital camera, and the images were processed by Image Pro Plus v1.5 (Media Cybernetics, Rockville, MD, USA). The wound healing rate was described with the percentage of repair area: Wound healing rate=(Area of before treatment − Area of observation time/Area of before treatment)×100%.
Statistical analysis
All of the data are presented as the mean±standard error ( ±s). Data processing was performed using SPSS version 13.0. A t-test and analysis of variance (ANOVA) were applied to test the difference between groups. A p value of <0.05 was considered as a significant statistical difference.
±s). Data processing was performed using SPSS version 13.0. A t-test and analysis of variance (ANOVA) were applied to test the difference between groups. A p value of <0.05 was considered as a significant statistical difference.
Results
The wound healing time was related to the immune state of the mice
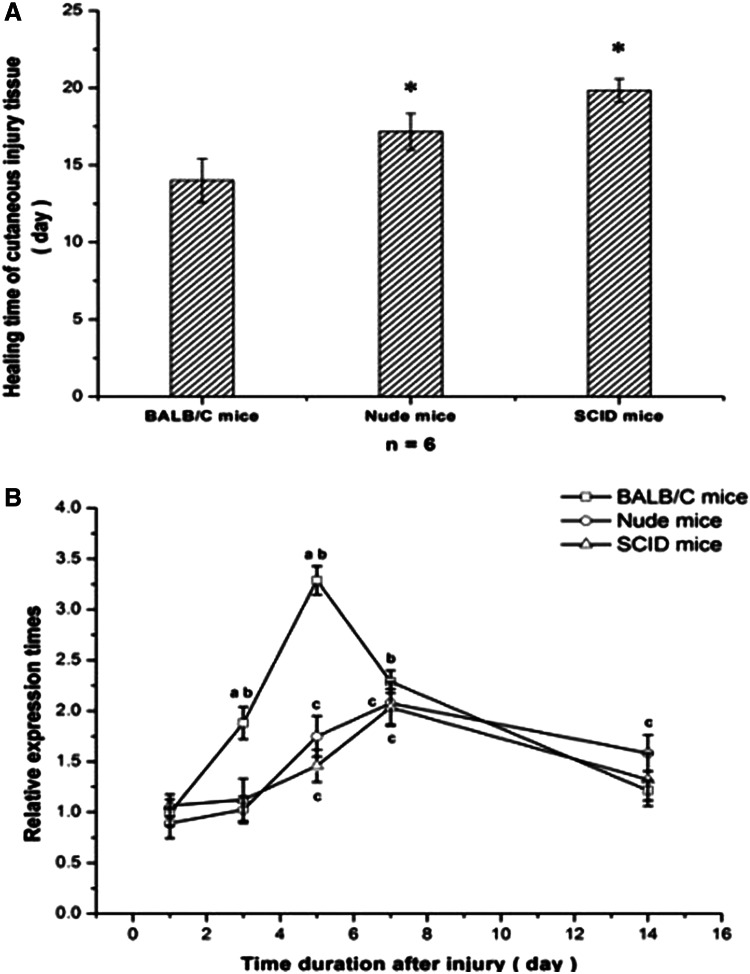
All mice survived the radiation injury and the skin-cutting model successfully. On the first day after surgery, the wound surfaces of BALB/c mice were clean and dry, and then began to become encrusted 48 h later. The wound surface shrunk obviously after the 7th day until primary healing at 15 days (14.00±1.41). In the nude and SCID mice, the encrusting time of nude mice and SCID mice, respectively, started at the 4th day and 5th day, and the shrinking of surface was delayed to 10 days, with an average healing time of 17.16±1.17 days and 19.83±0.76 days, respectively, which was obviously prolonged compared with the BALB/c mice (p<0.05) (Fig. 2A).
FIG. 2.
The relationship between healing time and SDF-1 expression in injured tissue of BALB/c, nude, and SCID mice after injury. (A) (*) Compared with BALB/c mice, the wound healing time was obviously delayed (P<0.05). (B) (a) BALB/c compared with nude or SCID mice, P<0.05. (b) BALB/c compared with its own control group, which had been irradiated with 60Co but had no skin wounding. (c) Nude and SCID mice compared with their respective control groups (P<0.05).
Expression of the SDF-1 gene in wound areas was affected by the different immune states of the mice
During the course of wound healing, SDF-1 gene expression of the BALB/c mice increased at the first day and reached a peak value until the 5th day (p<0.01) after the surgery; a gradual decrease followed and approached that of the unwounded mice in the 14th day, when the wounded surface was basically healed. The SDF-1 gene expression of the nude and SCID mice began to rise gradually and reached a postponed peak value in the 7th day after injury within the experimental time points (p<0.01); on the 14th day, with still unhealed wound surfaces, SDF-1 gene expression was still higher than in the respective unwounded mice. A direct comparison of the three different mice strains showed that the SDF-1 expression of BALB/c mice on the 5th day was double than that of the other two strains, but all mice had almost the same values at the 7th day; SDF-1 expression started to decline until the 14th day in all mice (Fig. 2B).
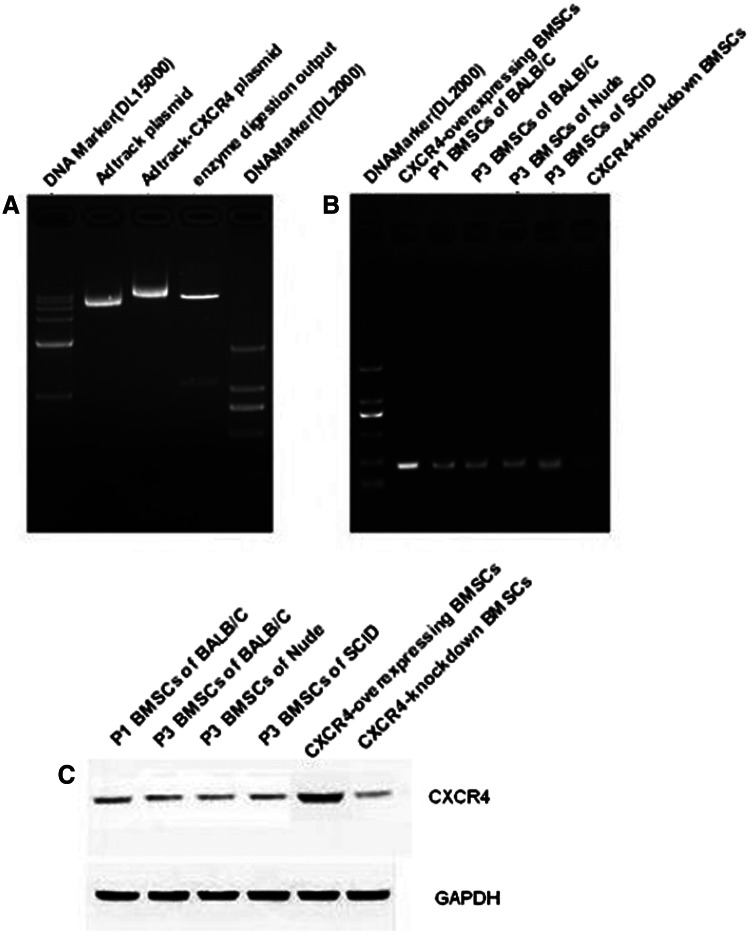
Although the relationship of immune states and SDF-1 expression is obvious, we have some interest in whether the expression of CXCR4 in BMSCs should be affected by the animals' immune states. For this question, detection for normal BMSCs was determined by RT-PCR and western blotting. The results showed there was no significant difference in expression of CXCR4 in BMSCs among the three mouse types (Fig. 3).
FIG. 3.
CXCR4 gene and protein expression in the BMSCs. There are no obvious differences of CXCR4 BMSCs in BALB/c, nude, and SCID mice. CXCR4 gene/protein expression can be overexpressed by Adv-CXCR4 and knocked down by CXCR4 shRNA lentiviral particles.
CXCR4-overexpressing BMSCs can preferentially migrate via a concentration gradient of SDF-1 in vitro and in vivo
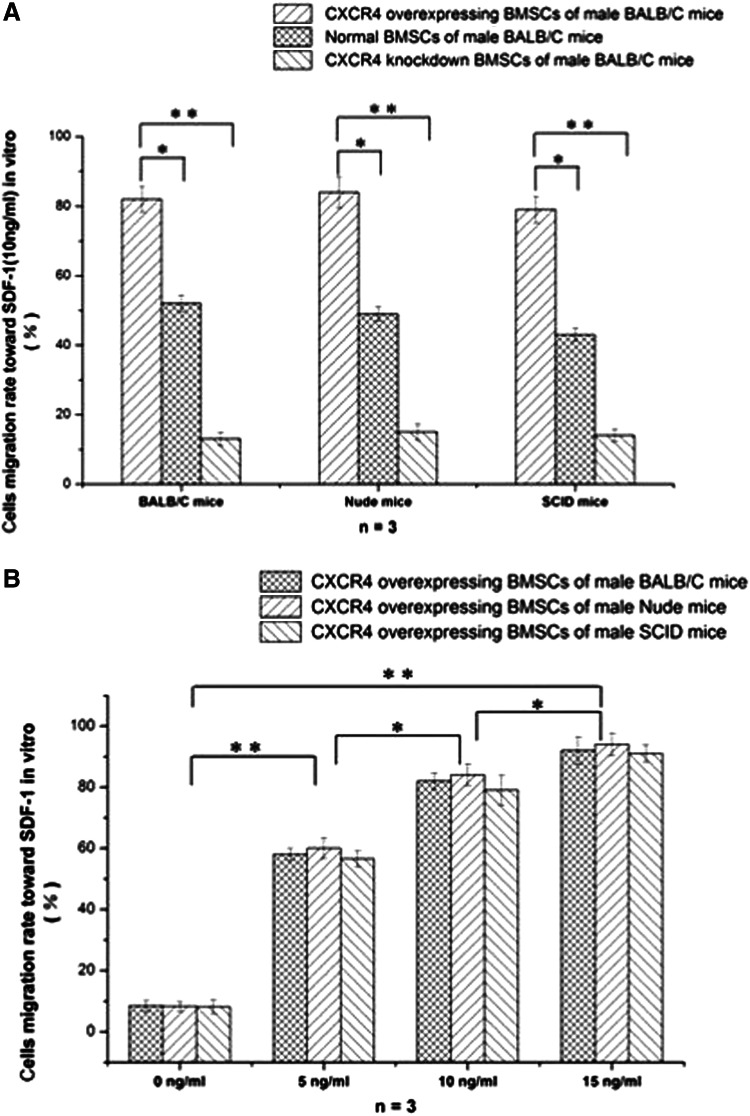
CXCR4-overexpressing BMSCs and CXCR4-suppressing BMSCs were constructed with genetically modified adenovirus and lentivirus, and were confirmed by levels of transcription and protein expression (Fig. 3). We compared the migration of CXCR4-overexpressing, CXCR4-suppressing, and normal BMSCs that interact with 10 ng/mL SDF-1 in vitro. As shown in Figure 4A, the migration can obviously increase with heightening of CXCR4 expression within the group of the same mice. Compared with normal BMSCs, CXCR4-overexpressing migration increases significantly (p<0.05). In the same vein, CXCR4 suppression decreases significantly (p<0.05). These results regarding the migration of CXCR4-overexpressing BMSCs with the concentration gradient of SDF-1 indicate that these two elements are positively correlated with each other, and this migration is not an obvious difference within the groups of different mice (Fig. 4B).
FIG. 4.
The effect of CXCR4 in BMSC migration with chemotaxis of SDF-1 in vitro. (A) CXCR4-overexpressing BMSCs have better chemotaxis with the invariable condition of SDF-1. (B) The immune state cannot change chemotaxis of BMSCs with the variable condition of SDF-1.
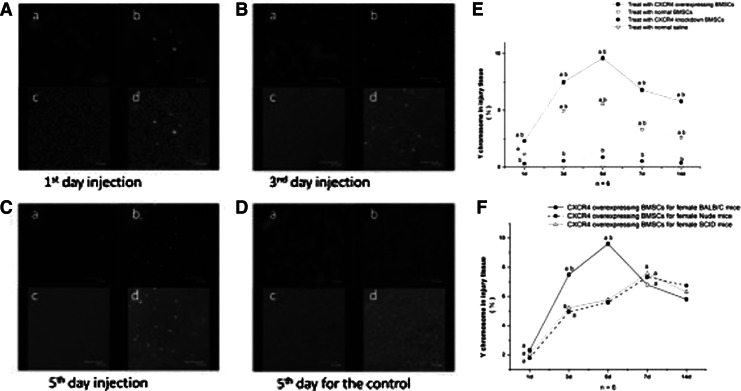
Finally, whether or not exogenous BMSCs could migrate into the host's injured tissue became our concern. The laser confocal microscopy result showed exogenous CXCR4-overexpressing BMSCs expressed in injured tissue of BALB/c mice. The 1st day after the skin injury, few CXCR4-overexpressing BMSCs with GFP were observed in the wound area (Fig. 5A); on the 3rd day, the number of positive cells increased in the wound (Fig. 5B), with a further increase of GFP signals on the 5th day after injury (Fig. 5C). On the 14th day, the wounded surface completely coalesced, and under laser confocal microscopy fluorescent GFP signals were still visible scattered within the tissue; however, no positive signals appeared in the control group that was treated with normal saline (Fig. 5D).
FIG. 5.
Detection for exogenous CXCR4-overexpressing BMSCs migrating into injured tissue. (A–D) Observation by laser confocal microscope. (a) DAPI staining of cell nuclei. (b) GFP signals of Adv-CXCR4–transfected male BMSCs. (c) Bright-field tissue image. (d) Overlay of a and b. (A) The group of wounds on the first day after injury. (B) The group of wounds on the third day after injury. (C) The group of wounds on the 5th day after injury. (D) The control group on the 5th day. (E and F) Observation by qRT-PCR. (a) Intragroup comparison (P<0.05). (b) Comparison among groups (P<0.05).
At same time, quantitative results of the sex-determining region Y (SRY) were also detected for BMSCs migrating into the injured tissue. In the final data display (Fig. 5E), the 1st day after the injury, Y chromosome signals were detected in groups A, B, and C injured tissue in female BALB/c mice; the peak time appeared at the 5th day after injury (p<0.01) with a consistent further appearance for 14th day. The Y chromosome of the CXCR4-overexpressing group was always obviously higher than the normal group. However, the CXCR4-suppressing group showed the opposite tendency with knockdown of CXCR4 genes. Another coincidence was that the implant and expression tendency of the Y chromosome in the CXCR4-overexpressing group was associated with expression of SDF-1 within the different groups of mice (Fig. 5F).
Exogenous BMSCs implanted in injuries can enhance the cutaneous wound healing of the host
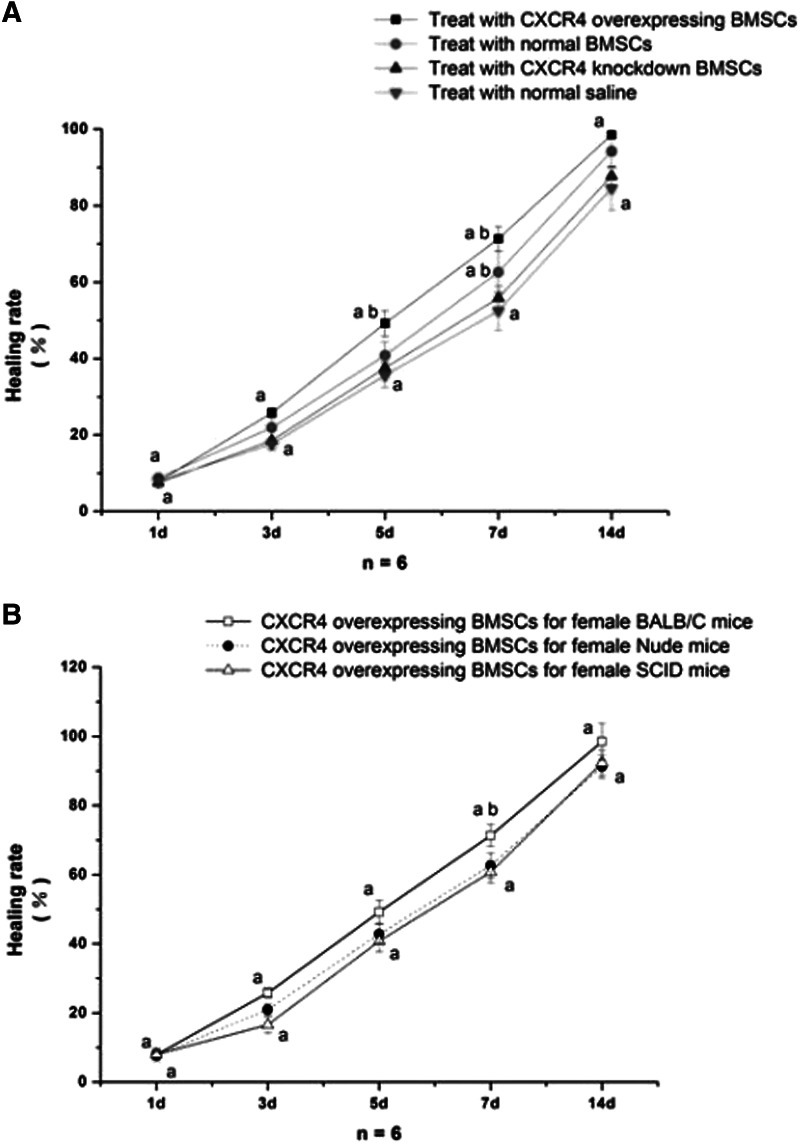
After the cells were caudally injected into the irradiated female skin-injured BALB/c mice, the animals were in good condition and the wounds began to encrust after 24–36 h. After 5 days, the wound surface could be observed to be obviously shrinking, and it healed primarily after 13 days for CXCR4-overexpressing BMSCs, with an average wound surface healing time of 12.46±1.17 days, which was sooner than BALB/c mice treated with nontransfected BMSCs or with saline (Fig. 6A). Most interesting is that CXCR4-overexpressing BMSCs can cover the insufficiency or delay of SDF-1 in cutaneous injured tissue of immunodeficient nude mice/SCID mice to enhance wound healing (Fig. 6B).
FIG. 6.
Comparison of wound healing among different treatments and mice strains. (A) BALB/c mice treated with CXCR4-overexpressing BMSCs, normal BMSCs, CXCR4-knockdown BMSCs, and normal saline. (B) CXCR4-overexpressing BMSCs can effectively improve wound healing for immunodeficiency. Note: (a) Intragroup comparison (P<0.05). (b) Comparison among groups (P<0.05).
Discussion
The healing response begins the moment the tissue is injured, and the entire course occurrs in very orderly and efficient phases—hemostasis, inflammation, proliferation, and remodeling (Gottrup, 2008). As the blood components spill into the site of injury, the platelets come into contact with exposed collagen and other elements of the extracellular matrix. Each of the contributing cell types has a crucial function present at the wound site during the phases of proliferation, migration, matrix synthesis, and contraction, as well as growth factor and matrix signaling (Schierle et al., 2009). Serious injuries or long-term chronic diseases lead to damage of many kinds of repair cells that participate in the edge and basal regions of healing wounds and cause the repair cell number and function to decline, leading to slow or refractory wound repair, because these cells cannot play their physiological function of endogenous repair.
Stem cells derived from adult cells (ASCs) have great therapeutic potential (Attinger et al., 2006), good biological capacity of tissue repair, and the potential of directional multiplex differentiation into many different cell types (Krause et al., 2001; Shiota et al., 2007). Besides, ASCs also secrete nutritional factors related to the regulating growth characteristics in local microenvironments (Gniecchi et al., 2008; Liu et al., 2006). Thus, these characteristics of ASCs may provide a possibility of repairing wound surfaces and improving their healing. It is well known that the bone marrow is not only one of ASCs storage organs, but also plays a role in mobilization of ASCs to wounds, because BMSCs derived from hematopoietic stem cells (Delorme et al., 2006) are capable of producing growth factors that play a critical role in healing of the damaged tissue (Liu et al., 2006; Minguell et al., 2001).
The signaling function of chemokines secreted from skin wounds leads to directed chemotactic migration of endogenous BMSC repair cells through their specific chemokine receptor interactions, allowing these cells to integrate into the wound surface and promote the wound healing process. The levels of chemokine expression determine the activity and functional state of repair cells and have important effects on wound repair (Mescher and Neff, 2005). Previous studies showed that SDF-1 is one of the strongest chemokines for chemotactic effects on BMSCs so far, and combined with the SDF-1 receptor CXCR4, the SDF-1/CXCR4 biological axis plays an important role in the process of tissue/organ wound repair Ceradini et al, 2004; Shiba et al., 2007). Healing of organs, i.e., heart, liver, and kidney, shows a relationship between SDF-1 concentration and the chemoattractant activity for ASCs, which means the local concentration variance of SDF-1 in wound areas can affect ASCs mobilization, homing, and aggregation (Kucia et al., 2006; Tang et al., 2005; Togel et al., 2005). However, primary BMSCs express high levels of CXCR4 in vitro, but with the extended passages the BMSCs gradually reduce their surface CXCR4 expression (Brenner et al., 2004; Wynn et al., 2004) to low or even no expression, which impairs their the migration toward SDF-1 (Honczarenko et al., 2006).
Our experiments show that SDF-1 showed a single-peak when the mouse skin tissue suffered damage, but BALB/c mice express the SDF-1 peak 2 days ahead and stronger than immunodeficient SCID or nude mice. These results suggest that the inflammatory response has started once BALB/c mice were injured, first by induction of neutrophil macrophage effusion and aggregation activated by numerous proinflammatory cytokines and chemokines, and then followed by secretion of large amounts of tissue repair factors (Martinez et al., 2009). Due to alternatively activated macrophages in the following 3–5 days, the fibroblasts and endothelial cells proliferated (Mantovani et al., 2009), becoming the main source for increased SDF-1 expression. Furthermore, with constant completion of repair, the reduced number of fibroblasts and completed wound angiogenesis as well as improved hypoxia, SDF-1 expression declined to control levels (Fig. 2) when the wound healed for 14 days. However, in nude and SCID mice, due to their immune deficiencies, the early inflammatory responses showed a lack or delay (Moseley et al., 2004), and resulted in delayed and low SDF-1 expression. Moreover, our results implied that the extent of BMSC migration into the wound surface is dependent on SDF-1 expression and positively related with wound healing. Low SDF-1 expression might fail to attract BMSCs and thereby delay wound healing, which is consistent with a report of Ponte et al. (2007), who suggested that BMSCs migration and homing to injured tissue depends on the systemic and local inflammatory state.
Kahn et al. (2004) reported that CXCR4 overexpression in human CD34+ progenitor cells using a lentiviral gene transfer technique helped navigate these cells to the murine bone marrow and spleen in response to SDF-1 signaling. Cells overexpressing CXCR4 exhibited significant increases in SDF-1–mediated chemotaxis and actin polymerization, leading to improved SDF-1–induced migration and proliferation/survival, and finally resulted in significantly higher levels of in vivo repopulation in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Brenner et al., 2004; Kahn et al., 2004). Moreover, other reports suggest that it is feasible to use CXCR4 gene-modified BMSCs for experimental research (Lien et al., 2009; Zhang et al., 2009). In this study, we used SDF-1 and CXCR4 interactions and gene cloning technology to construct CXCR4-overexpressing BMSCs, and found that high expression of SDF-1 enhances the migration of BMSCs to wound surfaces, thereby accelerating the repair of damaged tissue.
In conclusion, our findings provide a theoretical basis for wound healing in which high expression of SDF-1/CXCR4 leads to high BMSC migration into skin wounds with concomitant accelerated wound healing. The level of CXCR4 expression in BMSCs appears to be a critical factor for this healing mechanism.
Acknowledgments
We especially thank Dr. Y.A. Shan from the Southwest Hospital, the Third Military Medical University for technical assistance, and the Experimental Animal Center of Daping Hospital for animal supply and careful animal breeding.
This work was supported by a grant of the National Key Basic Research and Development Plan of PR China (No. 2011CB964701), the “863 Projects” of the Ministry of Science and Technology of the P.R. China (No. 2011AA020114), and the Natural Scientific Foundation of Chongqing (CSTC, no. 2008BC5004).
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Attinger C.E. Janis J.E. Steinberg J. Schwartz J. Al-Attar A. Couch K. Clinical approach to wounds: Debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast. Reconstr. Surg. 2006;117(7 Suppl):72S–109S. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]
- Besse A. Trimoreau F. Praloran V. Denizot Y. Effect of cytokines and growth factors on the macrophage colony-stimulating factor secretion by human bone marrow stromal cells. Cytokine. 2000;12:522–525. doi: 10.1006/cyto.1999.0580. [DOI] [PubMed] [Google Scholar]
- Brenner S. Whiting-Theobald N. Kawai T. Linton G.F. Rudikoff A.G. Choi U., et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–1133. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- Ceradini D.J. Kulkarni A.R. Callaghan M.J. Tepper O.M. Bastidas N. Kleinman M.E., et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Delorme B. Chateauvieux S. Charbord P. The concept of mesenchymal stem cells. Regen. Med. 2006;1:497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- Gnecchi M. Zhang Z. Ni A. Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottrup F. Trends in surgical wound healing. Scand. J. Surg. 2008;97:220–225. doi: 10.1177/145749690809700302. discussion 225–226. [DOI] [PubMed] [Google Scholar]
- Honczarenko M. Le Y. Swierkowski M. Ghiran I. Glodek A.M. Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- Kahn J. Byk T. Jansson-Sjostrand L. Petit I. Shivtiel S. Nagler A., et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- Krause D.S. Theise N.D. Collector M.I. Henegariu O. Hwang S. Gardner R., et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Kucia M. Wojakowski W. Reca R. Machalinski B. Gozdzik J. Majka M., et al. The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an SDF-1-, HGF-, and LIF-dependent manner. Arch. Immunol. Ther. Exp. (Warsz) 2006;54:121–135. doi: 10.1007/s00005-006-0015-1. [DOI] [PubMed] [Google Scholar]
- Lien C.Y. Chih-Yuan Ho K. Lee O.K. Blunn G.W. Su Y. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J Bone Miner Res. 2009;24:837–848. doi: 10.1359/jbmr.081257. [DOI] [PubMed] [Google Scholar]
- Liu Y. Dulchavsky D.S. Gao X. Kwon D. Chopp M. Dulchavsky S., et al. Wound repair by bone marrow stromal cells through growth factor production. J. Surg. Res. 2006;136:336–341. doi: 10.1016/j.jss.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Sica A. Sozzani S. Allavena P. Vecchi A. Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martinez F.O. Helming L. Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mescher A.L. Neff A.W. Regenerative capacity and the developing immune system. Adv. Biochem. Eng. Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Minguell J.J. Erices A. Conget P. Mesenchymal stem cells. Exp. Biol. Med. (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- Moseley R. Hilton J.R. Waddington R.J. Harding K.G. Stephens P. Thomas DW. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen. 2004;12:419–429. doi: 10.1111/j.1067-1927.2004.12406.x. [DOI] [PubMed] [Google Scholar]
- Ponte A.L. Marais E. Gallay N. Langonne A. Delorme B. Herault O., et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J. Song S. Cardozo-Pelaez F. Hazzi C. Stedeford T. Willing A., et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Schierle C.F. De la Garza M. Mustoe T.A. Galiano R.D. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–359. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- Sharma M. Afrin F. Satija N. Tripathi R.P. Gangenahalli G.U. Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 2010;20:933–946. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- Shiba Y. Takahashi M. Yoshioka T. Yajima N. Morimoto H. Izawa A., et al. M-CSF accelerates neointimal formation in the early phase after vascular injury in mice: the critical role of the SDF-1–CXCR4 system. Arterioscler. Thromb. Vasc. Biol. 2007;27:283–289. doi: 10.1161/01.ATV.0000250606.70669.14. [DOI] [PubMed] [Google Scholar]
- Shichinohe H. Kuroda S. Yano S. Hida K. Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–147. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- Shiota M. Heike T. Haruyama M. Baba S. Tsuchiya A. Fujino H., et al. Isolation and characterization of bone marrow-derived mesenchymal progenitor cells with myogenic and neuronal properties. Exp. Cell Res. 2007;313:1008–1023. doi: 10.1016/j.yexcr.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Tang Y.L. Qian K. Zhang Y.C. Shen L. Phillips M.I. Mobilizing of haematopoietic stem cells to ischemic myocardium by plasmid mediated stromal-cell-derived factor-1alpha (SDF-1alpha) treatment. Regul. Pept. 2005;125:1–8. doi: 10.1016/j.regpep.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Togel F. Isaac J. Hu Z. Weiss K. Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- Wynn R.F. Hart C.A. Corradi-Perini C. O'Neill L. Evans C.A. Wraith J.E., et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Ou L. Cheng Z. Jia X. Gao N. Kong D. Genetic modification of bone marrow mesenchymal stem cells with human CXCR4 gene and migration in vitro. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009;26:595–600. [PubMed] [Google Scholar]