Abstract
The first haematopoietic stem cells share a common origin with the dorsal aorta and derive from putative adult haemangioblasts in the dorsal lateral plate (DLP) mesoderm. Here we show that the transcription factor (TF) stem cell leukaemia (Scl/Tal1) is crucial for development of these adult haemangioblasts in Xenopus and establish the regulatory cascade controlling its expression. We show that VEGFA produced in the somites is required to initiate adult haemangioblast programming in the adjacent DLP by establishing endogenous VEGFA signalling. This response depends on expression of the VEGF receptor Flk1, driven by Fli1 and Gata2. Scl activation requires synergy between this VEGFA-controlled pathway and a VEGFA-independent pathway controlled by Fli1, Gata2 and Etv2/Etsrp/ER71, which also drives expression of the Scl partner Lmo2. Thus, the two ETS factors Fli1 and Etv6, which drives the VEGFA expression in both somites and the DLP, sit at the top of the adult haemangioblast gene regulatory network (GRN). Furthermore, Gata2 is initially activated by Fli1 but later maintained by another ETS factor, Etv2. We also establish that Flk1 and Etv2 act independently in the two pathways to Scl activation. Thus, detailed temporal, epistatic measurements of key TFs and VEGFA plus its receptor have enabled us to build a Xenopus adult haemangioblast GRN.
Keywords: Gene regulatory network, Haemangioblast, Haematopoietic stem cell, Scl, VEGF, ETS
INTRODUCTION
Bone marrow haematopoietic stem cells (HSCs) that sustain adult blood production are created during embryogenesis. In the mouse, HSC activity is first detected in the aorta, gonads and mesonephros (AGM) region of mid-gestation embryos (reviewed by Medvinsky et al., 2011). Recent tissue imaging and molecular analysis have revealed that HSCs are generated from specialised endothelial cells that acquire blood potential, the haemogenic endothelium, and are localised in the ventral wall of the single medial dorsal aorta (DA) (reviewed by Medvinsky et al., 2011). The intimate association between the DA and HSC ontogeny is conserved throughout vertebrate evolution, including in model organisms such as the zebrafish and Xenopus (reviewed by Ciau-Uitz et al., 2010a). In addition, the signalling events involved in the specification of the DA also play crucial roles in HSC generation (Gering and Patient, 2005; Ciau-Uitz et al., 2010b). Thus, a better knowledge of the origins and development of the DA could greatly enhance our understanding of the generation of HSCs in vivo.
The DA derives from lateral plate (LP) angioblasts that, in the mouse and the chick, assemble initially to form bilateral vessels, the paired dorsal aortae, on either side of the midline. The paired dorsal aortae subsequently fuse at the midline to create the DA (Garriock et al., 2010). The LP origin of the DA has been further confirmed by lineage labelling using a HoxB6-Cre line, crossed with a Rosa26 reporter line, that uses the LP enhancer element of HoxB6 (Wasteson et al., 2008). Using this transgenic line, it has been demonstrated that all DA endothelial cells of the mid-gestation embryo and adult mouse derive from LP mesoderm (Wasteson et al., 2008; Zovein et al., 2010). Importantly, it was also determined that virtually the entire bone marrow haematopoietic system derives from the LP (Zovein et al., 2010). However, neither the exact location of the DA mesodermal precursors in the LP nor their genetic programming is known.
In zebrafish and Xenopus embryos, we and others have previously described bilateral populations of LP mesodermal precursors that contribute to the DA and HSCs (reviewed by Ciau-Uitz et al., 2010a). In the zebrafish, these precursors are intermingled with haemangioblasts that differentiate into primitive blood, hampering their characterisation at this stage of development. By contrast, in Xenopus embryos, the DA LP precursors and embryonic blood emerge in separate anatomical locations and their ontogeny is distinct (Ciau-Uitz et al., 2000). This clear anatomical separation offers a unique opportunity for the genetic characterisation of the earliest precursor of the DA and HSCs.
Transplantation experiments carried out in Xenopus embryos have shown that HSC precursors are localised exclusively in the dorsal lateral plate (DLP) mesoderm proximal to the pronephros (Maeno et al., 1985a; Maeno et al., 1985b). Furthermore, lineage-labelling experiments have demonstrated that the DLP and blood vessels are patterned in an anterior to posterior manner, in which more anterior vessels derive from more dorsal blastomeres of the 32-cell stage embryo (Mills et al., 1999). Thus, the DLP proximal to the pronephros originates from medial blastomeres and gives rise to the DA encompassing the trunk, the site where HSCs emerge in the Xenopus embryo. By contrast, the DLP distal to the pronephros originates from more ventral blastomeres and gives rise to the tail artery, which does not produce HSCs (Mills et al., 1999; Ciau-Uitz et al., 2010a). Additionally, we and others have previously shown that the DLP, immediately adjacent to the ventral edge of the developing somites, contains cells co-expressing haematopoietic and endothelial genes; and that cells from the DLP migrate to the midline to coalesce and form the DA and, eventually, give rise to haemogenic endothelium and HSCs (Cleaver and Krieg, 1998; Ciau-Uitz et al., 2000; Walmsley et al., 2002). However, how these adult haemangioblasts are programmed is poorly understood. We have previously shown that BMP signalling is involved in their development (Walmsley et al., 2002), although the timing of that input is unknown. We have also shown that members of the ETS family of transcription factors (TFs), namely Fli1 and Etv6, are key players in the establishment of the adult haemangioblast programme (Liu et al., 2008; Ciau-Uitz et al., 2010b). However, although our data place Fli1 at the top of the genetic hierarchy, the position adopted by Etv6 is less clear. We have shown that Etv6 drives VEGFA signalling, both cell-autonomously and non-cell-autonomously in the somites (Ciau-Uitz et al., 2010b), but which of these acts first, how they interact and how they impact on the programme are unknown. Finally, how all these inputs relate to each other and to other key regulators, such as Scl and Lmo2, needs to be understood.
In this work, using Xenopus as a model, we establish the genetic cascade specifying the emergence of adult haemangioblasts and build a gene regulatory network (GRN) for the programming of these cells. This GRN shows that paracrine VEGFA signalling from the somites acts early and is essential for the establishment of VEGFA signalling in the adult haemangioblast precursors and therefore that the somites act as a signalling centre essential for the initiation of the specification of the HSC lineage. Once autonomous VEGFA signalling is established, synergism with a cell-autonomous, VEGFA-independent cascade regulated by Fli1, Gata2 and Etv2 is required to achieve Scl expression and adult haemangioblast programming. This GRN highlights unique functions for three members of the ETS family of TFs. Importantly, we show that Etv6 in the somites and Fli1 in lateral plate mesoderm sit at the top of the transcriptional cascade specifying the adult haemangioblast. We also show a dynamic temporal regulation of the key haematopoietic factor, Gata2, which is dependent on a relay of ETS TF activity, as it is initially activated by Fli1 but later requires Etv2 for its maintenance. We also demonstrate that a key function of Etv2 is to regulate the Scl transcriptional partner Lmo2. The GRN reveals that Etv2 and Flk1 act independently during adult haemangioblast programming, an issue that has been somewhat contradictory in the literature.
MATERIALS AND METHODS
Morpholinos
All morpholino oligonucleotides (MOs) were obtained from Gene Tools, LLC. MOs were used as follows: 20 ng/embryo of Scl MO (5′-TGTCTGTGCCCGGTCTCTCCATCAT-3′, targeting the translation initiation ATG); 40 ng/embryo of Etv6 MO (Ciau-Uitz et al., 2010b), 60 ng/embryo of Flk1 MO (Ciau-Uitz et al., 2010b), 25 ng/embryo of VegfA MO (Kälin et al., 2007), 25 ng/embryo of Etv2 MO (Salanga et al., 2010), 50 ng/embryo of Fli1 MO (Inui et al., 2006) and 50 ng/embryo of Gata2 MO (Dalgin et al., 2007). Scl MO was injected into the VMZ of four-cell stage embryos; all other MOs were injected into both blastomeres of the two-cell stage embryo. Two more MOs targeting Scl were designed: 5′-CTCTCCCCTCCTACTGACACTTTAC-3′ (Scl MO2), which largely overlaps with Scl MO but contains one more mismatch (supplementary material Fig. S2A); and 5′-TCTCCATCATCTTTAGGGACATTGC-3 (Scl MO3), which has a target sequence overlapping the ATG but encompasses an area poorly conserved between the two Scl alleles (supplementary material Fig. S2A). These MOs produced no phenotype and were used as control MOs (data not shown). Specificity of the Etv6 MO was confirmed by injection of a second MO targeting the UTR (Ciau-Uitz et al., 2010b); the phenotype was also rescued with either injection of exogenous VegfA mRNA or Etv6 mRNA resistant to the MOs. VegfA MO specificity was assessed using a previously reported five mismatches MO (Kälin et al., 2007), which gave no phenotype, and the MO phenotype was rescued with exogenous VegfA mRNA. The Flk1 MO phenotype is indistinguishable from the VegfA MO phenotype and its effects could not be rescued by exogenous VegfA mRNA, indicating that VEGFA could only signal through this targeted receptor. Etv2 MO was controlled by injection of a previously reported mismatch MO, which gave no phenotype (Salanga et al., 2010); additionally, a second partially overlapping MO (Neuhaus et al., 2010) produced an identical phenotype. The starting ATG codons for the two alleles of Gata2, as well as their 5′ UTRs, are not conserved and therefore the two alleles were targeted with specific MOs. Thus, for Gata2 depletion, these two MOs were co-injected; injection of a single MO, targeting only one allele, produced no phenotype, indicating a high degree of targeting specificity. We were unable to rescue Gata2 morphants with Gata2 re-expression owing to the embryos' high sensitivity to exogenous Gata2 mRNA.
Embryo manipulation, in situ hybridisation procedures and western blot analysis
Wild-type Xenopus laevis embryos were obtained and cultured as described (Walmsley et al., 2005) and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). Embryo microinjection and whole mount in situ hybridisation was performed as previously described (Walmsley et al., 2005). Before photography, embryos were cleared in benzyl benzoate:benzyl alcohol (2:1). Phenotypic analysis and interpretations were based on gene expression changes observed in the DLP proximal to the pronephros, the region from which HSC precursors originate (Maeno et al., 1985a; Maeno et al., 1985b). For details of probes used see supplementary material Table S1. Probes have been deposited into the European Xenopus Resource Centre, University of Portsmouth, UK. Protein extraction and western blot analysis were as previously described (Afouda et al., 2005); Scl protein was detected using an anti-Scl antibody from Santacruz, Tal1(C21) (Cat. No. SC12984), at a 1:1000 dilution. Network schematics were generated using Biotapestry and figures were prepared using Photoshop. All animal work was carried out according to UK Home Office regulations under the appropriate project licence.
Cloning and rescue experiments
Gata2FL-GR-HA was made by amplifying full-length Gata2 (GenBank accession no. BC108544) with the primers indicated in supplementary material Table S2 and fusing it to the hormone-binding domain of human glucocorticoid receptor (GR) by inserting it into the BglII site of pSP64T-GR (Tada et al., 1997). To generate mRNA for injections, Gata2FL-GR-HA was linearised with BamHI and transcribed with SP6 RNA polymerase using the mMESSAGE mMACHINE Kit (Ambion). Gata2FL-GR-HA (100 pg) was co-injected with Fli1 MO at the two-cell stage and activated with Dexamethasone (10 μM final concentration) at the desired stage of development.
RNA extraction and qPCR analysis
Total RNA from stage 26 excised DLPs was extracted using TRI Reagent (Sigma) and purified using the RNeasy Micro Kit (Qiagen). cDNA synthesis, SYBR green (Applied Biosystems) qPCR, data collection and analysis were performed as previously indicated (Ciau-Uitz et al., 2010b). Primers used are indicated in supplementary material Table S2.
RESULTS
Expression profile of adult haemangioblasts in the DLP mesoderm
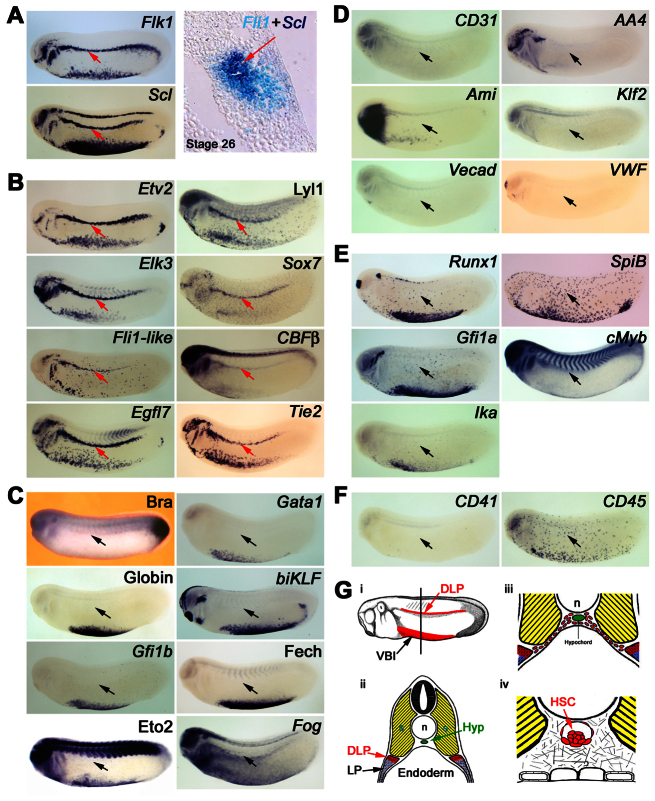
In Xenopus embryos, the DA and HSCs derive from precursors migrating from the DLP (Fig. 1G) (Cleaver and Krieg, 1998; Ciau-Uitz et al., 2000) and the DLP contains a discrete cell population co-expressing blood and endothelial genes (Fig. 1A) (Walmsley et al., 2002; Ciau-Uitz et al., 2010a) as well as VegfA (supplementary material Fig. S1C). To further characterise these adult haemangioblasts, expression of key blood and endothelial genes, as well as markers for haemogenic endothelium and mature blood and endothelium, was analysed (Fig. 1; supplementary material Fig. S1). In addition to the blood and endothelial genes previously reported (Walmsley et al., 2002; Ciau-Uitz et al., 2010a; Ciau-Uitz et al., 2010b) in the DLP (Fli1, Gata2, Flk1, Etv6, VegfA, Scl, Lmo2, Flt4, Flt1, Erg1 and Hex), we found that the TFs, Etv2, Lyl1, Elk3, Sox7, Fli1-like and CBFβ, together with the transmembrane proteins, Egfl7 and Tie2, are also expressed in the DLP (Fig. 1B). By contrast, neither the early mesodermal marker, Brachyury, nor genes expressed in mature erythrocytes (Gata1, Globin, BiKlf, Gfi1b, Fech, Eto2 and Fog; Fig. 1C) or mature endothelium (CD31, AA4, Ami, Klf2, Vecad and VWF) are expressed in the DLP (Fig. 1D). Similarly, some ETS TFs known to be involved in blood or endothelial development (Ets1, Ets2, Elf1, Elf2, Elf3, Etv7 and Elk4) are also absent in the DLP (supplementary material Fig. S1A). Furthermore, HSC/haemogenic endothelium genes, such as Runx1, SpiB, Gfi1a, cMyb and Ikaros (Fig. 1E), are not expressed in the DLP nor are CD41 or CD45, which mark haematopoietic commitment (Fig. 1F). All negative probes gave positive signals in other tissues (supplementary material Fig. S1B), sometimes in co-stained embryos.
Fig. 1.
Characterisation of DLP mesoderm adult haemangioblasts. (A) Adult haemangioblasts localise in the DLP adjacent to the somites and are the earliest HSC progenitors detectable by co-expression of VEGFR2, Flk1, and the stem cell leukaemia gene (Scl), which marks the emergence of haemangioblasts. The panel on the right shows co-expression of the endothelial gene, Fli1, and Scl on a 10 μM section. Note that no morphological differences are observed between adult haemangioblasts and surrounding tissues. (B) Expression analysis revealing novel haematopoietic, Lyl1, Sox7 and CBFβ, as well as novel endothelial, Etv2, Elk3, Fli1-like, Egfl7 and Tie2, gene expression in adult haemangioblasts. (C) Adult haemangioblasts do not express the early mesodermal marker, Brachyury (Bra), nor the erythrocyte differentiation genes, Gata1, Globin, biKLF, Gfi1b, Ferrochelatase (Fech), Eto2 and Fog. (D) Adult haemangioblasts do not express genes associated with mature blood vessels, CD31, AA4, Ami, Klf2, Vecad and VWF. (E) Adult haemangioblasts do not express key HSC-associated genes such as Runx1, SpiB, Gfi1a, cMyb and Ikaros (Ika). (F) Expression of CD41, a gene associated with haematopoietic commitment, and CD45, which is associated with haematopoietic differentiation, are undetectable in adult haemangioblasts. The DLP is indicated by the red or black arrows. All embryos were hybridised as whole mounts and are shown in lateral view, with anterior to the left and dorsal to the top. All embryos are shown at stage 26. (G) Schematic representation of the location of the DLP adult haemangioblast population and its derivatives. (i) Representation of a stage 26 embryo showing in red the Scl expression domains, the ventral blood island and the DLP mesoderm. (ii) Cross section of a stage 26 embryo, at the level indicated by the line in panel i, showing the location of the DLP. Note that adult haemangioblasts (red tissue) lies immediately ventral to the somites (yellow tissue) but at some distance from the hypochord (green tissue), where the DA and HSCs eventually emerge. (iii) Picture showing DA/HSC progenitors migrating from the DLP to the hypochord, a process taking place from stage 28 to 31. (iv) Schematic representation of HSCs emerging in association with the ventral wall of the DA; these haematopoietic clusters are found from stage 42 to 44, 4 days after the specification of adult haemangioblasts in the DLP. Hyp, hypochord; n, notochord; s, somites; VBI, ventral blood island.
In summary, the DLP expression profile strongly supports the view that this Flk1+ cell population represents haemangioblasts rather than haemogenic endothelium or committed/differentiated endothelial or blood cells because these cells do not express essential HSC/haemogenic endothelium genes, such as Runx1 and CD41, nor genes expressed in mature endothelium (Vecad, CD31, VWF) or mature blood cells (Gata1 and Globin). These adult haemangioblasts localise immediately adjacent to the somites (Fig. 1Gi,ii) and subsequently give rise to angioblastic progenitors no longer expressing blood genes (Scl, Gata2 and Lmo2) (Ciau-Uitz et al., 2000), which migrate to the midline (Fig. 1Giii) where they coalesce to form the DA, become haemogenic and later produce HSCs (Fig. 1Giv) (Ciau-Uitz et al., 2000).
Scl is required for the programming of adult haemangioblasts
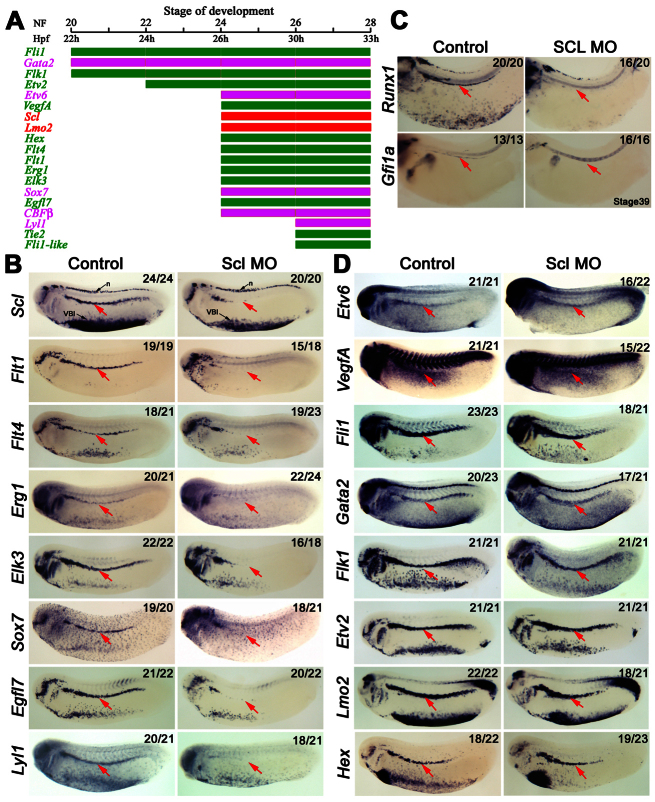
The adult haemangioblasts of the DLP represent the earliest identifiable precursors to haemogenic endothelium and haematopoietic cells derived from the haemogenic endothelium. How these precursors are specified is poorly understood, although we have previously shown the ETS TFs Fli1 and Etv6 to be crucial (Liu et al., 2008; Ciau-Uitz et al., 2010b). To better understand haemangioblast programming, we carried out a timecourse of expression for blood and endothelial regulators in the DLP (Fig. 2A). This analysis revealed four discrete steps in adult haemangioblast development: (1) the first genes expressed at stage 20, in agreement with a position at the top of the hierarchy (Liu et al., 2008), are Fli1 and Gata2 together with the VEGFA receptor, Flk1; (2) by stage 22 the ETS TF Etv2 has also been turned on; (3) many more genes are activated at stage 24, including Etv6 and its target, VegfA, as well as Scl and Lmo2; (4) finally, at stage 26, the full complement of blood and endothelial genes known to be expressed in the DLP before migration are expressed, suggesting that haemangioblast programming may have reached completion (Fig. 2A).
Fig. 2.
Scl marks and is essential for the emergence of adult haemangioblasts in the DLP. (A) Bar chart summarising the haematopoietic (magenta and red) and endothelial (green) expression hierarchy in the DLP reveals that adult haemangioblast programming takes place in four discrete transcriptional steps. Expression profiles were obtained by analysis of embryos subjected to whole-mount in situ hybridisation. Stages of development by morphological traits (NF) and hours post fertilisation (Hpf) are indicated at the top and are as indicated by Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). (B) Expression analysis showing haematopoietic (Scl, Sox7 and Lyl1) and endothelial (Flt1, Flt4, Egr1, Elk3 and Egfl7) genes dependent on Scl for their expression in the DLP (arrows). (C) Expression analysis showing that haemogenic endothelium, as indicated by Runx1 and Gfi1a, fails to be established in the DA of Scl morphants. The DA is indicated by red arrows. (D) Expression analysis showing that the haematopoietic genes, Gata2, Lmo2 and Etv6; and the endothelial genes, Fli1, Flk1, Etv2 and Hex, in the DLP, as well as the expression of the growth factor, VegfA, are not dependent on Scl. The DLP is indicated by the red arrow. All embryos were hybridised as whole mounts and are shown in lateral view, with anterior to the left and dorsal to the top. Embryos in B and D were analysed at stage 26, whereas those in C are stage 39 embryos. Numbers of embryos represented by each panel, out of the number analysed, are indicated in the top right corner. n, neurons, VBI, ventral blood island.
Scl is essential for HSC development (Porcher et al., 1996; Robb et al., 1996) and its expression marks the emergence of primitive haemangioblasts from Flk1+ cells differentiated from mouse embryonic stem cells (ESCs) (Chung et al., 2002; Park et al., 2004). Consistent with these observations, zebrafish Scl has been shown to be required for dorsal aorta as well as blood development in vivo (Patterson et al., 2005). Emergence of the blast colony-forming cell (BL-CFC), previously thought to be the in vitro equivalent of the primitive haemangioblast in the differentiating mouse ESC system but more likely to represent an early mesodermal precursor, does not require Scl (D'Souza et al., 2005). However, in the absence of Scl, this BL-CFC only has the potential to form smooth muscle at this point in its development, consistent with Scl specifying blood and endothelial potential from these cells. It therefore appears that Scl is crucial for both blood and endothelial development, acting at the stage when progenitors with blood and endothelial potential are being generated. However, the function of Scl in adult haemangioblasts has not been investigated because of the hitherto non-availability of the population for study in ESC cultures or in embryos.
To investigate the role of Scl in adult haemangioblast development, Scl was depleted using a translation-blocking morpholino oligonucleotide, Scl MO (supplementary material Fig. S2A). Western blot analysis indicates that this Scl MO efficiently blocks endogenous Xenopus Scl production when injected into embryos (Scl, supplementary material Fig. S2B) but does not block the translation of exogenous Scl protein produced from an mRNA without the Scl MO target sequence (2xHA-SCL, supplementary material Fig. S2B). Furthermore, this MO efficiently blocks the development of the primitive erythroid programme in the ventral blood island (supplementary material Fig. S2C). When adult haemangioblast specification was analysed, we found that most of the genes initiating expression at stage 24, when Scl is activated, or later, were absent in the DLP of Scl morphants (Fig. 2B). Consistent with a crucial role for Scl and its targets in programming the HSC, no Runx1 or Gfi1a staining was seen in the DA later (Fig. 2C). In contrast to these Scl-dependent genes, the upstream regulators of Scl transcription, Etv6 and VegfA (Ciau-Uitz et al., 2010b), were unaffected in Scl-depleted embryos (Fig. 2D). Likewise, as expected from their expression before Scl, Fli1, Gata2, Flk1 and Etv2 expression was also unaffected in Scl morphants (Fig. 2D). Interestingly, the expression of Scl itself was downregulated in the DLP but not in neurons or the ventral blood island, suggesting an autoregulatory loop specifically operating in the DLP haemangioblast (Fig. 2B).
Scl therefore marks the generation of the haemangioblast in the DLP, is required for the activation of a number of genes essential for blood and endothelial development, and is thus essential for adult haemangioblast programming.
Etv6 and thereby VEGFA expression in adult haemangioblasts is dependent on Fli1 and Gata2
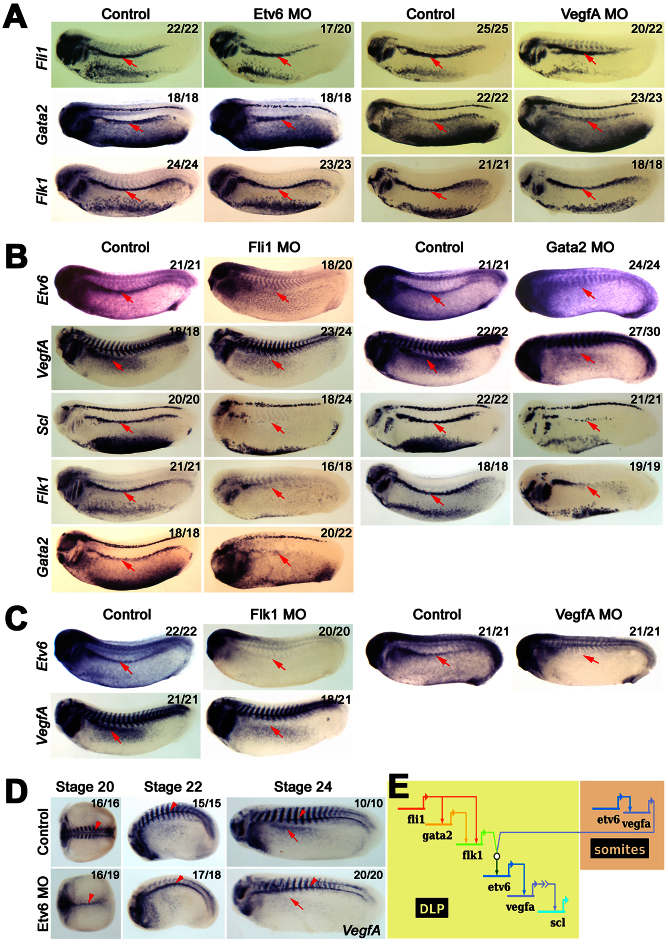
Because Scl is a key regulator of adult haemangioblast programming, we next investigated how Scl is itself regulated at the transcriptional level. We have previously shown that Fli1 regulates Gata2 expression in the DLP and that both of them are required for Scl expression (Liu et al., 2008). More recently, we have shown that both autocrine and paracrine VEGFA signalling is essential for Scl activation in the DLP, and that VegfA expression in both cases is controlled by the ETS TF Etv6 (Ciau-Uitz et al., 2010b) (supplementary material Fig. S1C). We therefore wanted to clarify the epistatic relationships between Fli1/Gata2 and Etv6/VEGFA. To this end, embryos injected with Etv6 MO or VEGFA MO were probed for Fli1, Gata2 and Flk1 expression (Fig. 3A). As previously reported, none of these genes were affected in the DLP of Etv6 or VEGFA morphants even though Scl expression was abrogated in the same experiment (Fig. 3A) (Ciau-Uitz et al., 2010b) (data not shown), indicating that Etv6/VEGFA are either acting in parallel to Fli1/Gata2 or downstream of them. To investigate whether Etv6/VEGFA are regulated by Fli1 and Gata2, their expression was analysed in Fli1 and Gata2-deficient embryos. Our results show that Etv6, VegfA and Scl expression in the DLP are absent in both Fli1 and Gata2 morphants (Fig. 3B). In the same experiment, we confirmed that Gata2 expression in the DLP is dependent on Fli1 (Fig. 3B). We have, therefore, established that Fli1 regulates Etv6, VegfA and Scl expression, possibly in part through Gata2. These data reinforce the position of Fli1 at the top of the adult haemangioblast transcriptional hierarchy.
Fig. 3.
Fli1 and Gata2 regulate Etv6/VEGFA signalling in the DLP to control the establishment of adult haemangioblasts. (A) Expression analysis showing that the expression of Fli1, Gata2 and Flk1 in the DLP (arrows) are not dependent on Etv6/VegfA signalling. (B) Expression analysis showing that the expression of Etv6, VegfA, Scl and Flk1 in the DLP (arrows) are dependent on both Fli1 and Gata2 transcriptional activities and that Gata2 expression is controlled by Fli1. (C) Expression analysis showing that the expression of Etv6 and VegfA in the DLP (arrows) is dependent on VEGFA signalling. (D) Expression analysis in staged embryos shows that Etv6 controls the expression of VegfA in the DLP (arrows) and in the somites (striped staining) but not in the hypochord (arrowheads). Note that VegfA expression in the somites is initially completely dependent on Etv6 but that by stage 24 some recovery is observed. (E) Diagram summarising the genetic cascade regulating Scl expression in the DLP. Relationships between genes are depicted by an arrow from the regulating gene to the regulated genes. This regulatory network shows that, although Fli1 and Gata2 are required for Etv6, VegfA and Scl expression, the establishment of endogenous VEGFA signalling and Scl expression in the DLP can only happen when VEGFA is produced in the somites, that paracrine VEGFA signalling is absolutely required for the initiation of the adult haemangioblast programme. The open circle indicates the interaction between the VEGFA receptor, Flk1, and VEGFA ligand from the somites. The arrow with chevrons indicates that VEGFA activates Scl indirectly, and that an intermediate factor controlled by VEGFA signalling is required. All embryos were hybridised as whole mounts and are shown in lateral view, with anterior to the left and dorsal to the top. All embryos are shown at stage 26, unless otherwise indicated. Numbers of embryos represented by each panel, out of the number analysed, are indicated in the top right corner.
The establishment of cell-autonomous VEGFA signalling in adult haemangioblasts is dependent on paracrine VEGFA signalling from the somites
Together with Fli1 and Gata2, Flk1 is at the top of DLP expression hierarchy (Fig. 2A) and it is also required for Scl expression (Ciau-Uitz et al., 2010b). In ESCs (Lugus et al., 2007) and endothelial cells (Mammoto et al., 2009), Flk1 expression is controlled by Gata2. Here we show that Flk1 expression is not initiated in the DLP of Gata2 morphants (Fig. 3B; Table 1); and, in agreement with Gata2 regulation by Fli1, Flk1 is also absent in the DLP of Fli1-deficient embryos (Fig. 3B). Thus, consistent with their position at the top of the adult haemangioblast transcriptional hierarchy, Fli1 and Gata2 control the expression of both VegfA and its receptor, Flk1, in the DLP mesoderm.
Table 1.
Transcriptional regulation of blood and endothelial genes in the DLP
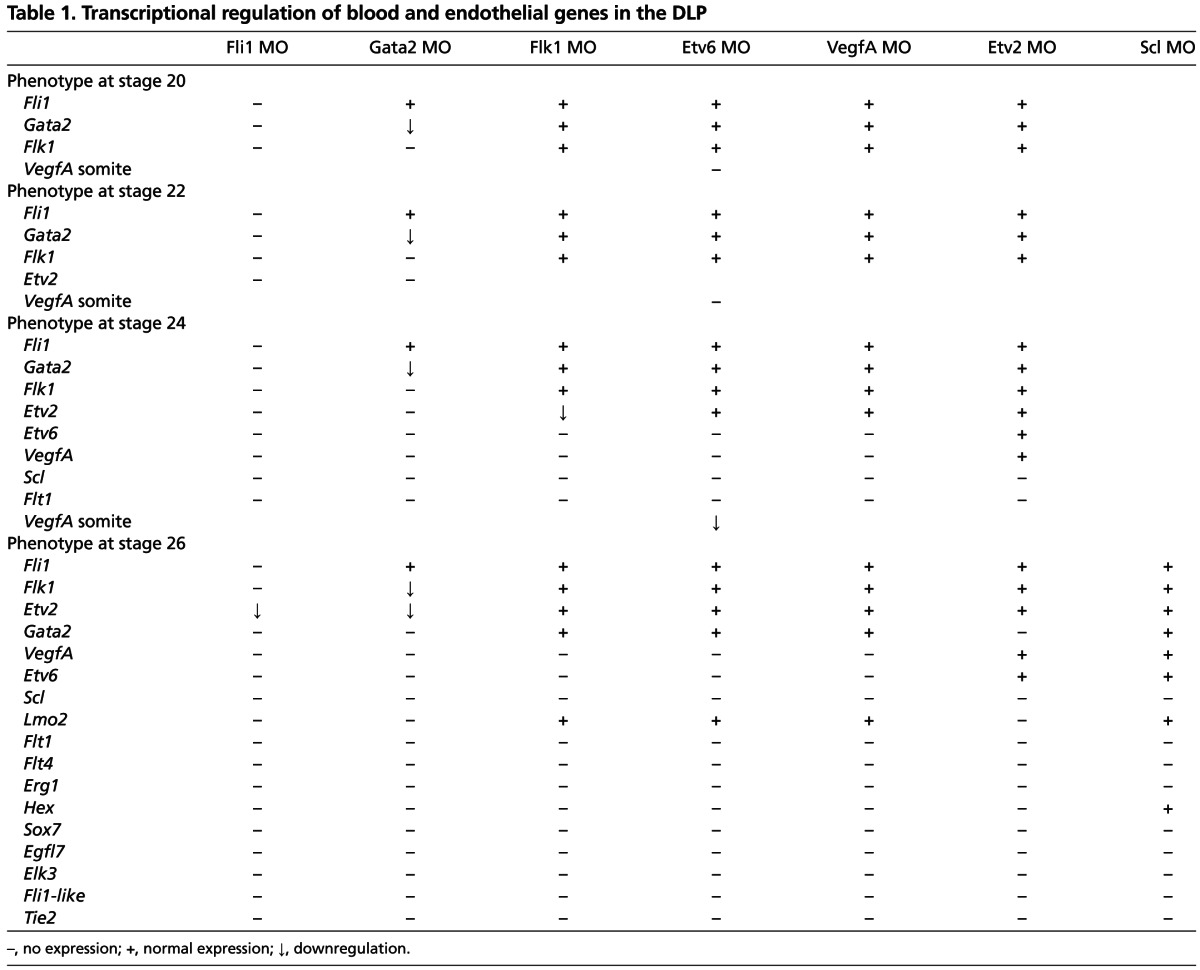
Analysis of Flk1 morphants showed that Etv6 and VegfA expression in the DLP is absent (Fig. 3C). Absence of Etv6 expression in VegfA-deficient embryos confirmed that VEGFA signalling is required for Etv6 expression in the DLP (Fig. 3C). We have previously reported that both endogenous and paracrine VEGFA (from the adjacent somites) are required for Scl expression in the DLP, and that both endogenous and paracrine VegfA expression are controlled by Etv6 (Ciau-Uitz et al., 2010b) (supplementary material Fig. S1C). Analysis of VegfA expression in Etv6 morphants shows that VegfA expression in the somites is initially almost completely dependent on Etv6 expression (stages 20 and 22; Fig. 3D). Later, by stage 24, VegfA expression in the somites has partially recovered but it is still insufficient for VegfA activation in the DLP (Fig. 3D). Taken together, our results strongly indicate that paracrine VEGFA signalling before stage 24 of development is essential for the establishment of endogenous VEGFA signalling in the DLP. In summary, establishment of Flk1 expression in the DLP and production of VEGFA in the somites are the first essential steps in the genetic cascade programming adult haemangioblasts in the DLP mesoderm (Fig. 3E). These data demonstrate the importance of VEGF ligand/receptor interactions between the DLP and adjacent somites.
Etv2 regulates Scl expression in adult haemangioblasts in synergy with Etv6/VEGFA
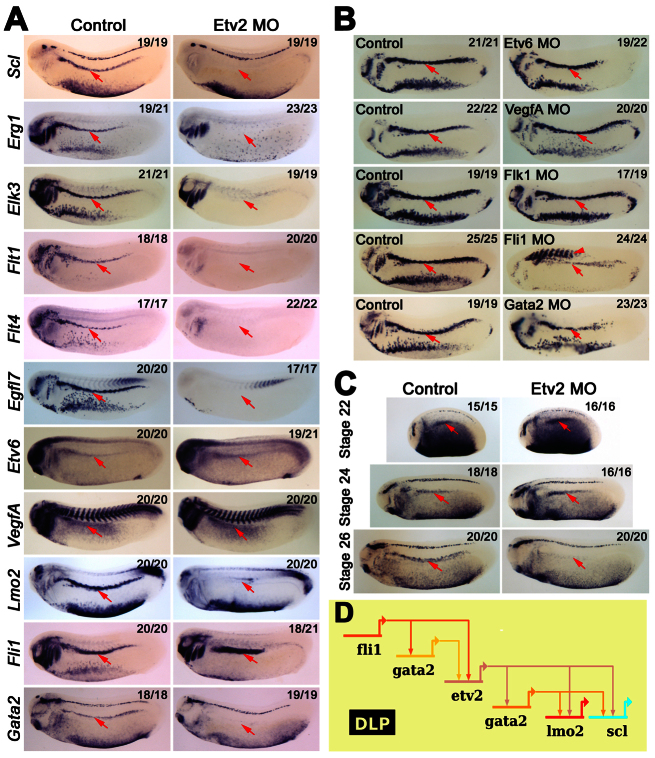
In recent years, the ETS TF Etv2 has emerged as a major regulator of yolk sac haematopoiesis and endothelial cell development in the mouse (Lee et al., 2008; Kataoka et al., 2011). In zebrafish and Xenopus embryos, however, although Etv2 is essential for vascular and primitive myeloid development, it is not required for primitive erythroid development (Sumanas and Lin, 2006; Liu and Patient, 2008; Sumanas et al., 2008; Salanga et al., 2010). Nevertheless, Etv2 can rescue both haematopoietic and vascular development in zebrafish cloche mutant embryos (Xiong et al., 2008), suggesting that its role in erythroid development may be covered by another member of the family in zebrafish. The precise role played by Etv2 in the emergence of HSCs is currently unknown beyond the complete lack of blood vessels, including the DA, in Etv2-deficient embryos. We have shown that Etv2 is expressed in the DLP and that its expression initiates after Flk1 but before Etv6 expression (Fig. 1; Fig. 2A), suggesting that it may play a role in adult haemangioblast programming. Indeed, Scl expression, together with the expression of the transcriptional targets of Scl (Egr1, Elk3, Flt1, Flt4, Egfl7), was found to be absent in the DLP of Etv2 morphants (Fig. 4A). In addition, expression of the Scl haemangioblast partner Lmo2 (Patterson et al., 2007) is also dependent on Etv2 (Fig. 4A). Thus Etv2 is an essential regulator of adult haemangioblast specification and HSC programming.
Fig. 4.
Etv2 acts downstream of Fli1 to control Gata2 and adult haemangioblast specification. (A) Expression analysis showing that Etv2 is a major regulator of gene expression in the DLP (arrows). Note that Etv6 and VegfA expression in the DLP is not dependent on Etv2. (B) Analysis of the transcriptional regulation of Etv2 in the DLP. Expression analysis showing that Etv2 expression in the DLP (arrows) is not dependent on Etv6/VEGFA signalling but dependent on Fli1 and Gata2. Note Etv2 ectopic expression in Fli1 morphants (arrowhead). (C) Expression analysis in staged embryos showing that Gata2 expression in the DLP (arrows) is initially independent of Etv2's transcriptional regulation but is controlled by Etv2 after stage 24 of development. All embryos were hybridised as whole mounts and are shown in lateral view, with anterior to the left and dorsal to the top. All embryos are shown at stage 26, unless otherwise indicated. Numbers of embryos represented by each panel, out of the number analysed, are indicated in the top right corner. (D) Diagram summarising an endogenous cell-autonomous and VEGFA-independent genetic cascade required for Scl expression in the DLP. This cascade is initiated by Fli1 and maintained by Etv2.
As Etv6 is also a key regulator of Scl expression in the DLP, we investigated the epistatic relationship between Etv2 and Etv6. Interestingly, although Etv2 is expressed before Etv6 in the DLP (Fig. 2A), expression of both Etv6 and VegfA were unaffected in the DLP of Etv2 morphants (Fig. 4A). Furthermore, Etv2 expression was largely unaffected in Etv6- and VegfA-depleted embryos (Fig. 4B), indicating that Etv2 expression in the DLP is largely independent of VEGFA signalling. This conclusion was further confirmed when Etv2 expression was assessed in Flk1-depleted embryos. In these morphants Etv2 expression initiated at the correct stage of development and only a slight decrease in its expression levels was observed (Fig. 4B). Etv2 has been reported to regulate Flk1 expression in mouse, zebrafish and Xenopus (Lee et al., 2008; Sumanas and Lin, 2006; Salanga et al., 2010). Here we have shown that Etv2 expression in the DLP initiates after Flk1 (Fig. 2A; supplementary material Fig. S2), and therefore Etv2 appears dispensable for Flk1 activation; nevertheless, we wondered whether Etv2 is required for Flk1 maintenance. However, Flk1 expression in Etv2-deficient embryos is unaffected (Table 1; Fig. 4; supplementary material Fig. S3); thus, Flk1 and Etv2 show no interdependent regulation during adult haemangioblast programming and therefore the Flk1 downregulation at later stages of development (Salanga et al., 2010) (supplementary material Fig. S3) is likely to be due to a complete lack of vascular endothelial cells. Therefore, both Etv2 and Etv6 are required for Scl expression, but they appear to control parallel genetic cascades that synergistically activate Scl in the DLP.
We have shown that Etv2 transcriptional activation in the DLP is predominantly independent of VEGFA signalling. To investigate whether Fli1 or Gata2 is involved in Etv2 regulation in the DLP, Etv2 expression was assessed in Fli1 and Gata2 morphants. Etv2 expression was absent at stages 22 and 24 (Table 1; supplementary material Fig. S4) and still significantly downregulated at stage 26 in the DLP of both Fli1 and Gata2-deficient embryos (Fig. 4B). This indicates that Fli1 regulates Etv2, possibly through Gata2. This further confirms that Fli1 together with Gata2 act at the top of the adult haemangioblast TF hierarchy and that they control haemangioblast programming by activating both VEGFA-dependent and VEGFA-independent pathways.
Etv2 regulates Scl through the maintenance of Gata2 expression
So far we have demonstrated that Fli1 and Gata2 are hierarchically upstream of Etv2. In agreement with this, Fli1 expression in the DLP of stage 26 Etv2 morphants was unaffected (Fig. 4A). Surprisingly, however, Gata2 expression at stage 26 was completely dependent on Etv2 (Fig. 4A), indicating that Fli1 was no longer sufficient for Gata2 expression. We reasoned that this could be explained if Gata2 is initially regulated by Fli1 during the early stages of haemangioblast specification but becomes dependent on Etv2 in the later stages of haemangioblast programming. Therefore, we carried out a timecourse of Gata2 expression in Etv2 morphants and indeed found that Gata2 expression in the DLP initiates normally, and that it is expressed up to stage 24 in the DLP of Etv2-deficient embryos but that expression was no longer detected by stage 26 (Fig. 4C). Thus, it appears that Fli1 is crucially important for the initiation of the adult haemangioblast programme, activating Gata2 and together regulating Flk1 and Etv2 in the DLP (Fig. 4D). After stage 24, Gata2 becomes dependent on Etv2, even with sustained Fli1 expression. Finally, Etv2 is essential for Gata2 maintenance and Scl expression, as well as for that of the Scl haemangioblast partner Lmo2 (Fig. 4D), and thereby for the programming of adult haemangioblasts.
The GRN controlling adult haemangioblast programming
The synergistic relationships worked out above are summarised in Table 1 and can be represented using a network diagram (Davidson, 2010) (Fig. 5). The timing and tissue of first expression are denoted by the position of the gene on the page, whereas its inputs into downstream targets are depicted by arrows. Where two gene products need to interact to influence the downstream gene, as for VegfA and its receptor, Flk1, or Scl and Lmo2, their output lines converge on chevrons or a circle, respectively. Most genes appear once in the diagram, the exceptions being Etv6 and VegfA, which are expressed in both somites and the DLP, and Gata2, which is duplicated at the stage when its expression control switches from Fli1 to Etv2.
Fig. 5.
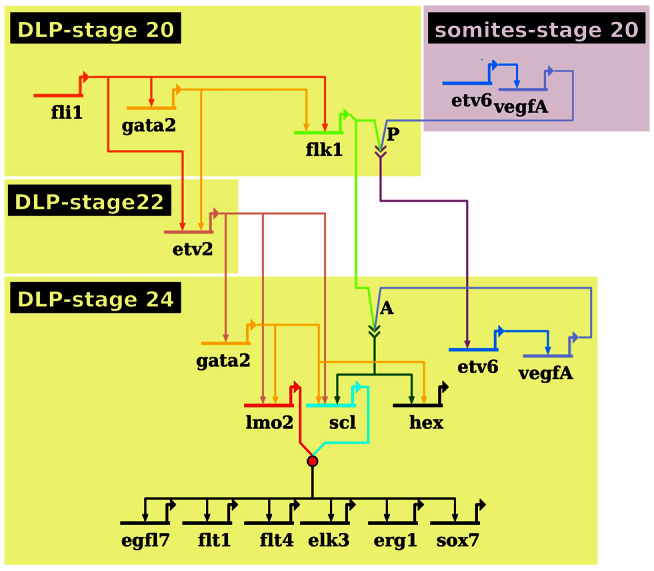
The adult haemangioblast genetic regulatory network. Network summarising the genetic interactions programming adult haemangioblasts in the DLP as determined by MO knockdown. Strikingly, somitic VEGFA is absolutely essential for Flk1 receptor activation in haemangioblast precursors; this activation results in the establishment of endogenous, cell-autonomous VEGFA signalling. Importantly, Scl expression and haemangioblasts cannot be specified unless this endogenous VEGFA signalling synergises with a VEGFA-independent pathway controlled by Fli1 and Etv2. The timing and tissue of expression is denoted by the position of the gene on the network, whereas its relationships with genes expressed at the same time or later is depicted by an arrow from the regulating gene to the regulated gene. Arrows with chevrons indicate the interaction between VEGFA and its receptor, Flk1. P indicates paracrine VEGFA signalling, whereas A indicates endogenous autocrine signalling. The red circle represents the formation of a well-characterised protein complex between Scl and Lmo2.
The GRN presented in Fig. 5 shows that, in the DLP itself, Fli1 sits at the top of the TF hierarchy and that some of its activity might be mediated by its downstream target, Gata2. To confirm this, a hormone-inducible form of Gata2, Gata2FL-GR-HA, was co-injected with Fli1 MO; Gata2FL-GR-AH was activated at stage 20 and Flk1, Etv2 and Scl expression in the DLP was analysed at stages 22, 24 and 26, respectively (supplementary material Fig. S5). Although Gata2 could rescue Scl expression in the ventral blood island to some extent (arrowheads), expression of Flk1, Etv2 and Scl could not be rescued in the DLP (supplementary material Fig. S5). Together with the results presented above, these indicate that Gata2, although required for gene expression in the DLP, is not sufficient, and that synergy between Fli1 and Gata2 is required for adult haemangioblast programming. To further confirm the interactions presented in Table 1, qPCR analysis was carried out on excised DLPs from stage 26 Fli1, Gata2 and Etv2 morphants (supplementary material Fig. S6). Particular attention was paid to the less than all-or-nothing effects, represented by downward-pointing arrows in Table 1. The qPCR data, however, confirmed the impacts on expression indicated in Table 1, further supporting the GRN presented in Fig. 5.
Confirmation that the genetic interactions presented in the GRN are direct will require biochemical analysis, using methods such as chromatin immunoprecipitation sequencing (ChIP-seq), which will require purification of large numbers of cells, something difficult to achieve currently in developing embryos. Nevertheless, close temporal monitoring of gene expression in normal and depleted embryos strongly suggests that most of the targets depicted in the diagram are direct. For example, Fli1 and Gata2 both initiate expression at stage 20, and loss of Fli1 prevents initiation of Gata2 expression. Such a direct interaction has support in the literature: several ETS-binding sites have been described in the regulatory sequences of Gata2, which are required for its expression in endothelial and blood cells (Pimanda et al., 2007), and Fli1 is the only ETS TF expressed in the DLP at this stage of development (Fig. 1; supplementary material Fig. S1), making it the only ETS TF available to activate Gata2. Similar timing arguments can be made for Gata2 and Flk1, and Fli1 and Flk1, and these are supported in the literature by seven Gata-binding sites and two ETS-binding sites having been shown to be crucial for Flk1 expression (Kappel et al., 2000; Minami et al., 2004; Ishitobi et al., 2011). Importantly, Gata2 has been shown to be required for Flk1 expression in endothelial cells and ESCs (Lugus et al., 2007; Mammoto et al., 2009) and, as already stated, Fli1 is the only ETS TF available at this stage of development. In the case of Gata2 and Etv2, when activating each other at different times, the maximum difference in their expression times is 2 hours, which is also likely to be too short for intermediate synthesis, especially for an organism that develops at low temperatures. In support of this, Gata- and ETS-binding sites are required for Etv2 expression in endothelial cells (Veldman and Lin, 2012; De Val et al., 2008); and Gata2 is the only Gata factor available for Etv2 activation and Fli1 the only ETS TF available. Finally, as Scl and Lmo2 require Etv2, which is required for contemporaneous Gata2 expression, the inputs of both into these genes are also likely to be direct, a conclusion supported in the case of Etv2 on Lmo2 by recent binding assays, albeit in the primitive haemangioblast (Koyano-Nakagawa et al., 2012).
Such a network diagram is an efficient way of summarising regulatory information: the hierarchical positions of the regulators can be seen at a glance and the order of events can be clearly depicted. For the first time we can visualise the genetic programming of the adult haemangioblast and identify the roles played by key players.
DISCUSSION
We describe a GRN for a population of cells in the LP mesoderm that contains precursors to the DA and the HSC: namely, adult haemangioblasts. The features of this GRN are described below, providing insight into the ontogeny of these cells and their derivatives, the DA and the HSC.
We have previously shown that the adult haemangioblast programme is dependent on VEGFA signalling driven by Etv6 in both the somites and the DLP itself (Ciau-Uitz et al., 2010b). Here, by carrying out careful timecourses of expression in normal and knockdown embryos, we show that the signal from the somites precedes that within the DLP itself (Fig. 5). Thus, VEGFA signalling from the somites is a key initiator of the haemangioblast programme in the adjacent LP mesoderm. An eventual response to this signalling is to establish VEGFA production in the DLP itself, rendering it cell autonomous. This cell-autonomous activity appears to be by a ‘private’ mechanism whereby VEGFA does not leave the cell, as also seen in HSCs (Ciau-Uitz et al., 2010b; Gerber et al., 2002).
This GRN also clearly shows that, in parallel to the establishment of autocrine VEGFA signalling, a VEGFA-independent genetic cascade has to be established in order to programme adult haemangioblasts in the DLP. Etv2 and Gata2, driven by Fli1, play pivotal roles in this pathway which culminates with the activation, in synergy with the VEGFA-dependent pathway, of Scl. Critically, this Etv2/Gata2 regulatory loop also controls the expression of Lmo2, a key Scl partner in haematopoietic cells and in haemangioblast programming (Yamada et al., 1998; Yamada et al., 2000; Patterson et al., 2007). Therefore, the VEGFA-independent pathway ensures that Lmo2 is in place to interact with Scl (Fig. 5, red circle) and thus achieve adult haemangioblast programming.
An important requirement for the early programming of the adult haemangioblast by VEGF is the expression of its receptor, Flk1, which enables the cells to respond. We have shown that the expression of this gene is dependent on Fli1 together with Gata2 (Liu et al., 2008) (this study). Fli1 is also at the top of the VEGF-independent pathway, so clearly it is important to determine how Fli1 expression is initiated in the DLP.
Many genes expressed in blood and endothelium contain ETS-binding sites in their regulatory regions (Dejana et al., 2007; Sato, 2001; Wilson et al., 2010). The GRN presented here reveals crucial and distinct roles, despite sharing a common binding sequence, for three ETS factors: namely Fli1, Etv2 and Etv6. Fli1 is the master regulator controlling both VEGF-dependent and VEGF-independent responses, whereas Etv6 drives VEGF production in both the somites and the DLP, with Etv2 being crucial for Lmo2 and maintenance of Gata2 expression in the VEGF-independent pathway to Scl expression. Thus, these three ETS factors control key check points in the two synergistic regulatory paths to Scl and thereby the full haemangioblast programme. Further ETS factors are turned on as part of this programme.
The epistatic relationship between Flk1 and Etv2 in haemangioblast development has been unclear. Both Flk1-deficient (Shalaby et al., 1995) and Etv2-deficient (Lee et al., 2008) mice exhibit severe defects in blood and blood vessel development, suggesting that both may have essential functions in the generation of haemangioblasts. Etv2 was initially believed to function above Flk1 in the haemangioblast hierarchy, because Flk1 expression is absent in E7.5 Etv2-deficient embryos, and because Etv2 overexpression in ESCs increases the number of Flk1+ cells and blood and endothelium produced (Lee et al., 2008). In zebrafish embryos, Flk1 is dependent on Etv2, and Etv2 overexpression induces Flk1 expression even in cloche mutants (Sumanas and Lin, 2006; Liu and Patient, 2008; Sumanas et al., 2008). In Xenopus embryos, exogenous Etv2 can activate Flk1 expression in the presence of cyclohexamide, indicating that Etv2 directly activates Flk1 (Salanga et al., 2010). However, more recently, it has been shown that VEGF potently induces Etv2 expression in Flk1+ ES-derived cells (Kataoka et al., 2011), indicating that Flk1 is actually upstream of Etv2. Furthermore, during gastrulation in the mouse, whereas the number of Flk1+ cells is only modestly affected in Etv2-deficient embryos, Etv2 expression is severely reduced in Flk1-deficient embryos (Rasmussen et al., 2012). Thus, a feasible scenario would be that VEGF is required for the initial expression of Etv2 but that continued expression of the VEGF receptor, Flk1, requires Etv2-positive feedback. All these observations relate primarily to the primitive haemangioblast, but, where it has been looked at, the DA is missing in these defective embryos too. We demonstrate here that, during adult haemangioblast specification, although both Flk1 and Etv2 are regulated by Fli1 and Gata2, they do not regulate each other. Our results indicate that Flk1 and Etv2 regulation may only become interdependent after adult haemangioblasts are specified and that the loss of Flk1 expression in Etv2-deficient embryos is due to the absence of endothelial cells (supplementary material Fig. S3).
A conserved feature of vertebrate developmental haematopoiesis is the emergence of HSCs from haemogenic endothelium localised in the ventral wall of the DA (reviewed by Medvinsky et al., 2011). However, the location of the LP mesodermal precursors giving rise to both DA haemogenic endothelium and bone marrow HSCs has not yet been determined. In this work we describe the presence of adult haemangioblasts in the LP mesoderm and characterise their genetic programming. Our analysis demonstrates that key haematopoietic TFs, such as Fli1, Gata2, Etv6, Etv2 and Scl, are crucially required for this programming. Misprogramming of these adult haemangioblasts disrupts HSC emergence in the DA, strongly suggesting that the first crucial step in the generation of HSCs is the programming of adult haemangioblasts. Characterisation of this cell population is therefore essential to understand how HSCs are first generated. The GRN presented here will act as a seed network for future studies. As new regulators are discovered they can be grafted on to this framework.
Supplementary Material
Acknowledgements
We are grateful to Matt Loose, Rachael Nimmo and Catherine Porcher for critical reading of the manuscript, and to Catherine Porcher for providing anti-Scl antibodies.
Footnotes
Funding
This work was supported by the UK Medical Research Council and the EuTRACC Consortium of the European Union Framework 7 Programme. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.090829/-/DC1
References
- Afouda B. A., Ciau-Uitz A., Patient R. (2005). GATA4, 5 and 6 mediate TGFbeta maintenance of endodermal gene expression in Xenopus embryos. Development 132, 763-774 [DOI] [PubMed] [Google Scholar]
- Bertwistle D., Walmsley M. E., Read E. M., Pizzey J. A., Patient R. K. (1996). GATA factors and the origins of adult and embryonic blood in Xenopus: responses to retinoic acid. Mech. Dev. 57, 199-214 [DOI] [PubMed] [Google Scholar]
- Chung Y. S., Zhang W. J., Arentson E., Kingsley P. D., Palis J., Choi K. (2002). Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development 129, 5511-5520 [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A., Walmsley M., Patient R. (2000). Distinct origins of adult and embryonic blood in Xenopus. Cell 102, 787-796 [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A., Liu F., Patient R. (2010a). Genetic control of hematopoietic development in Xenopus and zebrafish. Int. J. Dev. Biol. 54, 1139-1149 [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A., Pinheiro P., Gupta R., Enver T., Patient R. (2010b). Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev. Cell 18, 569-578 [DOI] [PubMed] [Google Scholar]
- Cleaver O., Krieg P. A. (1998). VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development 125, 3905-3914 [DOI] [PubMed] [Google Scholar]
- Cleaver O., Tonissen K. F., Saha M. S., Krieg P. A. (1997). Neovascularization of the Xenopus embryo. Dev. Dyn. 210, 66-77 [DOI] [PubMed] [Google Scholar]
- D'Souza S. L., Elefanty A. G., Keller G. (2005). SCL/Tal-1 is essential for hematopoietic commitment of the hemangioblast but not for its development. Blood 105, 3862-3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgin G., Goldman D. C., Donley N., Ahmed R., Eide C. A., Christian J. L. (2007). GATA-2 functions downstream of BMPs and CaM KIV in ectodermal cells during primitive hematopoiesis. Dev. Biol. 310, 454-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H. (2010). Emerging properties of animal gene regulatory networks. Nature 468, 911-920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Val S., Chi N. C., Meadows S. M., Minovitsky S., Anderson J. P., Harris I. S., Ehlers M. L., Agarwal P., Visel A., Xu S. M., et al. (2008). Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell 135, 1053-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Taddei A., Randi A. M. (2007). Foxs and Ets in the transcriptional regulation of endothelial cell differentiation and angiogenesis. Biochim. Biophys. Acta 1775, 298-312 [DOI] [PubMed] [Google Scholar]
- Garriock R. J., Czeisler C., Ishii Y., Navetta A. M., Mikawa T. (2010). An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development 137, 3697-3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H. P., Malik A. K., Solar G. P., Sherman D., Liang X. H., Meng G., Hong K., Marsters J. C., Ferrara N. (2002). VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417, 954-958 [DOI] [PubMed] [Google Scholar]
- Gering M., Patient R. (2005). Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev. Cell 8, 389-400 [DOI] [PubMed] [Google Scholar]
- Inui M., Fukui A., Ito Y., Asashima M. (2006). Xapelin and Xmsr are required for cardiovascular development in Xenopus laevis. Dev. Biol. 298, 188-200 [DOI] [PubMed] [Google Scholar]
- Ishitobi H., Wakamatsu A., Liu F., Azami T., Hamada M., Matsumoto K., Kataoka H., Kobayashi M., Choi K., Nishikawa S., et al. (2011). Molecular basis for Flk1 expression in hemato-cardiovascular progenitors in the mouse. Development 138, 5357-5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Broadbent J., Thomas P. Q., Smith J. C., Beddington R. S. (1999). An anterior signalling centre in Xenopus revealed by the homeobox gene XHex. Curr. Biol. 9, 946-954 [DOI] [PubMed] [Google Scholar]
- Kälin R. E., Kretz M. P., Meyer A. M., Kispert A., Heppner F. L., Brändli A. W. (2007). Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev. Biol. 305, 599-614 [DOI] [PubMed] [Google Scholar]
- Kappel A., Schlaeger T. M., Flamme I., Orkin S. H., Risau W., Breier G. (2000). Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood 96, 3078-3085 [PubMed] [Google Scholar]
- Kataoka H., Hayashi M., Nakagawa R., Tanaka Y., Izumi N., Nishikawa S., Jakt M. L., Tarui H., Nishikawa S. (2011). Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRα+ primitive mesoderm. Blood 118, 6975-6986 [DOI] [PubMed] [Google Scholar]
- Koyano-Nakagawa N., Kweon J., Iacovino M., Shi X., Rasmussen T. L., Borges L., Zirbes K. M., Li T., Perlingeiro R. C., Kyba M., et al. (2012). Etv2 is expressed in the yolk sac hematopoietic and endothelial progenitors and regulates Lmo2 gene expression. Stem Cells 30, 1611-1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Park C., Lee H., Lugus J. J., Kim S. H., Arentson E., Chung Y. S., Gomez G., Kyba M., Lin S., et al. (2008). ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2, 497-507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Patient R. (2008). Genome-wide analysis of the zebrafish ETS family identifies three genes required for hemangioblast differentiation or angiogenesis. Circ. Res. 103, 1147-1154 [DOI] [PubMed] [Google Scholar]
- Liu F., Walmsley M., Rodaway A., Patient R. (2008). Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr. Biol. 18, 1234-1240 [DOI] [PubMed] [Google Scholar]
- Lugus J. J., Chung Y. S., Mills J. C., Kim S. I., Grass J., Kyba M., Doherty J. M., Bresnick E. H., Choi K. (2007). GATA2 functions at multiple steps in hemangioblast development and differentiation. Development 134, 393-405 [DOI] [PubMed] [Google Scholar]
- Maeno M., Tochinai S., Katagiri C. (1985a). Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid-triploid or interspecific chimeras. Dev. Biol. 110, 503-508 [DOI] [PubMed] [Google Scholar]
- Maeno M., Todate A., Katagiri C. (1985b). The localization of precursor cells for larval and adult hematopoietic-cells of Xenopus-laevis in 2 regions of embryos. Dev. Growth Differ. 27, 137-148 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Connor K. M., Mammoto T., Yung C. W., Huh D., Aderman C. M., Mostoslavsky G., Smith L. E., Ingber D. E. (2009). A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows S. M., Salanga M. C., Krieg P. A. (2009). Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development 136, 1115-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A., Rybtsov S., Taoudi S. (2011). Embryonic origin of the adult hematopoietic system: advances and questions. Development 138, 1017-1031 [DOI] [PubMed] [Google Scholar]
- Meyer D., Stiegler P., Hindelang C., Mager A. M., Remy P. (1995). Whole-mount in situ hybridization reveals the expression of the Xl-Fli gene in several lineages of migrating cells in Xenopus embryos. Int. J. Dev. Biol. 39, 909-919 [PubMed] [Google Scholar]
- Mills K. R., Kruep D., Saha M. S. (1999). Elucidating the origins of the vascular system: a fate map of the vascular endothelial and red blood cell lineages in Xenopus laevis. Dev. Biol. 209, 352-368 [DOI] [PubMed] [Google Scholar]
- Minami T., Murakami T., Horiuchi K., Miura M., Noguchi T., Miyazaki J., Hamakubo T., Aird W. C., Kodama T. (2004). Interaction between hex and GATA transcription factors in vascular endothelial cells inhibits flk-1/KDR-mediated vascular endothelial growth factor signaling. J. Biol. Chem. 279, 20626-20635 [DOI] [PubMed] [Google Scholar]
- Neuhaus H., Müller F., Hollemann T. (2010). Xenopus er71 is involved in vascular development. Dev. Dyn. 239, 3436-3445 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1967). Normal Table of Xenopus laevis (Daudin). A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. Amsterdam: North-Holland Publishing Company; [Google Scholar]
- Park C., Afrikanova I., Chung Y. S., Zhang W. J., Arentson E., Fong Gh G., Rosendahl A., Choi K. (2004). A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development 131, 2749-2762 [DOI] [PubMed] [Google Scholar]
- Patterson L. J., Gering M., Patient R. (2005). Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood 105, 3502-3511 [DOI] [PubMed] [Google Scholar]
- Patterson L. J., Gering M., Eckfeldt C. E., Green A. R., Verfaillie C. M., Ekker S. C., Patient R. (2007). The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood 109, 2389-2398 [DOI] [PubMed] [Google Scholar]
- Pimanda J. E., Ottersbach K., Knezevic K., Kinston S., Chan W. Y., Wilson N. K., Landry J. R., Wood A. D., Kolb-Kokocinski A., Green A. R., et al. (2007). Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc. Natl. Acad. Sci. USA 104, 17692-17697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C., Swat W., Rockwell K., Fujiwara Y., Alt F. W., Orkin S. H. (1996). The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 86, 47-57 [DOI] [PubMed] [Google Scholar]
- Rasmussen T. L., Shi X., Wallis A., Kweon J., Zirbes K. M., Koyano-Nakagawa N., Garry D. J. (2012). VEGF/Flk1 signaling cascade transactivates Etv2 gene expression. PLoS ONE 7, e50103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L., Elwood N. J., Elefanty A. G., Köntgen F., Li R., Barnett L. D., Begley C. G. (1996). The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 15, 4123-4129 [PMC free article] [PubMed] [Google Scholar]
- Salanga M. C., Meadows S. M., Myers C. T., Krieg P. A. (2010). ETS family protein ETV2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the Xenopus embryo. Dev. Dyn. 239, 1178-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y. (2001). Role of ETS family transcription factors in vascular development and angiogenesis. Cell Struct. Funct. 26, 19-24 [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T. P., Gertsenstein M., Wu X. F., Breitman M. L., Schuh A. C. (1995). Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62-66 [DOI] [PubMed] [Google Scholar]
- Sumanas S., Lin S. (2006). Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 4, e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S., Gomez G., Zhao Y., Park C., Choi K., Lin S. (2008). Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood 111, 4500-4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., O'Reilly M. A., Smith J. C. (1997). Analysis of competence and of Brachyury autoinduction by use of hormone-inducible Xbra. Development 124, 2225-2234 [DOI] [PubMed] [Google Scholar]
- Tracey W. D., Jr., Pepling M. E., Horb M. E., Thomsen G. H., Gergen J. P. (1998). A Xenopus homologue of aml-1 reveals unexpected patterning mechanisms leading to the formation of embryonic blood. Development 125, 1371-1380 [DOI] [PubMed] [Google Scholar]
- Veldman M. B., Lin S. (2012). Etsrp/Etv2 is directly regulated by Foxc1a/b in the zebrafish angioblast. Circ. Res. 110, 220-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley M. E., Guille M. J., Bertwistle D., Smith J. C., Pizzey J. A., Patient R. K. (1994). Negative control of Xenopus GATA-2 by activin and noggin with eventual expression in precursors of the ventral blood islands. Development 120, 2519-2529 [DOI] [PubMed] [Google Scholar]
- Walmsley M., Ciau-Uitz A., Patient R. (2002). Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development 129, 5683-5695 [DOI] [PubMed] [Google Scholar]
- Walmsley M., Ciau-Uitz A., Patient R. (2005). Tracking and programming early hematopoietic cells in Xenopus embryos. Methods Mol. Med. 105, 123-136 [DOI] [PubMed] [Google Scholar]
- Wasteson P., Johansson B. R., Jukkola T., Breuer S., Akyürek L. M., Partanen J., Lindahl P. (2008). Developmental origin of smooth muscle cells in the descending aorta in mice. Development 135, 1823-1832 [DOI] [PubMed] [Google Scholar]
- Wilson N. K., Foster S. D., Wang X., Knezevic K., Schütte J., Kaimakis P., Chilarska P. M., Kinston S., Ouwehand W. H., Dzierzak E., et al. (2010). Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532-544 [DOI] [PubMed] [Google Scholar]
- Xiong J. W., Yu Q., Zhang J., Mably J. D. (2008). An acyltransferase controls the generation of hematopoietic and endothelial lineages in zebrafish. Circ. Res. 102, 1057-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Warren A. J., Dobson C., Forster A., Pannell R., Rabbitts T. H. (1998). The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc. Natl. Acad. Sci. USA 95, 3890-3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Pannell R., Forster A., Rabbitts T. H. (2000). The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 97, 320-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A. C., Turlo K. A., Ponec R. M., Lynch M. R., Chen K. C., Hofmann J. J., Cox T. C., Gasson J. C., Iruela-Arispe M. L. (2010). Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood 116, 3435-3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.