Abstract
Objectives:
To assess the characteristics of patients with primary hyperparathyroidism (PHPT) treated with cinacalcet and to evaluate its efficacy in reducing serum calcium and parathyroid hormone (PTH) concentrations after 1 year of treatment.
Methods:
The study included 20 patients with PHPT who had completed at least 12 months of treatment with cinacalcet (eight patients for refusal of parathyroidectomy, three for surgery not possible due to comorbidities and nine for progressive hypercalcemia prior to surgery). We recorded clinical and biochemical data at baseline, and after 3, 6 and 12 months of treatment. We also monitored adverse events. Cinacalcet was administered in increasing doses until normal serum calcium was reached or side effects preventing a further increase occurred.
Results:
After 3 months of treatment, serum calcium significantly decreased (11.73 ± 0.85 versus 10.71 ± 1.63 mg/dl, p < 0.001) and serum phosphorus significantly increased (2.41 ± 0.48 versus 2.63 ± 0.70 mg/dl, p = 0.004) while no significant change occurred in PTH (181.91 ± 102.37 versus 195.47 ± 111.71 pg/ml, p = 0.695). No further variation was observed after 6 months compared with 3 months of follow up. However, after 12 months of treatment, there was a significant decrease in PTH concentrations compared with baseline (181.91 ± 102.37 versus 152.47± 70.16 pg/ml, p = 0.028) as well as serum calcium (11.73 ± 0.85 versus 10.20± 0.95 mg/dl, p < 0.001); serum phosphorus significantly increased (2.41 ± 0.48 versus 2.71 ± 0.43 mg/dl, p = 0.01). Normocalcemia (S-Ca < 10.2 mg/dl) was achieved in 55% of patients. The medication was usually well tolerated (83.4%). Most common adverse events were nausea and vomiting, especially at the beginning of therapy.
Conclusion:
Cinacalcet rapidly reduced serum calcium in patients with PHPT and this reduction remained stable after 1 year of treatment. We also observed a decrease in PTH. Cinacalcet is an effective alternative in nonsurgical treatment of PHPT and may be useful in the preoperative hypercalcemia management.
Keywords: calcimimetics, cinacalcet, hypercalcemia, primary hyperparathyroidism
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrinological disease, characterized by overproduction of parathyroid hormone (PTH) and elevated serum calcium. The goal of therapy in patients with PHPT is to normalize serum calcium and PTH levels, and to improve the associated clinical manifestations [Bilezikian et al. 2009].The only intervention able to cure the disease is parathyroidectomy [Rubin et al. 2008; Utiger, 1999]. However, there are few medical alternatives for patients whose PHPT is unsolved by surgery, or in those with contraindications for surgery or who refuse the procedure.
The discovery of calcium-sensing receptors (CaSRs), which regulate PTH secretion according to extracellular calcium concentrations, has allowed specific antiparathyroid drugs called calcimimetics to be designed. Cinacalcet hydrochloride is an allosteric modulator of CaSRs that increases sensitivity to extracellular calcium and downregulates PTH secretion [Nemeth et al. 2004]. Treatment with cinacalcet has proven to be a reasonable alternative for several patient subgroups [Shoback et al. 2003; Peacock et al. 2005; Cetani et al. 2012]: patients who are not surgical candidates or who refuse surgery; those with persisting PHPT after failed surgery; or patients with inoperable disease due to comorbidities [Rothe et al. 2011; Khan et al. 2009].
Cinacalcet was approved by the European Medicines Agency in June 2008 for the treatment of hypercalcemia in patients with PHPT for whom parathyroidectomy is indicated but surgery is clinically inappropriate or is contraindicated.
In this context, the objectives of our study were to examine the characteristics of patients with PHTP treated with cinacalcet and to evaluate its efficacy in reducing serum calcium and PTH concentrations after 1 year of treatment.
Patients and methods
Study population
Our open-label prospective study included 34 patients (29 women and 5 men, aged 31–85 years) with PHPT who received cinacalcet. From April 2010 to December 2011 we consecutively recruited patients who had been diagnosed with PHTP at the Endocrinology Department and who had initiated treatment with cinacalcet. Cinacalcet was administered in increasing doses until normal serum calcium was reached or side effects preventing a further increase occurred. Exclusion criteria were chronic diseases affecting bone apart from PHPT (familial hypocalciuric hypercalcemia, Paget’s disease, rheumatoid arthritis, hypercortisolism, malignant tumors, renal bone disease and chronic liver disease) and creatinine clearance less than 60 ml/min.
The study was approved by the ethical review board of our hospital and conformed to the ethics guidelines for research in humans. All the participants in the study provided written informed consent.
Clinical data and serum measurements
Epidemiological data (age and sex) and medical history (duration of PHPT, concomitant illnesses and treatments) were recollected. Anthropometric data measured were body mass index calculated by the Quetelet formula (weight in kilograms divided by the square of height in meters), systolic blood pressure and diastolic at baseline and during follow up.
Samples of venous blood were taken in the morning after fasting overnight at baseline and after 3, 6 and 12 months of treatment. Calcium, phosphorus, albumin and creatinine were measured using standard automated laboratory techniques. Glomerular filtration rate was estimated by the Cockcroft–Gault equation. Serum intact PTH was measured using a two-site immunoassay for iPTH (Roche Diagnostics SL, Barcelona, Spain; intra- and inter-assay variability of 3%) and 25-hydroxyvitamin D (25-OH-D) was measured using radioimmunoassay (DiaSorin, Stillwater, Minnesota, USA).
Statistical analysis
Statistical analysis was performed with specific software, SPSS 15.0. Data for continuous variables are presented as mean ± standard deviation. Data for categorical variables are presented as numbers or percentages. Analysis of changes from baseline was performed using the Wilcoxon signed rank test. Number and percentage of subjects achieving a reduction in serum calcium to below the upper limit of the normal range (<10.2 mg/dl) were determined. Statistical significance was set at p < 0.05 (two tailed).
Results
Baseline characteristics of the study population
Mean age at diagnosis was 67.15 ± 14.8 years. Arterial hypertension was present in 67.6% of patients, type 2 diabetes in 29.4% and 8.82% had basal impaired glucose levels. Two patients had kidney stones and one had a hip fracture.
A total of 76.47% were receiving treatment with vitamin D (25-OH-D3) and 50% with bisphosphonates. The duration of PHPT at baseline was 12.22 ± 21.37 months (range 0–101) and the mean duration of treatment was 18 ± 11 months (0.1–42). Reasons for starting cinacalcet treatment were refusal to have parathyroidectomy (n = 8), surgery not possible due to comorbidities (n = 5) and progressive hypercalcemia prior to surgery (n = 21).
Twenty patients (17 women and 3 men) completed 12 months of treatment. Nineteen patients had sporadic PHPT and one had multiple endocrine neoplasia type 1 syndrome.
Dose and tolerance of cinacalcet treatment
The initial dose of cinacalcet was 30 mg/12 h in 94% and 30 mg/24 h in 6% and the mean daily dose was 60 mg (range 30–180). Forty-one percent of patients (n = 14) required dose adjustment, seven patients required a dose escalation due to persistent hypercalcemia and in four patients a reduction in the dose was made because of adverse events (two nausea, one vomiting and one myalgia). Treatment was discontinued in 13 patients, 4 because of adverse events and 9 when surgery was performed.
A total of 17.6% of patients experienced at least one mild to moderate adverse event. The adverse events most commonly reported were nausea and vomiting, especially at the beginning of therapy.
Biochemical changes during follow up and treatment
Table 1 shows the biochemical parameters of the study population during the study period. After 3 months of treatment, serum calcium was significantly decreased (11.73 ± 0.85 versus 10.71 ± 1.63 mg/dl, p < 0.001) and serum phosphorus increased (2.41 ± 0.48 versus 2.63 ± 0.70 mg/dl, p = 0.004) while no significant change occurred in PTH concentrations (181.91 ± 102.37 versus 195.47 ± 111.71 pg/ml, p = 0.695). No further variation was observed after 6 months compared with 3 months of follow up. However, after 12 months of treatment, PTH levels were significantly decreased compared with baseline (181.91 ± 102.37 versus 152.47 ± 70.16 pg/ml, p = 0.028) (Figure 1) as well as serum calcium (11.73 ± 0.85 versus 10.20± 0.95 mg/dl, p < 0.001); serum phosphorus was significantly increased (2.41 ± 0.48 versus 2.71 ± 0.43 mg/dl, p = 0.01) (Figure 2). Normocalcemia (S-Ca < 10.2 mg/dl) was achieved in 55% of patients. No significant changes occurred in 25-OH-D concentrations during the study period.
Table 1.
Biochemical parameters during period of study.
| Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|
| Basal | ||||
| Calcium (mg/dl) | 11.20 | 13.50 | 11.73 | 0.85 |
| Phosphorus (mg/dl) | 1.40 | 3.70 | 2.41 | 0.48 |
| Creatinine (mg/dl) | 0.55 | 1.30 | 0.84 | 0.17 |
| 25-OH-D (mg/dl) | 4.60 | 95.00 | 25.63 | 18.85 |
| PTH (pg/ml) | 78.18 | 451.10 | 181.91 | 102.37 |
| 3 months | ||||
| Calcium (mg/dl) | 9.00 | 16.70 | 10.71** | 1.63 |
| Phosphorus (mg/dl) | 0.87 | 4.10 | 2.63** | 0.70 |
| Creatinine (mg/dl) | 0.63 | 1.44 | 0.90* | 0.23 |
| 25-OH-D (mg/dl) | 4.00 | 80.10 | 30.12ns | 17.43 |
| PTH (pg/ml) | 79.35 | 511.00 | 195.47ns | 111.71 |
| 6 months | ||||
| Calcium (mg/dl) | 8.30 | 12.30 | 10.17** | 0.97 |
| Phosphorus (mg/dl) | 1.90 | 4.00 | 2.76** | 0.59 |
| Creatinine (mg/dl) | 0.56 | 1.58 | 0.83ns | 0.25 |
| 25-OH-D (mg/dl) | 9.20 | 64.20 | 28.95ns | 12.57 |
| PTH (pg/ml) | 66.72 | 502.00 | 191.25ns | 120.79 |
| 12 months | ||||
| Calcium (mg/dl) | 8.70 | 12.40 | 10.20** | 0.95 |
| Phosphorus (mg/dl) | 2.10 | 3.80 | 2.71* | 0.43 |
| Creatinine (mg/dl) | 0.55 | 1.96 | 0.88* | 0.30 |
| 25-OH-D (mg/dl) | 10.70 | 70.60 | 29.06ns | 14.88 |
| PTH (pg/ml) | 53.24 | 276.10 | 152.47* | 70.16 |
p < 0.05 compared with baseline values; **p < 0.01 compared with baseline values; ns, not significant.
2-OH-D, 25-hydroxyvitamin D; PTH, parathyroid hormone; SD, standard deviation.
Figure 1.
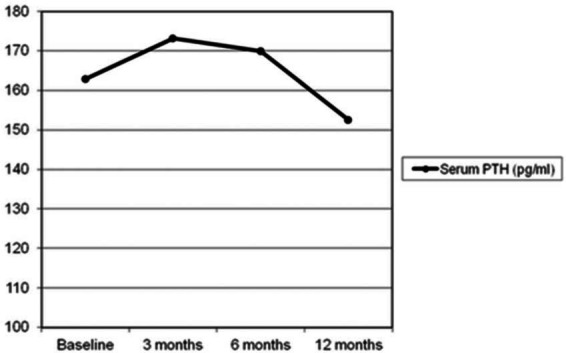
Mean serum parathyroid hormone (PTH) levels during study period.
Figure 2.
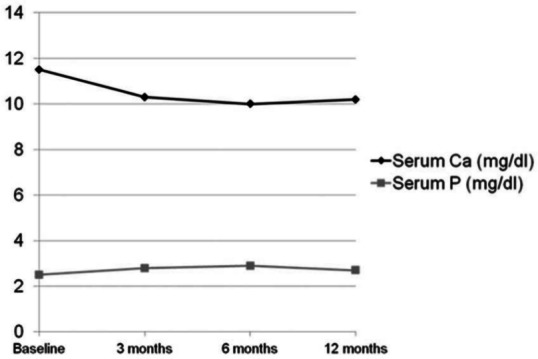
Mean serum calcium (Ca) and phosphorus (P) levels during study period.
Discussion
In this study, cinacalcet consistently decreased serum calcium and increased serum phosphorus in patients with PHPT. Patients experienced a rapid decrease in hypercalcemia after 3 months of treatment, with normalization in 55% of cases and stabilization of these changes at 12-month follow up. There was also a decrease in PTH concentrations, achieving normalization in 5% of patients after 12 months of treatment.
Several studies have demonstrated that cinacalcet effectively controls hypercalcemia while only modestly reduces PTH levels in a heterogeneous sample of patients with PHPT, including patients with and without an indication for parathyroidectomy and patients with a history of failed parathyroidectomy [Marcocci et al. 2009; Marcocci and Cetani, 2012; Peacock et al. 2011; Silverberg et al. 2007]. Our results are consistent with previous studies and demonstrate the stabilization of serum calcium in the medium term. Moreover, we found a greater reduction in PTH levels after 1 year of treatment and normalization was achieved in 5% of patients.
Previous pharmacokinetics studies demonstrated that, unlike serum calcium, predose plasma PTH does not accurately reflect the total changes in plasma PTH which decreases after each dose and returns to baseline by 8–12 h [Shoback et al. 2003]. The difference in serum calcium and PTH responses may be due to cinacalcet’s actions on CaSRs in tissues other than parathyroid glands, such as bone and particularly kidneys where it directly reduces tubular reabsorption of calcium [Peacock et al. 2005].
We included a high proportion of patients with progressive hypercalcemia prior to surgery given the long waiting time for parathyroidectomy at our hospital (12–18 months). In these patients, cinacalcet also effectively controlled biochemical abnormalities associated with PHPT. We consider that this drug may be applied as an interim solution before surgery or when comorbidities and other risk factors require optimal control [Duntas and Stathatos, 2011].
Cinacalcet was well tolerated in our patients and doses required were similar to previous studies. Some patients experienced gastrointestinal symptoms at the beginning of therapy with progressive tolerance after decreasing dosage. Finally, treatment was only discontinued in three patients due to adverse events. The initial dose was adjusted in less than half of patients. Therefore, cinacalcet is a safe and effective drug.
This study has certain limitations. First, we did not evaluate bone turnover markers or bone mineral density. In addition, a follow-up time of 1 year may be relatively short but we found significant results in the parameters analyzed. The strengths of our study are the sample size, which is superior to similar studies, and the inclusion of a high proportion of patients awaiting surgery.
Conclusion
Cinacalcet rapidly reduced serum calcium in patients with PHPT and this reduction remained stable after 1 year of treatment. There was also a decrease in PTH concentrations. Cinacalcet is an effective alternative in the nonsurgical treatment of PHPT and may be useful in preoperative hypercalcemia management.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Inés Luque-Fernández, Hospital Virgen de la Salud, Avda. Barber n 30, Toledo, Spain.
Antonia García-Martín, Hospital Comarcal del Noroeste, Caravaca de la Cruz, Spain.
Alessandra Luque-Pazos, Hospital Virgen de la Salud, Toledo, Spain.
References
- Bilezikian J., Khan A., Potts J., Jr (2009) Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 94: 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetani F., Saponaro F., Banti C., Cianferotti L., Vignali E., Chiavistelli S., et al. (2012) Cinacalcet efficacy in patients with moderately severe primary hyperparathyroidism according to the European Medicine Agency prescription labeling. J Endocrinol Invest 35: 655–660 [DOI] [PubMed] [Google Scholar]
- Duntas L., Stathatos N. (2011) Cinacalcet as alternative treatment for primary hyperparathyroidism: achievements and prospects. Endocrine 39: 199–204 [DOI] [PubMed] [Google Scholar]
- Khan A., Bilezikian J., Potts J., Jr (2009) Asymptomatic primary hyperparathyroidism: a commentary on the revised guidelines. Endocr Pract 15: 494–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci C., Cetani F. (2012) Update on the use of cinacalcet in the management of primary hyperparathyroidism. J Endocrinol Invest 35: 90–95 [DOI] [PubMed] [Google Scholar]
- Marcocci C., Chanson P., Shoback D., Bilezikian J., Fernandez-Cruz L., Orgiazzi J., et al. (2009) Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J Clin Endocrinol Metab 94: 2766–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E., Heaton W., Miller M., Fox J., Balandrin M., Van Wagenen B., et al. (2004) Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 308: 627–635 [DOI] [PubMed] [Google Scholar]
- Peacock M., Bilezikian J., Bolognese M., Borofsky M., Scumpia S., Sterling L., et al. (2011) Cinacalcet HCl reduces hypercalcemia in primary hyperparathyroidism across a wide spectrum of disease severity. J Clin Endocrinol Metab 96: E9–E18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock M., Bilezikian J., Klassen P., Guo M., Turner S., Shoback D. (2005) Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 90: 135–141 [DOI] [PubMed] [Google Scholar]
- Rothe H., Liangos O., Biggar P., Petermann A., Ketteler M. (2011) Cinacalcet treatment of primary hyperparathyroidism. Int J Endocrinol 2011: 415–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M., Bilezikian J., McMahon D., Jacobs T., Shane E., Siris E., et al. (2008) The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 93: 3462–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoback D., Bilezikian J., Turner S., McCary L., Guo M., Peacock M. (2003) The calcimimetic cinacalcet normalizes serum calcium in subjects with primary hyperparathyroidism. J Clin Endocrinol Metab 88: 5644–5649 [DOI] [PubMed] [Google Scholar]
- Silverberg S., Rubin M., Faiman C., Peacock M., Shoback D., Smallridge R., et al. (2007) Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab 92: 3803–3808 [DOI] [PubMed] [Google Scholar]
- Utiger R. (1999) Treatment of primary hyperparathyroidism. N Engl J Med 17: 1301–1302 [DOI] [PubMed] [Google Scholar]


