Abstract
Objectives:
Bendamustine is a unique cytotoxic agent active against various human malignancies, including chronic lymphocytic leukemia (CLL). In vitro studies suggest that cytotoxic activity of bendamustine on CLL-derived cells is synergized by rituximab. A retrospective chart review was conducted to characterize treatment-naïve outpatients and those with relapsed disease aged 70 years and over with CLL receiving bendamustine (with or without rituximab) and to evaluate real-world patterns of care, safety, and effectiveness.
Methods:
Using McKesson Specialty Care/US Oncology Network iKnowMed databases, 91 outpatients with at least two recorded visits and at least two cycles of bendamustine monotherapy or bendamustine–rituximab combination therapy were identified and included. Mean age at diagnosis and start of first therapy was 70.3 and 77.4 years respectively, and 63.7% of patients were men.
Results:
Observed overall response rate was 56.3% in pooled treatment-naïve patients [n = 9; complete response (CR) 18.8%; partial response (PR) 37.5%; nodular partial response (nPR) 0%] and 58.7% in pooled patients with relapsed disease (n = 44; CR 13.3%; PR 44.0%; nPR 1.3%). Median time to progressive disease has not been reached for the 16 treatment-naïve patients (median follow up 15.1 months), and was 18.4 months for those with relapsed disease (n = 73). No unexpected toxicities were observed. Overall rate of blood/bone marrow toxicities (all grades) was 40.7%; grade 3/4 rates were 18.8% in treatment-naïve patients and 25.3% in those with relapsed disease. Most frequent nonhematologic adverse events were fatigue and rash.
Conclusion:
In this retrospective chart review of 91 outpatients with CLL aged 70 years and over, bendamustine (with or without rituximab) was an effective therapeutic option with manageable toxicity.
Keywords: bendamustine, chart review, chemoimmunotherapy, chronic lymphocytic leukemia, retrospective, rituximab
Introduction
Chronic lymphocytic leukemia (CLL), the most common adult leukemia in Western countries [Kim et al. 2010; Knauf et al. 2009], is a progressive hematopoietic disorder characterized by the expansion of neoplastic lymphocytes in the blood, bone marrow, spleen, and lymph nodes [Wierda et al. 2008]. The incidence of CLL is two times higher in men than women, and nearly 70% of patients with CLL are older than age 65 years at the time of diagnosis [Gribben, 2010]. Notably, in 2010, CLL was estimated to account for 14,990 new cancer cases and 4390 deaths in the USA [Jemal et al. 2010].
Although allogeneic stem-cell transplantation has been shown to provide a durable response in select patients with CLL [Dreger et al. 2010; Gribben et al. 2005], many patients with CLL are considered poor candidates for allogeneic stem-cell transplantation due to age, comorbidities, and vulnerability to treatment-related toxicities [Hallek, 2009; Gribben et al. 2005]; thus, for many patients, the disease remains incurable with a highly variable clinical course [Gribben, 2010; Foon and Hallek, 2010]. Many patients remain asymptomatic for years and face a lengthy disease course [Wierda et al. 2008]. At present, patients with CLL are generally managed with a ‘watch and wait’ strategy until an indication for treatment emerges [Gribben, 2010]. Treatment is recommended for active/progressive disease, which may be indicated by constitutional symptoms attributable to CLL (e.g. fever, night sweats, severe fatigue, or involuntary weight loss) or cytopenias; massive, painful, or progressive adenopathy or splenomegaly; or rapidly progressive lymphocytosis [Cheson et al. 1996; Hallek et al. 2008].
Choice of initial therapy is based on several factors, including individual patient characteristics, prognostic markers, disease burden, and the rate of disease progression [Foon and Hallek, 2010]. In recent years, a deeper understanding of CLL and the introduction of purine analogues and anti-CD20 monoclonal antibodies as treatment options have switched the focus of management from a palliative approach to one directed at improving progression-free survival (PFS) and overall survival [Gribben, 2010; Foon and Hallek, 2010].
Bendamustine, a bifunctional derivative of mechlorethamine [Tageja and Nagi, 2010], is a unique cytotoxic agent [Cheson and Rummel, 2009] that has demonstrated activity against various human malignancies [Leoni et al. 2008], including CLL [Knauf et al. 2009], non-Hodgkin’s lymphoma [Cheson et al. 2010], multiple myeloma [Cheson et al. 2010], and solid tumors [Cheson et al. 2010]. In 2008, the US Food and Drug Administration (FDA) approved bendamustine for the treatment of CLL [Hallek, 2009] on the basis of data from a phase III trial in treatment-naïve patients in which the overall response rate (ORR) and PFS were significantly greater with bendamustine than chlorambucil [Knauf et al. 2009].
Rituximab is a chimeric monoclonal immunoglobulin G1 κ antibody to CD20, containing murine light and heavy chain variable region sequences and human constant region sequences [Robak et al. 2010b]. Since receiving FDA approval in 1997, rituximab in combination with chemotherapy has become the standard of care for several B-cell malignancies [Robak et al. 2010b].
Recent studies in treatment-naïve patients with CLL or those with relapsed disease have shown improved efficacy with the addition of rituximab to purine analogues and cyclophosphamide [Hallek et al. 2010; Robak et al. 2010a]. In vitro studies suggest that the cytotoxic activity of bendamustine on CLL-derived cell lines is synergistically enhanced by the addition of rituximab [Rummel et al. 2002]. Furthermore, studies conducted recently by the German Chronic Lymphocytic Leukemia Study Group (GCLLSG) have reported favorable findings with the use of bendamustine combined with rituximab in both treatment-naïve patients with CLL and those with relapsed/refractory disease [Fischer et al. 2011, 2012].
The National Comprehensive Cancer Network (NCCN) consensus guidelines include the use of bendamustine combined with rituximab as a suggested treatment regimen in most treatment-naïve patients with CLL and those with relapsed/refractory disease [NCCN, 2012]. In patients without del(17p), the guidelines recommend bendamustine plus rituximab for the following: as first-line therapy in patients aged at least 70 years or younger patients with comorbidities; first-line therapy in patients aged under 70 years or older patients without significant comorbidities; and therapy for relapsed/refractory disease in patients with short duration of response (e.g. <2 years based on clinical judgment) who are aged under 70 years or are older without comorbidities [NCCN, 2012]. The guidelines also include the use of bendamustine, with or without rituximab, as therapy for relapsed/refractory disease in patients with short response who are aged at least 70 years without del(17p) [NCCN, 2012].
This retrospective chart review sought to characterize outpatients aged at least 70 years with CLL receiving bendamustine (with or without rituximab) and evaluate patterns of care, safety, and real-world effectiveness outside of the controlled environment of clinical trials. The purpose was to examine whether real-world patient populations and results were consistent with results from carefully controlled clinical trials.
Methods
Overview
The current retrospective chart review was designed to provide a descriptive analysis of treatment-naïve outpatients with CLL and those with relapsed disease aged at least 70 years receiving bendamustine (with or without rituximab) and to evaluate patterns of care, safety, and real-world effectiveness outside of the controlled clinical trial environment. The protocol and related documents received US Oncology Network Institutional Review Board approval. Patient confidentiality was maintained according to US Oncology Network policies, and only US Oncology Network staff members were involved in data handling and analysis. Because of these controls and because the potential for patient risk or benefit related to this retrospective study is minimal, waiving informed consent does not adversely affect patients’ rights.
Data sources
Demographic, clinical, and treatment data used in the current retrospective chart review were extracted from McKesson Specialty Care/US Oncology Network iKnowMed (iKM) record databases, which include more than 16 million patient records and are used by 900 oncologists. The iKM databases contain information on outpatients under care in the US Oncology Network, including (but not limited to) laboratory results, diagnosis, therapy administration, line of therapy, staging, comorbidities, and performance status. Vital-status data from the Social Security Administration Death Index were supplemented with iKM data to determine mortality.
Patient population and time period
The current retrospective chart review included outpatients with CLL who received bendamustine alone or in combination with rituximab during the period between 1 March 2008 and 31 May 2010, and met prespecified inclusion and exclusion criteria. The chart-review index date was defined as the date on which the first treatment with the target drugs was initiated. Patients were followed until the end of the data stream or date of death, whichever occurred first.
Patients enrolled in a clinical trial and those being treated for another tumor type during the chart-review time period were excluded from evaluation. Patients were evaluated if they received care at a site that used the complete electronic medical record capabilities of iKM before the start of therapy, had at least two visits recorded, had received at least two cycles of bendamustine monotherapy or combination therapy with bendamustine plus rituximab, were aged at least 70 years, and met the inclusion criteria. The final patient population was divided into four groups based on previous therapy (treatment naïve or relapsed) and therapy received during the chart-review period (bendamustine monotherapy or combination therapy with bendamustine plus rituximab).
Objectives
The design of the retrospective chart review was based on five objectives. The strategy for identifying required data files included a review of each of the objectives to optimize data retrieval, and the minimum number of variables was extracted from data files to accomplish analysis goals. Only data from the chart-review time period were used in the analysis. All results were reported in the aggregate.
The first objective was to provide a descriptive analysis of the patient population with respect to demographics and clinical/treatment characteristics, with a focus on the following variables: age, sex, disease stage, performance status, median lactate dehydrogenase levels, first-line/relapsed therapy, and monotherapy/combination therapy. The Charlson comorbidity index was used to calculate weights for comorbidities, which were identified through programmatic queries of iKM databases for each patient.
The second objective was to determine response rates for all patients by subgroup, with a focus on complete response (CR), partial response (PR), nodular partial response (nPR), stable disease, and progressive disease. Chart reviews were conducted to provide detailed response rate information.
The third objective was to describe patterns of care in terms of median dose, median number of cycles, previous treatments, relative dose intensity, dose delay, dose reduction, and proportion of patients receiving a granulocyte colony-stimulating factor (GCSF), antiemetics, and antibiotics.
The fourth objective was to determine time to progressive disease (change in line of therapy, relapse after remission, or death due to any cause was used as a proxy for PFS), relapse after remission, or death from any cause. Patients who did not die, did not have relapsed disease, did not have progressive disease during the observation period, or were lost to follow-up were censored.
The final objective was to determine the incidence of adverse events (AEs) associated with bendamustine monotherapy or combination therapy with bendamustine plus rituximab. Treatment-related AEs were reported and graded according to the Common Terminology Criteria for AEs (version 4). Chart reviews were conducted to provide detailed information on AEs.
Statistical methods
Statistical analyses were performed using Statistical Analysis Software (v. 9.1). Estimates for continuous variables (e.g. age) were reported using mean, standard deviation, and ranges, and those for categorical variables [e.g. gender and Eastern Cooperative Oncology Group (ECOG) status] were reported using frequencies and proportions. Estimates for response rate variables were provided using frequencies and proportions, and 95% confidence intervals (CIs) were reported for each of the estimates. Kaplan–Meier curves were provided for estimates of PFS in the prespecified patient subgroups; median survival and 95% CIs were also reported.
Results
Patient demographics and characteristics
A total of 465 patients with CLL receiving bendamustine between 1 March 2008 and 31 May 2010 were screened for the retrospective chart review. Of these, 42 patients were excluded because of enrollment in a clinical trial, and 15 patients were excluded because they were being treated for another tumor type during the chart-review period. Only 91 of the remaining 408 patients met all inclusion criteria (i.e. at least two recorded visits, availability of complete electronic medical records, completion of at least two cycles of bendamustine, and age ≥70 years) (Figure 1).
Figure 1.
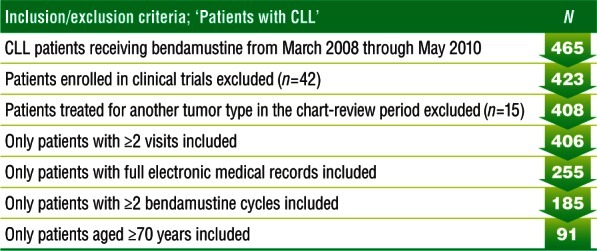
Patient inclusion and exclusion results. Eligibility screening based on prespecified inclusion and exclusion criteria.
CLL, chronic lymphocytic leukemia.
The median age of the 91 patients at the start of first therapy was 75.1 years (range 70.2–89.9), and the median age at diagnosis was 69.6 years (range 55.0–86.9); 63.7% of the patients were men (Table 1). Of those patients with a recorded Rai stage (60/91) and ECOG score (82/91), 40.0% and 23.3% of patients were in Rai stage I and II respectively, and almost 99% of patients had an ECOG score of 0 or 1 at initiation of first therapy. B symptoms were absent in over 75% of patients. The most common comorbidities were rheumatologic disease (n = 14), chronic pulmonary disease (n = 12), and diabetes (n = 9). Baseline laboratory values are presented in Table 2.
Table 1.
Patient demographics and baseline characteristics.
| Variable | All (N = 91) | Treatment naïve |
Relapsed or ≥second line |
||
|---|---|---|---|---|---|
| Bendamustine monotherapy (n = 10) | Bendamustine + rituximab combination therapy (n = 6) | Bendamustine monotherapy (n = 20) | Bendamustine + rituximab combination therapy (n = 55) | ||
| Median age at diagnosis (range), years | 69.6 (55.0–86.9) | 72.3 (63.5–83.4) | 72.9 (67.8–85.3) | 68.3 (58.9–79.5) | 68.89 (55.0–87.0) |
| Median age at beginning of first therapy (range), years | 75.1 (70.2–89.9) | 75.4 (71.1–83.5) | 74 (70.8–85.7) | 74.7 (70.5–89.2) | 75.5 (70.3–89.9) |
| Gender, n (%) | |||||
| Women | 33 (36.3) | 3 (30.0) | 4 (66.7) | 7 (35.0) | 19 (34.5) |
| Men | 58 (63.7) | 7 (70.0) | 2 (33.3) | 13 (65.0) | 36 (65.5) |
| Mean BMI at treatment baseline (SD) | 26.3 (4.4) | 27.2 (3.3) | 29.2 (5.7) | 24.9 (3.7) | 26.3 (4.5) |
| Rai stage at diagnosis, n (%) | |||||
| 0 | 7 (7.7) | 1 (10.0) | 0 | 4 (20.0) | 2 (3.6) |
| I | 24 (26.4) | 1 (10.0) | 3 (50.0) | 8 (40.0) | 12 (21.8) |
| II | 14 (15.4) | 0 | 0 | 2 (10.0) | 12 (21.8) |
| III | 7 (7.7) | 0 | 1 (16.7) | 1 (5.0) | 5 (9.1) |
| IV | 8 (8.8) | 0 | 0 | 2 (10.0) | 6 (10.9) |
| Unknown | 11 (12.1) | 0 | 2 (33.3) | 1 (5.0) | 8 (14.8) |
| Missing | 20 (22.0) | 8 (80.0) | 0 | 2 (10.0) | 10 (18.5) |
| ECOG score at initiation of first therapy, n (%) | |||||
| 0 | 42 (46.1) | 5 (50.0) | 5 (83.4) | 8 (40.0) | 24 (43.6) |
| 1 | 39 (42.9) | 3 (30.0) | 0 | 12 (60.0) | 24 (43.6) |
| 2 | 1 (1.0) | 0 | 0 | 0 | 1 (1.8) |
| Missing | 9 (10.0) | 2 (20.0) | 1 (16.6) | 0 | 6 (11.0) |
| B symptoms, n (%) | |||||
| Absent | 70 (76.9) | 8 (80.0) | 5 (83.3) | 16 (80.0) | 41 (74.5) |
| Present | 14 (15.4) | 1 (10.0) | 1 (16.7) | 3 (15.0) | 9 (16.4) |
| Missing | 7 (7.7) | 1 (10.0) | 0 | 1 (5.0) | 5 (9.1) |
| Most frequent comorbidities, n (%) | |||||
| Rheumatologic disease | 14 (15.4) | 1 (10.0) | 1 (16.6) | 3 (15.0) | 9 (16.4) |
| Chronic pulmonary disease | 12 (13.2) | 1 (10.0) | 0 | 2 (10.0) | 9 (16.4) |
| Diabetes | 9 (9.9) | 1 (10.0) | 1 (16.6) | 4 (20.0) | 3 (5.5) |
| Prior MI | 7 (7.7) | 2 (20.0) | 1 (16.6) | 1 (5.0) | 3 (5.5) |
| Ulcer disease | 6 (6.6) | 0 | 0 | 3 (15.0) | 3 (5.5) |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; MI, myocardial infarction; SD, standard deviation.
Table 2.
Baseline laboratory values.
| Variable | All (N = 91) | Bendamustine monotherapy (n = 10) | Bendamustine + rituximab combination therapy (n = 6) | Bendamustine monotherapy (n = 20) | Bendamustine + rituximab combination therapy (n = 55) |
|---|---|---|---|---|---|
| Hemoglobin (mg/dl) | |||||
| Mean (SD) | 11.5 (1.7) | 12.3 (2.2) | 11.7 (1.4) | 11.1 (1.5) | 11.5 (1.6) |
| Median (range) | 11.4 (7.5–16.3) | 11.8 (9.8–16.3) | 12.5 (9.2–13.0) | 11.1 (8.3–15.2) | 11.7 (7.5–14.2) |
| Hematocrit (%) | |||||
| Mean (SD) | 34.5 (4.9) | 37.3 (7.1) | 34.6 (4.3) | 33.3 (4.4) | 34.5 (4.7) |
| Median (range) | 34.5 (22.9–48.8) | 36.4 (28.3–48.8) | 35.9 (27.7–38.4) | 33.2 (24.9–46.4) | 35.1 (22.9–42.4) |
| Missing | 1 | 0 | 0 | 0 | 1 |
| Creatinine (mg/dl) | |||||
| Mean (SD) | 1.2 (0.4) | 1.2 (0.3) | 1.2 (0.28) | 1.1 (0.3) | 1.2 (0.4) |
| Median (range) | 1.1 (0.5–2.7) | 1.08 (0.87–1.8) | 1.1 (0.87–1.6) | 1.04 (0.58–2.0) | 1.1 (0.67–2.7) |
| Missing | 1 | 0 | 1 | 0 | 0 |
| Lactate dehydrogenase (U/liter) | |||||
| Mean (SD) | 247.6 (108.9) | 301.3 (146.3) | 299 (126.8) | 199.1 (78.9) | 254.8 (107) |
| Median (range) | 232.5 (95–636) | 295 (109–577) | 286 (189–435) | 184 (95–354) | 242.5 (110–636) |
| Missing | 13 | 2 | 2 | 0 | 9 |
| White blood cell count (× 103/µl) | |||||
| Mean (SD) | 61.8 (74.4) | 144.2 (121.4) | 40.2 (37.0) | 63.6 (58.9) | 49.3 (63.1) |
| Median (range) | 39 (2.5–395) | 98.5 (37.8–395) | 26.1 (8.0–90.5) | 40.6 (5.0–191) | 20 (2.5–266.5) |
| Missing | 9 | 1 | 0 | 4 | 4 |
| Neutrophils (× 103/µl) | |||||
| Mean (SD) | 6.4 (10.2) | 13.1 (19.8) | 5.9 (3.7) | 6.4 (10.7) | 5.4 (7.9) |
| Median (range) | 3.5 (0.5–62.0) | 6.3 (3.8–62.0) | 4.8 (3.0–13.1) | 3.3 (0.9–44.1) | 3.0 (0.5–44.5) |
| Missing | 16 | 2 | 0 | 5 | 9 |
| Lymphocytes (× 103/µl) | |||||
| Mean (SD) | 49.2 (59.8) | 97.0 (82.2) | 33.9 (30.0) | 55.2 (52.4) | 40.6 (57.4) |
| Median (range) | 32.3 (0.7–276.4) | 56.9 (29.3–276.5) | 34.2 (3.8–66.9) | 36.0 (2.8–163.4) | 13.9 (0.7–206.4) |
| Missing | 17 | 2 | 1 | 5 | 9 |
| Platelets (× 103/µl) | |||||
| Mean (SD) | 139.8 (66.7) | 127.3 (43.2) | 163.6 (71.9) | 118.3 (67.1) | 146.5 (67.9) |
| Median (range) | 126.5 (16.0–351) | 134 (70.0–179.0) | 143.5 (74.0–262.0) | 106 (16.0–281.0) | 132.5 (47.0–351.0) |
| Missing | 7 | 3 | 0 | 1 | 3 |
SD, standard deviation.
Treatment patterns
Sixteen of the 91 patients (17.6%) included in the chart review were treatment naïve, whereas 75 (82.4%) had relapsed disease after prior treatment. All patients received a median of three cycles of treatment, with the exception of treatment-naïve patients receiving bendamustine monotherapy, who received a median of two cycles (Table 3). Of the 16 treatment-naïve patients, 10 received three doses of bendamustine monotherapy once per cycle and 32 doses of monotherapy twice per cycle (median 97.9 mg/m2 dose); and six patients received three doses of bendamustine combined with rituximab once per cycle and 25 doses of the combination therapy twice per cycle (median 76.1 mg/m2 dose). Of the 75 patients with relapsed disease, 20 received seven doses of bendamustine monotherapy once per cycle and 62 doses of monotherapy twice per cycle (median 98.1 mg/m2 dose), and 55 received 29 doses of the combination therapy once per cycle and 172 doses of the combination therapy twice per cycle (median 82.0 mg/m2 dose). Nearly two-thirds of patients with relapsed disease had previously received rituximab alone or in combination therapy, and more than one-third of these patients had received combination therapy with cyclophosphamide (Figure 2).
Table 3.
Treatment patterns.
| Variable | All (N = 91) | Treatment naïve |
Relapsed or ≥second line |
||
|---|---|---|---|---|---|
| Bendamustine monotherapy (n = 10) | Bendamustine + rituximab combination therapy (n = 6) | Bendamustine monotherapy (n = 20) | Bendamustine + rituximab combination therapy (n = 55) | ||
| Median dose (range), mg/m2 | 89.6 (21.8–119.4) | 97.9 (72.6–101.9) | 76.1 (60.4–100.2) | 98.1 (40.2–117.7) | 82.0 (21.8–119.4) |
| Median number of cycles administered (range) | 3 (1–11) | 2 (2–6) | 3 (1–7) | 3 (1–11) | 3 (1–8) |
| Median number of cycles planned (range) | 6 (2–14) | 6 (2–6) | 6 (6–8) | 6 (2–14) | 6 (2–10) |
| Median number of previous treatments (range) | 2 (0–5) | 0 (0–0) | 0 (0–0) | 2 (1–5) | 2 (0–5) |
| Relative dose intensity (%) | |||||
| Mean (SD) | 44.0 (12.2) | 47.4 (8.6) | 38.6 (12.2) | 40.6 (13.9) | 45.1 (12.0) |
| Median (range) | 45.0 (5.4–100.0) | 45.6 (38.5–68.4) | 43.1 (15–48.3) | 44.4 (5.4–61.2) | 46.0 (18.1–100.0) |
| 25th–75th percentile | 38.1–50.3 | 41.4–50.5 | 36.5–45.5 | 32.8–49.8 | 37.8–50.5 |
| Relative dose intensity cut points, n (%) | |||||
| ≤30% | 8 (8.8) | 0 | 1 (16.7) | 3 (15.0) | 4 (7.3) |
| 31–50% | 59 (64.8) | 6 (60.0) | 5 (83.3) | 13 (65.0) | 35 (63.6) |
| ≥51% | 24 (26.4) | 4 (40.0) | 0 | 4 (20.0) | 16 (29.1) |
| Reasons for discontinuation, n (%) | |||||
| No response to treatment | 3 (3.3) | 0 | 0 | 1 (5.0) | 2 (3.6) |
| Patient refused due to toxicity | 1 (1.1) | 0 | 1 (16.7) | 0 | 0 |
| Toxicity, medically required | 10 (11.0) | 2 (20.0) | 0 | 2 (10.0) | 6 (11.0) |
| Patient-specific reason | 4 (4.4) | 0 | 1 (16.7) | 1 (5.0) | 2 (3.6) |
| Progression/relapse | 2 (2.2) | 0 | 0 | 0 | 2 (3.6) |
| Treatment completed as scheduled | 19 (20.9) | 1 (10.0) | 1 (16.7) | 4 (20.0) | 13 (23.6) |
| Other | 7 (7.7) | 0 | 0 | 2 (10.0) | 5 (9.1) |
| Unknown | 42 (46.1) | 7 (70.0) | 3 (50.0) | 10 (50.0) | 22 (40.0) |
| Patients who did not discontinue* | 3 (3.3) | 0 | 0 | 0 | 3 (5.5) |
| Maximum cycle delay, n patients (%) | |||||
| None | 36 (39.5) | 6 (60.0) | 3 (50.0) | 6 (30.0) | 21 (38.2) |
| 1–5 days | 5 (5.5) | 0 | 1 (16.7) | 1 (5.0) | 3 (5.5) |
| 5–10 days | 16 (17.6) | 2 (20.0) | 0 | 4 (20.0) | 10 (18.1) |
| 10–15 days | 16 (17.6) | 1 (10.0) | 0 | 6 (30.0) | 9 (16.4) |
| >15 days | 18 (19.8) | 1 (10.0) | 2 (33.3) | 3 (15.0) | 12 (21.8) |
| Maximum cycle delay, n doses (%) | |||||
| 1–5 days | 7 (8.9) | 0 | 1 (14.3) | 1 (5.0) | 5 (10.6) |
| 5–10 days | 23 (29.1) | 1 (20.0) | 0 | 8 (40.0) | 14 (29.8) |
| 10–15 days | 24 (30.4) | 3 (60.0) | 1 (14.3) | 8 (40.0) | 12 (25.5) |
| >15 days | 25 (31.6) | 1 (20.0) | 5 (71.4) | 3 (15.0) | 16 (34.1) |
| Total | 79 | 5 | 7 | 20 | 47 |
| Doses with no delay | 545 | 62 | 47 | 111 | 325 |
| Number of cycles with dose reduction, n patients (%) | |||||
| 1 | 26 (47.3) | 6 (85.7) | 0 | 6 (66.7) | 14 (40.0) |
| 2 | 14 (25.5) | 1 (14.3) | 2 (50.0) | 1 (11.1) | 10 (28.6) |
| 3 | 6 (10.9) | 0 | 0 | 1 (11.1) | 5 (14.3) |
| 4 | 6 (10.9) | 0 | 2 (50.0) | 0 | 4 (11.4) |
| 5 | 3 (5.4) | 0 | 0 | 1 (11.1) | 2 (5.7) |
| Total | 55 (60.4) | 7 (70.0) | 4 (100) | 9 (45.0) | 35 (63.6) |
| Dosing | |||||
| 1 per cycle | 42 | 3 | 3 | 7 | 29 |
| 2 per cycle | 291 | 32 | 25 | 62 | 172 |
| 3 per cycle | 0 | 0 | 0 | 0 | 0 |
| Patients receiving a granulocyte colony-stimulating factor, n (%) | 61 (67.0) | 2 (20.0) | 4 (66.7) | 12 (60.0) | 43 (78.2) |
| Patients receiving an IV/PO antiemetic, n (%) | 91 (100) | 10 (100) | 6 (100) | 20 (100) | 55 (100) |
| Patients receiving an IV/PO antibiotic during treatment$, n (%) | 9 (9.9) | 1 (10.0) | 1 (16.7) | 2 (10.0) | 5 (9.1) |
| Patients receiving an IV/PO anti-infective for prophylaxis prior to bendamustine administration, n (%) | |||||
| Yes | 13 (14.3) | 0 | 0 | 3 (15.0) | 10 (18.2) |
| No | 64 (70.3) | 5 (50.0) | 6 (100) | 17 (85.0) | 36 (65.5) |
| Missing | 14 (15.4) | 5 (50.0) | 0 | 0 | 9 (16.4) |
IV, intravenous; PO, oral; SD, standard deviation.
These patients did not have stop dates for their first bendamustine regimen, and had approximately 3 months of follow up.
Patients receiving intravenous antibiotics in a hospital (out of US Oncology Network) were not captured.
Figure 2.
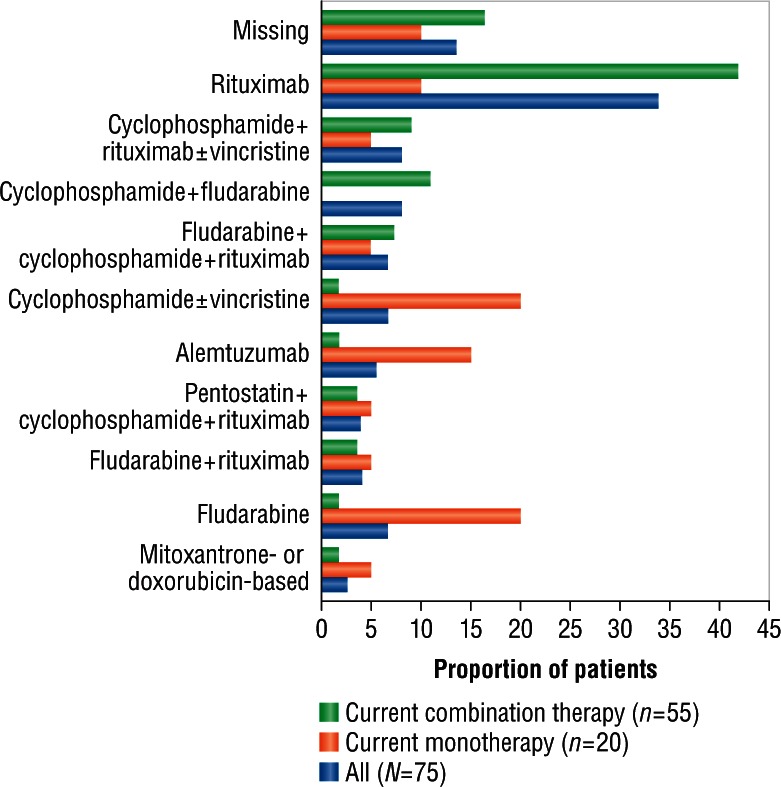
Prior therapy in patients receiving current bendamustine monotherapy and current combination therapy with bendamustine plus rituximab.
Nearly 40% of patients experienced no cycle delay, and nearly 20% of patients experienced a maximum cycle delay of more than 15 days. During the chart-review period, 100% of patients received an antiemetic, 67% a GCSF, and 9.9% an oral or intravenous antibiotic (Table 3).
Safety
No unexpected toxicities were observed. The overall rate (all grades) of blood/bone marrow AEs was 40.7% (Table 4). Grade 3/4 blood/bone marrow AEs occurred in 18.8% and 25.3% respectively of treatment-naïve patients and those with relapsed disease. Other grade 3/4 AEs included upper respiratory infection and abdominal pain in patients with relapsed disease, as well as rash and sepsis in both patient groups. The most commonly occurring nonhematologic AEs (≥5%, any grade) were fatigue (33.0%), weight loss (11.0%), infection (9.9%), gastrointestinal events (8.8%), fever (8.8%), pulmonary disease (6.6%), and rash (5.5%). All were grade 0–2, except for two cases each of grade 3/4 sepsis (one in the monotherapy group and the other in the combination therapy group) and rash (both in the monotherapy group) and one case each of grade 3/4 abdominal pain and upper respiratory infection (both in the combination therapy group).
Table 4.
Adverse events*.
| Toxicity category | All (N = 91) | Treatment naïve |
Relapsed or ≥second line |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Bendamustine monotherapy (n = 10) |
Bendamustine + rituximab combination therapy (n = 6) |
Bendamustine monotherapy (n = 20) |
Bendamustine + rituximab combination therapy (n = 55) |
||||||
| Total AEs (all grades) | All grades (n) | Grade 3/4 (n) | All grades (n) | Grade 3/4 (n) | All grades (n) | Grade 3/4 (n) | All grades (n) | Grade 3/4 (n) | |
| Weight loss | 10 | 1 (grade 0) | 0 | 0 | 0 | 2 (grade 0) | 0 | 7 (grade 0) | 0 |
| Pulmonary | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 1 |
| URI (1) | URI (1)$ | URI (1)$ | |||||||
| Dyspnea (2) | Dyspnea (2) | ||||||||
| Pleural effusion (1) Worsened pulmonary function (1) | Pleural effusion (1) Worsened pulmonary function (1) | ||||||||
| Other (1) | Other (1) | ||||||||
| Cardiac | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Mild CHF | Mild CHF | ||||||||
| Blood/bone marrow | 37 | 5 | 2 | 2 | 1 | 13 | 6 | 17 | 13 |
| Hematologic (1) | Thrombocytopenia (1)$ | Thrombocytopenia (1)$ | Cytopenia (1) | Hematologic (1)$ | Thrombocytopenia (1)$ Cytopenia (3) | Thrombocytopenia (1)$ | Thrombocytopenia (2) Cytopenia (6) | Thrombocytopenia (1)$Cytopenia (5)$Neutropenia (5)$Myelosuppression(1)$Hemolysis (1)$ | |
| Thrombocytopenia (4)Cytopenia (13)Anemia (4)Neutropenia (10)Pancytopenia (2)Myelosuppression (1)Hemolysis (2) | Cytopenia (3)Anemia (1) | Cytopenia (1)$ | Hematologic (1)$ | Anemia (2)Neutropenia (4)Pancytopenia (2)Hemolysis (1)$ | Neutropenia (3)$ | Anemia (1) Neutropenia (6) | |||
| Pancytopenia (1)$ | Myelosuppression (1)$ | ||||||||
| Hemolysis (1)$ | Hemolysis (1)$ | ||||||||
| Skin | 5 | 2 | 1 | 0 | 0 | 1 | 1 | 2 | 0 |
| Rash (5) | Rash (2) | Rash (1)$ | Rash (1)$ | Rash (1)$ | Rash (2) | ||||
| Fever (no infection) | 8 | 2 | 0 | 0 | 0 | 1 | 0 | 5 | 0 |
| Gastrointestinal | 8 | 0 | 0 | 1 | 0 | 2 | 0 | 5 | 1 |
| Nausea/vomiting (4)Constipation (1) | Nausea/vomiting (1) | Nausea/vomiting (2) | Nausea/vomiting (1)Constipation (1) | Abdominal pain (1)$ | |||||
| Abdominal pain (1) | Mucositis (1) | ||||||||
| Mucositis (1) | Diarrhea (1) | ||||||||
| Diarrhea (1) | Abdominal pain (1)$ | ||||||||
| Neurologic | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Blood pressure | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Hypertension (4) | Hypertension (4) | ||||||||
| Hemorrhage | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Infection | 9 | 1 | 1 | 0 | 0 | 2 | 0 | 6 | 1 |
| Herpes zoster (2) | Klebsiella | Klebsiella sepsis (1) | Other (2) | Herpes zoster (2) | Cryptococcal sepsis (1) | ||||
| Cryptococcal | sepsis (1) | Cryptococcal | |||||||
| sepsis (1) | sepsis (1) | ||||||||
| Klebsiella sepsis (1) | Pneumonia (1) | ||||||||
| Pneumonia (1) | |||||||||
| Other (4) | Other (2) | ||||||||
| General | 34 | 3 | 0 | 1 | 0 | 9 | 0 | 21 | 0 |
| Fatigue (30) | Fatigue (3) | Fatigue (1) | Fatigue (6) | Fatigue (20) | |||||
| Weakness (3) | Weakness (2) | Weakness (1) | |||||||
| Anorexia (1) | Anorexia (1) | ||||||||
AE, adverse event; CHF, congestive heart failure; URI, upper respiratory infection.
Frequency is counted by type of events; each patient may have had multiple events.
Adverse event(s) led to dose reduction/delay or discontinuation of bendamustine.
Real-world effectiveness
Among all 91 patients, the ORR was 58.2% (95% CI 47.4–68.5), with CR, PR, and nPR rates of 14.3%, 42.9%, and 1.1% respectively. Fourteen patients (15.4%) had stable disease, and 19 (20.9%) had progressive disease. Data were missing for five patients (5.5%) (Table 5).
Table 5.
Response rates.
| Variable, n (%) | All (N = 91) | Treatment naïve |
Relapsed or ≥second line |
||
|---|---|---|---|---|---|
| Bendamustine monotherapy (n = 10) | Bendamustine + rituximab combination therapy (n = 6) | Bendamustine monotherapy (n = 20) | Bendamustine + rituximab combination therapy (n = 55) | ||
| Overall response rate* [95% CI] | 53 (58.2) [47.4–68.5] | 5 (50.0) [18.7–81.3] | 4 (66.7) [22.3–95.6] | 9 (45.0) [23.0–68.4] | 35 (63.6) [49.5–76.2] |
| Complete response [95% CI] | 13 (14.3) [7.8–23.2] | 1 (10.0) [0.25–44.5] | 2 (33.3) [4.3–77.7] | 4 (20.0) [5.7–43.6] | 6 (10.9) [4.1–22.2] |
| Partial response [95% CI] | 39 (42.9) [32.5–53.6] | 4 (40.0) [12.1–73.7] | 2 (33.3) [4.3–77.7] | 5 (25.0) [8.6–49.1] | 28 (50.9) [37.0–64.6] |
| Nodular partial response | 1 (1.1) | 0 | 0 | 0 | 1 (1.8) |
| Stable disease | 14 (15.4) | 2 (20.0) | 2 (33.3) | 5 (25.0) | 5 (9.1) |
| Progressive disease | 19 (20.9) | 1 (10.0) | 0 | 5 (25.0) | 13 (23.6) |
| Missing | 5 (5.5) | 2 (20.0) | 0 | 1 (5.0) | 2 (3.6) |
CI, confidence interval.
Overall response rate = complete response + partial response + nodular partial response.
The observed ORR for the pooled treatment-naïve patients was 56.3% (n = 9; CR 18.8%; PR 37.5%; and nPR 0%); 6.3% had progressive disease. Among all treatment-naïve patients (n = 16), the ORR was 50.0% for those receiving bendamustine monotherapy (95% CI 18.7–81.3) and 66.7% for those receiving combination therapy with bendamustine plus rituximab (95% CI 22.3–95.6); the rate of progressive disease was 10.0% and 0% respectively (Table 5).
The observed ORR for the pooled patients with relapsed disease was 58.7% (n = 44; CR 13.3%; PR 44.0%; and nPR 1.3%); 24.0% had progressive disease. Among all patients with relapsed disease (n = 75), the ORR was 45.0% for those receiving bendamustine monotherapy (95% CI 23.0–68.4) and 63.6% for those receiving combination therapy with bendamustine plus rituximab (95% CI 49.5–76.2); the rate of progressive disease was 25.0% and 23.6% respectively (Table 5).
Among 89 patients for whom data were available, median time to progressive disease was 19.1 months (95% CI 14.9–21.3), with a median length of follow up of 17.8 months (Table 6). Progression was reported in 57 patients, and 32 patients were censored.
Table 6.
Time to progressive disease.
| Variable | All (N = 89) | Treatment naïve |
Relapsed or ≥second line |
||
|---|---|---|---|---|---|
| Bendamustine monotherapy (n = 10) | Bendamustine + rituximab combination therapy (n = 6) | Bendamustine monotherapy (n = 19) | Bendamustine + rituximab combination therapy (n = 54) | ||
| Time to progressive disease, months | |||||
| Median (95% CI) | 19.1 (14.9–21.3) | 26 (20.3–NA) | – | 21.6 (19.1–24.3) | 14.5 (12.9–18.8) |
| Mean (SE)* | 19 (1.23) | 20.3 (3.5) | – | 22.8 (2.0) | 14.8 (1.1) |
| Patients with event, n | 57 | 4 | 0 | 16 | 37 |
| Patients censored, n | 32 | 6 | 6 | 3 | 17 |
| Median length of follow up, months | 17.8 | 17.7 | 15.1 | 24.5 | 16.8 |
CI, confidence interval; NA, not available; SE, standard error.
Mean and SE were provided because median time to next treatment was not reached for some strata. Mean and SE may be underestimated because the largest observation was censored, and the estimation was restricted to the largest event.
Median time to progressive disease had not been reached for 16 treatment-naïve patients (median follow up 15.1 months). Among 73 patients with relapsed disease, median time to progression was 18.4 months (95% CI 12.9–21.6), 21.6 months (95% CI 19.1–24.3) for those receiving bendamustine monotherapy, and 14.5 months (95% CI 12.9–18.8) for those receiving combination therapy with bendamustine plus rituximab. Kaplan–Meier estimates for disease progression over time in individual and pooled groups are shown in Figure 3.
Figure 3.
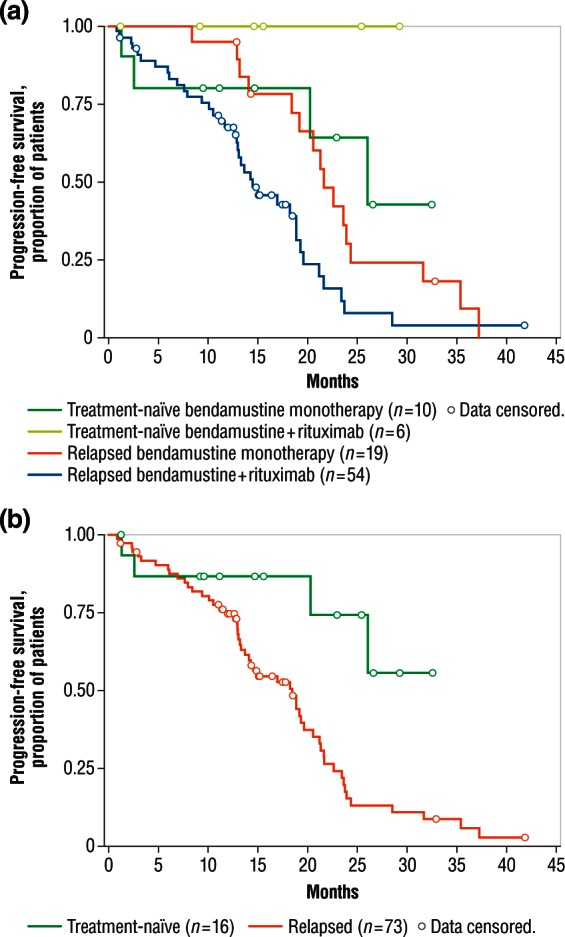
Estimates of time to progressive disease. (a) Kaplan–Meier estimates of time to progressive disease by individual patient groups. (b) Kaplan–Meier estimates of time to progressive disease by pooled treatment-naïve and relapsed patient groups.
Discussion
CLL is predominantly a disease of older people, with a median age at diagnosis of 72 years [Gribben, 2010]. Given the advanced age of affected individuals, management must often be tailored to accommodate specific patient characteristics, such as physical fitness, comorbidities, and degree of tolerability for treatment [Hallek, 2009]. A comprehensive understanding of the rapidly changing CLL therapeutic landscape is therefore especially critical for the optimal management of older patients with CLL [Hallek, 2009].
Rituximab as a combination therapy has been investigated in numerous clinical trials [Hallek, 2009]. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) has been shown to improve PFS and overall survival in treatment-naïve patients with CLL [Hallek et al. 2010] and to prolong PFS in patients with relapsed/refractory CLL [Robak et al. 2010a]. In the open-label, multicenter, randomized, phase III REACH (Rituximab in the Study of Relapsed CLL) study of patients with relapsed/refractory CD20-positive CLL, PFS was 30.6 months for 276 patients who received FCR versus 20.6 months for 276 patients who received fludarabine and cyclophosphamide [hazard ratio (HR) 0.65; 95% CI 0.51–0.82; p < 0.001) [Robak et al. 2010a]. In a GCLLSG study of treatment-naïve patients with CD20-positive CLL, 65% of 408 patients who received FCR were free of progression at 3 years after randomization, compared with 45% of 409 patients who received fludarabine and cyclophosphamide (HR 0.56; 95% CI 0.46–0.69; p < 0.0001); the overall survival rate was 87% versus 83% respectively (HR 0.67; 95% CI 0.48–0.92; p = 0.012) [Hallek et al. 2010].
Although FCR is currently considered to be the most active first-line therapeutic regimen in CLL [Tam et al. 2008], the regimen is associated with considerable toxicity, which may be especially problematic in older patients with CLL who may also have numerous comorbidities [Hallek, 2009]. Indeed, data suggest that older patients with CLL have less tolerance for FCR chemoimmunotherapy and experience more infectious complications than younger patients [Badoux et al. 2011].
The results of the current retrospective chart review suggest that bendamustine (with or without rituximab) was an effective therapeutic strategy in CLL in 91 patients with a mean age of 77.4 years at the start of first therapy. The ORRs were 58.7% among patients with relapsed disease and 56.3% among treatment-naïve patients. Median time to progressive disease was 18.4 months for the 73 patients with relapsed disease and had not yet been reached for 16 treatment-naïve patients at a median follow up of 15.1 months.
Bendamustine monotherapy has shown clinical efficacy in several phase I/II trials in patients with advanced relapsed/refractory CLL [Kath et al. 2001; Aivado et al. 2002; Bergmann et al. 2005; Lissitchkov et al. 2006]. Additionally, bendamustine was found to be more effective than chlorambucil in a prospective, randomized, phase III trial in 319 patients with advanced treatment-naïve CLL who had a mean age of about 63 years and a median of six treatment cycles in both arms [Knauf et al. 2009]. The ORR and median PFS were 68% and 21.6 months respectively for 162 patients who received bendamustine, compared with 31% and 8.3 months in 157 patients who received chlorambucil (p < 0.0001 for both measures) [Knauf et al. 2009]. Although the population in this study was too small to draw definitive conclusions, the observed ORR in treatment-naïve patients receiving bendamustine monotherapy was 50%, with a median PFS of 26 months (95% CI 20.3 to not reached).
Two multicenter phase II studies of bendamustine plus rituximab in treatment-naïve patients with CLL and those with relapsed disease were conducted by the GCLLSG [Fischer et al. 2011, 2012]. The first study investigated the safety and effectiveness of bendamustine (70 mg/m2 on days 1 and 2) combined with rituximab (375 mg/m2 on day 0 of the first cycle and 500 mg/m2 on day 1 of all subsequent cycles) in 78 patients with relapsed or refractory CLL [Fischer et al. 2011]. The median age was 66.5 years; 37% of the study population was aged 70 years or over. The ORR in the intent-to-treat population (n = 46) was 59.0% (95% CI 47.3–70.0), with CR, PR, and nPR rates of 9.0%, 47.4%, and 2.6% respectively. Median PFS at 24 months was 14.7 months.
In the second GCLLSG study, 117 treatment-naïve patients with advanced CLL (median age 64 years; 26% ≥70 years) received bendamustine (90 mg/m2 on days 1 and 2) combined with rituximab (375 mg/m2 on day 0 of the first cycle and 500 mg/m2 on day 1 of all subsequent cycles). The ORR, CR, and PR rates were 88.0%, 23.1%, and 64.9% respectively [Fischer et al. 2012]. Follow up was 36 months. Median event-free survival was 33.9 months and 106 (91%) patients were still alive at a median follow up of 27 months.
Bendamustine treatment was well tolerated in both of the GCLLSG studies [Fischer et al. 2011, 2012]. Given these promising results, GCLLSG has initiated a phase III trial investigating the safety and efficacy of FCR versus bendamustine combined with rituximab in treatment-naïve patients with CLL [Clinicaltrials.gov identifier: NCT00769522].
In this study, the median ORR among patients with relapsed disease treated with combination bendamustine and rituximab was 63.6%, and PFS was 14.5 months, but again, the small size of the cohort precludes drawing a definitive conclusion. Of note in relation to the GCLLSG study in treatment-naïve patients [Fischer et al. 2012], the median ORR was 66.7% for the six treatment-naïve patients who received the combination therapy but PFS was not reached.
In the GCLLSG study, grade 3/4 hematologic toxicities ranged from 17% for anemia to 28% for thrombocytopenia in patients with relapsed disease receiving combination therapy [Fischer et al. 2011]. For the present chart review, the overall rate of grade 3/4 hematologic toxicities among patients with relapsed disease receiving combination therapy was 24%; cytopenia and neutropenia were the most common, occurring in less than 10% of the study cohort.
Real-world safety and tolerability studies of bendamustine with or without rituximab in patients aged 70 years and over are sparse. A recent review of 20 frail patients (median age 72 years) with aggressive B-cell non-Hodgkin’s lymphoma and a median international prognostic index score of 3 showed a low incidence of grade 3/4 AEs [Horn et al. 2012]. In a total of 68 treatment cycles, grade 3/4 hematologic toxicities occurred in five cycles (leukopenia and anemia in two cycles each and thrombocytopenia in one), grade 3 infection occurred in two cycles, and grade 4 thromboembolism and grade 3 nausea/emesis occurred in one cycle each.
As for patients with CLL, no special findings regarding toxicities were reported for patients aged at least 70 years in the GCLLSG studies [Fischer et al. 2011, 2012].
Potential limitations of the current chart review are acknowledged. In addition to its retrospective nature, the chart review is limited in its ability to identify and stratify risk, as iKM databases do not capture IgHV mutation status, Rai stage at the time of treatment initiation, or cytogenetics. Furthermore, iKM data are collected with an intent-to-treat approach (i.e. the data are collected for clinical practice not research purposes), which may impede the standardization of collection methods and instruments, as well as the reporting practices of physicians. It should also be noted that not all community oncology practices are included in iKM datasets and that not all US Oncology Network clinics use full iKM electronic medical records. Finally, inaccurate or inadequate codes in iKM databases could potentially have introduced bias into the results. Despite these potential limitations, it is important to note that the reported outcomes reflect a median of only three cycles of therapy.
Conclusion
Among all 91 patients analyzed in this retrospective chart review, the ORR was 58.2%, with CR, PR, and nPR rates of 14.3%, 42.9%, and 1.1% respectively. Among all 89 patients for whom data were available, median time to progressive disease was 19.1 months, and the median length of follow up was 17.8 months. No unexpected toxicities were observed, and treatment was generally well tolerated. These findings suggest that bendamustine (with or without rituximab) may be an effective therapeutic strategy with manageable toxicity in older patients with CLL.
Acknowledgments
Presented in part at the 2011 American Society of Hematology Annual Meeting and Exposition, 10–13 December 2011, San Diego, CA, USA.
Footnotes
Funding: This research was sponsored by and conducted in collaboration with Teva Branded Pharmaceutical Products R&D, Inc. Funding for editorial assistance was provided to The Curry Rockefeller Group, LLC, Tarrytown, NY.
Conflict of interest statement: Kathryn S. Kolibaba received research funding from Teva Branded Pharmaceutical Products R&D, Inc. James A. Sterchele had an employment/leadership position with Teva Branded Pharmaceutical Products R&D, Inc. Avani D. Joshi received research funding from Teva Branded Pharmaceutical Products R&D, Inc. Michael Forsyth received research funding from Teva Branded Pharmaceutical Products R&D, Inc. Erin Alwon received research funding from Teva Branded Pharmaceutical Products R&D, Inc. Hooman Beygi had an employment/leadership position with Teva Branded Pharmaceutical Products R&D, Inc. and ownership interest with Teva Branded Pharmaceutical Products R&D, Inc. Gerard T. Kennealey had an employment/leadership position with Cephalon, Inc. at the time this review was conducted (now Teva Branded Pharmaceutical Products R&D, Inc.).
Contributor Information
Kathryn S. Kolibaba, Compass Oncology, 120 SE 136th Avenue, Vancouver, WA 98684, USA
James A. Sterchele, Teva Branded Pharmaceutical Products R&D, Inc., Frazer, PA, USA
Avani D. Joshi, Abbott Laboratories (formerly of McKesson Specialty Care, The Woodlands, TX, USA)
Michael Forsyth, US Oncology, The Woodlands, TX, USA.
Erin Alwon, OptumHealth, Inc. (formerly of McKesson Specialty Care, The Woodlands, TX, USA).
Hooman Beygi, Teva Branded Pharmaceutical Products R&D, Inc., Frazer, PA, USA.
Gerard T. Kennealey, Formerly of Cephalon, Inc. (now Teva Branded Pharmaceutical Products R&D, Inc.), Frazer, PA, USA
References
- Aivado M., Schulte K., Henze L., Burger J., Finke J., Haas R. (2002) Bendamustine in the treatment of chronic lymphocytic leukemia: results and future perspectives. Semin Oncol 29(4 Suppl. 13): 19–22 [DOI] [PubMed] [Google Scholar]
- Badoux X., Keating M., Wang X., O’Brien S., Ferrajoli A., Faderl S., et al. (2011) Fludarabine, cyclophosphamide and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood 117: 3016–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M., Goebeler M., Herold M., Emmerich B., Wilhelm M., Ruelfs C., et al. (2005) Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica 90: 1357–1364 [PubMed] [Google Scholar]
- Cheson B., Bennett J., Grever M., Kay N., Keating M., O’Brien S., et al. (1996) National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 87: 4990–4997 [PubMed] [Google Scholar]
- Cheson B., Rummel M. (2009) Bendamustine: rebirth of an old drug. J Clin Oncol 27: 1492–1501 [DOI] [PubMed] [Google Scholar]
- Cheson B., Wendtner C., Pieper A., Dreyling M., Friedberg J., Hoelzer D., et al. (2010) Optimal use of bendamustine in chronic lymphocytic leukemia, non-Hodgkin lymphomas, and multiple myeloma: treatment recommendations from an international consensus panel. Clin Lymphoma Myeloma Leuk 10: 21–27 [DOI] [PubMed] [Google Scholar]
- Dreger P., Döhner H., Ritgen M., Böttcher S., Busch R., Dietrich S., et al. (2010) Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood 116: 2438–2447 [DOI] [PubMed] [Google Scholar]
- Fischer K., Cramer P., Busch R., Böttcher S., Bahlo J., Schubert J., et al. (2012) Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 30: 3209–3216 [DOI] [PubMed] [Google Scholar]
- Fischer K., Cramer P., Busch R., Stilgenbauer S., Bahlo J., Schweighofer C., et al. (2011) Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 29: 3559–3566 [DOI] [PubMed] [Google Scholar]
- Foon K., Hallek M. (2010) Changing paradigms in the treatment of chronic lymphocytic leukemia. Leukemia 24: 500–511 [DOI] [PubMed] [Google Scholar]
- Gribben J. (2010) How I treat CLL up front. Blood 115: 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribben J., Zahrieh D., Stephans K., Bartlett-Pandite L., Alyea E., Fisher D., et al. (2005) Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood 106: 4389–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek M. (2009) State-of-the-art treatment of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 440–449 [DOI] [PubMed] [Google Scholar]
- Hallek M., Cheson B., Catovsky D., Caligaris-Cappio F., Dighiero G., Döhner H., et al. (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111: 5446–5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek M., Fischer K., Fingerle-Rowson G., Fink A., Busch R., Mayer J., et al. (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376: 1164–1174 [DOI] [PubMed] [Google Scholar]
- Horn J., Kleber M., Hieke S., Schmitt-Gräff A., Wäsch R., Engelhardt M. (2012) Treatment option of bendamustine in combination with rituximab in elderly and frail patients with aggressive B-non-Hodgkin lymphoma: rational, efficacy, and tolerance. Ann Hematol 91: 1579–1586 [DOI] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300 [DOI] [PubMed] [Google Scholar]
- Kath R., Blumenstengel K., Fricke H., Höffken K. (2001) Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. J Cancer Res Clin Oncol 127: 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Jung Y., Chen J., Alhasan A., Kaewsaard P., Zhang Y., et al. (2010) Evidences showing wide presence of small genomic aberrations in chronic lymphocytic leukemia. BMC Res Notes 3: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf W., Lissitchkov T., Aldaoud A., Liberati A., Loscertales J., Herbrecht R., et al. (2009) Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 27: 4378–4384 [DOI] [PubMed] [Google Scholar]
- Leoni L., Bailey B., Reifert J., Bendall H., Zeller R., Corbeil J., et al. (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 14: 309–317 [DOI] [PubMed] [Google Scholar]
- Lissitchkov T., Arnaudov G., Peytchev D., Merkle K. (2006) Phase-I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre-treated patients with B-chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy. J Cancer Res Clin Oncol 132: 99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN (National Comprehensive Cancer Network) (2012) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™): Non-Hodgkin’s Lymphomas. Version 3.2012. Available at: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf (accessed 4 December 2012).
- Robak T., Dmoszynska A., Solal-Céligny P., Warzocha K., Loscertales J., Catalano J., et al. (2010a) Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 10: 1756–1765 [DOI] [PubMed] [Google Scholar]
- Robak T., Lech-Maranda E., Robak P. (2010b) Rituximab plus fludarabine and cyclophosphamide or other agents in chronic lymphocytic leukemia. Expert Rev Anticancer Ther 10: 1529–1543 [DOI] [PubMed] [Google Scholar]
- Rummel M., Chow K., Hoelzer D., Mitrou P., Weidmann E. (2002) In vitro studies with bendamustine: enhanced activity in combination with rituximab. Semin Oncol 29(4 Suppl. 13): 12–14 [DOI] [PubMed] [Google Scholar]
- Tageja N., Nagi J. (2010) Bendamustine: something old, something new. Cancer Chemother Pharmacol 66: 413–423 [DOI] [PubMed] [Google Scholar]
- Tam C., O’Brien S., Wierda W., Kantarjian H., Wen S., Do K., et al. (2008) Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 112: 975–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda W., Keating M., O’Brien S. (2008) Chronic lymphocytic leukemias. In DeVita V., Jr, Lawrence T., Rosenberg S. (eds), DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology, 8th ed., vol. 2 Philadelphia: Lippincott Williams & Wilkins, pp. 2278–2292 [Google Scholar]


