Abstract
Peripheral T-cell lymphomas (PTCLs) are a diverse family of lymphoid neoplasms with poor prognosis. They represent approximately 6–10% of non-Hodgkin lymphomas with significant geographic variation. The median age at diagnosis varies with histology, however the majority of patients with PTCL are in their fifth or sixth decade of life. Until recently clinical development of new agents for PTCL was slow due to difficulties in making the correct diagnosis, lack of uniform classification and combination of rarity and biologic diversity of the group. In the last 5 years, significant advances were made to overcome these obstacles, leading to the approval of three new agents for relapsed and refractory PTCL by the Food and Drug Administration, based on well conducted prospective studies. Pralatrexate, a unique antifol, was the first agent granted approval, followed by romidepsin, a histone deacetylase inhibitor, and brentuximab vedotin, an immunoconjugate. Owing to the unique nature of these agents, durable responses were seen in patients with highly refractory disease, and some of these responses are long lasting after discontinuation of therapy. Accumulating data indicate that these novel agents have little cumulative toxicity and can be administered continuously to patients who are not candidates for consolidative stem-cell transplantation (SCT), with little impact on quality of life. They might also provide a new salvage option for patients eligible for SCT with no impact on autologous stem-cell collection or subsequent engraftment. New studies are underway to evaluate efficacy and safety of new agents in combination regimens for both newly diagnosed and relapsed/refractory PTCL. Several other investigational drugs showed promise in recent trials. This review focuses on novel therapies for T-cell lymphomas, their place in current treatment paradigms and future directions.
Keywords: brentuximab vedotin, pralatrexate, romidepsin, T-cell lymphoma, therapy
Introduction
The World Health Organization (WHO) 2008 classification of hematologic malignancies defines peripheral T-cell lymphomas (PTCLs) as lymphoid neoplasms originating from mature, post-thymic T cells. Corresponding to the complexity of the T-cell compartment of the immune system, PTCLs are diverse with distinct biologic subtypes and variable clinical behavior. Three clinical subfamilies are frequently described: nodal, extra-nodal and leukemic. However, this distinction is arbitrary and does not take into account the pathophysiology and molecular signatures of particular entities. For example, extranodal nasal(-type) natural killer (NK)-cell lymphoma and aggressive NK-cell leukemia are likely to share some or many biologic features as well as etiology (Ebstein–Barr virus), but ascribed to different groups, while peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) are placed in the same group of nodal type, but have very dissimilar molecular profiles and natural history. Future classifications of PTCLs will likely be based on genomic studies and will better predict clinical behavior and responses to therapy.
Table 1 summarizes the current classification of PTCLs and the frequency of specific subtypes. PTCL-NOS, AITL and anaplastic large cell lymphoma (ALCL) are more common in North America and Europe, while extranodal nasal NK-cell lymphoma and adult T-cell leukemia/lymphoma (ATL) are prevalent in Southeast Asia, Japan and the Caribbean basin [Anderson et al. 1998; Armitage et al. 2008]. The two latter subtypes are associated with viral etiology, Ebstein–Barr virus and human T-lymphotropic virus type-I (HTLV-I) respectively and targeting viral agents along with systemic chemotherapy is an attractive hypothesis that is being tested in clinical trials.
Table 1.
WHO 2008 classification of PTCLs and their frequency*.
| PTCL subtype | Frequency (%) |
|---|---|
| T-cell prolymphocytic leukemia | NA |
| T-cell large granular lymphocytic leukemia | 2–5% |
| Aggressive NK-cell leukemia | <1 |
| Adult T-cell leukemia/lymphoma | 9.6 |
| Extranodal NK/T-cell lymphoma, nasal type | 10.4 |
| Enteropathy-associated T-cell lymphoma | 4.7 |
| Hepatosplenic T-cell lymphoma | 1.4 |
| Subcutaneous panniculitis-like T-cell lymphoma | 0.9 |
| Peripheral T-cell lymphoma, not otherwise specified | 25.9 |
| Angioimmunoblastic T-cell lymphoma | 18.5 |
| Anaplastic large cell lymphoma, ALK+ | 6.6 |
| Anaplastic large cell lymphoma, ALK− | 5.5 |
Primary cutaneous T-cell lymphomas are excluded. Frequency shown is among PTCLs.
ALK, anaplastic lymphoma kinase; NA, not available; NK, natural killer; PTCL, peripheral T-cell lymphoma; WHO, World Health Organization.
Hepatosplenic T-cell lymphoma (HSTL), entero-pathy-associated T-cell lymphoma (EATL) and aggressive NK-cell leukemia are extremely rare (< 0.1% of non-Hodgkin lymphomas), and little is known about their biology and best therapeutic approach. International collaboration and ongoing T-cell lymphoma registry studies will hopefully shed more light on pathophysiology and identify new therapeutic targets to improve dismal outcomes in these subtypes.
Knowledge about clinical outcomes in PTCL is mostly derived from earlier studies of aggressive lymphomas that included both B-cell and T-cell histologies. Several retrospective analyses compared the outcomes of PTCLs and large B-cell lymphomas (LBCLs) [Morabito et al. 2004; Rudiger et al. 2002; Lopez-Guillermo et al. 1998; Savage et al. 2004; Schmitz et al. 2010]. When controlled for International Prognostic Index (IPI), the 5-year survival was significantly lower in patients with PTCL than in those with LBCL treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like therapies (Table 2). Addition of etoposide to CHOP (CHOEP) slightly improved the event-free survival for younger patients with newly diagnosed PTCLs in a recent ad hoc analysis of several German High-Grade Non-Hodgkin Lymphoma Study Group trials [Schmitz et al. 2010]. However, the benefit was most pronounced for patients with ALCL and those with low IPI-risk disease. The majority of patients with IPI greater than 1 relapsed and died of the disease within 2 years of the diagnosis. Intensified alternating chemotherapy regimens (hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone [CVAD]) did not significantly improve the outcomes for patients with PTCL [Escalon et al. 2005; Simon et al. 2010]. Similarly, several studies reported on the benefit of high-dose therapy and autologous stem-cell transplantation (SCT) in first remission of patients with PTCL [Corradini et al. 2006; Reimer et al. 2009; d’Amore et al. 2012]. While the outcomes in these studies appear slightly better than historical controls, the absence of control arms and patient selection bias makes these data hard to interpret. There is an obvious need for developing new PTCL-specific therapies.
Table 2.
Selected studies of PTCL outcomes.
| Study | Patients (n) | Disease subtypes included | OS % | ORR % |
|---|---|---|---|---|
| Morabito et al. [2004] | 297 | PTCL-U | 42 (5 years) | 76 |
| Rudiger et al. [2002] | 96 | PTCL-U, ENKL, AITL, EATL, HSTCL, ATLL | 26 (5 years) | NR |
| Lopez-Guillermo et al. [1998] | 174 | ALCL, PTCL-U, AITL, EATL | NR | 64 |
| Savage et al. [2004] | 199 | PTCL-U, ALCL, ENKL, SPTCL, AITL, EATL | 22–43 (5 years) | 78–90 |
| Simon et al. [2010] | 88 | PTCL-U, AITL, ALCL | 41 (2 years) | 62 |
| Schmitz et al. [2010] | 289 | PTCL-U, AITL, ALCL, ALK | 54 (3 years) 67 (3 years) 62 (3 years) |
NRNRNR |
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ATLL, adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma; ENKL, extranodal nasal NK-cell lymphoma; HSTCL, hepatosplenic T-cell lymphoma; NR, not reported; OS, overall survival; ORR, objective response rate; PTCL-U, peripheral T-cell lymphoma, unspecified; SPTCL, subcutaneous panniculitis-like T-cell lymphoma.
This review describes new therapies for T- and NK-cell lymphomas that emerged from recent international trials with particular focus on US Food and Drug Administration (FDA)-approved agents, pralatrexate, romidepsin and brentuximab vedotin (BV).
New FDA-approved agents
Pralatrexate
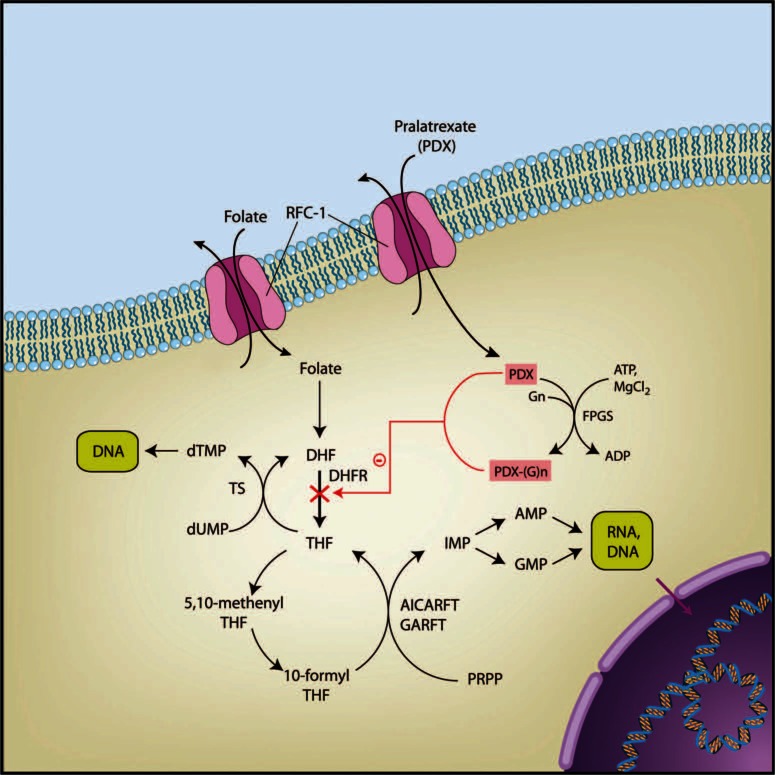
Pralatrexate, or 10-propargyl-10-deazaaminopterin, is a member of a new class of rationally designed antifolates developed in an attempt to improve upon the efficacy of its predecessors, particularly methotrexate (Figure 1). The design aimed and succeeded to improve membrane transport and polyglutamation by reduced folate carrier type 1 (RFC-1) and folylpolyglutamylsynthase (FPGS), respectively [Sirotnak et al. 1984a, 1987]. The substantially greater activity over methotrexate in murine tumor models and human tumor xenografts appears to be due to the more effective RFC-1 mediated influx and intracellular retention of polyglutamated metabolites [Schmid et al. 1985; Sirotnak et al. 1984b, 1998]. Biochemically (influx Vmax/Km), the pralatrexate incorporation rate through RFC-I is 14 times higher than that of methotrexate. Similarly, Vmax/Km for the FPGS suggests that pralatrexate is 10 times more efficiently polyglutamated compared with methotrexate. Polyglutamation, in turn, assures greater intracellular ‘trapping’ of the metabolites and enhanced cytotoxicity.
Figure 1.
Mechanism of action of pralatrexate. Pralatrexate is transported into the tumor cell via reduced folate carrier type 1 (RFC-1) membrane transporter; inside the cell pralatrexate is polyglutamated by FPGS; pralatrexate-Glu metabolite accumulates inside the cell with ensuing blockade of DHFR and DNA synthesis. Illustration courtesy of Alessandro Baliani© (2013).ADP, adenosine diphosphate; AMP, adenosine monophosphate; AICARFT, 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase; ATP, adenosine triphosphate; DHFR, dihyfdrofolate reductase; dTMP, de-oxy-thymidine monophosphate; dUMP, de-oxy-uridine monophosphate; FPGS, folylpolyglutamyl synthase; GARFT, glycinamide ribonucleotide formyltransferase; GMP, guanosine monophosphate; G, glutamate; IMP, imidazole phosphate; PRPP, phosphoribosyl pyrophosphate; THF, tetrahydropholate; TS, thymidine synthase.
RFC-1 is a fetal oncoprotein highly expressed on the majority of embryonic cells during early development to facilitate DNA synthesis via incorporation of reduced folates [Kuhnel et al. 2000; Sirotnak and Tolner, 1999]. The expression subsides dramatically in adults with the exception of rapidly proliferating tissues (i.e. hematopoietic cells and basal layer of intestinal mucosa). Various oncogenes, including H-ras and c-myc, can upregulate the RFC-1 expression in various malignancies corresponding to the high DNA synthesis demand of rapidly proliferating tumors. It is not surprising therefore that methotrexate, an old antifolate, has been used for decades to treat various malignancies, including breast, bladder and head/neck cancers, leukemias, lymphomas and others. The 10-deazaaminopterins, including pralatrexate, are therefore not a new class of antineoplastic agents, but rather an attempt to improve upon the efficacy of previous antifolates and overcome resistance mechanisms (including downregulation of RFC-1 and FPGS).
The first clinical experience with pralatrexate was reported in patients with non-small cell lung cancer (NSCLC) [Krug et al. 2000]. Mucositis was found to be the main dose-limiting toxicity, and the maximum tolerated dose was established at 170 mg/m2 every 2 weeks. Few responses and stable disease were seen in heavily pretreated patients. In the subsequent phase II trial in patients with NSCLC, mucositis was once again prominent at the starting dose of 150 mg/m2 every 2 weeks and the treatment dose was reduced for the last 10 (out of total 39) patients to 135 mg/m2 every 2 weeks [Krug et al. 2003]. For the entire study population the incidence of mucositis was 15% for grade 3 and 5% for grade 4. It is noteworthy that supplementation with vitamin B12 and folic acid was not given during pralatrexate therapy, and investigators did not find a correlation between serum homocystein or methylmalonic acid (MMA) levels and the severity of mucositis.
The first report of the safety and efficacy of pralatrexate in hematologic malignancies came from O’Connor and colleagues who conducted a dose/schedule finding study in relapsed and refractory lymphomas [O’Connor et al. 2009]. Interestingly, the initially used dose of 135 mg/m2 every 2 weeks, established in NSCLC, revealed a higher incidence of mucositis in patients with lymphoma, and in contrast to the previous report, mucositis severity was greatest in patients with elevated homocystein and MMA levels and in patients with low pralatrexate clearance. These observations led to a new dose and schedule for patients with lymphoma and the requirement for folate and vitamin B12 supplementation prior to and during pralatrexate therapy. The maximum tolerated dose was established at 30 mg/m2 weekly for 6 out of 7 weeks, and the use of vitamin supplementation significantly reduced incidence of stomatitis. Phase II of this trial, as well as another report [O’Connor et al. 2007], demonstrated significant activity of pralatrexate in patients with PTCLs. When analyzed by the lymphoma lineage, the overall response rates were 10% and 54% in patients with B-cell and T-cell lymphomas respectively. All eight patients who achieved complete response (CR) had T-cell lymphoma. Interestingly, two of the patients with T-cell lymphomas who achieved a CR had previously failed to respond to treatment with methotrexate, suggesting a lack of cross resistance between the two antifolates. Responses were seen across all histologic subtypes of T-cell lymphomas, and two of the patients experienced tumor lysis-like syndrome from rapid response to pralatrexate. Remarkable responses seen in patients with T-cell lymphoma treated on this phase II–I–II trial led to the development and execution of the registrational prospective phase II trial of pralatrexate in patients with relapsed and refractory T-cell lymphomas.
PROPEL was the first international prospective study of novel therapeutic agents in patients with PTCL [O’Connor et al. 2011]. The dose and schedule of pralatrexate was 30 mg/m2 weekly for 6 out of 7 consecutive weeks and was based on the described phase II–I–II trial above. Out of 109 heavily pretreated patients (median of three prior therapies) with various histologies of PTCL, 12 achieved CR and 20 achieved partial response for the overall and CR rates of 29% and 11% respectively. The duration of response was 10.1 months and median progression-free and overall survivals were 3.5 and 14.5 months respectively. Responses were generally rapid, with 66% of responses seen after the first cycle of therapy (6 weeks). When analyzed by histology, patients with angioimmunoblastic T-cell lymphoma had fewer responses (1 out of 13 patients) than other subtypes. Overall, there was no difference in the response rate between patients with one and those with multiple prior therapies. However, in patients who received CHOP as the only prior treatment (N = 15), the response rate was 47% and the CR rate was 30%, suggesting that the response rate might be higher if pralatrexate is used earlier in the disease course (second line of therapy). Of interest is the fact that eight patients who achieved a response were able to proceed to either allogeneic or autologous SCT as a consolidative treatment with curative intent. The latter observation suggests that pralatrexate could be used as a single-agent salvage therapy in selected patients prior to SCT.
Unique activity of pralatrexate in transformed mycosis fungoides (tMF) deserves particular attention. tMF is a rapidly progressive and clinically challenging malignancy. It is characterized by primary chemotherapy refractoriness in the majority of patients, limited activity of current therapeutic agents and short median survival between 12 and 24 months from the time of transformation, despite the use of aggressive treatments. Foss and colleagues recently reported on 12 heavily pretreated patients with tMF who participated in the PROPEL trial and received single-agent pralatrexate [Foss et al. 2012]. According to investigator assessment (which was likely more accurate for these patients due to difficulties with photodocumentation of skin lesions), the overall response rate was 58%, and the median progression-free and overall survivals were 5.3 and 13 months respectively. These findings suggest an important role of pralatrexate as a treatment option for this very challenging patient population.
The toxicity of pralatrexate observed in the PROPEL study was predictable. The most prominent adverse events were mucositis and thrombocytopenia. Mucositis occurred in 70% of patients, but severe events were uncommon, with 17% of patients experiencing grade 3 and 4% grade 4 events. Treatment delays and the dose reduction specified by protocol allowed the majority of patients with mucositis to remain on treatment. Thrombocytopenia was reversible and was seen in 41% of patients, 14% grade 3 and 19% grade 4. Importantly, there appears to be no cumulative myelosuppression with prolonged use of pralatrexate in patients with long-lasting responses. Overall, mucositis and thrombocytopenia led to discontinuation of therapy in 6% and 5% of patients respectively. Other toxicities were uncommon and the majority were grade 1–2.
Given the efficacy and toxicity profile of pralatrexate reported in the PROPEL trial, it seems reasonable to consider the use of this agent in the following clinical settings: in patients with relapsed T-cell lymphoma who are not candidates for aggressive salvage protocols with curative intent (i.e. autologous or allogeneic SCT); in patients with relapsed T-cell lymphoma who are candidates for aggressive salvage protocols with curative intent who failed standard salvage therapy; in patients with T-cell lymphoma who relapsed after autologous or allogeneic SCT; in patients who have primary refractory disease not responding to standard salvage therapies. The use of pralatrexate in first salvage of relapsed or refractory disease in SCT candidates is controversial at this time and should be individualized to a particular clinical scenario.
Current studies are focusing on evaluating the role of pralatrexate in frontline therapy for PTCLs in combination with other agents, and in combination with new biologic agents for relapsed and refractory disease.
Romidepsin (depsipeptide)
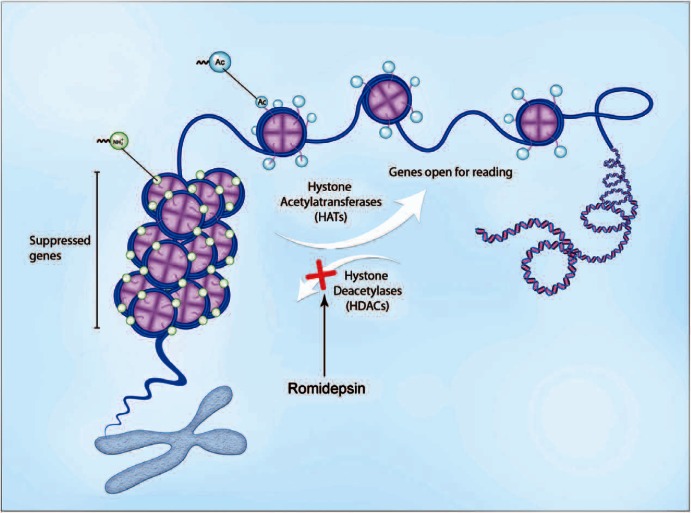
Romidepsin is a new potent class I selective histone deacetylase (HDAC) inhibitor (Figure 2), isolated from the fermentation broth of Chromo-bacterium violaceum [Ueda et al. 1994b; Nakajima et al. 1998]. It has demonstrated potent cytotoxic activity in vitro against human tumor cell lines and in vivo against human tumor xenografts and murine tumors [Ueda et al. 1994a, 1994b]. While romidepsin was found to be a Pgp-1 (product of multidrug resistance, MDR, gene) substrate, it has shown lack of cross resistance with cytotoxic agents, including vincristine, 5-fluorouracil, mitomycin C and cyclophosphamide. Laboratory studies have demonstrated that romidepsin, like other HDAC inhibitors, induces expression of specific subsets of genes linked to inhibition of growth and induction of differentiation and apoptosis [Van Lint et al. 1996; Della Ragione et al. 2001]. Romidepsin, however, is structurally distinct from other known HDAC inhibitors and may have other mechanisms of cytotoxic action.
Figure 2.
Mechanism of action of romidepsin. Binding of romidepsin to intracellular and intranuclear histone deacetylase (HDAC) leads to hyperacetylation of histones and DNA unfolding. Increase in DNA accessibility facilitates binding of the transcription factors and changes the activity of numerous genes affecting cell growth, proliferation and apoptosis, resulting in cell death in sensitive tumors. Illustration courtesy of Alessandro Baliani© (2013).
In preclinical studies of romidepsin, greater activity was observed with intermittent rather than with daily administration due to better ability of the host to tolerate the higher doses. In addition, short (<4 min) or prolonged (>24 h) infusions were associated with the greatest toxicity, while 1–4 h infusions produced the least toxicity and allowed for the highest individual doses. Two potentially serious toxicities were observed in preclinical studies. Cardiac toxicity, including elevation of cardiac enzymes, was seen with some dosing schedules. In addition, local inflammation and necrosis was noted at catheter insertion cites. Based on these observations, 4 h infusions and an intermittent schedule was chosen for early human studies.
The first phase I study of romidepsin in refractory neoplasms included 37 patients with a variety of solid tumors [Sandor et al. 2002]. Dose escalation proceeded from 1 mg/m2 (infused over 4 h) on days 1 and 5 of a 21-day cycle and maximum tolerated dose was defined at 17.8 mg/m2. Reversible ST/T changes and mild reversible arrhythmias were seen on post-treatment electrocardiograms, and there were no clinically significant changes in left ventricular ejection fraction. Dose-limiting toxicities were fatigue, nausea/vomiting, thrombocytopenia and cardiac arrhythmia. In the additional early trials of romidepsin, encouraging responses were observed in the peripheral and cutaneous T-cell lymphomas [Piekarz et al. 2001, 2004] and further clinical development focused on this group of hematologic malignancies.
The first phase II trial of romidepsin in PTCLs enrolled 47 heavily pretreated patients with various histologic subtypes [Piekarz et al. 2011]. The median number of prior therapies was three (range 1–11) and 38% of patients had disease that failed to respond to prior autologous SCT. All but two patients were treated at the dose of 14 mg/m2 on days 1, 8 and 15 of the 28-day cycle. The overall response rate was 38% with complete remission achieved in 18% of evaluable patients. The median duration of response was 8.9 (range 2–74) months. Responses were seen in all subtypes, with six responses observed among the 18 patients with prior SCT. These results warranted further development of romidepsin for PTCL.
In the registrational trial of romidepsin for relapsed and refractory PTCL, 130 patients were treated at 14 mg/m2 with a 4 h intravenous infusion on days 1, 8 and 15 of a 28-day cycle [Coiffier et al. 2012]. The median number of prior systemic therapies was two, and 21 (16%) patients had disease that failed to respond to prior autologous SCT. Overall, treatment was well tolerated and only 13 (10%) patients discontinued therapy due to treatment-related adverse events. The most common grade 3 and higher adverse events were thrombocytopenia (24%), neutropenia (20%) and infections (all types, 19%). No infectious prophylaxis was used during treatment. The objective response rate was 25% (33 out of 130), including 15% (19 out of 130) with CR/complete response, unconfirmed (CRu). The median duration of response for all the responders was 17 months. Interestingly, of the 19 patients achieving CR, 17 (89%) did not experience disease progression at the median follow up of 13.4 months.
Several observations were made in the pivotal trial of romidepsin. Patients with PTCL who achieved complete remission were able to proceed to high-dose therapy and autologous or allogeneic SCT as definitive curative treatment. In some of these patients autologous stem cells were collected after prolonged romidepsin therapy without compromise of the cellular composition of the graft or impact on engraftment [Shustov et al. 2010]. Patients who achieved CR/CRu and were not eligible for autologous SCT were able to continue prolonged romidepsin ‘maintenance’ without showing cumulative toxicity. Patients with different subtypes of PTCL had similar responses. There was no correlation between responses and any of the following: patient characteristics, prior SCT, number or type of prior therapy, or response to last prior therapy.
In conclusion, the reported primary objectives (efficacy and safety) as well as the observations mentioned above make romidepsin a suitable agent to use in patients with relapsed and refractory PTCL in both the pretransplant setting and for palliative management of patients who have had a post-transplant relapse or those who are ineligible for transplant. Current studies are focusing on combination treatments with other agents and incorporation of romidepsin into SCT protocols.
Brentuximab vedotin (SGN-35)
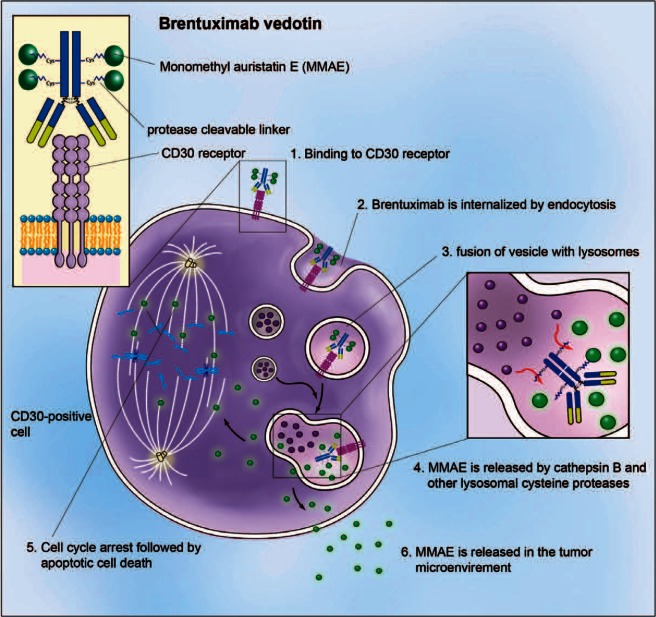
BV is an immunoconjugate combining a potent antitubulin agent, monomethyl auristatin E (MMAE), with a CD30-specific chimeric immunoglobulin G1 monoclonal antibody [Doronina et al. 2003; Hamblett et al. 2004]. A serum protease-resistant linker ensures minimal drug fall off while in the plasma. Upon binding the CD30 receptor, the antibody–drug conjugate is rapidly internalized and incorporated into a lysosome complex, in which the peptide linker is cleaved. MMAE is then released, leading to a preferential accumulation of the antitubulin agent within the tumor cells. The resultant blockade of the tubulin network causes cell cycle arrest between the G2 and M phase (G2/M) and apoptosis (Figure 3). Significant in vitro activity against CD30+ tumor cell lines and in vivo activity in murine models of Hodgkin lymphoma and ALCL was demonstrated in preclinical studies [Oflazoglu et al. 2008].
Figure 3.
Mechanism of action of brentuximab vedotin (BV). BV binds to surface CD30 leading to endocytosis; incorporation of BV–CD30 into the lysosomal complex leads to cleavage of the peptide linker and release of monomethyl auristatin E (MMAE) inside the cell; binding of the MMAE to the tubulin network leads to cell cycle arrest in the G2/M phase and apoptosis. Illustration courtesy of Alessandro Baliani© (2013).
CD30 is a member of the tumor-necrosis factor receptor family and is amply expressed on ALCL and to a smaller degree on other subtypes of PTCLs [Falini et al. 1995; Inghirami et al. 2011]. It is therefore an attractive therapeutic target. A phase I study of BV in patients with relapsed and refractory CD30+ lymphomas (primarily Hodgkin lymphoma and ALCL) was recently reported [Younes et al. 2010]. A total of 45 eligible patients were treated with escalating doses of BV on day 1 of a 21-day cycle. Patients had received a median of three prior systemic therapies and 73% had undergone autologous SCT. The maximum tolerated dose was established at 1.8 mg/kg administered every 3 weeks. The most common adverse events were fatigue, pyrexia, diarrhea, nausea, neutropenia and peripheral sensory neuropathy. Objective responses were observed in 17 patients (38%), including 11 complete remissions.
A phase II registrational trial of BV in patients with relapsed and refractory ALCL (ALK+ and ALK–) enrolled 58 patients with a median of two prior systemic therapies and with 26% having disease that failed to respond to prior autologous SCT [Pro et al. 2012]. Half of the patients had refractory disease and 22% had disease that showed no response to any prior therapy. In these patients with highly refractory disease, 86% achieved an objective response and 57% achieved CR. The median duration of response was 12.6 months. Similar responses were seen in both ALK+ and ALK– types of ALCL. Grade 3 and higher adverse events seen in over 10% of patients were neutropenia (21%), thrombocytopenia (14%) and peripheral sensory neuropathy (12%). The overall incidence of peripheral neuropathy was 53%, with 14% grade 3 and no grade 4 events. The median time to onset was 13.3 weeks (four cycles) for any grade of neuropathy and 28.4 weeks for grade 3 (nine cycles). Resolution or some improvement of peripheral neuropathy was observed in 81% of cases, with the median time to improvement of 9.9 weeks (range 0.3–32.9). Complete resolution of all types of neuropathy was achieved in 48% of patients. The remarkable activity demonstrated in this phase II trial led to the accelerated approval of BV by the FDA for patients with relapsed and refractory ALCL. The efficacy and safety profile of BV make it a highly suitable agent for both palliative treatment and pretransplant salvage therapy.
It is important to point out that durable remissions achieved with BV in some patients with relapsed and refractory ALCL allowed for successful transition to curative therapy using allogeneic or autologous SCT. Preliminary data indicate that neither the composition of the autologous graft nor the post-transplant clinical course was negatively impacted by pretransplant therapy with BV [Shustov et al. 2012].
Encouraging results of the phase II trial in relapsed and refractory ALCL led to a recently completed study of BV combined with chemotherapy in untreated patients with newly diagnosed ALCL and CD30+ PTCL. Preliminary results of this study were reported and are encouraging, with an overall response rate of 100% and an acceptable safety profile. Follow up of this trial continues and mature results are eagerly awaited.
New combination regimens
SMILE
Besides the emergence of new agents for PTCL, encouraging activity of older agents and their combinations were recently demonstrated for specific histologic subtypes. The most notable advances were reported for extranodal NK-cell lymphoma, nasal/nasal type. While up to 60% of patients with localized stage I–II disease can be cured with radiotherapy as a primary treatment [Li et al. 2006, 2012; Wang et al. 2012], patients with relapsed disease and advanced stage disease have a dismal prognosis when CHOP-like therapies are used for initial treatment. High expression of the MDR1 gene product, pgp, is thought to be at least partially responsible for this resistance [Yamaguchi et al. 1995]. In a search for an effective alternative regimen, the NK-Cell Tumor Study Group, comprising Japanese and Asian subgroups, has formulated a novel chemotherapeutic regimen, consisting of corticosteroid (dexamethasone), methotrexate, ifosfamide, etoposide, and L-asparaginase (SMILE). The design of SMILE was based on several considerations. Etoposide has demonstrated activity against NK-cell neoplasms both in vitro and in vivo [Uno et al. 2001]. L-Asparaginase induces apoptosis of NK-lymphoma cells in vitro and was reported to have significant activity as a single agent and in combination in clinical trials [Nagafuji et al. 2001; Yong et al. 2003]. Dexamethasone has higher potency, longer half life and is better in ameliorating the adverse drug effects of L-asparaginase than prednisolone [Nowak-Gottl et al. 2003]. Finally, methotrexate and ifosfamide are resistant to MDR-mediated efflux and have reported efficacy in NK-cell lymphomas [Lee et al. 2006]. In the phase I study of SMILE in advanced stage, relapsed or refractory NK-cell lymphoma, the dose-limiting toxicities were neutropenia and serious infections [Yamaguchi et al. 2008]. In the two subsequent phase II trials, conducted by Japanese (study 1) [Yamaguchi et al. 2011] and Asian lymphoma study groups (study 2) [Kwong et al. 2012], 38 and 87 patients, respectively, were treated with the SMILE regimen. Both trials included patients with newly diagnosed advanced stage and relapsed/refractory disease.
The SMILE treatment was associated with significant toxicity, predominantly hematologic and infectious. In study 1, grade 4 neutropenia was observed in 92% of the patients; the most common grade 3 and higher nonhematologic toxicity was infection (61%). In study 2, grade 3 and higher neutropenia was seen in 66% of patients and grade 3 and higher thrombocytopenia was seen in 41% of patients; there were five sepsis-related deaths. Nephrotoxicity was common in study 2 and developed in 17% of patients.
The efficacy of the SMILE regimen was encouraging in both phase II studies. In study 1, the overall response and CR rates after two cycles were 79% and 45% respectively. The 1-year overall survival rate was 55%. Out of 28 patients who completed the protocol treatment, 19 underwent SCT. In study 2, the rates of overall response and CR were 81% and 66% respectively and 5-year overall survival was 50%. In both studies there was no difference in responses between newly diagnosed and relapsed/refractory disease.
The GELA (Groupe d’Etude des Lymphomes de l’Adulte) and GOELAMS (Groupe Ouest Est d’Etude des Leucémies et Autres Maladies du Sang) Intergroup recently reported on a combination regimen that is conceptually similar but less intense than SMILE in patients with extranodal NK-cell lymphomas [Jaccard et al. 2011]. In this phase II prospective clinical trial 19 patients with relapsed/refractory disease received therapy with L-asparaginase, dexamethasone and methotrexate combination (AspaMetDex). A total of 14 out of 18 evaluable patients had an objective response, 11 entering complete remission for overall response and CR rates of 78% and 61% respectively. The 1-year overall survival was 50%. The regimen was well tolerated despite the fact that the median age of the patients was 60 years (range 45–76). Grade 3 and higher neutropenia was only observed in 8% of patients and the remaining grade 3 and higher adverse events were seen in less than 5% of patients.
The outcomes reported in these two prospective clinical trials compare very favorably to the historical data. Therefore, the SMILE protocol should be preferred to the previously used regimens for patients with advanced stage and relapsed/refractory extranodal NK-cell lymphomas. Close monitoring for infections and aggressive management of myelosuppression is necessary. Multitargeted infectious disease prophylaxis is recommended. In older and less fit patients, AspaMetDex offers an acceptable alternative.
DeVIC
Approximately half of the patients with localized extranodal NK-cell lymphoma will be cured with primary radiotherapy. In an attempt to improve on these results, the Japan Clinical Oncology Group conducted a phase II study of concurrent chemoradiotherapy using a combination of dexamethasone, etoposide, ifosfamide and carboplatin (DeVIC) [Yamaguchi et al. 2009, 2012]. The rationale for this combination was similar to that previously described, namely, to use non-MDR efflux susceptible agents and etoposide. In this 33-patient prospective trial, 77% achieved CR after therapy and the 2-year overall survival was 78%, which compares favorably to previous reports of radiotherapy alone. The most common grade 3 and higher nonhematologic toxicity was mucositis related to radiation (30%).
DeVIC with concurrent radiotherapy should therefore be considered for patients with early localized stage nasal NK-cell lymphoma, especially those with high risk features (‘B’ symptoms, elevated lactate dehydrogenase, regional lymph node involvement, stage II disease).
Investigational agents in peripheral T-cell lymphomas
Several new investigational agents showed promise and are being evaluated in patients with PTCLs (Table 3). Two of these agents deserve specific mentioning. Belinostat, a novel pan-HDAC inhibitor, was evaluated in a phase II trial for patients with relapsed and refractory PTCL and cutaneous T-cell lymphoma. Belinostat was given at a dose of 1000 mg/m2 on days 1–5 of the 21-day cycle. A total of 20 patients with various histologies of PTCL were treated. Seven patients responded, with two patients achieving complete remission for an overall response rate of 25%. The median duration of response was over 159 days (range 1–504). Treatment was well tolerated with four grade 3 and higher adverse events, including pruritis, edema and ileus. These promising results led to a multicenter, international trial of belinostat in relapsed/refractory PTCL that was recently completed having accrued 151 patients. The results of this study have not been reported yet.
Table 3.
Novel agents in trials for PTCL.
| Agent | Mechanism of action | Stage of development | Agent type/route |
|---|---|---|---|
| Belinostat | HDAC inhibition | Phase II | SMI/IV |
| Dasatinib | ABL, SRC, Ckit, EPHA, PDGFb | Phase I/II | SMI/oral |
| Tipifarnib | Farnesyl transferase inhibition | Phase II | SMI/oral |
| Plitidepsin | Activation of JNK and MAPK | Phase II | Peptide/IV |
| KW-0761 | Anti-CCR4 antibody | Phase II | MoAB/IV |
| Lenalidomide | Immunomodulation | Phase II | SMI/oral |
| Zanolimumab | Anti-CD4 antibody | Phase II | MoAB/IV |
| A-dmDT390-bisFv | Anti-CD3-diftitox | Phase I | ADC/IV |
| Crizotinib | ALK-1 inhibition | Phase I | SMI/oral |
| Forodesine | PNP inhibition | Phase I | NA/oral |
| Alisertib | Aurora kinase inhibition | Phase II/III | SMI/oral |
Patients proceeding to autologous or allogeneic SCT after achieving response with single agent therapy.
ADC, antibody drug conjugate; ALK-1, activin receptor-like kinase-1; EPHA, ephrin-A; HDAC, histone deacetylase; IV, intravenous; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MoAB, monoclonal antibody; NA, not applicable; PDGFb, platelet-derived growth factor b; PNP, purine nucleoside phosphorylase; PTCL-U, peripheral T-cell lymphoma ; SMI, small molecule inhibitor.
Aurora kinase inhibitors are a new class of antineoplastic agents currently under investigation for various malignancies. Alisertib, an oral selective aurora A inhibitor, was evaluated in a pilot study of patients with refractory T-cell and B-cell lymphomas. Treatment was given at a dose of 50 mg twice daily and was generally well tolerated, and the most common grade 3/4 adverse events were neutropenia (63%), thrombocytopenia (31%), stomatitis (15%) and fatigue (6%). Dose reduction to 40 mg twice daily was required in 24 patients (50%). Out of eight patients with PTCL, four achieved an objective response (57%). The current Southwest Oncology Group clinical trial is accruing patients with relapsed and refractory PTCL and is looking to confirm these initial encouraging results.
Summary
PTCLs are diverse and clinically aggressive lymphoid neoplasms with poor prognosis. They are distinct from mature B-cell and precursor-cell lymphomas. Frontline and salvage therapies, as well as treatment paradigms used for PTCLs in the last two decades, were developed primarily for aggressive B-cell lymphomas. Retrospective studies indicated inferior outcomes when these therapies are used for PTCLs and new approaches are needed. Development of a new classification system, advances in diagnostic tools and international collaboration of academic institutions in recent years have resulted in the approval of three new agents with specific indication for PTCLs. These agents are pralatrexate, romidepsin and BV (summarized in Table 4). These agents belong to three different classes of antineoplastic drugs but share several features. First, they demonstrate impressive activity in patients with heavily pretreated, highly refractory disease, some of whom have failed to respond to prior therapy with autologous SCT. Second, none of these agents demonstrated a propensity to significant cumulative toxicity with little impact on quality of life, making them suitable for future trials of long-term maintenance therapy. Third, there is accumulating although anecdotal evidence that all three agents might have no impact on the ability to collect autologous stem cells or on subsequent engraftment in patients who have undergone autologous HCT after attainment of durable remission in described trials. Hence, any of these agents can be used as salvage therapy in patients who are candidates for a definitive curative approach. Fourth, there is no apparent cross resistance between these new drugs, making them suitable for sequential single-agent therapy as an alternative to more toxic combination treatments in a noncurative setting. The last feature is particularly important for older and debilitated patients who cannot tolerate aggressive protocols.
Table 4.
Summary of FDA-approved novel agents for PTCL.
| Agent | Patients (n) | Histology | Dose/schedule | ORR/CR % | DOR (months) | SCT* (n) |
|---|---|---|---|---|---|---|
| Pralatrexate | 109 | All PTCL | 30 mg/m2 IV Weekly 6/7 weeks |
29/11 | 10.1 | 6 |
| Romidepsin | 131 | All PTCL | 14 mg/ m2 IV Weekly 3/4 weeks |
25/15 | 17 | 6 |
| B-vedotin | 58 | ALCL* | 1.8 mg/kg IV Every 3 weeks |
86/57 | 12.6 | 11 |
Patients proceeding to autologous or allogeneic SCT after achieving response with single agent therapy.
ALCL, anaplastic large cell lymphoma; CR, complete response; DOR, duration of response; FDA, US Food and Drug Administration; IV, intravenously; ORR, objective response rate; SCT, stem-cell transplantation; PTCL-U, peripheral T-cell lymphoma.
Current and future studies will further define the role of the approved and still experimental agents for various clinical indications and in combination regimens. These efforts have begun and will continue improving the curability in patients with PTCLs.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Consultant and Honoraria: SPECTRUM Pharmaceuticals, Celgene, Seattle Genetics, Inc.
References
- Anderson J., Armitage J., Weisenburger D. (1998) Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s lymphoma classification project. Ann Oncol 9: 717–720 [DOI] [PubMed] [Google Scholar]
- Armitage J., Vose J., Weisenburger D. (2008) International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol 26: 4124–4130 [DOI] [PubMed] [Google Scholar]
- Coiffier B., Pro B., Prince H., Foss F., Sokol L., Greenwood M., et al. (2012) Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30: 631–636 [DOI] [PubMed] [Google Scholar]
- Corradini P., Tarella C., Zallio F., Dodero A., Zanni M., Valagussa P., et al. (2006) Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia 20: 1533–1538 [DOI] [PubMed] [Google Scholar]
- d’Amore F., Relander T., Lauritzsen G., Jantunen E., Hagberg H., Anderson H., et al. (2012) Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 30: 3093–3099 [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Criniti V., Della Pietra V., Borriello A., Oliva A., Indaco S., et al. (2001) Genes modulated by histone acetylation as new effectors of butyrate activity. FEBS Lett 499: 199–204 [DOI] [PubMed] [Google Scholar]
- Doronina S., Toki B., Torgov M., Mendelsohn B., Cerveny C., Chace D., et al. (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21: 778–784 [DOI] [PubMed] [Google Scholar]
- Escalon M., Liu N., Yang Y., Hess M., Walker P., Smith T., et al. (2005) Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M.D. Anderson Cancer Center experience. Cancer 103: 2091–2098 [DOI] [PubMed] [Google Scholar]
- Falini B., Pileri S., Pizzolo G., Durkop H., Flenghi L., Stirpe F. (1995) CD30 (ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 85: 1–14 [PubMed] [Google Scholar]
- Foss F., Horwitz S., Coiffier B., Bartlett N., Popplewell L., Pro B., et al. (2012) Pralatrexate is an effective treatment for relapsed or refractory transformed mycosis fungoides: a subgroup efficacy analysis from the PROPEL study. Clin Lymphoma Myeloma Leuk 12: 238–243 [DOI] [PubMed] [Google Scholar]
- Hamblett K., Senter P., Chace D., Sun M., Lenox J., Cerveny C., et al. (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 10: 7063–7070 [DOI] [PubMed] [Google Scholar]
- Inghirami G., Pileri S. and European T-Cell Lymphoma Study Group (2011) Anaplastic large-cell lymphoma. Semin Diagnost Pathol 28: 190–201 [DOI] [PubMed] [Google Scholar]
- Jaccard A., Gachard N., Marin B., Rogez S., Audrain M., Suarez F., et al. (2011) Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 117: 1834–1839 [DOI] [PubMed] [Google Scholar]
- Krug L., Azzoli C., Kris M., Miller V., Khokhar N., Tong W., et al. (2003) 10-Propargyl-10-deazaaminopterin: an antifolate with activity in patients with previously treated non-small cell lung cancer. Clin Cancer Res 9: 2072–2078 [PubMed] [Google Scholar]
- Krug L., Ng K., Kris M., Miller V., Tong W., Heelan R., et al. (2000) Phase I and pharmacokinetic study of 10-propargyl-10-deazaaminopterin, a new antifolate. Clin Cancer Res 6: 3493–3498 [PubMed] [Google Scholar]
- Kuhnel J., Chiao J., Sirotnak F. (2000) Contrasting effects of oncogene expression on two carrier-mediated systems internalizing folate compounds in fisher rat 3T3 cells. J Cell Physiol 184: 364–372 [DOI] [PubMed] [Google Scholar]
- Kwong Y., Kim W., Lim S., Kim S., Tang T., Tse E., et al. (2012) SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood 120: 2973–2980 [DOI] [PubMed] [Google Scholar]
- Lee K., Yun T., Kim D., Im S., Kim T., Yoon S., et al. (2006) First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/− radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma 47: 1274–1282 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang H., Jin J., Wang W., Liu Q., Song Y., et al. (2012) Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys 82: 1809–1815 [DOI] [PubMed] [Google Scholar]
- Li Y., Yao B., Jin J., Wang W., Liu Y., Song Y., et al. (2006) Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 24: 181–189 [DOI] [PubMed] [Google Scholar]
- Lopez-Guillermo A., Cid J., Salar A., Lopez A., Montalban C., Castrillo J., et al. (1998) Peripheral T-cell lymphomas: Initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. classification. Ann Oncol 9: 849–855 [DOI] [PubMed] [Google Scholar]
- Morabito F., Gallamini A., Stelitano C., Callea V., Guglielmi C., Neri S., et al. (2004) Clinical relevance of immunophenotype in a retrospective comparative study of 297 peripheral T-cell lymphomas, unspecified, and 496 diffuse large B-cell lymphomas: experience of the Intergruppo Italiano Linformi. Cancer 101: 1601–1608 [DOI] [PubMed] [Google Scholar]
- Nagafuji K., Fujisaki T., Arima F., Ohshima K. (2001) L-Asparaginase induced durable remission of relapsed nasal NK/T-cell lymphoma after autologous peripheral blood stem cell transplantation. Int J Hematol 74: 447–450 [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kim Y., Terano H., Yoshida M., Horinouchi S. (1998) FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp Cell Res 241: 126–133 [DOI] [PubMed] [Google Scholar]
- Nowak-Gottl U., Ahlke E., Fleischhack G., Schwabe D., Schobess R., Schumann C., et al. (2003) Thromboembolic events in children with acute lymphoblastic leukemia (BFM protocols): prednisone versus dexamethasone administration. Blood 101: 2529–2533 [DOI] [PubMed] [Google Scholar]
- O’Connor O., Hamlin P., Portlock C., Moskowitz C., Noy A., Straus D., et al. (2007) Pralatrexate, a novel class of antifol with high affinity for the reduced folate carrier-type 1, produces marked complete and durable remissions in a diversity of chemotherapy refractory cases of T-cell lymphoma. Br J Haematol 139: 425–428 [DOI] [PubMed] [Google Scholar]
- O’Connor O., Horwitz S., Hamlin P., Portlock C., Moskowitz C., Sarasohn D., et al. (2009) Phase II–I–II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol 27: 4357–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor O., Pro B., Pinter-Brown L., Bartlett N., Popplewell L., Coiffier B., et al. (2011) Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 29: 1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oflazoglu E., Kissler K., Sievers E., Grewal I., Gerber H. (2008) Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol 142: 69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarz R., Frye R., Prince H., Kirschbaum M., Zain J., Allen S., et al. (2011) Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood 117: 5827–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarz R., Robey R., Sandor V., Bakke S., Wilson W., Dahmoush L., et al. (2001) Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood 98: 2865–2868 [DOI] [PubMed] [Google Scholar]
- Piekarz R., Robey R., Zhan Z., Kayastha G., Sayah A., Abdeldaim A., et al. (2004) T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood 103: 4636–4643 [DOI] [PubMed] [Google Scholar]
- Pro B., Advani R., Brice P., Bartlett N., Rosenblatt J., Illidge T., et al. (2012) Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 30: 2190–2196 [DOI] [PubMed] [Google Scholar]
- Reimer P., Rudiger T., Geissinger E., Weissinger F., Nerl C., Schmitz N., et al. (2009) Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol 27: 106–113 [DOI] [PubMed] [Google Scholar]
- Rudiger T., Weisenburger D., Anderson J., Armitage J., Diebold J., MacLennan K., et al. ; Non-Hodgkin’s Lymphoma Classification Project (2002) Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the non-Hodgkin’s lymphoma classification project. Ann Oncol 13: 140–149 [DOI] [PubMed] [Google Scholar]
- Sandor V., Bakke S., Robey R., Kang M., Blagosklonny M., Bender J., et al. (2002) Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res 8: 718–728 [PubMed] [Google Scholar]
- Savage K., Chhanabhai M., Gascoyne R., Connors J. (2004) Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 15: 1467–1475 [DOI] [PubMed] [Google Scholar]
- Schmid F., Sirotnak F., Otter G., DeGraw J. (1985) New folate analogs of the 10-deaza-aminopterin series: Markedly increased antitumor activity of the 10-ethyl analog compared to the parent compound and methotrexate against some human tumor xenografts in nude mice. Cancer Treat Rep 69: 551–553 [PubMed] [Google Scholar]
- Schmitz N., Trumper L., Ziepert M., Nickelsen M., Ho A., Metzner B., et al. (2010) Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-grade Non-Hodgkin Lymphoma Study Group. Blood 116: 3418–3425 [DOI] [PubMed] [Google Scholar]
- Shustov A., Gopal A., Lupinacci T., Johnson D., Zecha G., Cherian S. (2010) Successful peripheral blood hematopoietic cell mobilization and compositional autograft analysis in a patient with peripheral T-cell lymphoma treated with single agent romidepsin. Meeting Proceedings, International T-cell Lymphoma Forum 2010, Lahaina, HI, USA Abstract TR_2. [Google Scholar]
- Shustov A., Rosenblatt J., Kennedy D., Fanale M. (2012) Peripheral blood stem cell (PBSC) mobilization and engraftment after brentuximab vedotin treatment in anaplastic large cell lymphoma (ALCL) patients. Ann Oncol 23(Suppl. 9): ix353, abstract 1083P. [Google Scholar]
- Simon A., Peoch M., Cassasus P., Deconick E., Colombat P., Desablens B., et al. (2010) Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T-cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haemtol 151:159–166 [DOI] [PubMed] [Google Scholar]
- Sirotnak F., DeGraw J., Colwell W., Piper J. (1998) A new analogue of 10-deazaaminopterin with markedly enhanced curative effects against human tumor xenografts in mice. Cancer Chemother Pharmacol 42: 313–318 [DOI] [PubMed] [Google Scholar]
- Sirotnak F., DeGraw J., Moccio D., Samuels L., Goutas L. (1984a) New folate analogs of the 10-deaza-aminopterin series. basis for structural design and biochemical and pharmacologic properties. Cancer Chemother Pharmacol 12: 18–25 [DOI] [PubMed] [Google Scholar]
- Sirotnak F., DeGraw J., Schmid F., Goutas L., Moccio D. (1984b) New folate analogs of the 10-deaza-aminopterin series. further evidence for markedly increased antitumor efficacy compared with methotrexate in ascitic and solid murine tumor models. Cancer Chemother Pharmacol 12: 26–30 [PubMed] [Google Scholar]
- Sirotnak F., Schmid F., Samuels L., DeGraw J. (1987) 10-Ethyl-10-deaza-aminopterin: structural design and biochemical, pharmacologic, and antitumor properties. NCI Monogr (5): 127–131 [PubMed] [Google Scholar]
- Sirotnak F., Tolner B. (1999) Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr 19: 91–122 [DOI] [PubMed] [Google Scholar]
- Ueda H., Manda T., Matsumoto S., Mukumoto S., Nishigaki F., Kawamura I., et al. (1994a) FR901228, a novel antitumor bicyclic depsipeptide produced by chromobacterium violaceum no. 968. III. Antitumor activities on experimental tumors in mice. J Antibiot 47: 315–323 [DOI] [PubMed] [Google Scholar]
- Ueda H., Nakajima H., Hori Y., Fujita T., Nishimura M., Goto T., Okuhara M. (1994b) FR901228, a novel antitumor bicyclic depsipeptide produced by chromobacterium violaceum no. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J Antibiot 47: 301–310 [DOI] [PubMed] [Google Scholar]
- Ueda H., Nakajima H., Hori Y., Goto T., Okuhara M. (1994) Action of FR901228, a novel antitumor bicyclic depsipeptide produced by chromobacterium violaceum no. 968, on ha-ras transformed NIH3T3 cells. Biosci Biotechnol Biochem 58: 1579–1583 [DOI] [PubMed] [Google Scholar]
- Uno M., Tsuchiyama J., Moriwaki A., Noguchi T., Mizoguchi K., Ogino T., et al. (2001) In vitro induction of apoptosis for nasal angiocentric natural killer cell lymphoma-derived cell line, NK-YS, by etoposide and cyclosporine A. Br J Haematol 113: 1009–1014 [DOI] [PubMed] [Google Scholar]
- Van Lint C., Emiliani S., Verdin E. (1996) The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr 5: 245–253 [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li Y., Wang W., Jin J., Dai J., Wang S., et al. (2012) Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys 82: 1115–1121 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kita K., Miwa H., Nishii K., Oka K., Ohno T., et al. (1995) Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76: 2351–2356 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kwong Y., Kim W., Maeda Y., Hashimoto C., Suh C., et al. (2011) Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-cell Tumor Study Group study. J Clin Oncol 29: 4410–4416 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Suzuki R., Kwong Y., Kim W., Hasegawa Y., Izutsu K., et al. (2008) Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci 99: 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M., Tobinai K., Oguchi M., Ishizuka N., Kobayashi Y., Isobe Y., et al. (2009) Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan clinical oncology group study JCOG0211. J Clin Oncol 27: 5594–5600 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Tobinai K., Oguchi M., Ishizuka N., Kobayashi Y., Isobe Y., et al. (2012) Concurrent chemoradiotherapy for localized nasal natural Killer/T-cell lymphoma: an updated analysis of the Japan Clinical Oncology Group study JCOG0211. J Clin Oncol 30: 4044–4046 [DOI] [PubMed] [Google Scholar]
- Yong W., Zheng W., Zhang Y., Zhu J., Wei Y., Zhu D., et al. (2003) L-Asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol 78: 163–167 [DOI] [PubMed] [Google Scholar]
- Younes A., Bartlett N., Leonard J., Kennedy D., Lynch C., Sievers E., et al. (2010) Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363: 1812–1821 [DOI] [PubMed] [Google Scholar]