Summary
The role of microcirculation in skeletal muscle is to provide the supply of oxygen and various nutrients and to remove waste products of muscle metabolism. As skeletal muscles are composed of different fibre types, this review tries to elucidate the question of capillary supply and flow with respect to these. It reviews the current knowledge of structure of microcirculation and its nervous, hormonal, and local (myogenic, metabolic and endothelial) control. It also discuss factors involved in the increase in blood flow and changes in microcirculation occurring during muscle contractions, exercise training, muscle hypertrophy and atrophy, hypoxia, ageing, hypertension, diabetes and limited blood supply.
Keywords: arterioles, blood flow, capillaries, endothelium, muscle fibres, shear stress
Structure of microcirculation
Most initial studies on microcirculation were performed on thin muscles which could be transilluminated such as spinotrapezius or cremaster where the microvessels form arcades. It is now known that the arrangement is similar in other transilluminated thin muscles where muscle fibres and capillaries run in parallel (hamster retractor, rat gracilis or rabbit or cat tenuissimus), or in thicker muscles such as extensor digitorum longus in rats or gluteus in mice using epi-illumination. Microcirculation in most muscles branches from one or more feed arteries into a system or arterioles (classified according diameters and authors from the largest (arteriole A1) to smallest (A 4, 5 or more) with the terminal arteriole supplying usually up to 20 capillaries. Blood returns to collecting venules which merge to form larger venules, arranged in a similar manner to arterioles and veins (Fig. 1) (1). Smooth muscle cells are present in several layers in larger arterioles and in one layer in the terminal arterioles but not in venules. All microvessels are lined with endothelial cells which are connected with smooth muscle cells by gap and myoendothelial junctions. Capillaries have about 30% of their surface covered by pericytes, cells which may regulate endothelial proliferation and are important as precursors of smooth muscle cells in transformation of capillaries into arterioles (arteriolarization) during growth and remodeling of the microcirculation (2).
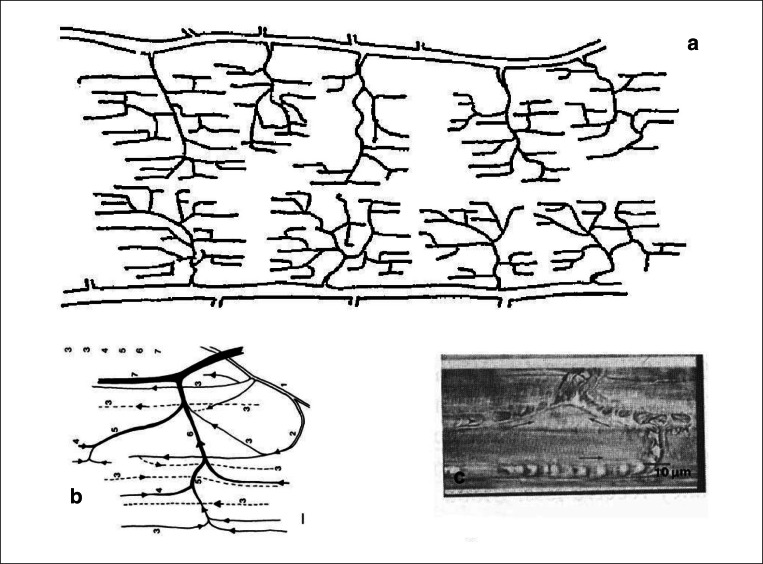
Figure 1.
a) scheme of microcirculation in the hamster retractor muscle (modified from 1) b) arterioles 1–3, capillaries 3, venules 4–6, collecting vein 7 c) picture of red blood cells in capillaries entering a venule (top); arrows indicate flow.
Capillary flow in skeletal muscles is heterogeneous and this led to a concept of nutritive and non-nutritive flow. Capillary flow in other organs is regulated by precapillary sphincters but there is no morphological evidence for their presence in skeletal muscle. The non-nutritive flow supplies connective tissue and tendons rather than muscle fibres although there are some experiments indicating a possibility of different pathways of capillary flow (3). The most likely explanation of non uniformity of capillary flow is in the variability of capillary lengths, velocity of red blood cells (0.018–0.324 mm.sec −1) and capillary haematocrit with some capillaries with almost stationary flow. This results in different transit times of red blood cells (RBC) which is important for delivery of oxygen to muscle fibres (2).
Most muscles have fibres with different contractile and metabolic properties. The division is important, from the point of view of microcirculation, only into glycolytic (fast) and oxidative (fast and slow) fibres. Motor units are composed of fibres of similar type which are located randomly in most muscles. Thus for a long time it was difficult to explain the regulation of flow in microvascular units which are not parallel to the arrangements of motor units. Few muscles have either predominantly oxidative (soleus in several mammalian species) or predominantly glycolytic (e.g. surface of rat tibialis anterior) fibres. Capillaries in these muscle differ not only according to their density but also according to their shape (Fig. 2) Observation of microcirculation in these muscles revealed that capillaries in tibialis anterior have faster velocity of red blood cell (Vrbc) with shorter red cells transit time than in soleus. Vrbc in these capillaries increases more during contractions (4) to carry away the metabolites such as lactic acid produced in glycolytic fibres during muscle contractions (5).
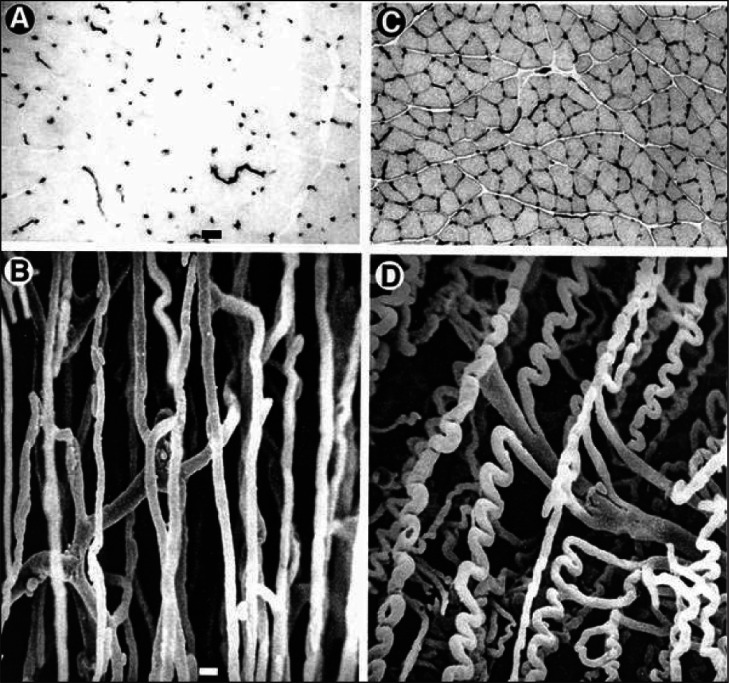
Figure 2.
Capillary bed in rat fast extensor digitorum longus (A,B) and slow soleus muscle (C,D). A and C are cross sections with capillaries stained for alkaline phospatase shown as black dots, B and D are vascular casts.
Control of microcirculation
Microcirculation in skeletal muscle is controlled by general mechanisms activated during whole body response (hormonal or nervous) and by local mechanisms related to changes in individual muscles (myogenic, metabolic and endothelial).
Nervous control
All arterial branches of microcirculation are supplied by adrenergic sympathetic nerve fibres with α and β receptors while venous microvessels are not innervated (6). Basal sympathetic constrictor tone in skeletal muscle is relatively high at rest which is demonstrated by increase in flow after pharmacological adrenergic blockade or interruption of sympathetic innervation. Vessels supplying slow oxidative muscles are less responsive to sympathetic activation or to catecholamines than those in fast muscles and this enables to maintain blood flow in these postural muscles during fight or flight situations. Activation of sympathetic nerves enables the diversion of blood from skeletal muscles to other organs in life threatening situations such as haemorrhage or shock. Constriction of large arterioles helps to maintain the blood pressure while dilatation in distal arterioles (due to release of metabolites) promotes improved oxygen delivery (7). Vessels in some species have cholinergic sympathetic innervation which was supposed to produce dilatation in muscles in preparation for fight or flight. There are reports on peptidergic (neuropeptide Y) and purinergic innervation, although their function has yet to be specified (see 2).
Hormonal control
Catecholamines, mainly adrenaline, which are released from the adrenal medulla in response to exercise, fight or flight situation, hypotension or hypoglycaemia, are most important.
Adrenaline acts on β receptors in muscle vessels and causes dilatation while noradrenalin (released only in smaller amounts systemically) acts on α receptors and is much more important locally (see above). High levels of circulating angiotensin II, in response to activation of the reninangiotensin system by e.g. haemorrhage or cardiac failure cause vasoconstriction. Arginine vasopressin (AVP) can dilate microvessels in muscles via V2 receptors, but during high stress states such as hemorrhagic hypotension or congestive heart failure, may have a predominantly vasoconstrictor effect via V1 receptors to support arterial pressure. By contrast, atrial natriuretic peptide (ANP) can antagonise α1-mediated vasoconstriction in large arterioles. Skeletal muscle microcirculation is significantly affected by insulin. Systemic administration of insulin caused arteriolar dilation in rat spinotrapezius and increased velocity of red blood cells both in arterioles and capillaries in rat cremaster. Thyroxine increases both muscle blood flow and metabolism (see 2).
Local control
Myogenic
Myogenic response of vascular smooth muscle in the skeletal muscle microcirculation, i.e. vessel constriction in response to increased pressure and dilation in response to decreased pressure, is relatively strong. Increased pressure leads to membrane depolarization, increased intra-cellular Ca2+ concentration in smooth muscle cells and their contraction (reviewed in 8). Myogenic response also explains Vasomotion i.e. regular contraction or dilatation of terminal arterioles which contributes to temporal inhomogeneity of capillary flow. Myogenic responses in microvessels can be attenuated by accumulation of metabolites during light exercise.
Metabolic
Metabolic control of muscle blood flow is related to the metabolic rate of the muscle. In spite of hundreds of papers reporting various substances as responsible for the increase in muscle blood flow during muscle contractions (decreased pH, increased PCO2, increased osmolality, increased adenosine and / or adenosine nucleotides, potassium, phosphates, kinins, prostaglandins or nitric oxide (NO), none alone can account for increased blood either in response to muscle contraction (functional hyperaemia) or after temporary limitation of blood flow (reactive hyperaemia). Joyner & Proctor (9) have recently reiterated the limitations of attempting to ‘isolate’ a potential vasodilator metabolite. Thomas & Segal (10) suggest that K+ which is released from contracting muscle fibres causes hyperpolarization of vascular smooth muscle with subsequent inhibition of Ca++ influx. Armstrong et al. (11) showed indeed that stimulation of only very few muscle fibres causes dilatation of the supplying arterioles which was absent when different K+ channels were inhibited. The metabolites are presumed to act on all segments of the microvascular network within the muscle with the largest dilation in response to stimulation-evoked contractions in pre-terminal and terminal arterioles [7–13 μm diameter] (12), but dilatation includes also the feed arteries (13).
Endothelial
Endothelial cells are the first cells sensing changes occurring inside (changes in flow velocity and consequently shear stress, i.e. friction between the red blood cells and endothelial lining) ) as well outside ( metabolic changes occurring in muscle fibres and/or changes in capillary wall tension due to length/diameter changes of surrounding muscle fibres) the vessels. They release relaxing [such as nitric oxide (NO), prostaglandins (PGs), endothelial-derived hyperpolarizing factor (EDHF)] as well as constricting factors [such as endothelin (ET)] in response to chemical and physical signals. Inhibition of NO synthesis by competitive inhibitors increases vascular resistance more in larger than smaller (< 25 μm) vessels and decreases blood flow in muscles at rest. Similarly, cyclooxygenase inhibitors of prostaglandin synthesis such as indomethacin cause vasoconstriction of arterioles in resting muscle (see 2). Endothelial NO and PG release may also be agonist-induced, by catecholamines, acetylcholine (ACh), histamine or bradykinin (14). Dilatation induced by these agonists cannot be eliminated either nitric oxidase synthase or cyclooxygenase blockers. It is abolished by several calcium dependent potassium channel (KCa) blockers (15). Potocnik et al. (15) also demonstrated the presence of myoendothelial cell junctions which explain the transmission of signal from the endothelium to smooth muscle cells. Endothelin, a potent endothelial-derived vasoconstrictor, may antagonize dilator mechanisms in skeletal muscle (16).
The endothelium is important in the transmission of signals between different segments of the microvascular network. If an agonist such as acetylcholine or noradrenalin is applied locally to an arteriole, the vasoactive response is not confined to the site of stimulation but is propagated along the vessel for a distance of several millimetres independently of innervation or flow.
This mechanism, termed ‘conducted’ dilation or constriction, appears to depend on direct coupling between endothelia-endothelial and endothelial-vascular smooth muscle cells via gap junctions formed by connexins and electrotonic spread of changes in membrane potential (reviewed in 17). It is very important in the explanation of increased blood flow during muscle contractions (see later) Spreading vasodilatation can be limited by activation of alpha receptors (18).
Microcirculation during functional hyperaemia
About 90% of capillaries are perfused at rest, but contain a different number of red blood cells with different velocity of flow. Capillary perfusion increases with only few contractions. This is due partly due to increase concentration of interstitial K+ and possibly other substances which are released from contracting muscle fibres (see above) and partly due to increased shear stress and release of NO from capillary endothelium (Fig. 3), as Vrbc increases already with the first contraction (18). The signals are spreading via gap junctions and KATP channels to arterioles and even feed arteries (19) thus enabling increase in blood flow to larger regions.
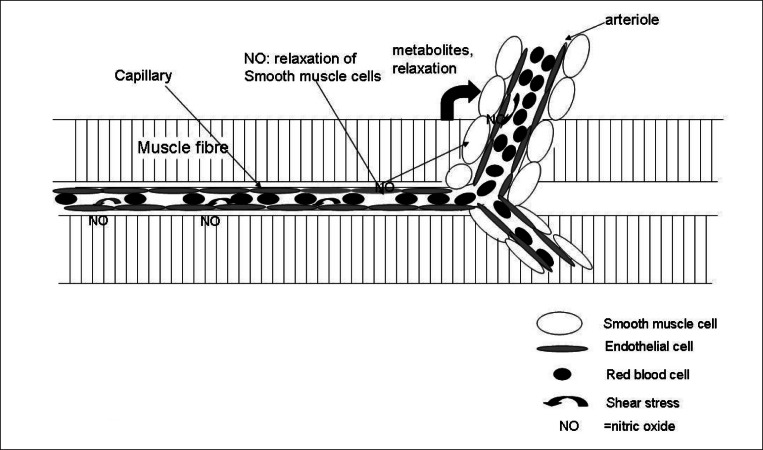
Figure 3.
Interaction between capillary shear stress, metabolites released from muscle fibres, endothelial cells and smooth muscle cells. Increased shear stress due to the increased velocity of flow causes release of NO from endothelial cells which contribute to the relaxation of smooth muscle cells. On a long-term basis shear stress and NO also contribute to the upregulation of VEGFR2 (vascular endothelial growth factor receptor 2) and VEGF and thus stimulate capillary growth.
The microvascular units (arteriole-capillaries-venule) do not supply distinct motor units The spreading vasodilatation can explain the long-lasting puzzle about the different anatomical arrangement of microvascular units with length in the order of several hundreds mm with the activation of muscle fibres long several cm within one motor unit. When the motor unit is activated, the signals from capillaries supplying the contracting muscle fibres spread towards the nearest arteriolar network. With many such networks activated at the same time the dilatation spreads towards the supplying arteries and blood flow increases.
LONG-TERM CHANGES IN MICROCIRCULATION UNDER PHYSIOLOGICAL CONDITIONS
Training and muscle stimulation
Long term increase in muscle activity over several weeks induced either by training or by chronic electrical stimulation results in growth of capillaries and arterioles (2). Capillary growth in endurance trained muscles is related to the intensity of muscle activity and is usually but not always preceded by an increase in oxidative enzymes. The increase occurs predominantly around oxidative fibres (20) as these are preferentially recruited and receive increased blood flow during voluntary activity (21). Endurance training in humans leads to increased capillary supply in particular muscles involved in the training protocol, e.g. deltoid muscle in rowers and swimmers, vastus lateralis in cyclists (22). Fast glycolytic fibers are only activated during supramaximal contractions of short duration and capillary growth was observed in the glycolytic parts of gastrocnemius muscle in rats trained by sprint training (23) possibly linked with increased blood flow to this region (Fig. 4). Training also stimulates growth of arterioles: both the number and diameter of terminal arterioles (24), diameter and flow in arterioles < 25 μm (25) and diameter of feed artery (26) are higher in trained rats. Capillary growth induced by training is not due to proliferation of endothelial cells (27) but to elongation of existing capillaries and probably capillary splitting(28).
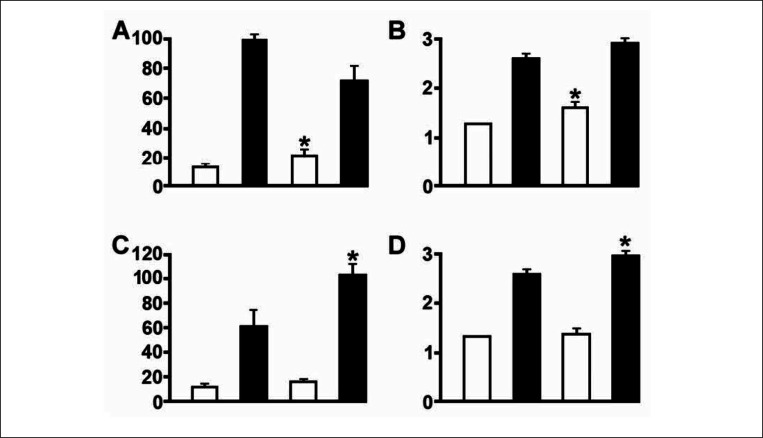
Figure 4.
The effect of training on blood flow (a.c) and capillary supply (b,d) in different skeletal muscle after high intensity sprint training (top panels) and endurance training (bottom panels). White columns predominantly glycolytic fibres, black columns predominantly oxidative muscles. The first two columns in each panels represent control values, the second two columns values after training. The difference after training is denoted by*. Note that sprint training alters values in glycolytic and endurance in oxidative muscles.
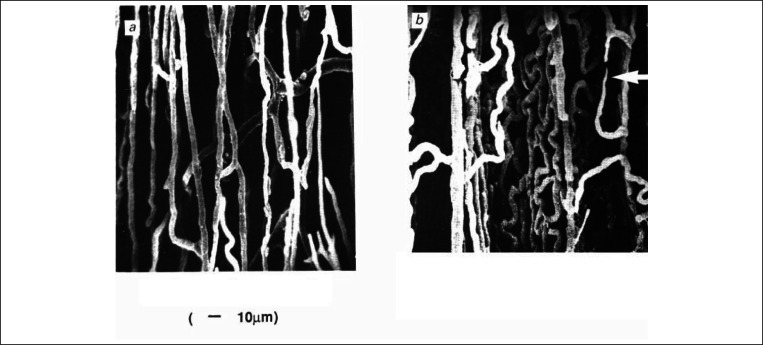
With endurance training growth of vessels usually appears after many weeks, but training by running to exhaustion accelerated this process (29). Increasing muscle activity in selected muscle groups (hind-limb ankle flexors in rats or rabbits) by chronic electrical stimulation induced capillary growth within only 4 days and doubled C: F ratio within a month (31). Capillaries formed a very complex network with multiple intercapillary connections and sprouts (Fig. 5) (32). Growth of vessels induced by increased activity can be initiated by growth factors secreted from muscle, or by mechanical factors such as shear stress and/or wall tension. Vascular endothelial growth factor (VEGF) is involved (33) but other factors, such as metalloproteinases or NO are also engaged (34) and there is obviously an interplay of different growth factors as well as anti-angiogenic factors necessary to initiate and maintain capillary growth (35). Capillary growth in muscles whose activity was increased by chronic electrical stimulation was very probably initiated by shear stress with subsequent activation of endothelial nitric oxide synthase (eNOS), generation of NO and increased protein of Vascular Endothelial Growth Factor Receptor 2 (VEGFR2). As stimulation continued, shear stress and generation of NO by activation of eNOS became less important, but it is possible that NO was generated by nNOS activation (36) as nonspecific inhibitor of NOS LNNA inhibited capillary growth in the absence of elevated shear stress (37).
Figure 5.
Cast of the microvasculature from control (a) and chronically stimulated (8 h/day,7 days) muscle. Arrow indicates capilary sprouts (40).
Capillaries can grow by sprouting or longitudinal splitting. While shear stress results in capillary growth by splitting and stretch by sprouting, repeated muscle contractions combine both factor (38).Capillary growth in stimulated muscles is accompanied by growth of arterioles and the size of the whole vascular bed estimated by corrosion casts is therefore enlarged (39). Transforming growth factor-β1 (TGF β1) and platelet derived growth factor (PDF) are probably involved in this growth (2).
Muscle hypertrophy
Heavy resistance training leads to muscle hypertrophy which is not accompanied by increased number of capillaries (40). In contrast, muscles hypertrophied as a result of muscle overload due to extirpation of synergistic muscles show capillary and arteriolar growth (41) due to mechanical forces acting on the capillary wall (stretch and possibly increased capillary wall tension), but not due to shear stress as blood flow in these muscles is not increased. Capillaries are tethered to muscle fibres and when these are stretched the basement membrane is disturbed and activity of metallo proteinases (mainly MMP 2) is increased with resulting capillary sprouting (42). The expression of VEGF is increased, but NO is not involved (33).
Ageing
Numerous data on capillary supply and arrangement of microvasculature do not show any substantial changes with increasing age (reviewed in 43). However, both vasodilator and vasoconstrictor responses decrease with advancing age due to endothelial dysfunction in arterioles – decreased production of NO and prostacyclin (44) Spreading vasodilatation so important in the mediation of functional hyperaemia, is impaired and consequently post contraction vasodilatation of arterioles in attenuated (45). These changes affect more vessels supplying highly oxidative muscles and their lower blood flow can explain the difficulties to maintain posture in old age which is dependent to a great deal on good function of the anti gravitational muscles. There shift of blood flow from oxidative towards glycolytic muscles is particularly evident during exercise (19).
Hypoxia
Acute systemic hypoxia leads to increased diameter of arterioles and venules which is due to the action of adenosine and mediated by NO (46). Muscles in chronically hypoxic rats had larger diameter of capillaries, higher red cell flux and higher capillary density (47) all of which together with growth of new arterioles (48) help maintain oxygen supply to muscle fibres in spite of low values of oxygen in arterial blood .Growth of new vessels is due to enhanced expression of VEGF which is activated by hypoxia-inducible factor-1alpha (HIF-1alpha) although other mechanisms may be involved (49).
MICROCIRCULATION IN PATHOLOGICAL STATES
Muscle atrophy
The most common causes of muscle atrophy are either prolonged inactivity, denervation or degenerative muscle diseases. Immobilization by plaster casts had relatively small effect on capillary supply. On the other hand, muscles exposed to prolonged inactivity in animals suspended in a position which prevented anti gravitational muscles to support body weight had lower C:F ratio (50). In addition smaller capillary diameter and their lower tortuosity result in lower capillary volume. These changes are linked with apoptosis of capillary endothelial cell (51).
Muscles undergoing atrophy after denervation or tenotomy could be expected to have higher density of capillaries due to decreased fiber size. This has indeed been described not only in denervated muscles but also in muscles where atrophy was due to tenotomy (reviewed in 23). However, blood flow referred to muscle weight was higher during the first two months after denervation or tenotomy (possibly due to dilatation of arterioles in response to metabolites released from atrophying muscle fibres) with better capillary perfusion (reviewed in 52). With long lasting denervation (2–18 months) capillaries degenerated and many disappeared, such that some parts of the affected muscles became avascular (53). One reason for capillary regression in long-term denervated muscles might be the decreased expression of mRNA for VEGF and its receptors and also decreased expression of angiopoetin (54). Degeneration and loss of capillaries in the later stages may also be due to lack of perfusion and / or lack of mechanical stretch as the muscles are not capable of contractions. Limited perfusion might be explained by degeneration of vascular smooth muscle in arterioles (55) which would render the vessels less responsive to vasodilator stimuli.
Although changes in microcirculation have been considered as an important factor in the pathogenesis of Duchenne muscular dystrophy, there no evidence for changes in capillary supply, muscle blood flow (2) or reactivity of arterioles (56). However, ultrastructural studies showed swelling of endothelial cells and thickening of the basement membrane (57) which could cause impairment of oxygen and solute diffusion.
Muscle ischaemia
Deindl & Schaper (58) reviewed compensatory changes including development of collateral circulation and remodeling of arteriolar and capillary vascular bed in chronic ischemia. Collateral vessels usually develop by enlargement of preexisting vessels due to shear stress and some growth of arterioles (59). This is possibly due to up-regulation of VEGF mRNA expression and expression of mRNA for fibroblast growth factor-1 receptors (FGFR-1) which may mediate signal transduction in proliferating smooth muscle cells and thus contribute to arteriolar growth. However, neither FGF-1 nor FGF-2-mRNA were changed in either muscles or arteries, so that their role in vascular remodeling in chronic ischemia is questionable. Diameters of arterioles and venules in muscles remote from the site of iliac artery ligation were unaffected.
Diameters of capillaries were smaller due to capillary endothelial swelling; this would increase the barrier thickness for diffusive exchange of oxygen and metabolites and contribute to the impaired muscle function. However, since capillary Vrbc was slightly higher, capillary shear stress was unchanged and this could help to maintain endothelial function and the integrity of the capillary bed. Whether chronic ischemia leads to capillary growth in the more remote muscles or not is still unresolved. In spite of numerous papers, the reports on the capillary supply are controversial. Perfusion of the capillary bed in ischemic muscles at rest is no different from normal muscles for up to 5 weeks after ligation of the iliac artery. However, total muscle blood flow, capillary Vrbc and red cell flux do not increase during contractions and arterioles do not dilate. The latter is likely due to endothelial dysfunction: arteriolar dilation to an endothelium-independent agonist is more or less preserved while responses to endothelium-dependent dilators are either absent or severely attenuated. Microcirculation in chronically ischemic muscles can be improved by exercise or by chronic electrical stimulation which increased capillary supply and furthermore restored the ability of arterioles to dilate and improved total muscle blood flow and performance (2,60).
Diabetes and obesity
Patients with type I (insulin dependent) diabetes have higher muscle blood flow at rest as well as during exercise, normal capillary density and higher capillary diffusion capacity in spite of thicker capillary basement membrane. In contrast, capillary density was lower in patients with type II (insulin independent) diabetes. Data on microcirculation in animals with streptozotocin-induced diabetes showed a decrease in C:F ratio with longer time after inducement. Capillaries branch less and are straighter and narrower with decreased proportion of continuously flowing capillaries compared to control animals. Capillary Vrbc, red cell flux and proportion of continuously flowing capillaries were all lower (reviewed in 2).The percentage of capillaries perfused with erythrocytes was lower also in an animal model of type II diabetes (61). Decreased branching was observed already in the early stages after the onset of diabetes (62). Attenuated dilalatation in response to endothelium dependent agonist (acetylcholine) indicates endothelium dysfuction (63).All these findings contribute to the explanation of muscle fatigue in diabetes.
Microvessels studied in animal models of obesity (which often precedes diabetes in humans) have smaller lumen, thinner wall and reduced distensibility (64). This, together with attenuated response to endothelium dependent agonists explain impaired perfusion in response to increased metabolic demand.
Hypertension
Numerous studies of different models of hypertension in animals ( mostly rats) showed rarefaction (decreased numbers) of arterioles and venules, but not capillaries. Together with increased arterial media / lumen ratio (due to both hyperplasia and hypertrophy of the smooth muscle cells), reduced vasodilator capacity (due to impaired endothelial NO production) and increased vasoconstriction of arterioles in response to noradrenalin and endothelin is the basis of higher peripheral resistance and leads to limited capillary perfusion (2). Treadmill training increased capillary density and normalized arteriolar wall/lumen ratio, changes which were initiated by increased level of VEGF (65) and obviously improved capillary perfusion.
Heart failure
Endothelial cells dysfunction in large and small vessels in cases of chronic heart failure (CHF) result in perfusion deficit, alterations in microcirculation and increased skeletal muscle fatigue (66). Arterioles are narrower than in controls, dilate less in response to endothelial dependent (Ach) agonist and constrict more in response to noradrenalin. Although muscle C:F ratio is not changed (67) their perfusion is impaired: the percentage of capillaries with intermittent flow is higher and capillary Vrbc and red cell flux lower (68). C:F ratio was significantly lower in patients with established CHF and their basement membrane thicker (68). All these changes impair oxygen delivery and may explain muscle fatigue and metabolic changes observed in skeletal muscles following heart failure. Endothelial dysfunction in CHF may be alleviated by interventions such as L-arginine treatment or training (69). Chronic electrical muscle stimulation prevented the narrowing of arterioles and resulted in increased C:F ratio in rats with myocardial infarction (70). Physical training in patients with CHF can reverse reduced microvascular density, although the mechanism is not known. Training does not change the expression of VEGF or endothelial NO synthase (71) may affect mechanical factors in microcirculation ( eg shear stress) due to training induced improvement of muscle blood flow.
Conclusions
Microcirculation in skeletal muscles is adapted to muscle metabolism: capillary supply is better and capillary perfusion in postural muscles under resting conditions is more homogeneous with longer transit times for RBC to ensure good supply of oxygen than in fast contracting muscles. However, during muscle contraction, velocity of flow is faster in fast muscles to ensure the clearance of metabolites. Vessels in postural muscles are less sensitive to noradrenalin and/or sympathetic stimulation. This helps to maintain their circulation even when flow is diverted from musculature to other organs under life threatening conditions. Endothelial cells in capillaries and arterioles play an important part in the regulation of microcirculation in health (they sense and conduct the initial impulse for dilatation during muscle contraction and thus enable adjustment of capillary perfusion with activation of different motor units) but also in diseased states, e.g. impairment of muscle microcirculation in heart failure. Microvascular bed (capillaries and arterioles) can grow in muscles exposed to increased activity and regress during long-lasting inactivity. Mechanical factors (such as shear stress, wall tension or stretch of vessels) as well as growth factors, mainly Vascular endothelial growth factor (VEGF) play an important part in these processes.
References
- 1.Emerson GG, Segal SS. Alignment of microvascular units along skeletal muscle fibers of hamster retractor. J Appl Physiol. 1997;82:42–48. doi: 10.1152/jappl.1997.82.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Hudlicka O, Brown MD, Egginton S. The microcirculation in skeletal muscle. In: Engel A, Franzini-Armstrong C, editors. Myology, Basic and Clinical. 3rd edition. McGraw-Hill; New York: 2004. pp. 511–533. [Google Scholar]
- 3.Clark MG, Rattigan S, Clerk LH, Vincent MA, Clark AD, Youd JM, Newman JM. Nutritive and non-nutritive blood flow: rest and exercise. Acta Physiol Scand. 2000;168:519–530. doi: 10.1046/j.1365-201x.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 4.Dawson JM, Tyler KR, Hudlicka O. A comparison of the microcirculation in rat fast glycolytic and slow oxidative muscles at rest and during contractions. Microvasc Res. 1987;33:167–182. doi: 10.1016/0026-2862(87)90015-x. [DOI] [PubMed] [Google Scholar]
- 5.Hudlicka O, Hoppeler H, Uhlmann E. Adaption of the size of the capillary bed to the oxidative capacity in skeletal muscle. Pflugers Arch. 1987;410:369–375. doi: 10.1007/BF00586513. [DOI] [PubMed] [Google Scholar]
- 6.Marshal JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle in the rat. J Physiol. 1982;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–34. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 8.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci. 1999;96:313–326. [PubMed] [Google Scholar]
- 9.Joyner MJ, Proctor DN. Muscle blood flow during exercise: the limits of reductionism. Med Sci Sports Exerc. 1999;31:1036–1040. doi: 10.1097/00005768-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581:841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd LR, Johnson PC. Diameter changes in arteriolar networks of contracting skeletal muscle. Am J Physiol. 1991;260:H662–H670. doi: 10.1152/ajpheart.1991.260.3.H662. [DOI] [PubMed] [Google Scholar]
- 13.Segal SS. Communication among endothelial and smooth muscle cells coordinates blood flow control during exercise. NIPS. 1992;7:156–156. [Google Scholar]
- 14.Monbouli JV, Vanhoutte PM. Endothelial dysfunction:from physiology to therapy. J Mol Cell Cardiol. 1999;31:61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- 15.Potocnik SJ, McSherry I, Ding H, et al. Endothelium-dependent vasodilation in myogenically active mouse skeletal muscle arterioles: role of EDH an K(+) channels. Microcirculation. 2009;16:377–390. doi: 10.1080/10739680902804042. [DOI] [PubMed] [Google Scholar]
- 16.Bakker EN, van der Linden PJ, Sipkema P. Endothelin-1 induced constriction inhibits nitric-oxide mediated dilation in isolated rat resistance arteries. J Vasc Res. 1997;34:418–424. doi: 10.1159/000159252. [DOI] [PubMed] [Google Scholar]
- 17.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 18.Moore AW, Bearden SE, Segal SS. Regional activation of rapid onset vasodilatation in mouse skeletal muscle: regulation through adrenoreceptors. J Physiol. 2010;588:3321–3331. doi: 10.1113/jphysiol.2010.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole DC, Ferreira LF. Oxygen exchange in muscle of young and old rats: muscle-vascular-pulmonary coupling. Exp Physiol. 2007;92:341–346. doi: 10.1113/expphysiol.2006.036764. [DOI] [PubMed] [Google Scholar]
- 20.Murrant CL, Sarelius IH. Coupling of metabolism and muscle blood flow in capillary units during contraction. Acta Physiol Scand. 2000;168:531–534. doi: 10.1046/j.1365-201x.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 21.Hudlicka O, Brown MD, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 22.Laughlin MH, Armstrong RB. Muscle blood flow during locomotory exercise. Exerc Sport Sci Rev. 1985;13:95–136. [PubMed] [Google Scholar]
- 23.Hudlicka O. The response of muscle to enhanced and reduced activity. Bailliere's Clinical Endocrinology and Metabolism. 1990;4:417–439. doi: 10.1016/s0950-351x(05)80063-1. [DOI] [PubMed] [Google Scholar]
- 24.Gute D, Laughlin MH, Amann JF. Regional changes in capillary supply of interval-sprint and low-intensity endurance trained rats. Microcirculation. 1994;1:183–193. doi: 10.3109/10739689409148273. [DOI] [PubMed] [Google Scholar]
- 25.Lash JM, Bohlen HG. Functional adaptations of rat skeletal muscle arterioles to aerobic exercise training. J Appl Physiol. 1992;72:2052–2062. doi: 10.1152/jappl.1992.72.6.2052. [DOI] [PubMed] [Google Scholar]
- 26.Binder LW, Murfee WL, Song J, Laughlin MH, Price RJ. Computational network model prediction of hemodynamic alterations due to arteriolar remodelling in interval spring trained skeletal muscle. Microcirculation. 2007;14:181–192. doi: 10.1080/10739680601139294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lash JM. Contribution of arterial feed vessels to skeletal muscle functional hyperemia. J Appl Physiol. 1994;76:1512–1519. doi: 10.1152/jappl.1994.76.4.1512. [DOI] [PubMed] [Google Scholar]
- 28.Ljungqvist A, Unge G. Capillary proliferation activity in myocardium and skeletal muscle of exercised rats. J Appl Physiol. 1997;43:306–307. doi: 10.1152/jappl.1977.43.2.306. [DOI] [PubMed] [Google Scholar]
- 29.Appell H-J. Morphological studies on skeletal muscle capillaries under conditions of high altitude training. Int J Sports Med. 1980;1:37–41. [Google Scholar]
- 30.Waters RE, Rotevain S, Annex BH, Yan Z. Voluntary running induces fibre-type specific angiogenesis in mouse skeletal muscle. Am J Physiol. 2004;287:C 1342–1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 31.Brown MD, Cotter MA, Hudlicka O, Vrbova G. The effect of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflügers Archiv. 1976;361:241–250. doi: 10.1007/BF00587288. [DOI] [PubMed] [Google Scholar]
- 32.Hansen-Smith FM, Hudlicka O, Egginton S. In vivo angiogenesis in adult rat skeletal muscle: early changes in capillary network architecture and ultrastructure. Cell Tissue Res. 286:123–136. doi: 10.1007/s004410050681. 196. [DOI] [PubMed] [Google Scholar]
- 33.Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle and their modification by muscle pathology. Frontiers in Bioscience. 2001;6:75–89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- 34.Egginton S. Invited review:activity induced angiogenesis. Pflugers Arch. 2009;457:963–977. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 35.Olfert IM, Birot O. Importance of antiangiogenic factors in the regulation of skeletal muscle angiogenesis. Microcirculation. 2011;18:316–330. doi: 10.1111/j.1549-8719.2011.00092.x. [DOI] [PubMed] [Google Scholar]
- 36.Copp SW, Hirai DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide in the modulating microvascular and contractile function in rat skeletal muscle. Microcirculation. 2011 doi: 10.1111/j.1549-8719.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- 37.Hudlicka O, Brown MD, May S, Zakrzewicz A, Pries AR. Changes in capillary shear stress in skeletal muscle exposed to long-term activity:role of nitric oxide. Microcirculation. 2006;13:249–259. doi: 10.1080/10739680600556951. [DOI] [PubMed] [Google Scholar]
- 38.Egginton S, Zhou A-L, Brown MD, Hudlicka O. Unorthodox angiogenesis. Cardiovasc Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 39.Hansen-Smith FM, Egginton S, Hudlicka O. Growth of arterioles in chronically stimulated adult skeletal muscle. Microcirculation. 1998;5:49–59. [PubMed] [Google Scholar]
- 40.Dawson JM, Hudlicka O. The effect of long-term activity on the microvasculature of rat glycolytic skeletal muscle. Int J Microcirc Clin Exp. 1989;8:53–69. [PubMed] [Google Scholar]
- 41.Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc. 1988;20:S132–134. doi: 10.1249/00005768-198810001-00008. [DOI] [PubMed] [Google Scholar]
- 42.Hansen-Smith F, Egginton S, Zhou A-L, Hudlicka O. Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. Microvascular Res. 2001;62:1–14. doi: 10.1006/mvre.2001.2308. [DOI] [PubMed] [Google Scholar]
- 43.Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6:1–14. doi: 10.1023/a:1025809808697. [DOI] [PubMed] [Google Scholar]
- 44.Poole DC, Behnke BJ, Musch TI. Capillary hemodynamics and oxygen pressures in the ageing microcirculation. Microcirculation. 2009;13:289–299. doi: 10.1080/10739680600618793. [DOI] [PubMed] [Google Scholar]
- 45.Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirculation. 2006;13:301–314. doi: 10.1080/10739680600619023. [DOI] [PubMed] [Google Scholar]
- 46.Bearden SE, PAyne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall JM. Role of adenosine in skeletal muscle during systemic hypoxia. Clin Exp Pharmacol Physiol. 2002;29:843–849. doi: 10.1046/j.1440-1681.2002.03734.x. [DOI] [PubMed] [Google Scholar]
- 48.Fisher AJ, Schrader NW, Klitzman B. Effect of chronic hypoxia on capillary flow and hematocrit in rat skeletal muscle. Am J Physiol. 1992;262:H1877–1883. doi: 10.1152/ajpheart.1992.262.6.H1877. [DOI] [PubMed] [Google Scholar]
- 49.Price RJ, Skalak TC. Arteriolar remodeling in skeletal muscles of rats exposed to chronic hypoxia. J Vasc Res. 1998;35:238–244. doi: 10.1159/000025589. [DOI] [PubMed] [Google Scholar]
- 50.Breen E, TAng K, Olfert MI, Knapp A, Wagner P. Skeletal muscle capillarity during hypoxia:VEGF and its activation. High Alt Med Biol, 2008;9:158–166. doi: 10.1089/ham.2008.1010. [DOI] [PubMed] [Google Scholar]
- 51.Desplanches D, Kayar SR, Sempore B, Flandrois R, Hoppeler H. Rat soleus muscle ultrastructure after limb suspension. J. Appl. Physiol. 1990;69:504–508. doi: 10.1152/jappl.1990.69.2.504. [DOI] [PubMed] [Google Scholar]
- 52.Fujino H, Kohzuki H, Takeda I, et al. Regression of capillary network in atrophied muscle induced by hindlimb unweighting. J.Appl.Pysiol. 2005;98:1407–1413. doi: 10.1152/japplphysiol.00961.2004. [DOI] [PubMed] [Google Scholar]
- 53.Hudlicka O. Do changes in the vascular bed contribute to the development of denervation atrophy in skelètal muscle? Basic Appl Myol. 2007;17:123–124. [Google Scholar]
- 54.Borisov AB, Huang SK, Carlson BM. Remodeling of the vascular bed and progressive loss of capillaries in denervated skeletal muscle. Anat Rec. 2000;258:292–304. doi: 10.1002/(SICI)1097-0185(20000301)258:3<292::AID-AR9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 55.Wagatsuma A, Tamaki H, Ogita F. Capillary supply and gene expression of angiogenesis-related factors in murine skeletal muscle following denervation. Exp Physiol. 2005;90:403–409. doi: 10.1113/expphysiol.2004.029769. [DOI] [PubMed] [Google Scholar]
- 56.Dedkov EL, Kostomirova TY, Borisov AB, Carlson BM. Resistance vessel remodelling and reparative angiogenesis in the microcirculatory bed of long-term denervated skeletal muscles. Microvasc Res. 2002;63:96–114. doi: 10.1006/mvre.2001.2372. [DOI] [PubMed] [Google Scholar]
- 57.Bagher P, Duan D, Segal SS. Evidence for impaired neuromuscular transmission in a murine model of Duchenne muscular dystrophy. J Appl Physiol. 2011;110:601–609. doi: 10.1152/japplphysiol.01106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miike T, Sugino S, Ohtani Y, Taku K, Yoshioka K. Vascular endothelial cell injury and platelet embolism in Duchenne muscular dystrophy at the preclinical stage. J Neurol Sci. 1987;82:67–80. doi: 10.1016/0022-510x(87)90007-4. [DOI] [PubMed] [Google Scholar]
- 59.Deindl E, Schaper W. Collateral and capillary formations – a comparison. In: Dormandy JA, Dole WP, Rubanyi GM, editors. The therapeutic angiogenesis. Springer; 1999. pp. 67–86. [Google Scholar]
- 60.Bailey AM, O'Neill TJ, 4th, Morris CE, Peirce SM. Arteriolar remodelling following ischemic injury extends from capillary to large arteriole in the microcirculation. Microcirculation. 2008;15:389–404. doi: 10.1080/10739680701708436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hudlicka O, Brown MD. Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, NO and VEGF. J Vasc Res. 2009;46:504–512. doi: 10.1159/000226127. [DOI] [PubMed] [Google Scholar]
- 62.Padilla DJ, McDonough P, Behnke BJ, et al. Effects of type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol. 2006;291:H2439–2444. doi: 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- 63.Benedict KF, Coffin GS, Barrett EJ, Skalak TC. Hemodynamic system analysis of capillary network remodelling during the progression of type 2 diabetes. Microcirculation. 2011;18:63–73. doi: 10.1111/j.1549-8719.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 64.Alsip NL, Schuschke DA, Miller FN. Microvascular responses in the skeletal muscle of the diabetic rat. J Lab Clin Med. 1996;128:429–437. doi: 10.1016/s0022-2143(96)80016-3. [DOI] [PubMed] [Google Scholar]
- 65.Stepp DW, Pollock DM, Frisbee JC. Low-flow vascular remodelling in the metabolic syndrome X. Am J Physiol. 2004;286:H964–970. doi: 10.1152/ajpheart.00836.2003. [DOI] [PubMed] [Google Scholar]
- 66.Amaral SL, Sanchez LS, Chang AJ, Rossoni LV, Michelini LC. The course of training induced microvasculatory changes and on VEGF expression in skeletal muscles of spontaneously hypertensive female rats. Braz Med Biol Res. 2008;41:424–431. doi: 10.1590/s0100-879x2008000500012. [DOI] [PubMed] [Google Scholar]
- 67.Drexler H, Coats AJS. Explaining fatigue in congestive heart failure. Annu Re. Med. 1996;47:241–256. doi: 10.1146/annurev.med.47.1.241. [DOI] [PubMed] [Google Scholar]
- 68.Thomas DP, Hudlicka O, Brown MD, Deveci D. Alterations in small arterioles precede structural and functional changes in limb skeletal muscle following myocardial infarction. Am J Physio. 1998;275:H1032–1039. doi: 10.1152/ajpheart.1998.275.3.H1032. [DOI] [PubMed] [Google Scholar]
- 69.Kindig CA, Musch TI, Basaraba RJ, Poole DC. Impaired capillary hemodynamics in skeletal muscle of rats in chronic heart failure. J Appl Physiol. 1999;87:652–660. doi: 10.1152/jappl.1999.87.2.652. [DOI] [PubMed] [Google Scholar]
- 70.Hambrecht R, Hilbrich L, Erbs S, et al. Correlation of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral L-arginine supplementation. J Am Coll Cardiol. 2000;35:706–713. doi: 10.1016/s0735-1097(99)00602-6. 2000. [DOI] [PubMed] [Google Scholar]
- 71.Thomas DP, Hudlicka O. Arteriolar reactivity and capillarization in chronically stimulated rat limb skeletal muscle post-MI. J Appl Physiol. 1999;87:2259–2265. doi: 10.1152/jappl.1999.87.6.2259. [DOI] [PubMed] [Google Scholar]
- 72.Testa M, Ennezat PV, Vikstrom KL, et al. Modulation of vascular endothelial gene expression by physical training in patients with chronic heart failure. Italian Heart J. 2000;1:426–430. [PubMed] [Google Scholar]