Summary
The primary aim of this prospective cohort study was to compare the incidence of Achilles tendinopathy symptoms in elite soccer players with and without baseline asymptomatic ultrasound abnormalities. This study also investigated the relationship between baseline tendon thickness and development of symptoms. Using ultrasonography, 18 players were examined in 2009 for the existence of hypoechoicity, paratenon blurring, focal thickening and/or neovascularisation, and anteroposterior tendon thickness was measured. Symptom development during the follow-up period was assessed by interview one year later. Baseline mid-tendon thickness was greater (p=0.041) in tendons that experienced symptoms [median (IQR): 0.53 (0.51–0.55) cm] in the following year than tendons remaining asymptomatic [0.48 (0.45–0.52) cm]. No association between the existence of baseline ultrasound signs and development of symptoms in the following year was observed (Chi-Square: 1.180, p=0.277). A thicker baseline mid-tendon thickness was identified as a risk indicator for the development of Achilles tendinopathy in elite soccer players.
Keywords: tendinopathy, sonography, ultrasound, football, Achilles
Introduction
Achilles tendinopathy (AT) is a common overuse injury amongst athletes, with an increasing incidence over the past 30 years (1,2). It is typified by pain that usually occurs at both the onset and cessation of activity (3), swelling around the tendon, morning stiffness, and performance impairment (4). It is often chronic, with one study reporting that only 37% of athletes felt their AT symptoms had improved after 2 years (6). In severe cases, AT can end a sporting career (2,6). The condition is therefore especially problematic to elite professional athletes.
Achilles tendinopathy is particularly prevalent in athletes who perform running and jumping (7), and is thus common in sports such as soccer. In the four principal soccer leagues in England, there are an average of 3.5 Achilles tendon-related injuries per week in preseason, and an average of one injury per week in the competitive season (8). There is a need to ensure that chronic and potentially career-ending AT injuries are detected at an early stage, because AT responds better to treatment when pathology is less severe (1,9). The ultrasound (US) signs of hypoechoic areas, spindle-shaped thickening, neovascularisation and paratenon blurring (10,11) have been shown to be associated with AT (12,13) and therefore may be potential predictors of future tendinopathy (6,7) when existing in asymptomatic individuals. Further, the time lost in sporting participation is especially important to address in professional athletes such as the sample in this study.
Fredberg and Bolvig (6) demonstrated that a sample of asymptomatic Danish soccer players with US-detected abnormalities had a 45% risk of developing AT over the following year compared to a 1% risk in asymptomatic players with no baseline US signs. A later and larger study (7), looking at a similar sample, demonstrated an almost 3-fold increase in the risk of developing symptoms in players with ultrasonographic abnormalities compared to those without. However, the only US abnormalities that were measured in these two studies were spindle-shaped thickening and hypoechoicity, and they may have therefore possibly underestimated any effect. Furthermore these Danish results (6,7) may not be applicable to players at the highest level of the sport, such as those in the current cohort, as playing and training conditions may be different.
In addition to detecting structural abnormalities, US can also be used to measure the dimensions of the tendon, which may also be potential predictors of future symptoms. Cross-sectional studies have shown a relationship between antero-posterior Achilles tendon thickening and symptoms (14), but it is not possible to determine from these studies whether tendon thickening predates symptoms. Although two longitudinal studies (6,7) have shown the existence of baseline spindle shaped thickening leads to an increased likelihood of future symptoms, no studies have analysed the mathematical relationship between baseline tendon thickness measurements and subsequent symptom development.
The primary aim of this study was to compare the risk of developing AT symptoms in elite professional soccer players with and without baseline asymptomatic sonographic signs. The secondary aim was to determine the relationship between baseline tendon thickness and the development of symptoms. These aims were chosen in order to guide sports and exercise medicine professionals working with footballers at the highest level.
Materials and Methods
Subjects
Twenty five male elite soccer players were recruited from an English Premier League first team squad in April 2009 via the club’s medical staff. The exclusion criteria were any age below eighteen, and Achilles tendinopathy symptoms on the day of the baseline test. The sample size was calculated with an online calculator (http://www.dssresearch.com/toolkit/sscalc/size_p2.as) using data from a previous study (6), an alpha of 0.05 and a power of 80%. This gave a sample size of 10 subjects required for each follow-up group (symptoms developed during the follow up year versus no symptoms developed). Ethical approval was granted by the Queen Mary Research and Ethics Committee, and informed consent was obtained from each participant.
Baseline procedures
Baseline measures were performed in late April 2009. All subjects were asked a question about present AT symptoms (“Do you have pain, tenderness or stiffness of your Achilles tendons today?”). Only players who were symptom free were allowed to continue participation in the study, which happened to be all 25 players recruited. Height and weight were measured and recorded, and age data were collected.
Ultrasound
Players were then examined sonographically prior to a training session. The US scanner used was the high-resolution 3–12 MHz linear array transducer (Voluson-I, GE Medical Systems, UK). All ultrasound scans were performed by a single operator (Sports and Exercise Medicine trainee) who had been adequately trained by an experienced radiologist in scanning Achilles tendons (15).
The procedure was performed with the subject lying prone on the examination bed, with their ankles in a relaxed position (approximately plantargrade). All subjects were imaged bilaterally, right leg first, in the sagittal plane. Probe pressure was minimised to prevent neovessel obliteration, and the probe was held perpendicular to the imaged tissues to minimise anisotropy (16). Three grey-scale US pictures were taken, which were of the calcaneal insertion (defined on US as the clearest image of the pre-Achilles bursa); the musculotendinous junction [the area found on US where the last soleus fibres attach to the tendon (17)]; and the midpoint of the two. The antero-posterior tendon thickness at these 3 points was measured offline using Image J software. The thickness at these three points was measured, rather than at the greatest width, because many of the tendons were parallel (i.e they did not have a thickest point) and it was desired to measure at consistent points along the tendon.
The tendon was rescanned for areas of hypoechoicity, spindle-shaped thickening and paratenon blurring. Fredberg and Bolvig’s (6) criteria of spindle-shaped thickening, defined as a thickness more than 0.1 cm in relation to the normal distal part of the tendon, and hypoechoic regions with a diameter of more than 0.1cm, were applied. Paratenon blurring was defined as an indistinct anterior or posterior tendon border (18). The tendon was also assessed using power Doppler for neovascularization at a gain setting established by reducing the gain until no random noise was produced deep to the cortical bone of the calcaneal tuberosity (19). Hypoechoicity, spindle-shaped thickening, paratenon blurring and neovascularisation were graded on either their presence or absence in a tendon. The presence of any one of these four US signs classified a tendon as ultrasonographically abnormal.
Follow up procedures
Follow-up occurred in May 2010, approximately 12 months after the baseline measures. Seven subjects were unable to attend follow up, due to having been transferred or on loan to another club, or away from training due to non-Achilles injury. Results therefore pertain to the eighteen subjects attending both baseline and follow-up. The outcome variable was the presence of any AT symptoms during the one year follow up period, assessed with the question: “Have you had any pain, tenderness or stiffness in either Achilles tendon at any time in the past 12 months?” This question was asked in an interview, and the player was encouraged to think back to any incidences, of any duration, where they had felt pain in the AT. This was facilitated by pointing to the players’ own Achilles tendon. The examiner conducting the interview had not carried out the baseline US examinations and was unaware of US scan results.
Data Analysis
The software SPSS version 18.0 (SPSS Inc, Chicago, USA) was used for analysis. Both tendons of each subject were treated as individual tendons in the sample, as is the case in previous studies (6,7, 20). Continuous data in each of the two follow-up groups (symptomatic during follow up versus asymptomatic during follow up) were plotted and inspected for normality. All anthropometric and thickness measurement data were non-parametrically distributed in the symptomatic during follow up group so the non-parametric Mann-Whitney U test was used to compare the two follow up groups for the potential confounders of age, height and weight, and for the baseline thickness variables. Pearson’s Chi-square tests were used to assess the strength of relationship between the existence of abnormal US signs in tendons at baseline and the development of symptoms in tendons at follow up. In all cases, p<0.05 was used to indicate significance.
RESULTS
Subjects
The 18 male professional soccer players who had not been lost to follow up led to a sample of 36 tendons at one year follow-up. All tendons had been asymptomatic on the day of testing at baseline. The potential confounders of age, height and weight were not significantly different between (p>0.05) tendons that were symptomatic during follow up and those that were not symptomatic (Tab. I).
Table 1.
Median (IQR) age, height and weight for tendons that were symptomatic and asymptomatic during the 12 month follow up period.
| Symptomatic during follow up (n=6) | Asymptomatic during follow-up (n=30) | p | |
|---|---|---|---|
| Age (years) | 24.0 (22.0–27.5) | 23.0 (22.0 – 26.0) | 0.307 |
| Height (m) | 1.78 (1.75–1.93) | 1.80 (1.78–1.86) | 0.885 |
| Weight (kg) | 73.0 (65.0 – 84.4) | 75.0 (72.8–78.0) | 0.442 |
Baseline US signs and development of symptoms
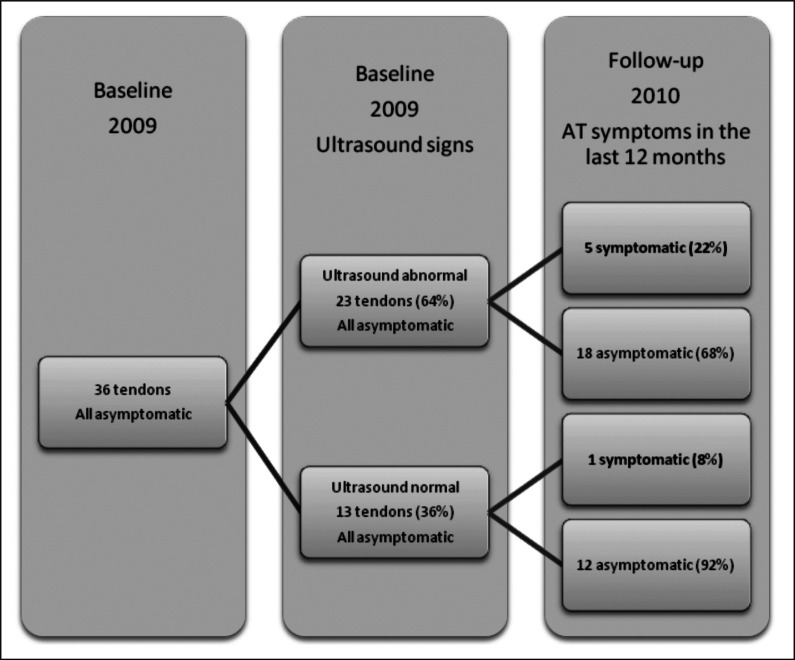
From the twenty-three ultrasonographically abnormal but asymptomatic tendons at baseline, five (22%) tendons subsequently developed AT symptoms during follow up compared to one tendon (8%) from the thirteen ultrasonographically normal asymptomatic tendons (Fig. 1). This difference in risk was non-significant [Pearson’s χ2 (1) = 1.180, p=0.277]. Furthermore, there was no significantly increased risk identified between any individual baseline US sign considered alone, and the development of AT symptoms in the subsequent twelve months [Neovascularisation: χ2 (1) = 1.44, p=0.23; Hypoechoicity: χ2 (1) = 0.22, p=0.88; Paratenon blurring: χ2 (1) = 0.51, p=0.47; Focal thickening: χ2 (1) = 0.66, p=0.42)].
Figure 1.
Baseline US findings in Achilles tendons, and symptom status at 12 months follow up.
Baseline thickness measurements and development of symptoms
Tendons that became symptomatic during the 12 month follow up had significantly thicker (p=0.041) mid-tendon thickness at baseline [median (IQR): 0.53 (0.51–0.55) cm] than tendons remaining asymptomatic [0.48 (0.45–0.52) cm]. Tendons symptomatic during follow-up showed a strong trend (p=0.053) of being thicker at the calcaneal insertion at baseline [0.50 (0.45–0.55) cm] than tendons asymptomatic during follow up [0.46 (0.43–0.48) cm]. No baseline difference between the two follow up groups was observed for baseline musculotendinous junction thickness (p=0.686) (Fig. 2).
Figure 2.
A box plot of baseline thickness (cm) in tendons which were symptomatic in the 12 month follow up period and in tendons which remained asymptomatic. Boxplots represent the median, interquartile range and full range (excluding outliers). Calc = calcaneal, MTJ=Musculotendinous junction. * = significantly greater in the symptomatic group (p=0.041).
Discussion
Statement of principal findings
A thicker mid-tendon at baseline, in the absence of symptoms, was significantly related to an increased likelihood of AT symptoms developing in elite professional soccer player tendons one year later. In contrast, there was no association between asymptomatic baseline ultrasonographic abnormalities (neovascularization, hypoechogenicity, paratenon blurring and focal thickening) and the development of subsequent AT symptoms.
Strengths and weaknesses of the study
This study is the first to use predominantly world class soccer players, and is thus the only study providing information relevant to players at the very highest levels of soccer. This is also the first study to analyse the relationship between Achilles tendon thickness measurements and the subsequent development of AT symptoms in soccer players. Since the potential confounders of height, age and weight were similar between those with and without subsequent symptom development, the differences in baseline thickness may represent a real risk factor for subsequent symptoms.
A longitudinal cohort design was used, which is optimum for a study attempting to assess causal relationships involving baseline variables that require objective measurement. In addition, the assessor collecting data on injuries during the follow up period was blinded to the baseline status of the participants, thus avoiding detection bias. One potential weakness was the possible under-powering of the part of the study dealing with the relationship between US abnormalities and symptom development. Post-hoc power calculation (http://www.openepi.com/OE2.3/Menu/OpenEpiMenu.htm) showed that our power was only 18%, which concurs with our failure to recruit enough subjects at baseline to reach our sample size analysis target. Another drawback was that any player recall inaccuracy could reduce the validity of the data on AT symptoms during the follow-up period. However, conducting the questionnaire as an interview allowed identification of the exact site of pain and therefore may have reduced any such inaccuracy. There was also the possibility of players under-reporting AT because of fear regarding team selection, but all players were clearly told about the confidentiality of results, and that club medical personnel would not be given any study information. Another limitation was that the timing of the US scan was not standardised, so some players might have just finished training when the scans were performed. This could have potentially affected the measure of neovascularisation, which has shown to be increased by activity (21). Finally, although all players were asymptomatic at baseline, this did not preclude the fact that some had suffered symptoms previously. It is therefore possible that some of the ultrasound signs or thickness measurements were influenced by past pathology, rather than these measures relating only to future symptoms.
Relation to other studies
Fredberg and Bolvig (6) did not analyse the relationship between continuous thickness measures and later symptoms. They observed a relationship between symptom development and the existence or not of spindle-shaped thickening, but, unlike our study, this did not permit any calculation of thickness thresholds associated with later development of symptoms. In the later study, Fredberg et al. (7) also did not analyse the relationship between continuous measures of thickness and subsequent symptom development.
In contrast to our study, Fredberg and Bolvig (6) showed that sonographically abnormal Achilles tendons at baseline led to a significantly increased risk of developing symptoms in Danish elite players. They noted that abnormal sonographic findings at baseline conferred a relative risk of 38.6 (95% CIs: 5.0, 301.1) for developing symptoms at follow up compared to the group with normal baseline sonographic findings. This is a much larger effect than our non-significant relative risk of 2.8 (95% CIs: 0.4, 21.7). Although our lack of statistical significance may relate to our smaller sample sizes, the difference in effect size is difficult to explain, given the same follow up duration.
Although the players in the Danish study (6) were likely to be at a lower level of excellence than our cohort, this difference could not explain the large difference in effect size. Another possible reason for the observed difference could be the time of season at which baseline measures were made. In our study, baseline measures were performed at the close of the season, whereas in the Danish study (6) they were performed pre-season. It is possible that end of season sonographic findings may not be as prognostic as those taken when players are relatively rested. Sonographic findings have been noted to occur as a non-pathological response to acute activity (21), and it is possible that a similar effect could occur in response to chronic fatigue. One other methodological difference was that Freberg and Bolvig (6) used only one measure of Achilles tendon ultrasonographic abnormality (the existence of spindle shaped thickening) in contrast to our four separate criteria. However this difference would probably have led to Fredberg and Bolvig (6) detecting less of an effect, if all four criteria are valid measures of tendon pathology. On the other hand, it is possible that if localised thickening is a more valid measure of tendon abnormality than the other criteria then this might explain their more pronounced findings; however when we analysed tendon abnormality specifically in terms of spindle-shaped thickening we did not observe a significant or strong effect size.
Interestingly, Fredberg and colleagues (7) observed a very similar relative risk to ours, at 2.6 (95% CI: 1.3, 5.1), although their finding was statistically significant. That analysis included hypoechoicity as a criterion of baseline Achilles tendon abnormality, in addition to the existence of spindle shaped thickening. However, as previously argued, this is unlikely to explain the difference to their results in the earlier study (6). Fredberg and colleagues (7) included data on participants exposed to a prophylactic intervention of eccentric exercise, but data from those participants are not included in the relative risk calculation given above, calculated by the author of the present paper from Fredberg and colleagues (7) data on control participants.
One relative weakness of the two Danish studies (6,7) was their failure to blind the assessor collecting data on the development of symptoms; however, potential selection bias was limited by the ongoing recording of injuries to confirm questionnaire findings. A relative strength of the Danish studies (6,7) compared to ours was their greater sample sizes, permitting greater power to detect significant differences.
Meaning of the study: possible mechanisms and implications for clinicians
The baseline Achilles tendon thickness associated with subsequent symptoms in the current study [median (IQR): 0.53 (0.51–0.55) cm] was much lower than that reported in previous studies of symptomatic patients scanned in a radiology departments [>0.59 cm] (14,22). This difference may reflect the early stage of (asymptomatic) pathology at which the tendons in our study existed at baseline. Recent studies have identified reactive followed by degenerative stages of tendon pathology (23,24). Reactive tendinopathy is characterized by less tendon anteroposterior thickening (25), is more common among young active athletes, and is less likely to contain Doppler signal (23,24). Given that only 2 of the 5 sonographically abnormal tendons that later became symptomatic contained Doppler signal, and that they were less thick than previous reports of symptomatic tendon, we may have identified subtle reactive tendon pathology at baseline. As Achilles tendons often swell in the mid-portion, this section of the tendon may be more sensitive to this initial and subtle pathology, explaining why only this site showed significant differences between the follow up groups. These findings need to be confirmed in larger studies but may have important implications for the detection of early tendon pathology and prevention of pain.
On a clincal level, these findings indicate that US screening could be appropriate. Once a screening programme is in place, then preventative measures could be taken for those players showing mid-tendon thickness over an appropriate threshold. Our data might suggest that a sonographically-measured mid tendon thickness of over 0.5 cm would indicate an increased risk of future symptoms. Consideration of the percentiles of our data in each group suggests that this mid-tendon thickness threshold of 0.5 cm would have a sensitivity of 90% and an approximate specificity of 60% for detecting future symptom development. Clinicians should consider paying extra attention to the emergence of such signs, and institute training and treatment modifications to reduce reactive tendinopathy at this stage.
Unanswered questions and future research
Our study probably has insufficient numbers to allow accurate or reliable thresholds of tendon thickness to be calculated. Larger scale studies with more normally distributed data are necessary before such calculations can derive clinically useful results. Further work therefore needs to gather data from more than one soccer club to ensure sufficient numbers of participants. Finally, variables such as height and levels of training load may influence tendon size as well (15), and further work will need to take this into consideration when calculating such thresholds.
To avoid problems with recall of injury during the follow-up period, future studies should also provide players with an injury diary during the follow-up period, where any twinges or pains, however mild, can be recorded as they happen. Finally, to avoid prior activity confounding ultrasonographic findings, future studies should conduct all scans before training begins.
Acknowledgments
We would like to convey particular gratitude to the players who agreed to participate in this study with so much enthusiasm and goodwill. David Wales, Ian Beasley, Zohra Shaikh and Arun Jangra are also acknowledged for their invaluable assistance.
References
- 1.Jarvinen TA, Kannus P, Maffulli N, Khan KM. Achilles Tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10:255–266. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Longo UG, Rittweger J, Garau G. No Influence of Age, Gender, Weight, Height, and Impact Profile in Achilles Tendinopathy in Masters Track and Field Athletes. Am J Sports Med. 2009;37:1400–1405. doi: 10.1177/0363546509332250. [DOI] [PubMed] [Google Scholar]
- 3.Longo UG, Ronga M, Maffuli N. Achilles Tendinopathy. Sports Med Arthrosc. 2009;17:112–126. doi: 10.1097/JSA.0b013e3181a3d625. [DOI] [PubMed] [Google Scholar]
- 4.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 5.Almekinders LC, Almekinders SV. Outcome in the treatment of chronic overuse sports injuries: A retrospective study. J Orthop Sports Phys Ther. 1994;19:157–161. doi: 10.2519/jospt.1994.19.3.157. [DOI] [PubMed] [Google Scholar]
- 6.Fredberg U, Bolvig L. Significance of ultrasonographically detected asymptomatic tendinosis in the patellar and Achilles tendons of elite soccer players-A longitudinal study. Am J Sports Med.. 2002;30:488–491. doi: 10.1177/03635465020300040701. [DOI] [PubMed] [Google Scholar]
- 7.Fredberg U, Bolvig L, Andersen NT. Prophylactic Training in Asymptomatic Soccer Players with Ultrasonographic Abnormalities in Achilles and Patella Tendons: the Danish Super League Study. Am J Sports Med. 2008;36:451–460. doi: 10.1177/0363546507310073. [DOI] [PubMed] [Google Scholar]
- 8.Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme:an audit of injuries in professional football—analysis of preseason injuries. Br J Sports Med. 2002;36:436–441. doi: 10.1136/bjsm.36.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rompe JD, Furia JP, Maffulli N. Mid-portion Achilles tendinopathy—current options for treatment. Disabil Rehabil. 2008;30:1666–1676. doi: 10.1080/09638280701785825. [DOI] [PubMed] [Google Scholar]
- 10.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001;9:233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 11.Alfredson H. The chronic painful Achilles and patellar tendon: research on basic biology and treatment. Scan J Med Sci Sports. 2005;15:252–259. doi: 10.1111/j.1600-0838.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 12.Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18:565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- 13.Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33:1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Richards PJ, Win T, Jones PW. The distribution of microvascular response in Achilles tendonopathy assessed by colour and power Doppler. Skeletal Radiol. 2005;34:336–342. doi: 10.1007/s00256-004-0834-2. [DOI] [PubMed] [Google Scholar]
- 15.Filippucci E, Unlu Z, Farina A, Grassi W. Sonographic training in rheumatology: a self teaching approach. Ann Rheum Dis. 2003;62:565–567. doi: 10.1136/ard.62.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNally EG. Practical Musculoskeletal Ultrasound. Philadelphia: Elsevier; 2005. Ultrasound of the foot and ankle. [Google Scholar]
- 17.Cove R, Weller D, Westwood M. The Achilles Musculotendinous Junction: A Survey of Orthopaedic Surgeons. The Foot and Ankle Online Journal. 2009;2:1–4. [Google Scholar]
- 18.Hughes TH. In: Imaging of Tendon Ailments. Chapter 7 in Tendon Injuries – Basic Science and Clinical Medicine. Maffulli N, Renstrom, Leadbetter WB, editors. Springer-Verlag; London: 2005. [Google Scholar]
- 19.Zanetti M, Metzdorf A, Kundert AP, et al. Achilles tendons: clinical relevance of neovascularisation diagnosed with power Doppler US. Radiology. 2003;227:556–560. doi: 10.1148/radiol.2272012069. [DOI] [PubMed] [Google Scholar]
- 20.Emerson C, Morrissey D, Perry M, Jalan R. Ultrasonographically detected changes in Achilles ten-dons and self reported symptoms in elite gymnasts compared with controls – An observational study. Man Ther. 2010;15:37–42. doi: 10.1016/j.math.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Cook JL, Khan KM. Etiology of tendinopathy. In: Woo SLY, Renström P, Arnoczky SP, editors. Tendinopathy in Athletes, Encyclopedia of Sports Medicine. Oxford, United Kingdom: Blackwell Publishing; 2007. pp. 10–28. [Google Scholar]
- 22.Astrom M, Gentz C, Nilsson P, Rausing A, Sjoberg S. Imaging in chronic achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skeletal Radiol. 1996;25:615. doi: 10.1007/s002560050146. [DOI] [PubMed] [Google Scholar]
- 23.Malliaras P, Richards PJ, Garau G, Maffulli N. Achilles Tendon Doppler Flow May Be Associated With Mechanical Loading Among Active Athletes. Am J Sports Med.. 2008;36:2210–2215. doi: 10.1177/0363546508319052. [DOI] [PubMed] [Google Scholar]
- 24.Malliaras P, Richards P, Garau G, Maffulli N, Cook J. Temporal sequence of gray-scale ultrasound changes and their association with neovascularity and pain in the patellar tendon. Br J Sports Med. 2010;44:944–947. doi: 10.1136/bjsm.2008.054916. [DOI] [PubMed] [Google Scholar]
- 25.Malliaras P, Richards PJ, Garau G, Maffulli N. Achilles tendon shape and echogenicity on ultrasound among active badminton players. Scand J Med Sci Sports. 2011 doi: 10.1111/j.1600-0838.2010.01156.x. [e pub before print] [DOI] [PubMed] [Google Scholar]