Summary
Muscle tissue damage might be related to metabolic and mechanical factors. Certain drugs have been associated with increased blood levels of creatin phospho kinase (CPK) and myoglobin that are biochemical markers of musculoskeletal damage. An increase of CPK plasma levels might suggest severe rhabdomyolysis with possible resulting renal failure. Telbivudine is an antiviral drug indicated for the treatment of chronic hepatitis B (CHB) in adult patients. An increase in CPK plasma levels has been recently described in some telbivudine-treated CHB patients without muscle-skeletal symptoms. In this paper we report a CHB patient that developed a severe increase of CPK plasma levels during telbivudine-treatment. Pharmacological evaluation, using the Naranjo probability scale, indicated a probable relationship between telbivudine and CPK increase, so telbivudine was discontinued and replaced with entecavir with a complete resolution of laboratory findings. In conclusion, telbivudine treatment can induce muscular damage in the absence of skeletal injury, therefore we suggest to closely monitor the muscular function of the patients treated with this drug in order to prevent possible major complications.
Keywords: muscular damage, CPK, telbivudine, chronic hepatitis B
Introduction
Muscle tissue damage is related to both metabolic and mechanical factors that allow to classify this condition in traumatic and non traumatic (4–8) (Tab. 1).
Table 1.
Traumatic and non traumatic causes able to induce muscle tissue damage.
| Traumatic causes | Non traumatic causes |
|---|---|
| Strenuous exercise | Infectious diseases (i.e. infectious sustained from influenza-A, coxsackie virus, CMV, EBV, rotavirus, enterovirus, HIV, Streptococcus beta haemoliticus, staphilococcus, Salmonella, Shigella, Legionella, Clostridium) |
| Direct muscle damage (i.e. injuries, delirium tremens, psycosis, seizures) | Metabolic diseases (ipokaliemia, ipophosphatemia, ipocalcemia) |
| Ischemic muscle damage (i.e. compartment syndrome, thrombosys, embolism) | Reumathic disorders (i.e polymyositis and dermatomyositis) |
| Myositis ossificans | Autoimmune diseases (myositis ossificans, rhabdomyolysis) |
| Neoplasms | Drug treatment (e.g. statins, theophilline, antiH1, benzodiazepines, amphotericine B, antidepressants) |
| Intramuscular abscess | Toxics (alcohol, cocaine, amphetamine, ecstasy) Radiation therapy Chronic denervation or disuse Sickle cell anemia |
Muscle damage is able to induce release in blood of both myoglobin and other biochemical markers such as creatin phospho kinase (CPK) (9). In particular, CPK that is present in musculoskeletal cells, heart and nervous cells is involved in ATP synthesis, and is the most used indicator of musculoskeletal damage (10).
However, an increase in CPK plasma levels has been also reported during drug treatment (11,12). In particular, statins as well as fluoroquinolones have been associated with muscle pain and weakness (11–13). Moreover, experimental study in rats documented a dose-dependent CPK increase during bupivacaine-treatment probably related to glutamate release (14).
Recently an increase in CPK plasma levels (>7 vs normal values) has also been described in 12.5% of telbivudine-treated chronic hepatitis B (CHB) patients about 56.9 weeks after beginning of telbivudine; the CPK increase disappeared after drug dismission (15,16). Telbivudine is a L-nucleoside analogue able to inhibits polymerase gamma responsible for mtDNA replication. More recently Finsterer and Ay (17) reported a case of telbivudine induced CPK-increase in a patient with a previous muscle damage and with myalgia, tiredness and reduced tendon reflexes. However, it is possible that an increase in CPK plasma levels may be present during telbivudine-treatment also in patients without history of muscle damage as in the following case.
Case presentation
In June 2008, a 67 years old Caucasian man without history of smoking or drinking presented to our observation with typical signs of HBV infection, HBeAg negative, HBV DNA positive (basal HBV DNA levels 65,760 IU/ml by real-time PCR), serum alanine aminotransferase (ALT) levels 120 IU/L (normal range < 41 IU/L), negative for anti-HDV, anti-HCV and anti-HIV antibodies.
At the basal screening, several possible causes of liver disease including autoimmune, thesaurismosic and metabolic origins were excluded.
On April 17th 2009, patient began antiviral therapy with telbivudine (LdT; 600 mg/day orally) with a satisfactory virologic suppression after 4 weeks (HBV DNA < 30 IU/ml; ALT 20 IU/L).
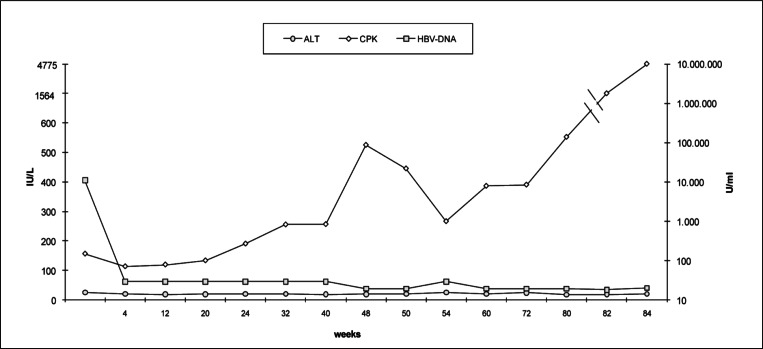
At follow up, 32 and 40 weeks after the beginning of telbivudine, blood tests documented a complete suppression of HBV DNA levels (<12 IU/ml), a normalization in ALT plasma values (20 IU/L) but an increase in CPK plasma levels (255 IU/L; normal values < 190 IU/L).
A new follow up, 48 weeks after the beginning of telbivudine, documented an increase in CPK plasma levels (525 IU/L; CPK-MB 16 IU/L, normal values < 25 IU/L; MB/CPK 3%, normal values < 6%); while echocardiographic evaluation showed the presence of medium tricuspidal damage, low pulmonary hypertension, left atrial dilatation, left ventricular hypertrophy with diastolic dysfunction (FE simpson 60%), without clinical symptoms.
A further increase in CPK plasma levels was recorded from the 50 weeks after the beginning of telbivudine (Fig. 1). Moreover, at 84 weeks an increase in CPK (4,775 IU/L) and either serum aspartate aminotransferase (147 IU/L), ALT (49 IU/L) and CPK-MB (56 IU/L) plasma levels was reported; while normal values in aldolase, creatinine, potassium, calcium, phosphorum, ureic acid and myoglobine were documented.
Figure 1.
ALT, CPK and HBV-DNA plasma levels during Telbivudine oral therapy (600 mg/die).
Pharmacological evaluation, using the Naranjo probability scale (18), indicated a probable relationship between telbivudine and CPK increase, so telbivudine was discontinued and replaced with entecavir (0.5 mg/day) with a complete resolution of laboratory findings (CPK, CPKMB and ALT plasma levels) in about 16 weeks and without in crease in HBV-DNA.
Material and Methods
Serum ALT, AST and CPK levels were routinely measured by automatic analyzer according to manufacturer instructions. Serum HBsAg, HBsAb, HBeAg, HBeAb and HBcAb were determined by electrochemiluminescence immunoassay (ECLIA) using Abbott Architect i2000 (ABBOTT, Wiesbaden, Germany), according to the manufacturer instructions. Serum HBV DNA levels were determined by real time TaqMan PCR technology (Roche).
Discussion
Creatine kinase, lactate dehydrogenase, aldolase, myoglobin, troponin, aspartate aminotransferase, and carbonic anhydrase CAIII are the most useful serum markers of muscle injury (9). Normal reference ranges for serum CK are 55 to 170 IU/L for males, and 30 to 135 IU/L for females (19). Total creatine kinase measurement in serum is used as index for detection and monitoring of skeletal muscle diseases. A recent study provided support for the validity of serum CPK measurement as an index of skeletal muscle injury caused by orthopaedic surgery (20).
Different human tissues exhibit varying distributions of cytoplasmic and mitochondrial isoenzymes of creatine kinase (21). Indeed, high CPK levels cannot specifically indicate skeletal muscle damage because of the presence of CPK isoforms at skeletal, cardiac and brain levels (CPK-MM, CPK-MB and CPK-BB) that are included in total CPK activity. These enzymes are normally strictly intracellular, and their increased activity in plasma reflects the escape via membrane structures. Thus, although the direct demonstration of muscle damage is histological, in practice the diagnosis is largely based on the measurement of plasma enzyme concentrations (22). A CPK activity >500 IU/l is considered a sign of skeletal muscle damage (23).
In our patient an increase of total CPK higher than 500 IU/L and the pathological increase of the MB isoform together suggest a skeletal muscular damage. The relationship between telbivudine and muscle damage has been confirmed by the Naranjo adverse probability scale (18), that indicated a probable relationship between muscle symptoms and drugs treatment. Indeed, we considered the development of muscle damage after telbivudine treatment, the improvement of adverse reaction after drug dismission, the absence of other drugs contemporarily assumed by the patient and the presence of objective evidence. Furthermore, it should be noted that no skeletal muscle damage injury was referred by the patient and the family history was negative for neuromuscular disorder.
Telbivudine is a L-nucleoside analogue able to inhibits polymerase gamma responsible for mtDNA replication. Previously an increase in CPK plasma levels and myalgia have been reported in patients during telbivudine treatment (15,16). More recently, Finsterer and Ay (17) reported a case of telbivudine induced CPK-increase in a patient with a previous muscle damage and with myalgia, tiredness and reduced tendon reflexes. Even tough profound CPK elevations have been reported without renal impairment (24), drastic increase of CPK and CPK-MB levels might suggest exertional rhabdomyolysis and patients should be hospitalized in order to prevent possible renal failure. A relationship between CPK elevation and the severity of disease has been established (>6000 IU/L predicts renal failure), however patients can have significant morbidity with only moderately elevated CPK levels (25, 26). In our patient the higher plasmatic CPK value recorded during telbivudine treatment was 4800 IU/L but the patient remained asymptomatic. Notably, this finding concurs with the clinical significance of rhabdomyolysis that is attributable to the often subtle initial manifestations to the extent of which serious sequel can occur (27). As such, early detection and diagnosis of extreme CPK increase is pivotal.
Conclusion
Telbivudine treatment can induce substantial muscular damage in the absence of skeletal injury. The possible absence of symptoms suggests to closely monitor the muscular function of the patients treated with this drug in order to prevent possible major complications.
References
- 1.Rigante D, Bersani G, Compagnone A, Zampetti A, De Nisco A, Sacco E, Marrocco R. Exercise-induced rhabdomyolysis and transient loss of deambulation as outset of partial carnitine palmityl transferase II deficiency. Rheumatol Int. 2011;31:805–807. doi: 10.1007/s00296-009-1221-z. [DOI] [PubMed] [Google Scholar]
- 2.Lee R. Ask the doctor. Can muscle damage from a statin, or from strenuous exercise, elevate creatinine even after I stopped taking the statin and exercising but continue to take Zetia and Diovan HCT? Harv Heart Lett. 2009;19:28. [PubMed] [Google Scholar]
- 3.Montero J, Lovesio C, Godoy MV, Ruiz G. Rhabdomyolisis caused by spinning in nine patients. Medicina (B Aires) 2009;69:153–156. [PubMed] [Google Scholar]
- 4.Tóth AR, Varga T. Myocardium and striated muscle damage caused by licit or illicit drugs. Leg Med (Tokyo) 2009;11(Suppl 1):S484–487. doi: 10.1016/j.legalmed.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 5.Fearnley RA, Lines SW, Lewington AJ, Bodenham AR. Influenza A-induced rhabdomyolysis and acute kidney injury complicated by posterior reversible encephalopathy syndrome. Anaesthesia. 2011;66:738–742. doi: 10.1111/j.1365-2044.2011.06752.x. [DOI] [PubMed] [Google Scholar]
- 6.Danis R, Akbulut S, Ozmen S, Arikan S. Rhabdomyolysis-induced acute renal failure following fenofibrate therapy: a case report and literature review. Case Report Med. 2010:537–818. doi: 10.1155/2010/537818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banasik M, Kuźniar J, Kusztal M, Porazko T, Weyde W, Klinger M. Myoglobinuria caused by exertional rhabdomyolysis misdiagnosed as psychiatric illness. Med Sci Monit. 2008;14:CS1–4. [PubMed] [Google Scholar]
- 8.Madrazo Delgado M, Uña Orejón R, Redondo Calvo FJ, Criado Jiménez A. Ischemic rhabdomyolysis and acute renal failure. Rev Esp Anestesiol Reanim. 2007;54:425–435. [PubMed] [Google Scholar]
- 9.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–767. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 10.Poels PJ, Gabreels FJ. Rhabdomyolysis: a review of the literature. Clin Neurol Neurosurg. 1993;95:175–192. doi: 10.1016/0303-8467(93)90122-w. [DOI] [PubMed] [Google Scholar]
- 11.Gallelli L, Ferraro M, Spagnuolo V, Rende P, Mauro GF, De Sarro G. Rosuvastatin-induced rhabdomyolysis probably via CYP2C9 saturation. Drug Metabol Drug Interact. 2009;24:83–87. doi: 10.1515/dmdi.2009.24.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, Hoppeler H, Breil F, Draeger A. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;181:E11–18. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durey A, et al. Levofloxacin-induced Achilles tendinitis in a young adult in the absence of predisposing conditions. Yonsei Med J. 2010;51:454–456. doi: 10.3349/ymj.2010.51.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherng CH, Wong CS, Wu CT, Yeh CC. Intramuscular bupivacaine injection dose-dependently increases glutamate release and muscle injury in rats. Acta Anaesthesiol Taiwan. 2010;48:8–14. doi: 10.1016/S1875-4597(10)60003-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XS, Jin R, Zhang SB, Tao ML. Clinical features of adverse reactions associated with telbivudine. World J Gastroenterol. 2008;14:3549–3553. doi: 10.3748/wjg.14.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liaw YF, Gane E, Leung N, GLOBE Study Group et al. 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Finsterer J, Ay L. Myotoxicity of telbivudine in preexisting muscle damage. Virol J. 2010;7:323. doi: 10.1186/1743-422X-7-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 19.Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med. 1988;148:1553–1557. [PubMed] [Google Scholar]
- 20.Kumbhare D, Parkinson W, Dunlop B. Validity of serum creatine kinase as a measure of muscle injury produced by lumbar surgery. J Spinal Disord Tech. 2008;21:49–54. doi: 10.1097/BSD.0b013e31805777fb. [DOI] [PubMed] [Google Scholar]
- 21.Wu AH, Perryman MB. Clinical applications of muscle enzymes and proteins. Curr Opin Rheumatol. 1992;4:815–820. [PubMed] [Google Scholar]
- 22.Coudreuse JM, Dupont P, Nicol C. Delayed post effort muscle soreness. Rev Sci Soc Fr Reeduc Fonctionnelle Readaptation Med Phys. 2004;47:290–298. doi: 10.1016/j.annrmp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Martínez Amat A, Marchal Corrales JA, Rodríguez Serrano F, Boulaiz H, Prados Salazar JC, Hita Contreras F, Caba Perez O, Carrillo Delgado E, Martín I, Aranega Jimenez A. Role of α-actin in muscle damage of injured athletes in comparison with traditional markers. Br J Sports Med. 2007;41:442–446. doi: 10.1136/bjsm.2006.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarkson PM, Kearns AK, Rouzier P, Rubin R, Thompson PD. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med Sci Sports Exerc. 2006;38:623–627. doi: 10.1249/01.mss.0000210192.49210.fc. [DOI] [PubMed] [Google Scholar]
- 25.Thompson PD, Clarkson PM, Rosenson RS, et al. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97:69C–76C. doi: 10.1016/j.amjcard.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 27.Palebani M. Skeletal muscle biomarkers: not new but still interesting diagnostic tools. Clinical Chemistry and Laboratory Medicine. 48:745–746. doi: 10.1515/CCLM.2010.170. 6. [DOI] [PubMed] [Google Scholar]