Summary
The purpose of this study was to determine the efficacy of platelet-rich plasma (PRP) 1-injection during an Achilles tendon rat tear model. 80 male adult imbreded rats (Wistar Kyoto), underwent under surgical tendon rupture. 40 Animal (PRP group rats) were given a local injection with 0,25 mL of PRP, and 40 animal (control group) were given the same quantity of control solution. The rats were sacrified at 1, 2, 4 and 6 weeks (each time point, 20 rats of the each group) after surgical tear and tendon tissue was analysed by macroscopic aspect, histology, immunostaining and Real Time (RT)-PCR to evaluate tissue repair. PRP improved tendon remodelling by better coordination of the reconstructive process with earlier formation of tendon-like continuity only in the first week after surgery. However, after 2,4 and 6 weeks, Achilles tendons in the PRP group had no difference compared to the control group. Immunostaining and RT-PCR did not show any difference between PRP treated and untreated group. Based on these findings a single injection of PRP appear not useful for Achilles rat tendon tear.
Keywords: growth factors, Achilles tendon tear, PRP, tendon repair
Introduction
Tendon injuries are a major cause of musculoskeletal morbidity affecting professional and recreational athletes but also sedentary middle-aged individuals (1,2). An estimated 30% to 50% of all sports-related injuries are tendon disorders, where Achilles tendon injury represent 52%. Achilles tendon injuries frequently lead to sport cessation for long periods and may interfere with activities of daily living (3).
Tendon repair can occur either intrinsically via the resident tenocytes or via extrinsic mechanisms, whereby cells from the surrounding sheath or synovium invade the tissue. Three biologically and temporally overlapping phases are described during tendon repair, namely inflammatory, reparative and remodeling. Various attempts to improve the autologous healing potential have involved the use of the growth factors to enhance cell proliferation, chemotaxis, aid angiogenesis, and influence cell differentiation during tendon healing (1).
PRP injections has been recently introduced in tendinopathy and tendon tears raised high expectations, although the rationale for use PRP is still unclear. However, as is recognized in treating tendon injuries, the healing pathways are extremely complex and not fully understood with regards to stimulatory, inhibitory, and regulatory influences on healing (4,5). In this setting, it is suggested that platelets derived from whole blood using simple cell-separating systems provide a release of various growth factors that participate in tissue repair processes, due to effects on angiogenesis and collagen synthesis (6,7). Despite many basic science and animal studies and some small case reports promising results of the use of PRP in tendinopathy, and tendon tears the level of scientific evidence of efficacy is lacking (8).
We performed an animal study, and analyzed the healing of 80 rat tendon wounded and surgically repaired through normally used techniques versus rat tendon treated with the same surgical technique to which PRP 1-injection was added. Moreover, we evaluated mRNA and protein expression of genes which play a pivotal role in tendon healing like PDGF and TGF-β, fundamental for collagen synthesis and matrix remodelling.
Materials And Methods
Animal Model
We performed an Achilles tendon tear model on 80 male adult imbreded rats (Wistar Kyoto) (300 g; Charles River Laboratories, Italy); 40 animals received PRP (PRP group), and 40 animals served as an untreated control (control group). Thereafter, 20 rat a time were sequentially euthanatized from each group at 1, 2, 4, and 6 weeks postoperatively.
Preparation of PRP
Whole blood was collected from male Wistar Kyoto rats: 3 donor rats were sufficient for an experiment on 10–20 recipient rats. The donor rats were anesthetized and then sacrificed by CO2 inhalation; the blood was collected from posterior vena cava and platelet concentrate was prepared using GPSII system (Biomet). 27 mL of whole blood was added to 3 mL of anticoagulant ACD-A (citrate dextrose solution A) and centrifuged at 3200 rpm for 15 minutes to obtain platelet rich plasma (PRP). Further 5 mL of whole blood was collected without anticoagulant and, after their coagulation, were centrifuged at 3200 rpm for 3 minutes to obtain activated autologous thrombin to which calcium chloride was added in the ratio of 1:5. PRP and activated thrombin were mixed together in the ratio of 10:1 to obtain platelet concentrate.
Surgical Procedure And Treatment
The rats were anesthetized with ketamine/xylazina in an anesthetic induction chamber and then in a mask. The skin was shaved and the operation performed under aseptic condition. A 1,5 cm transverse incision was made in the skin lateral to the right Achilles tendon; the surrounding fascia was cut longitudinally and the Achilles tendon exposed. Subsequently, the Achilles tendon was cut transversely 7 mm proximally to its calcaneal insertion. The plantaris muscle and tendon were left intact. The skin only was sutured with 4-0 monofilament nylon. PRP Group rats were given a local injection with 0,25 mL of PRP while control group were given the same quantity of control solution. After the operation, all rats were moved to housing room in standard conditions at Charles River Italia (Calco, Italy). Animals were let free to weight bearing and no postoperative immobilization was administered. Rats of both groups were sacrificed at 1, 2, 3 and 6 weeks post injury. The tendon was dissected from the attached calcaneal bone, kept in neutral tamponed formalin.
Ethics and euthanasia
After administrating sodiumthiopental (50 mg/Kg) to induce coma, pancuronium (Pavulon Ink Co. USA) (1mg/KG) was delivered in order to stop breathing to perform a comfortable euthanasia. The study was approved by the local ethics committee of our faculty in acceptance with the ethics standard of “principles” of Laboratory Animal care.
Histology and immunohistochemistry
Tendons for histological examination were prepared with routine methods for paraffin sections. For each paraffin inclusion 11 sections of 4–5 μM were cut: 1 for staining with Harris hematoxylin and eosin and 10 for immunocytochemical staining. Slides were pretreated with 3% H2O2 for 15 min to inhibit endogenous peroxidase and with blocking agent (Normal Reagent; Vector, Burlingame, CA) for 30 min to prevent background staining. Unmasking of the antigens was performed by incubating tissue sections in 10 mM sodium cytrate buffer pH=6 for 9 minutes. Incubation with primary antibodies was performed for 1 h at room temperature using the following monoclonal antibodies: Vascular Endothelial Growth Factor (VEGF1) (1:25; DakoCytomation, Denmark), anti-Human Collagen IV (CIV22) (1:50, DakoCytomation), anti-Human CD31 Endothelial Cell (JC70A) (1:20, DakoCytomation), anti-Human CD34 Class IIQBEnd-10 (1:50, DakoCytomation), Collagen I (COL1) (1:100; Abcam), anti-FGF2/basic (FGFb-FM2) (1:100, Upstate), Platelet-Derived Growth Factor- (PDGF) α and β (1:100, LabVision) and Collagen III (FH7A) (1:600, Abcam). Detection of the hybridization signal was performed with Dako EnVision kit using DakoCytomation Autostainer. Counterstaining was performed with Harris hematoxylin. Sections were examined independently by two pathologists. For routine histology, scores were assigned for two sections from each tendon (proximal and distal within the lesion). All tendon parameters were scored from 1 (normal) to 4 (severe changes) for: tenocyte shape, tenocyte density, free hemorrhage, neovascularization, collagen fiber linearity and collagen fiber uniformity. Scores from both segments (proximal and distal) and both observers were averaged. This grading scheme expands on previously described systems which utilize an eight-parameter, four-point score (9–11).
RNA Extraction From FFPE Tissue
A block of formalin-fixed, paraffin-embedded tissue was cut using a microtome, and 3 paraffin slides (10-mm thick) were placed directly into a sterile microfuge tube. Then 1 ml of limonene was added, after which the specimens were incubated for 10 minutes at room temperature and centrifuged for 5 minutes at maximum speed. Limonene was renewed to remove residual paraffin. After centrifugation, the tissues was washed in 1ml of 100% ethanol and centrifuged again. The wash was repeated using 90% ethanol and 70% ethanol. The tissue was dried at room temperature for 5 minutes. Total RNA was extracted using Absolutely RNA FFPE Kit (Stratagene) according to the manufacturer’s protocol which has been specifically optimized to remove the modifications made to the RNA molecule during tissue fixation that would render the RNA useless in downstream applications. After the end of the procedure, total RNA was eluted in 30 ml of DEPC treated water and then stored at −80°C.
cDNA Synthesis
In each case, cDNA was generated by reverse transcription of 2 mg of total RNA. Reverse transcription was performed by High-Capacity cDNA Archive kit (Applied Biosystems) which employs random primers and Multi-scribe RT enzyme. Samples were heated at 25°C for 10 minutes and then at 37°C for 2 hours. Total volume of each RNA sample was 100 μl and cDNA final concentration was 20 ng/ml. cDNA was stored at −20°C.
Semiquantitative PCR
For an initial evaluation of expression of collagen I, collagen III, PDGFα, PDGFβ and TGFβ3, a semiquantitative PCR was performed using primers (see table 1). Specific primers that span introns, thus precluding amplification of genomic DNA were designed using Oligo Express software (copyright© 2000–2002 Teemu Kuulasma) and synthesized by Primm (Milan). The PCR mixture contained 5 ml of cDNA (20 ng/ml concentrated): the reaction was performed with 5 ml of 10X buffer (Vivantis), 2 ml of 50 mM MgCl2 (2mM final concentration), 2 ml of dNTP (400 μM each), 1,5 ml of each primer (300 nM each), 0,4 ml (2 unit) of Taq DNA Polymerase (Vivantis) and PCR-grade H2O up to final volume of 50 ml. PCR was performed in a GeneAmp® PCR System 2700 (Applied Biosystems) for a initial denaturation of 2 minutes at 95°C, followed denaturation at 95°C for 20 seconds, annealing at 58°C for 45 seconds and extension at 72°C for 1 minute; the reaction was accomplished with a final extension at 72°C for 5 minutes. Serial sampling at 25, 30 and 35 cycles were also performed. PCR products were analyzed with 2% agarose gel electrophoresis; TBE buffer containing 0,08% ethidium bromide was used for gel staining.
Table 1.
RT-PCR oligonucleotides:
| PRIMERS | SEQUENCE 5′ → 3′ | TEMP. ANNEALING (°C) |
|---|---|---|
| GAPDH | Forward GGCTCTCTGCTCCTCCCTGT | 58 |
| Reverse AATGAAGGGGTCGTTGATGG | ||
| COLLAGEN III | Forward GGGAACAACTGATGGTGCTA | 58 |
| Reverse GACTCATAGGACTGACCAAGG | ||
| COLLAGEN I | Forward CCCCAGCCGCAAAGAGTCT | 58 |
| Reverse GCATACATCAGGTTTCCACG | ||
| PDGF-β | Forward CTATGAAATGCTGAGTGACCA | 56 |
| Reverse ACTCCAGAATGTGCTCGGG | ||
| PDGF-a | Forward GCACAAAGTTTCACCAGAGC | 58 |
| Reverse AGGCTAGAGTCAGGGAGGAG | ||
| TGF-β | Forward TGTACGGCAGTGGCTGAAC | 58 |
| Reverse ATTCATGTTGGACAACTGCTC |
RT-PCR
Quantitative PCR was used to study gene expression of PDGF, TGF-β3 and genes coding for different types of collagen (I and III). Real time PCR for TGF-β3, collagen I and collagen III was performed using the dsDNA binding fluorescent dye SYBR™ Green I while quantitative analysis of PDGFα and PDGFβ was performed using TaqMan probes. For genes analyzed by SYBR™ Green I method, Brilliant SYBR™ Green QPCR Master Mix (Stratagene) was added to 300 nM of each primer and cDNA (40 ng for collagen I and TGF-β3 and 80 ng for collagen III). A passive reference dye (ROX) was added to the reaction mix in the ratio of 1:50 to compensate for non-PCR variation in fluorescence. The thermal cycling program consisted of an initial denaturation step of 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds, 58°C for 1 minute and 72°C for 30 seconds. Melting curve analysis was also performed to ensure the amplification of a single PCR product. In the case of PDGFα and PDGFβ, we performed amplifications, 20 ml reactions using TaqMan Universal PCR MasterMix and 40 ng of cDNA. The thermal cycling program was: initial step of 50°C for 2 minutes and denaturation at 95°C for 15 minutes followed by 45 cycles of 65°C for 15 seconds and 60°C for 1 minute. Reaction with neither RT and nor template was included as a negative control; samples consisted in 3 replicates for every gene. All samples were calculated relative to the rat GAPDH gene which was also amplified for each sample. Quantitative PCR was performed using ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) and relative expression was calculated according to the 2^-DDCt method using only results with Ct<0,3 which were considered reliable.
Statistics
All results were expressed as mean ± standard deviation (SD). Significant differences among groups were evaluated using the Mann-Whitney U test. A difference of P ≤ .05 was considered to be statistically significant. Histological scores were compared using Kruskal-Wallis non-parametric ANOVA.
Results
Macroscopic Observation
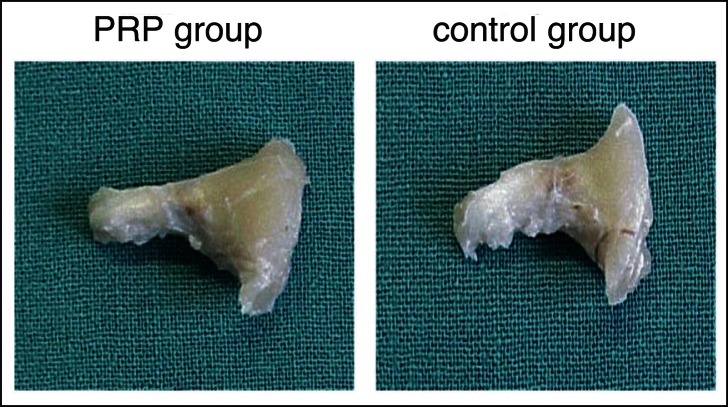
Macroscopic differences has been observed among PRP group and control group only one week after the surgical Achille tendon repair. We observed a greater homogeneity inside and around the lesion repair tissue in PRP treated tendons. In the PRP single injection rats treated group a good estate and a nearly normal calibre, a greater quantity of fibrotic-like granulation tissue which granted regular structural continuity to the tendon in site of tenotomy was noted. We did not observe any differences in the adherences between tendon and underlying tissues or a macroscopic area of hematoma around tendon in both the groups treated or untreated. No cases showed suture downfall or tendon retears (Fig. 1). At weeks 2, 4 and 6 PRP the two groups resulted homogeneous for macroscopic appearance. At 6 weeks all the groups appeared homogeneous and quite similar: PRP treated and untreated groups showed normal diameter although reduced as compared with control tendons at 1 or 2 weeks, similar to pre-surgical one.
Figure 1.
Macroscopic findings.
The lateral appearance of the Achilles tendon in the PRP group and the control group at 1 weeks after surgery.
Microscopic Observation
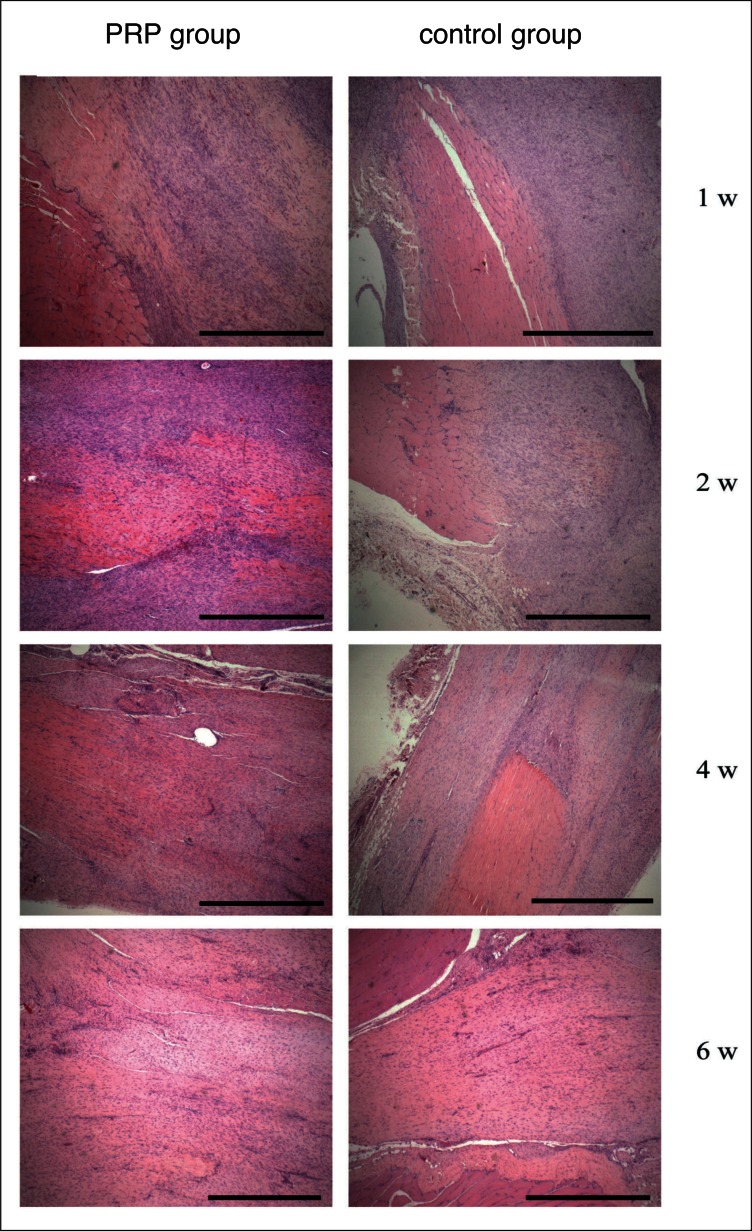
Histologically, differences between the two groups were observed at week one. On PRP treated tendons group, we observed the presence of more hyperplastic fibroblast. Erythrocytes were seen in intracellular matrix. Untreated tendons showed a greater cicatricial reaction and increase of number cell: therefore the structure resulted disorganized with exuberant proliferative reaction. Extracellular matrix appeared scanty. Cumulative histology scores were different (more normal) for PRP group compared control group (Fig. 2; Table 2). At week 2 we noted a slight difference of cellularity and PRP treated tendons showed more homogeneous in calibre, structure and reparation degree. At weeks four and 6 treated and untreated groups showed no differences (Fig. 2). Cumulative histology scores were no different for PRP group compared to control group at week 2, 4 and 6 (data not shown).
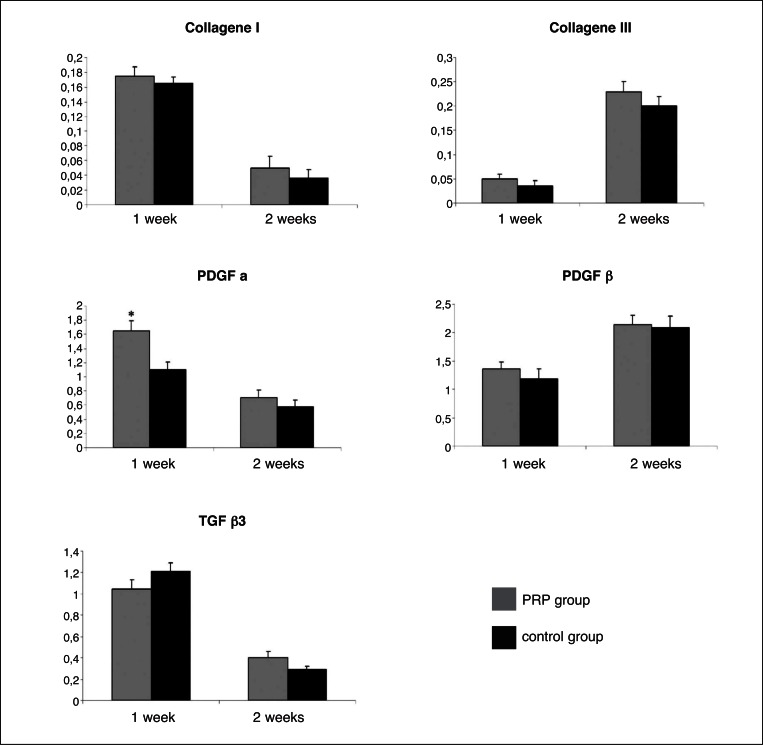
Figure 2.
Findings of RT-PCR analysis.
Collagene I, Collagene III, PDGF-α, PDGF-β and TGFβ3, expression was analyzed with RT-PCR, with GAPDH as the internal control for normalization. Results are given as the mean and standard deviation for 8 rats. Statistical analyses were performed with the unpaired Student t test: *p< 0.05.
Table 2.
Histologic scoring at week one:
| Tendon parameter | control Group | PRP Group | P-value |
|---|---|---|---|
| Cell shape | 1.0 (0.9 to 1.3) | 1.5 (1.3 to 2) | 0.1 |
| Cell density | 1.52 (1.0 to 2.0) | 2.35 (1.3 to 2.8) | 0.2 |
| Free hemorrhage | 1.3 (0.9 to 1.7) | 1.75 (1.1 to 2.4) | 0.1 |
| Neo-vascularization | 2.0 (1.4 to 2.2) | 2.0 (1.2 to 2.3) | 0.7 |
| Collagen linearity | 2.75 (1.3 to 2.1) | 2.75 (2.4 to 3.0) | 0.1 |
| Collagen uniformity | 2.0 (1.4 to 2.1) | 2.0 (1.8 to 3.1) | 0.1 |
| Cumulative Score | 10.5 (6 to 11.4) | 12.3 (9.1 to 15.6) | 0.2 |
Histologic scores for PRP group and the control group reported as median (95% confidence interval). The P-values are listed. (Significant differences P < 0.05).
RT-PCR
Quantitative PCR reactions were performed on collagen I and III, PDGFα, PDGFβ and TGFβ3, using SYBR Green I in the first three cases and TaqMan Probes in the last two cases. We chose to analyze only groups of the first and the second week because mRNA production is restricted to the early phases of healing process. We did not find any significant difference between PRP treated and control tendons at weeks 1 and 2. Collagen I was found to be down-regulated in PRP treated tendons at 2 weeks (p = 0.075) compared to controls at the same week (Fig. 2A). Collagen III did not show any significant difference between PRP treated and not-treated groups both at weeks 1 and 2 (Fig. 2B). PDGFα was up-regulated in control groups compared to PRP treated groups although the significance was found only in the first week (p = 0.035) (Fig. 2C). PDGFβ tended to be more expressed in control groups compared to PRP treated ones but we could not see any significance. (Fig. 2D). TGFβ3 seemed to be up-regulated in treated tendon at 1 week compared to controls at the same week although we did not find a significance (p = 0.07) (Fig. 2E).
Immunohistochemistry
There were no significant immunohistochemical differences between the groups in regards to VEGF1, FGFb, PDGFα and PDGFβ growth factors nor to collagen formation (Fig. 3)
Figure 3.
Findings of histological analysis of the Achilles tendon. H & E staining of the PRP group and control group at 1, 2, 4, 6 weeks after surgery. Magnification × 400. Bar scale = 100 μm.
Discussion
This study suggests which a single injection PRP in a ruptured tendon healed more rapidly compared with the controls only during the first week after injury. Our results have recorded in the 1° week macroscopic differences between the two groups, PRP group, in particular, developed better homogeneity of features; but after the second week, tendons of the PRP treated group and of the untreated group developed equivalent homogeneity. Microscopic examination in the PRP treated group shows in the hyperplastic, proliferating and active phases more abundant fibroblasts population compared to the control group but only during the first week. Even the extracellular matrix in the site of the lesion shows features of homogeneity and rearrangement superior to the untreated group only during the first week.
In our study by RT-PCR we found down-regulation of only PDGFα statistically significant and in the first week after injury in PRP treated tendons compared to controls; while PDGFβ, TGFβ3, collagen I and III, did not show any significant change between the two groups both in the first and in the second week. Since PDGF is one of the main component of PRP, we would expected to find it more expressed in tendon treated with platelet rich plasma even if several manuscripts reported an up-regulation of both PDGFα and PDGFβ in the first-second day after PRP injection and a rapid down-regulation in the following days (12–14). We can speculate that the analysis of PDGF expression after 7 days post injection missed detect on an augment of mRNA because the synthesis of PDGF was already started, but it was not confirmed with the immunohistochemical result. The data pertaining to other immunohistochemical markers reveal no significant difference between the PRP group and control group. Moreover, it would be useful to investigate soon after PRP injection (at least in the first 24 hours): mRNA synthesis in fact is the earlier process in response to modifications of normal body status (14–16).
A similar tear rat model with platelet concentrate injection has been reported by Aspenberg and Virchenko in the 2004 but differently they performed mechanical test and only histological score. The results appear in this manuscript promising unlikely the tests were performed just to 3 weeks postoperatively and no immunohistochemical investigations were reported, therefore our results appear to be in contrast (17).
In conclusion, platelet concentrate may be useful for the treatment of Achilles tendon ruptures although our results suggests that the use of a single injection of PRP is not an adjuvant to the complete recovery of functionality in this rat Achilles tendon tear model.
The healing process is a cascade of the interactions among cells, extracellular matrix and growth factors still unknown, therefore, further molecular and immunohistochemical are necessary to establish a PRP influence over the tendon-healing process immediately after its injection. Further clinical studies are also warranted for surgeons to better understand the use of PRP in patients who undergo surgical tendon repair. Despite the justified scientific rationale and the companies interest in the use of the growth factors, the literature is lacking of clinical prospective randomized studies that prove the effectiveness of this management modality (18).
Acknowledgments
We would like to thank Dr. A. Persico for her help. This study was partially supported by EON Medica S.r.l. Monza, Italy.
Footnotes
Conflict of interest disclosure:
All the Authors declare no conflict of interest. Antonina Parafioriti M.D., Anna C Berardi PhD
References
- 1.Maffulli N, Longo UG, Maffulli GD, Khanna A, Denaro V. Achilles tendon ruptures in elite athletes. Foot Ankle Int. 2011;32:9–15. doi: 10.3113/FAI.2011.0009. [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 3.Zafar MS, Mahmood A, Maffulli N. Basic science and clinical aspects of achilles tendinopathy. Sports Med Arthrosc. 2009;17:190–197. doi: 10.1097/JSA.0b013e3181b37eb7. [DOI] [PubMed] [Google Scholar]
- 4.Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215:837–845. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- 5.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39:258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 6.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bir SC, Esaki J, Marui A, et al. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50:870–879. doi: 10.1016/j.jvs.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 9.Movin T, Gad A, Reinholt FP, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand. 1997;68:170–175. doi: 10.3109/17453679709004002. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Longo UG, Franceschi F, Rabitti C, Denaro V. Movin and bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466:1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 12.McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27:1033–1042. doi: 10.1002/jor.20853. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 14.Oliva F, Gatti S, Porcellini G, Forsyth NR, Maffulli N. Growth factors and tendon healing. Scott Med J. 2010;55:35. doi: 10.1159/000328878. [DOI] [PubMed] [Google Scholar]
- 15.Andia I, Sanchez M, Maffulli N. Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther. 2010;10:1415–1426. doi: 10.1517/14712598.2010.514603. [DOI] [PubMed] [Google Scholar]
- 16.Mei-Dan O, Lippi G, Sánchez M, Andia I, Maffulli N. Autologous platelet-rich plasma: a revolution in soft tissue sports injury management? Phys Sportsmed. 2010;38:127–135. doi: 10.3810/psm.2010.12.1835. [DOI] [PubMed] [Google Scholar]
- 17.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75:93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez M, Anitua E, Azofra J, Andía I, Padilla S. Mujika Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. I. Am J Sports Med. 2007;35:245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]