Summary
Introduction:
Long head biceps (LHB) tendinopathy is a common cause of anterior shoulder pain. Isolated LHB pathology is most common among younger people who practise overhead sports. The authors conducted a short-term prospective randomised study to test the effectiveness of two different methods for the treatment of isolated LHB tendinopathy: biphasic oscillatory waves and hyperthermia.
Study design:
The study is a prospective randomised study (Level II).
Material and methods:
The authors identified 20 patients who had clinical and ultrasound (US) evidence of LHB tendinopathy. No patient was a high-level athlete. The patients were randomly assigned to two groups. Group A (10 patients) was treated with bi-phasic oscillatory waves, while Group B received hyperthermia. During the treatment period, no other electromedical therapy, injections with corticosteroids, oral analgesics or nonsteroidal anti-inflammatory drugs were allowed. All the patients were assessed at baseline (T0), immediately after the end of the treatment period (T1) and 6 months after the end of treatment (T2) using a visual analogic scale (VAS) and Constant-Murley Score (CMS). Furthermore, all patients underwent US examinations at T0 and at T1. All the US examinations were performed by the same radiologist.
Results:
The VAS scores showed a highly statistically significant reduction of pain at T1 both in Group A (65%; p=0,004) and in Group B (50%; p=0,0002). The CMS also showed a statistically significant improvement between the pre-intervention, the post-treatment and the short-term follow-up in both groups. In addition, the peritendinous fluid evident on US examination at T0 was no longer present in all cases at T1.
Conclusion:
These findings suggest that both bi-phasic oscillatory waves and hyperthermia are able to relieve pain in patients with isolated LHB tendinopathy. This is a Class II level of evidence.
Keywords: biphasic oscillatory waves, InterX, hyperthermia, long head biceps, rehabilitation, tendinopathy
Introduction
Long head biceps (LHB) tendinopathy is a common cause of anterior shoulder pain. In 95% of LHB tendinopathy it is associated with rotator cuff tear or a SLAP lesion (1,2). Usually LHB tendinopathy is frequent in patients between 18 and 35 years old and athletes, particularly throwing athletes, gymnasts, swimmers and participants in contact sports and martial arts (1), while isolated tendinopathy of the LHB only accounts for 5% of all LHB tendinopathy (3).
Hippocrates described dislocation of LHB tendon (4). In 1872 Duplay described LHB tendonitis (5), while Pasteur in 1932 (6) was the first to recognize LHB tendinopathy as an isolated condition. Prior to Neer’s chronic impingement syndrome theory tenotomy of LHB was the main surgical procedure used to treat a painful shoulder.
The natural history of progressive degeneration of the LHB can be summed up as: tenosynovitis, tendinosis, delamination, pre-rupture and finally rupture (7). Several treatments for tendinopathy of LHB have been described, depending on patients’ age, activity level, co-morbidities and extent of disability. Conservative management consists of physiotherapy, ice, non-steroidal anti-inflammatory drugs (NSAIDs) and physical therapy. Injections of corticosteroids and local anaesthetics can be used for diagnostic or therapeutic purposes (8). Surgical procedures, such as tenotomy or tenodesis, may be warranted if symptoms persist despite adequate conservative treatment or if intratendinous lesions are identified on MRI or US scans (7,8).
Electrical stimulation has been cited in several rehabilitation protocols and appears to be useful in pain management (9). Bi-phasic oscillatory waves therapy is a non-invasive, interactive electrical neurostimulation device, generating high-amplitude, pulsed, damped biphasic sinusoidal current, which is delivered directly to the soft tissue by two concentric electrodes (10,11). The waveform shape and energy delivered to the body changes as a function of the skin impedance and underlying tissue characteristics (10,11). Changes in tissue impedance are shown on a display as color changes and/or increased adherence of electrodes to the skin. The changes allow the therapist to locate and target the zone of treatment for each patient. Bi-phasic oscillatory waves differs from traditional transcutaneous electrical nerve stimulation (TENS) because the interactive waveform adjusts in response to change in skin impedance (Fig.1).
Figure 1.
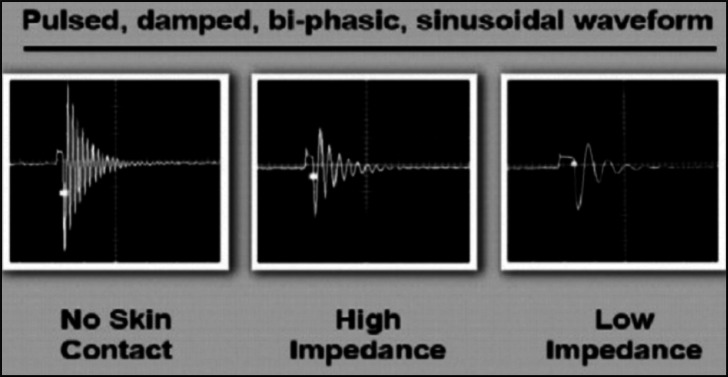
The waveform of the non-invasive neurostimulation device. The interactive waveform adjusts in response to changes in skin impedance. Electrodes are applied on the skin over the sites of low impedance which are specifically targeted. Conductive gel is not required.
Hyperthermia has been also recently introduced in rehabilitation (12). Hyperthermia is based on equipment that generates radio frequency waves. It combines a superficial cooling system and a deep-heating source with a microwave power generator at 434 MHz. Unlike many other forms of electrotherapy the current is not transmitted by direct contact but by the movement of attraction and repulsion of electric charges. It generates deep hyperthermia increasing internal temperature and the energy potential of cell membranes, rising tissue to therapeutic temperatures to a depth of several centimetres from the skin with no risk of overheating the superficial tissues. Hyperthermia is thought to increase the cellular metabolic processes and help restore tissue physiology, and it is theorized to reduce pain.
To our knowledge there are no studies detailing the conservative management of isolated LHB tendinopathy, as it usually occurs in combination with other pathologies (8). There are no studies on the use of any physical therapies for the treatment of isolated LHB tendinopathy in the literature. We have chosen hyperthermia as a control because a previous study on the treatment of supraspinatus tendinopathy has demonstrated its effectiveness at short-term follow-up (13).
The aim of this study was to evaluate prospectively the effectiveness of these two different rehabilitation methods in the treatment of isolated LHB tendinopathy.
Patients and Methods
Patients Selection
In the period 2007 to 2009, we examined 418 outpatients with shoulder pain. Of these, only 20 patients (4.7%), who had clinical and US evidence of LHB tendinopathy, were selected for this study. Thirteen patients were women and seven were men, and their mean age was 43.8 (S.D. 12.9) years. Although no patient was a high-level athlete, all were amateur athletes. Six patients had a dual diagnosis of adhesive capsulitis and tendinopathy of LHB. These patients were treated by a physiotherapist during the same session. One investigator (F.O.) was responsible for diagnosing the 20 patients. Another investigator (S.R.) randomized the patients, while a third orthopedic surgeon (A.G.V.) evaluated patients at pre-treatment, at the end of the treatment and at follow-up.
Inclusion criteria
Patients were included in this study only if they met the following criteria:
Exclusion criteria
Patients were excluded in this study if they presented:
- Clinical and US diagnosis of rotator cuff tears;
- Systemic diseases (neoplasia, diabetes, rheumatoid arthritis, epilepsy);
- Pacemaker use;
- Previous shoulder dislocation or shoulder instability.
Outcome measures
All the patients were assessed at baseline (T0), immediately after the end of the treatment period (T1) and 6 months after the end of treatment (T2). They were assessed by an orthopedic surgeon (A.G.V.) who had never seen the patients and was blinded to the treatment group. The following clinical measures were recorded at T0, T1 and T2:
- Visual Analog Scale (VAS) score: this score, which is a subjective measure of pain, ranges from 0 (no pain) to 10 (worst pain) (16).
- Constant-Murley Score (CMS): this score is based on four variables that are used to assess shoulder function (17). The subjective variables are pain and ADL (sleep, work, recreation/sport) and they give a maximum of 35 points. The objective variables are range of motion and strength which give a maximum of 65 points. The maximum overall score is thus 100 which indicates a normal shoulder.
All the patients also underwent US examination of the shoulder at T0 and T1. These examinations were always performed by the same operator.
Randomisation process and intervention
After eligibility for inclusion in the study had been determined and a diagnosis of LHB tendinopathy made, the eligible subjects gave their consent to enter the study. The study was a short-term prospective comparative study including 20 patients (13 women and 7 men), who were randomised into two groups, group A and B. The Group A patients had a mean age of 45.9 years (S.D. 15.4), while the mean age of the patients in Group B was 41.7 years (S.D. 10.4).
Intervention
The patients in Group A underwent bi-phasic oscillatory waves therapy, while those in Group B were treated with hyperthermia. Each group received a total of 10 treatment sessions, one session three times a week. The Group A patients were treated with bi-phasic oscillatory waves at the beginning of every session. For bi-phasic oscillatory waves, InterX 5002 (Neuro Resource Group, Enermedica S.r.l, Rome, Italy) was used. It was applied all over the course of LHB tendon. Patients of group B were treated with HCR 901 device for TECAR-therapy (Restek s.r.l., Rome, Italy). Hyperthermia treatment was administered at 434 MHZ, and the probe was placed over the LHB groove. After that patients with adhesive capsulitis were submitted by the physiotherapist to active and passive mobilization of the shoulder, pendular swinging in the prone position in flexion and extension of the shoulder and passive glenohumeral joint stretching exercises. During the treatment period, no other types of physical therapy, injections with corticosteroids, oral analgesics or NSAIDs were allowed.
Statistical Analysis
All continuous variables were analysed using a repeated-measures univariant analysis of variance (ANOVA) to determine significant differences in VAS and CMS between the patients belonging to group A and B at T0, T1 and T2, and between the two groups. A p-value of ≤0.01 was considered statistically significant. To confirm statistical significant results Bonferroni’s correction was implemented. The data were analysed by a statistician unaware of treatment allocation.
Results
The random allocation of the patients produced, at baseline, two groups that were well-matched for age, pain intensity and functional disability. In 70% of the patients, the LHB tendinopathy affected the dominant shoulder.
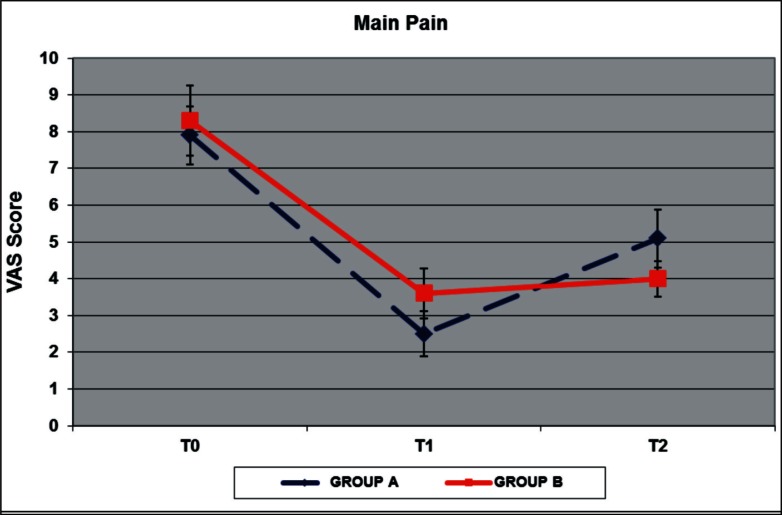
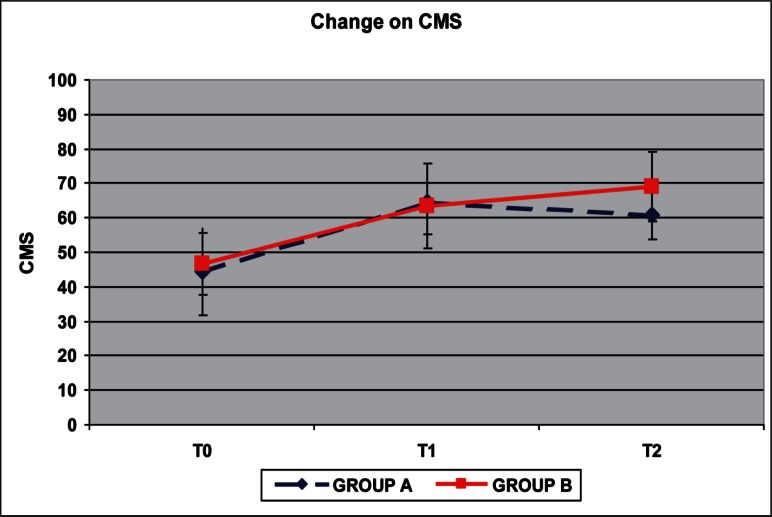
Pretreatment VAS and CMS showed that patients had a comparably high level of pain, with an average VAS score of 7.9 for group A (S.D. 1.1) and 8.3 for group B (S.D. 1.34) at T0, and similar functional limitations with average CMS of 44.5 for group A (S.D. 17.81) and 46.5 for group B (S.D. 12.51). We found a reduction of pain immediately after the treatment (T1) and at follow-up (T2) compared to the pretreatment scores in both groups (Fig. 2 and Tab.1). The ANOVA test applied between T0 and T1 VAS score and between T0 and T2 showed a statistically significant reduction of pain in group A (p= 0.004; p=0.002). The mean VAS score at T2 (average 5.1; S.D. 1.1) was greater than the mean VAS score at T1 (average 2.5; S.D. 0.85) in the group treated with Bi-phasic oscillatory waves, but the T2 mean score was lower than those at T0 (p=0.001). In group B we found a statistically significant reduction of pain from T0 to T1, and from T0 to T2 (p=0.0002 and p=0.007 respectively). The mean VAS score at T2 (average 4; S.D. 0.67) was greater than the mean VAS score at T1 (average 3,6; S.D. 0.97), but this difference is not statistically significant (p=0.295). The CMS measures showed an improvement between T0, T1 and T2 in both groups (Fig. 3 and Tab.1). In group treated with Bi-phasic oscillatory waves only the differences between T0 and T1 are statistically significant (p=0.009), while in group treated with hyperthermia the CMS showed an improvement only between T0 and T2 scores which was statistically significant (p=0.001). We found statistically significant differences between the two treatments from T1 and T2 measured both with VAS score (p=0.001) and with CMS (p=0.006), but none differences between T0 and T1, T0 and T2. We also observed that the peritendineus fluid of the tendon seen at US evaluation was no longer present in all cases at T1. No adverse reactions in both groups were reported.
Figure 2.
Results of mean VAS Score in group A and B. The reduction of pain at T1 and T2 was significant in both groups (P < 0.01).
Table 1.
VAS and Constant Scores
| VAS | CONSTANT | |||||||
|---|---|---|---|---|---|---|---|---|
| Group and time | Mean difference | S.D. | ANOVA | Bonferroni | Mean difference | S.D. | ANOVA | Bonferroni |
| Group A | ||||||||
| T0-T1 | 5.4 | 2.93 | p=0.004 | p=0.007 | 15.4 | 18.6 | p=0.009 | p=0.011 |
| T1-T2 | −2.6 | 1.64 | p=0.01 | p=0.001 | −3.6 | 11.21 | p>0.01 | p>0.01 |
| T0-T2 | 2.8 | 1.79 | p=0.002 | p=0.001 | 18.8 | 16.34 | p>0.01 | p>0.01 |
| Group B | ||||||||
| T0-T1 | 4.7 | 2.67 | p=0.0002 | p=0.002 | 17 | 17.04 | p>0.01 | p>0.01 |
| T1-T2 | −0.6 | 0.83 | p>0.01 | p=0.011 | 5.5 | 15.65 | p>0.01 | p>0.01 |
| T0-T2 | 4.3 | 2.43 | p=0.007 | p=0.001 | 22.5 | 17.44 | p=0.006 | p=0.007 |
Figure 3.
Results of mean CMS in group A and B. The improvement of shoulder function at T1 and T2 was significant in both groups (P < 0.01).
Discussion
The role of the LHB tendinopathy as a source of pain has received much attention in the orthopedic literature, although isolated LHB tendinopathy is a rare pathologic condition and poorly studied. LHB tendinopathy is increasingly being recognized and associated conditions, such as rotator cuff tear or glenohumeral instability, must be evaluated and treated appropriately. Pain is the first and most important symptom reported by patients with isolated LHB tendinopathy. Isolated tendinopathy of LHB may respond to conservative management. Rest, ice, nonsteroidal anti-inflammatory medication and intra-articular steroid injections are commonly used (18), even if the evidence of conservative treatment for LHB tendinopathy is limited. A Cochrane review published in 2003 looked at 26 different studies involving physical therapy for shoulder conditions and concluded that there was some evidence for mobilization and physiotherapy for rotator cuff disorders, such as rotator cuff tendinopathy, adhesive capsulitis, and calcific tendonitis, but none of these studies specifically evaluated the management of isolated LHB. This review also evaluated the use of therapeutic ultrasound, laser therapy and pulsed electromagnetic field in the treatment of these conditions and could not find any evidence to support their use (19). More recently some studies have been published about rehabilitation protocols of shoulder pain. In a study limited to supraspinatus tendinopathy (13), hyperthermia resulted in significantly less pain and improved function as compared with those patients receiving ultrasound or home exercises. No significant relief of pain was found when generalized shoulder pain was treated with low-energy laser therapy versus placebo laser (20), while high-intensity laser therapy was found superior to ultrasound (21). Pulsed electromagnetic field (PEMF) therapy has been suggested as treatment for musculoskeletal disorders because of its vasodilating and pain-relieving properties. Results from a recent double-blind, randomised control trial revealed no significant differences in shoulder pain or function between pulsed electromagnetic field and placebo (22). Although there is evidence supporting the use of several different physical therapies and their short-term benefits, further studies are strongly needed.
When conservative treatment fails, surgical solutions may be appropriate. A great deal has been written about LHB tendinopathy and the various surgical treatments available, but there is little consensus among the authors (8). LHB tendon decompression (23), subacromial decompression (24) and debridement of the intra-articular portion of the biceps tendon in cases of partial tears (25) have been described in the literature. There is a clear debate between tenotomy and tenodesis for the treatment of biceps tendinopathy (7,26). Maffulli suggests that biceps tenotomy be the preferred method because of its simplicity and velocity and because it needs less postoperative rehabilitation than tenodesis. Tenodesis instead should be preferentially performed in patients younger than 60 years old and active patients (26).
Bi-phasic oscillatory waves is a new generation of non-invasive health devices in pain management. It was developed in Russia in the 1980’s by A.A. Karasev. This technology was than developed in United States by Neuro Resource Group in 2004 and approved from Food and Drugs Administration for antalgic therapy (27). Now it is currently used in sport medicine and rehabilitation, even if there is not evidence on its effectiveness. The precise biochemical mechanism of the action for non-invasive neurostimulation is not yet completely known. The mechanism of pain relief is thought to include both segmental and descending neural inhibition (28), whereas the mechanism for reducing inflammation may be mediated by peripheral opiates and also animal studies have suggested that stimulation releases endogenous opioids (29). Bi-phasic oscillatory waves therapy has demonstrated to be effective in relieving pain and improving functionality in patients affected by trochanteric fracture of the femur and bimalleolar ankle fracture submitted to surgical osteosynthesis (10,11). Except these two studies, only a few case series have been reported in international meetings to our knowledge (30–32).
This study has several limitations. The small number of patients is usually intrinsic in this kind of pathology. Maybe a multicentric study could cover the epidemiologic lacking of data and improve future conclusions. The short-term follow-up is also a limitation of our study, but considering the small number of patients, we preferred not risk losing patients at a longer follow-up. The short-term follow-up also prevents us from determining the relapse rate. Furthermore the Constant score is a general shoulder clinical evaluation test and not specific for LHB tendinopathy. This theoretically may have affected the findings. Finally the lacking of statistical power due to the small sample size is another limitation of our study whereby we do not claim which our conclusions can be extended to the general population.
Conclusions
Both bi-phasic oscillatory waves and hyperthermia appear to be an effective treatment for isolated LHB tendinopathy. Bi-phasic oscillatory waves appears more effective than hyperthermia to relieve pain immediately after the end of the treatment, while hyperthermia shows better results at short term follow-up. This is to our knowledge the first study focusing on the conservative treatment of isolated LHB tendinopathy, therefore we advocate further studies with more patients and a longer term follow-up to confirm our results.
Acknowledgments
We are grateful Prof. Nicola Maffulli MD for his helpful discussions and Eng. Simone Richetta for his suggestions to review the study with ANOVA test.
References
- 1.Churgay CA. Diagnosis and treatment for biceps tendonitis and tendinosis. Am. Fam Physician. 2009;80:470–476. [PubMed] [Google Scholar]
- 2.Rockwood CA, Matsen FA. The Shoulder, vols 1 and 2. Philadelphia: WB Saunders; 1990. [Google Scholar]
- 3.Post D, Benca P. Primary tendinitis of the long head of the biceps. Clin Orthop. 1989;246:117–125. [PubMed] [Google Scholar]
- 4.Adams FL. The genuine works of Hippocrates, Vol 1 and 2. New York: William Wood; 1986. [Google Scholar]
- 5.Duplay S. On scapula-humeral periarthritis. Paris Clinical Lectures. Med Presse. 1900;69:571–573. [Google Scholar]
- 6.Pasteur F. Les algies de l'épaule et la physioterapie. La tenobursite bicipitale. J Radiol Electrol. 1932;16:419–429. [Google Scholar]
- 7.Boileau P, Baquè F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89:747–757. doi: 10.2106/JBJS.E.01097. [DOI] [PubMed] [Google Scholar]
- 8.Krupp RJ, Kevern MA, Gaines MD, Kotara S, Singleton SB. Long Head of the Biceps Tendon Pain: Differential Diagnosis and Treatment. Journal of orthopaedic & sports physical therapy. 2009;39:55–70. doi: 10.2519/jospt.2009.2802. [DOI] [PubMed] [Google Scholar]
- 9.Pope G, Mockett S, Write J. A survey of electrotherapeutic modalities: ownership and use in NHS in England. Physiotherapy. 1995;81:82–91. [Google Scholar]
- 10.Gorodetskyi IG, Gorodnichenko AI, Tursin PS, Reshetnyak VK, Uskov ON. Non-invasive interactive neurostimulation in the post-operative recovery of patients with a trochanteric fracture of the femur: a randomized, controlled trial. J Bone Joint Surg [Br] 2007;89:1488–1494. doi: 10.1302/0301-620X.89B11.19352. [DOI] [PubMed] [Google Scholar]
- 11.Gorodetskyi IG, Gorodnichenko AI, Tursin PS, Reshetnyak VK, Uskov ON. Use of Noninvasive Interactive Neurostimulation to Improve Short-Term Recovery in Patients with Surgically Repaired Bimalleolar Ankle Fractures: A Prospective, Randomized Clinical Trial. J Foot Ankle Surg. 2010;49:432–437. doi: 10.1053/j.jfas.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Giombini A, Casciello GF, Di Cesare MC, Di Cesare A, Dragoni S, Sorrenti DA. A controlled study on the effects of hyperthermia at 434 MHz and conventional ultrasound upon muscle injuries in sport. J Sports Med Phys Fitness. 2001;41:521–527. [PubMed] [Google Scholar]
- 13.Giombini A, Di Cesare A, Safran MR, Ciatti R, Maffulli N. Short-term effectiveness of hyperthermia for supraspinatus tendinopathy in athletes: a short-term randomized controlled study. Am J Sports Med. 2006;34:1247–1253. doi: 10.1177/0363546506287827. [DOI] [PubMed] [Google Scholar]
- 14.Bennet WF. specifity of Speed's Test: Arthroscopic technique for evaluating the biceps tendon at level of the bicipitale groove. Arthroscopy. 1998;14:789–796. doi: 10.1016/s0749-8063(98)70012-x. [DOI] [PubMed] [Google Scholar]
- 15.Holtbly R, Razmjou H. Accuracy of the Speed's and Yergason's tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy. 2004;20:231–236. doi: 10.1016/j.arthro.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 16.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesthesia & Analgesia. 86(1998):102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Edward H, Yian MD, Aarun J, Ramappa MD, Oernulf Arneberg MD, Christian Gerber MD. The constant score in normal shoulders. Journal of Shoulder and Elbow Surgery. 2005;142:128–133. doi: 10.1016/j.jse.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Patton WC, McCluskey GM., 3rd Biceps tendinitis and subluxation. Clin Sports Med. 2001;20:505–529. doi: 10.1016/s0278-5919(05)70266-0. [DOI] [PubMed] [Google Scholar]
- 19.Green S, Buchbinder R, Hetrick S. Physiotherapy intervention for shoulder pain. Cochrane database of systematic reviews. 2003. 2 Art. No. CD004258. [DOI] [PMC free article] [PubMed]
- 20.Bingol U, Altan L, Yurtkuran M. Low-power laser treatment for shoulder pain. Photomed Laser Surg. 2005;23:459–464. doi: 10.1089/pho.2005.23.459. [DOI] [PubMed] [Google Scholar]
- 21.Santamato A, Solfrizzi V, Panza F, et al. Short-term effects of high-intensity laser therapy versus ultrasound therapy in the treatment of people with subacromial impingement syndrome: a randomized clinical trial. Phys Ther. 2009;89:643–652. doi: 10.2522/ptj.20080139. [DOI] [PubMed] [Google Scholar]
- 22.Aktas I, Akgun K, Cakmak B. Therapeutic effect of pulsed electromagnetic field in conservative treatment of subacromial impingement syndrome. Clin Rheumatol. 2007;26:1234–1239. doi: 10.1007/s10067-006-0464-2. [DOI] [PubMed] [Google Scholar]
- 23.Murthi AM, Vosburgh CL, Neviaser TJ. The incidence of pathologic changes of the long head of the biceps tendon. J Shoulder Elbow Surg. 2000;9:382–385. doi: 10.1067/mse.2000.108386. [DOI] [PubMed] [Google Scholar]
- 24.Neer CS., 2nd Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 25.Barber FA, Byrd JB, Wolf EM, Burkhart SS. How would you treat the partially torn biceps tendon? Arthroscopy. 2001;17:636–639. doi: 10.1053/jars.2001.24852. [DOI] [PubMed] [Google Scholar]
- 26.Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of the pathologic lesions of the tendon of the long head biceps brachii. Am J Sport Med. 2009;37:828–833. doi: 10.1177/0363546508322179. [DOI] [PubMed] [Google Scholar]
- 27.Neuro Resource Group Inc Food and Drug Administration 510k database. Available at: http://www.ac-cessdata.fda.gov/cdrh_docs/pdf4/K042912.pdf. Accessed July 14, 2010. [Google Scholar]
- 28.Overgaard J, Gonzalez F, Hulshof MC, Arcangeli J, Dahl O, Mella O. Randomized trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet. 345(1995):540–543. doi: 10.1016/s0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 29.Hyun-Woo K, Dae-Hyun, Seo-Yeon Y, K, et al. The anti-inflammatory effects of low- and high-frequency electroacupuncture are mediated by peripheral opioids in a mouse air pouch inflammation model. J Altern Complement Med. 2006;12:39–44. doi: 10.1089/acm.2006.12.39. [DOI] [PubMed] [Google Scholar]
- 30.Maale G, Gamez MRN, Wild GMBa, Walker JRPT. The effect of an handheld, portable, neuro stimulator, using two concentric conductive electrodes with signals that are damped, bi-phasic oscillatory waveform, which use use skin as a conduit, in patients with chronic severe pain form large orthopedic surgical procedures. 18th Annual Symposium of the International Society for Technology in Arthroplasty; September 29 – October 2, 2005; Kyoto, Japan. [Google Scholar]
- 31.Pavoni PF, Possenti G, Marsiglia G, et al. A new device for microwave hyperthermia: from high quality physiotherapy to oncology. Paper presented at: 7th International Congress on Hyperthermia Oncology; April 9–13, 1996; Rome, Italy. [Google Scholar]
- 32.Coleman S. Knee injuries: InterX therapy to solve unsolved sport injuries. International Congress on Sports Rehabilitation and Traumatology; Bologna, Italy: 2005. [Google Scholar]