Summary
Graft necrosis following ACL reconstruction is often associated with the use of autologous grafts. Host cells rather than graft cells contribute to the repair of the tendon-bone interface and the remodeling of the autologous graft. The native tendon-bone interface is not recreated and the biomechanical properties are not restored back to native values. We examined the effects of introducing gaps within the tendon graft prior to ACL reconstruction in a rodent model. We hypothesised that gaps will make physical way for host cells to infiltrate and repopulate the graft and thus enhance healing. Animals were sacrificed at seven, fourteen, and twenty-eight days for biomechanical testing and histology. Our findings indicate that graft necrosis, usually observed in the initial two weeks of the healing process, is averted. Histological observations showed that tendon-bone healing stages were hastened however this didn’t translate into improved biomechanical properties.
Keywords: ACL reconstruction, graft cells, graft necrosis, host cells, rodent model, tendon-bone healing
Introduction
The native insertional site of the Anterior Cruciate Ligament (ACL) is classified as a fibrocartilaginous entheses and is characterized by four distinct zones. These regions gradually merge into one another and create a complex transitional structure that enables stress dissipation and smooth transition of forces across two materials with contrasting mechanical properties. The four zones that typify the fibrocartilaginous insertional site are morphologically separated into fibrous connective tissue, non-mineralized fibrocartilage, mineralized fibrocartilage and bone (1). Following surgical repair, particularly in the early postoperative periods, this highly specialized transitional zone is not recreated and normal mechanical properties are not achieved (2–4).
A strategy to improve the healing process and promote solid integration between tendon and bone in order to realize early biomechanical properties may hasten and improve rehabilitation and outcome.
The cell types that initiate and regulate the process of healing at this junction have not been concretely identified. It has been reported that in the first two weeks following ACL reconstruction in a rodent model, graft cells do not survive and the graft undergoes necrosis (5). It seems that the host cells rather than graft cells contribute to the repair of the interface and the remodeling of autologous grafts (6). These host cells could be of several types and the accumulation of these requires either recruitment of new cells from the circulatory system or mitogenesis of host cells found in the surrounding bone marrow, joint cavity, or the tendon graft (6). There is a need to facilitate the introduction of host cells into the grafted tendon in order to prevent early graft necrosis and initial weakness of the graft and further promote tendon-bone healing.
In this study we utilized a previously reported rodent ALC reconstruction model (6) to examine what effects the introduction of gaps within the tendon graft, prior to ACL reconstruction, may have on tendon-bone healing and graft necrosis. We hypothesized that introducing gaps will make physical way for host cells to infiltrate and repopulate the graft material post surgery thus prevent graft necrosis in the initial periods of the healing process and potentially improve biomechanical and histological properties of the tendon-bone interface.
Materials and Methods
Study approval was obtained from the local Animal Care and Ethics Committee.
Study Design
A total of 42 female Sprague Dawley rats (11 weeks of age) were used in this study. Animals were randomly allocated to 2 equal numbered groups (control reconstruction or reconstruction with artificially induced gaps within the tendon graft) and surgery was performed. Animals were sacrificed at 1 week (n = 6), 2 weeks (n = 18) and 4 weeks (n = 18) following surgery. Six rats in each group were culled for biomechanical testing at 2 and 4 weeks while the other 6 specimens at each time point were prepared for histological examination (3 per group). In order to test the hypothesis that gaps within the tendon do not compromise mechanical properties of the graft, an in vitro pilot study was completed prior to commencement of the animal study.
Animal Model and Surgery
A rodent ACL reconstruction model was adopted from Kawamura et al. (6) and slightly modified. Briefly, the animal was placed under general anesthesia and a longitudinal incision was made to the medial aspect of the distal leg and ankle. The flexor digitorum longus (FDL) tendon was identified and cut just distal to the ankle. A second incision was made over the knee a lateral parapatellar arthrotomy was performed and the native ACL excised. Using an 18-gauge needle (outer diameter 1.27 mm) a bone tunnel was made in the proximal tibia and the distal femur, entering the joint at the attachment sites of the ACL.
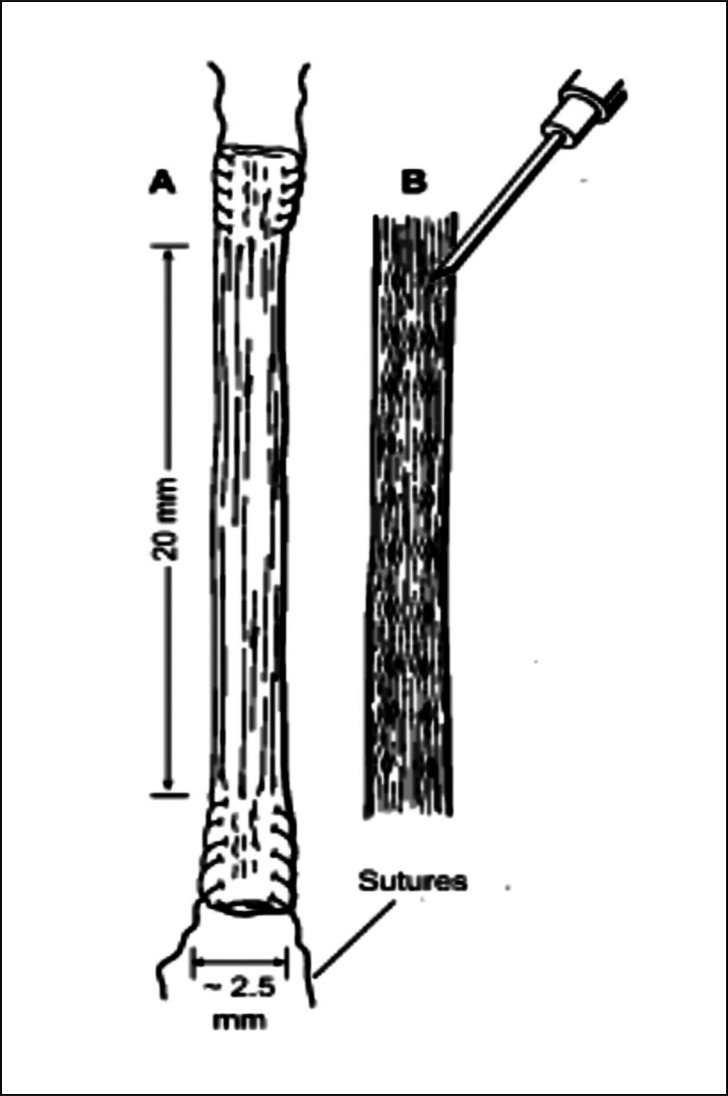
In the experimental group gaps were created between the collagen fascicles by the use of a 21-gauge needle (outer diameter 0.8 mm). 20 gaps were fashioned along two parallel rows (10 gaps within each) along the entire length of the graft (Fig. 1). The graft was left relaxed on top of a surgical swab while the gaps were created to prevent possible damage to the collagen fascicles. A 4-0 Ethibond suture (Ethicon, Somerville, NJ) was passed through each end of the previously-harvested tendon graft and then the graft was passed through the bone tunnels to replace the ACL. Both ends of the grafted tendon were secured to the surrounding periosteum at the extra-articular tunnel exit site at the distal femur and proximal tibia using 4-0 Ethibond suture. Muscle was closed with interrupted 4-0 Ethibond suture and skin was closed, as a separate layer to muscle, in a continuous fashion using 3-0 Vicryl suture (Ethicon, Somerville, NJ). Postoperatively, all animals were returned to their cage and activity was allowed ad libitum.
Figure 1.
Schematic drawing illustrating the FDL tendon graft (A) and positioning of gaps fashioned between the collagen fascicles (B). Gaps were created using a 21-gauge needle (outer diameter 0.8 mm). 10 gaps were created along each of the two parallel rows along the entire length of the graft.
Histology
Following sacrifice, right hindlimbs (from the femoral head to the distal tibia) were harvested. The skin and soft tissues were dissected away from the bones, with care taken not to disturb the knee joint. The femur-ACL graft-tibia specimens were fixed for 48 hours in 10% neutral buffered formalin. Tissues were transferred into a 10% formic acid-formalin solution for decalcification. Once adequate decalcification had been achieved (generally 2–3 days), the femur-ACL graft-tibia complex was sectioned axially, perpendicular to bone tunnels, into 8 sections. The axial sections were processed for paraffin histology using a tissue processing machine (Shandon Excelsior ES®, Thermo Fisher Scientific, Kalamazoo, USA). The segments were embedded in paraffin wax, cut into 5μm thick serial sections using a hand-operated microtome (Leica RM2165, Leica Instrument GmbH, Nussloch, Germany) and mounted onto glass slides for histology. Sections were stained using Harris Haematoxylin & Eosin (H&E).
Histology slides were assessed using an Olympus BX51 light microscope (Olympus, Sydney, Australia) under plain light. Histology was qualitatively assessed for integrity, quality, and viability of the graft, degree of tendon-bone integration, new bone formation, presence of fibrous tissue interface layer, cellular activity, and presence of Sharpey’s fibres. Assessments were made in a blinded fashion by three independent observers.
Histomorphology
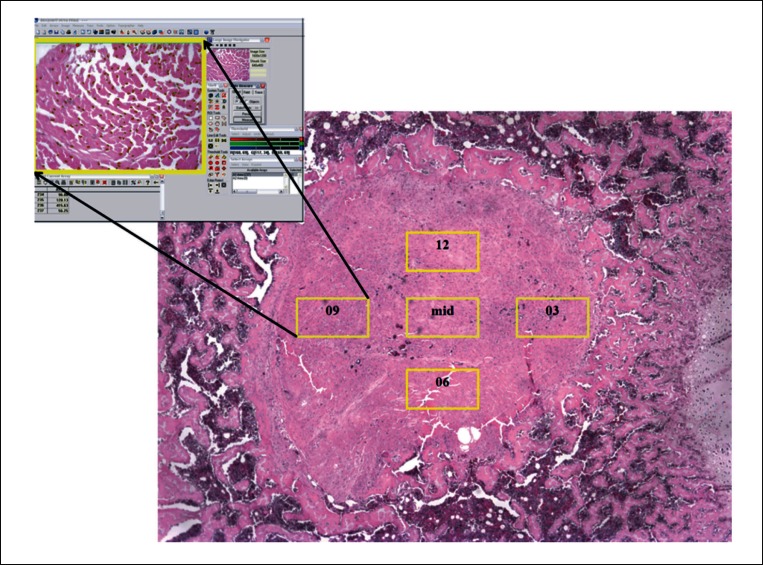
Histomorphometric assessment of cellularity was quantified using Bioquant software (BIOQUANT Image Analysis Corporation, Nashville, Tennessee). One tibial section per specimen, 5 mm below the tibial plate, was used for quantification of cellularity. Quantitative analysis involved counting the number of fibroblasts within a corresponding tibial section of all the specimens prepared for histology. Firstly, images containing the entire graft area (x4 magnification) were digitally captured (for reference purposes) using a camera (DP72, Olympus, Tokyo, Japan) attached to the microscope. The magnification of the microscope was increased to ×20 and a further five equivalent regions of interest (ROIs) were captured for fibroblast cell counting. The ROIs namely ‘03’, ‘06’, ‘09’, ‘12’ (referenced to analogous hours on a clock), and ‘mid’ were placed in corresponding regions of the graft across all specimens examined (Fig. 2). The captured images were then imported to the Bioquant software and processed to select only the fibroblastic cells within the ROI (Fig. 3). The cells were quantified for each ROI and the number of fibroblasts within one section of one specimen was estimated by the total number of cells within all five ROIs of that specimen.
Figure 2.
Histology section (×4 magnification) of ACL tendon graft within a tibial bone tunnel. Five regions of interest, 03, 06, 09, 12, and mid (×20 magnification) are seen within yellow rectangles. Each of the regions of interest was imported into Bio-quant software (represented in the top left corner) for fibroblast cell counting.
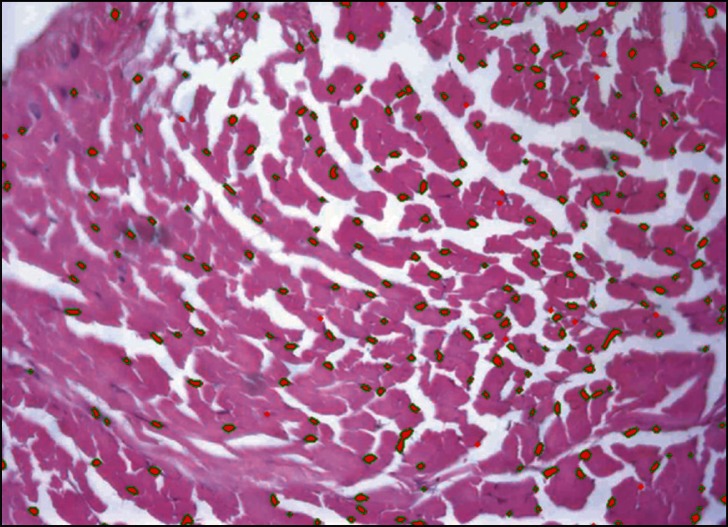
Figure 3.
ACL graft region of interest (one out of five) imported in the Bioquant software. Fibroblasts can be seen selected and highlighted in red color with green bordering. The cells were quantified for each ROI and the number of fibroblasts within one section of one specimen was estimated by the total number of cells within all five ROIs of that specimen.
Mechanical Testing
In vitro mechanical testing was performed on 16 rat FDL tendons (n = 8 control group & n = 8 experimental group). Gaps in the experimental group were created in identical fashion as the in vivo study. Mechanical testing parameters were kept constant.
Mechanical testing of the specimens from the in vivo study involved harvesting the femur-ACLgraft-tibia construct from each animal. The constructs were stripped of all soft tissue. Surgical loupes were used and extra care taken to ensure no damage was made to the ACL graft whilst dissecting the remaining knee ligaments. Sutures used to secure the graft were not removed from the specimens. Following complete dissection, the construct was embedded, in full extension, into custom made jigs using low point Wood’s metal to ensure secure fixation. Specimens were mounted onto a material testing machine 858 Bionix (MTS Systems, MN, USA). Mechanical testing was performed in force control with zero axial load. The construct was loaded to failure at a rate of 0.5mm/s. Failure mode of the specimen construct was recorded as the site of the graft failure; graft midsubstance, tibial tunnel, or femoral tunnel. Testing was performed at room temperature and tendon kept hydrated with Phosphate Buffered Saline (PBS) throughout.
Mechanical testing data was analyzed using MatLab 7.0 (The Math Works Inc, Natick, Massachusetts). Load-deformation curves were generated for each specimen and load and stiffness values obtained. The maximum load required for the construct to fail was defined as load-to-graft failure (Newton [N]). Stiffness was defined as the linear part of the slope of the load deformation curve with units of N/mm.
Statistical Analysis
For all quantitative parameters (peak load, stiffness, and cellularity) statistical comparisons between the groups were performed by 1-way ANOVA. Significance was set to an alpha level of p < 0.05. Analysis was performed using SPSS version 18.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
Results
The surgical procedure was well tolerated by all animals with normal cage activity resuming by the first post-operative day. At sacrifice, macroscopic dissection did not reveal any infection and all joint surfaces appeared normal. An intra-articular graft between the femur and tibia was present and appeared healthy in all specimens. Some thickened synovium was present at the anterior aspect of the intra-articular portion of the graft.
Histology
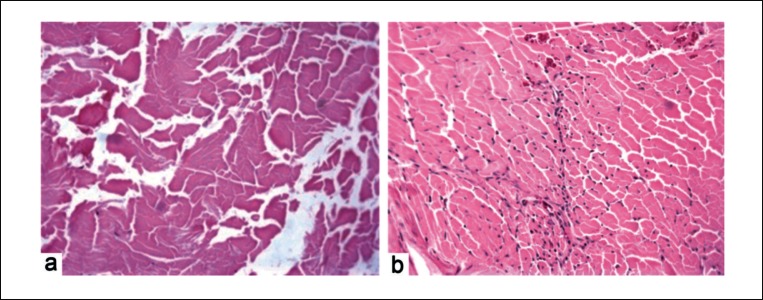
Slight visual differences were noted between the control and experimental groups at different time points. At week one post surgery microscopic evaluation of the healing tissue revealed formation of a loose and poorly organized fibrovascular interface layer between tendon and bone. Cellular composition of the interface consisted of neutrophils, fibroblasts, sparse osteoblasts on the outer edge of the bone tunnel and an influx of a high number of inflammatory cells. There were no differences noted between the groups. Graft necrosis was observed at the inner part of the tendon graft in the control group (Fig. 4a). Group with gaps within the tendon graft demonstrated an infiltration of host cells and a healthy live graft (Fig. 4b). Overall there was little or no evidence of structural incorporation of tendon into bone for either group. As time progressed, at the two week time period (Fig. 5), the fibrovascular interface layer demonstrated more collagen deposition and remodelling. It became more organized and dense. Differences were not noted between the control and treatment groups. In both groups, there was evidence of newly formed trabecular bone circumferentially around the bone tunnel. Some early remodelling of the tendon graft was seen at the outer portion of the tendon. Although a higher number of cells were observed in the control group at two weeks compared to four weeks post surgery, graft necrosis was still noted. In the treatment group the grafts looked healthy with cell numbers increasing steadily.
Figure 4.
Histology images (H&E staining) of tendon graft at one week post surgery. Control group (a) showed a necrotic graft while treatment group (b) showed a live graft, populated with cells (original magnification ×20). Significant difference was seen at one (p < 0.01) and two weeks (p < 0.05) post surgery.
Figure 5.
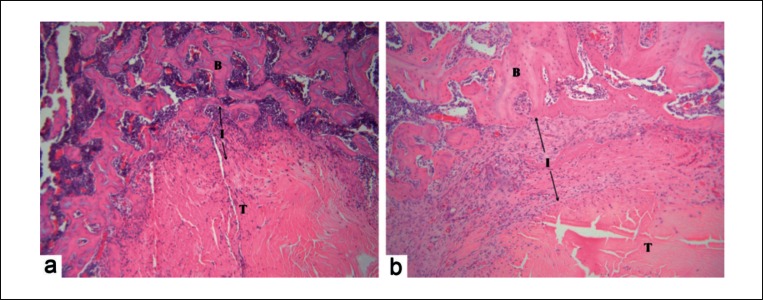
Histology images (H&E staining) of tendon-bone interface at two weeks post surgery B = Bone, I = Interface, T = Tendon, arrows denote the width of the interface layer. No major differences were noted between the interface layer and newly formed trabeculae surrounding the tendon graft across specimens from the control group (a) and treatment group (b) (original magnification ×10).
At four weeks post surgery more new woven bone was deposited along the bone tunnel in the treatment group than the control group. There was some evidence of indirect type tendon-to-bone insertion in the group that had gaps induced into the grafted tendon as noted by fibre (Sharpy’s-like fibers) continuity between tendon graft and lamellar bone as denoted by thin arrows in Figure 6. In the control group there were distinct margins between tendon and surrounding bone. Cell numbers had increased in both groups and graft necrosis was not seen. Abundant number of small blood vessels (denoted by thick arrows in Fig. 6) indicating greater graft vascularity, was seen in the treatment group.
Figure 6.
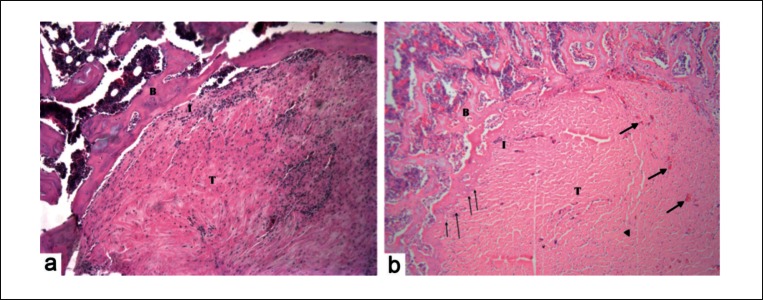
Histology images (H&E staining) of tendon-bone interface at four weeks post surgery B = Bone, I = Interface, T = Tendon, thin arrows denote Sharpy’s-like fibres, thick arrows denote small blood vessels. Less lamellar bone was seen in control group (A). Increased number of Sharpy’s-like fibers was seen protruding from the lamellar bone into the tendon graft in the treatment group (B). Greater graft vascularity, was also seen in the treatment group (original magnification ×10).
Histomorphology
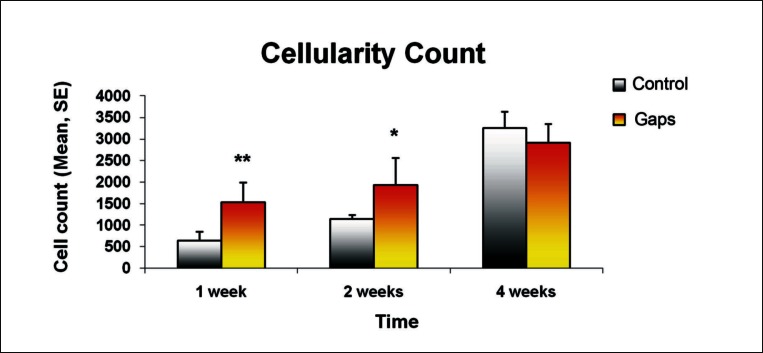
Histomorphology confirmed histological findings of graft necrosis within the inner portion of the tendon graft in the control group at one and two weeks post surgery. Significant difference in cellularity count was noted between the two groups at both one week (* = p < 0.01) and two weeks (** = p < 0.05) while there was no significant difference found at four weeks (Fig. 7).
Figure 7.
Significant difference in the cellularity count was noted between the two groups at both one week (** = p < 0.01) and two weeks (* = p < 0.05) while there was no significant difference found at four weeks. All values are expressed as mean ± standard error.
Mechanical Testing
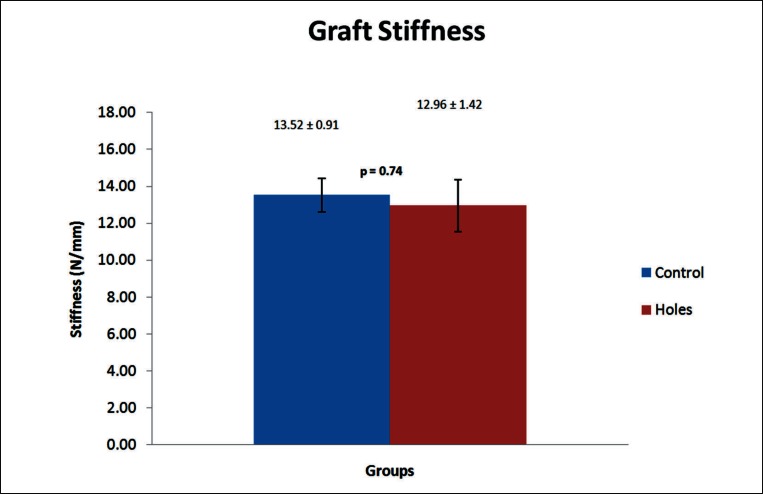
In vitro mechanical testing of the rat FDL tendons with and without gaps showed no significant difference in stiffness values between the groups (p = 0.74). Graphical representation of these results is depicted in Figure 8. Due to the size of the rat FDL tendon peak-load values were not recorded due to tendon slippage from the grips.
Figure 8.
Mean graft stiffness of in vitro tested FDL tendon grafts from the control and experimental groups. No significant difference was noted between the groups. Data is presented as mean ± standard error.
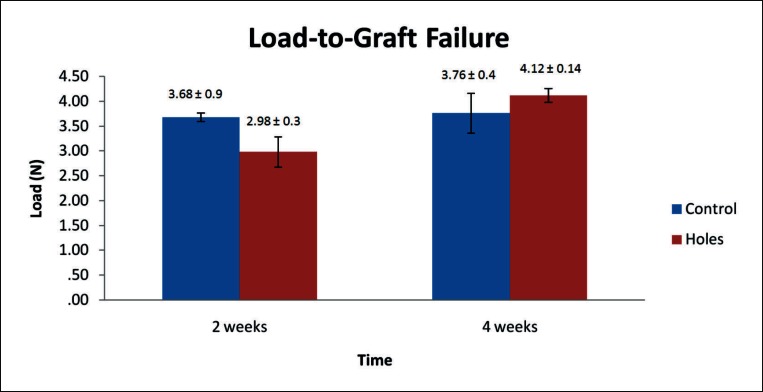
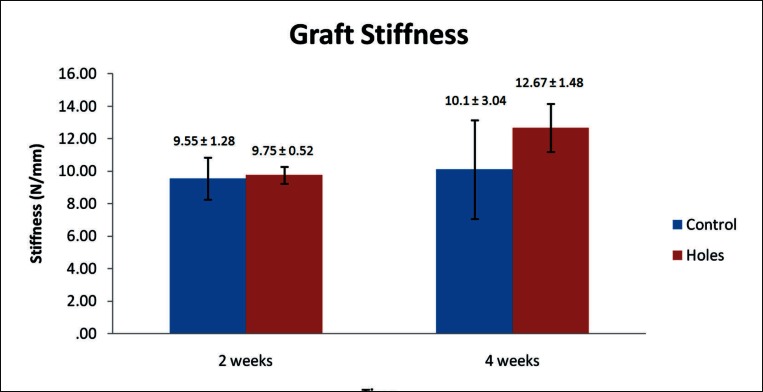
During mechanical testing of the in vivo study specimens two samples failed at the tibial growth plate and were discarded from the results. Mechanical data is summarized below and presented in Figure 9 (load-to-graft failure) and Figure 10 (graft stiffness). All values are expressed as mean ± standard error. At fourteen days post surgery load-to-graft failure was higher for the control than the experimental group (3.68 ± 0.9 N and 2.98 ± 0.3 N, respectively) while the graft stiffness was almost identical (9.55 ± 1.28 N/mm and 9.75 ± 0.52 N/mm, respectively). These differences were not found to be significant. At four weeks post surgery the load-to-graft failure values increased in both groups with the percentage increase lower in the control group than the treatment group. The treatment group peak load to failure was higher than the control (4.12 ± 0.14 N and 3.76 ± 0.4 N, respectively) but the difference was not found to be significant. Graft stiffness decreased between two and four weeks in the control group (10.1 ± 3.04 N/mm) and was lower than that of the treatment group (12.67 ± 1.48 N/mm) which itself increased. This difference was not found significant.
Figure 9.
Mean load-to-graft failure of control and experimental groups at two and four weeks post surgery. No significant difference was noted between the groups or within groups across time points. Data is presented as mean ± standard error.
Figure 10.
Mean graft stiffness of control and experimental groups at two and four weeks post surgery. No significant difference was noted between the groups or within groups across time points. Graft stiffness decreased between two and four weeks in the control group while an increase was seen in the experimental group. Data is presented as mean ± standard error.
No major differences were observed in failure modes between the groups. At two weeks in the treatment group, three specimens failed at midsubstance, one at the tibial tunnel, and two at the femoral tunnel while in the control group two specimens failed at each one of the failure sites. At four weeks, two specimens failed at midsubstance in the control group and four in the treatment group. No failures were seen at the tibial tunnel while three specimens failed at the femoral tunnel in the control group and one in the treatment group.
Discussion
It is widely acknowledged that the four zones typical of fibrocartilaginous type entheses, such as that present at the insertion of the ACL, are not regenerated following surgical repair (1, 7–10). This is thought to contribute to inferior mechanical properties of the interface, particularly in the early post-operative time periods, resulting in delayed rehabilitation. There is a need to hasten the early tendon-to-bone healing process and augment the mechanical strength of the interface between bone and tendon to facilitate an early return to competitive sport, work, and other regular daily activities.
Tendon-bone interfaces are classified as direct or indirect based on the orientation of the collagen fibers at the interface (11). Considering the geometry of a tendon graft in a bone tunnel, it is impossible to form a direct interface since the collagen fibers are perpendicular to the bone. The introduction of a gap between the collagen fascicles without compromising the mechanical properties of the graft could allow for more robust tissue integration and potentially a more robust tendon-bone interface.
Our histomorphology analysis and histology observations indicate that graft necrosis usually observed in the initial two weeks of the healing process is averted by inducing gaps into the tendon graft prior to ACL reconstruction. Furthermore, early infiltration of host cells and repopulation of the graft seems to initiate healing at an earlier stage, ultimately leading to a more profound tendon-bone interface as indicated by histology.
These positive differences did not translate into superior mechanical strength of the interface. Biomechanical testing revealed similar load-to-graft failure and graft stiffness values across both groups at both two and four week time points.
The in vitro mechanical testing performed on the rat FDL tendons with and without gaps found no significant difference in graft mechanical properties. As a result we excluded any possibility that gaps may compromise the mechanical integrity of the graft in this study. Hence, it was assumed that the collagen fascicles were not damaged, and the mechanical properties similarly not compromised by creating gaps in the graft. To further reduce the risk of collagen fascicle damage, gaps were not created under tension i.e. the graft was left relaxed and hydrated on top of a surgical gauze swab while the gaps were fashioned. It is important to note that in this study we induced gaps throughout the entire length of the tendon graft. In this animal model, due to the graft’s diminutive size it was extremely difficult to approximate the exact portion of the graft which was to correspond to the intraosseous portion. Thus gaps were induced throughout the entire length of the graft. Although we hypothesised desired effects of introducing gaps within the intraosseous portion of the graft, the gaps in the intra-articular part of the graft were constantly exposed to synovial fluid which could have had a negative effect on the mechanical strength of the tendon. Synovial fluid contains various matrix metalloproteinases (MMPs) which are a family of zinc dependant proteases that have been found to degrade components of extracellular matrix of tissues in which they are found in (12). Demirag et al. (13) found that tendon-bone healing in a rabbit ACL model can be enhanced by effectively blocking MMPs. Specimens which received an MMPs inhibiter showed a significantly greater load-to-failure at both two and five weeks post surgery. Greater number of Sharpy’s like fibres and a more mature interface tissue was also noted. Although results from this study did not demonstrate histological evidence of degeneration of the intra-articular portion of the graft, it is possible that mechanical properties of the graft were affected due to the influx of synovial fluid. In a clinical setting, using a human sized tendon graft it would be much more feasible to limit the gaps to the portions of the graft that are intended solely to the tibial and femoral bone tunnels. This would limit the potential degenerative problems associated with the influx of synovial fluid into the intra-articular portion of the graft while still providing benefits of gaps within the intraosseous part of the graft.
Another factor which could have impacted on the results of biomechanical testing was the graft preparation for the testing procedure. In order to avoid any graft damage in and around the bone tunnels upon harvest, the sutures used to secure the graft to the surrounding periosteum were not removed. This could have partly contributed to the measured mechanical strength of the tendon-bone interface. Furthermore, the femur-ACL graft-tibia complex was tested in full extension and the direction of force may not have been in direct alignment with the long axis of the graft, potentially biasing results. Lastly, during mechanical testing two specimens failed at the tibial growth plate. This was not considered the true strength of the interface and thus, these specimens were discarded. This limited the number of specimens that were tested at the four week time period to only five per group, which potentially reduced the statistical power.
Major differences were observed in terms of graft necrosis as measured by quantitative histomorphology and histological observations. Graft necrosis has previously been reported to occur following ACL reconstructive surgeries (14, 15). Kobayashi et al. (5) also studied the fate of host and graft cells in early healing of a tendon graft within a bone tunnel. They demonstrated that graft cells survived for the first week following transplantation but completely disappeared within two weeks while the host cells survived throughout all time periods. As there were only a few fibroblasts within the graft at one and two weeks post surgery the graft was deemed to be necrotic.
We found a significantly higher number of cells, progressively ingrowing into the grafted tendon, in the treatment group compared to the control group in the first two weeks. This correlated with our histological observation of graft necrosis in the inner portion of the FDL graft in the control group and a viable healthy graft in the treatment group. There were no differences in cell number and graft viability seen at four weeks post surgery and no differences in cell morphology across specimens within or across groups at any time point. We speculate that prevention of early graft necrosis is achieved by making physical way for the host cells to infiltrate and repopulate the grafted tendon. It is likely that local cells i.e. pluripotent cells of the bone marrow within the cancellous bone surrounding the intraosseous portion of the FDL tendon graft are the cells that infiltrate the grafted tendon. It has been demonstrated that these cells have the ability to differentiate in a variety of cell types which can subsequently form mesenchymal tissues, such as bone, tendon, and ligament (16–18). In this study the early infiltration of host cells proved to initiate healing at an earlier stage resulting in a more profound tendon-to-bone interface in the treatment group by the four week time period as indicated by histological characteristics observed. Rate of healing and remodelling was seen analogous in both groups at one and two weeks post surgery but differences in the tendon-bone interface were evident at four weeks. In the treatment group, more woven bone was seen circumferentially around and continuous with, the bone tunnel with some sporadic Sharpy’s-like fibers protruding from the bone to the tendon. These results indicate that the gradual remodeling process of tendon-to-bone healing can be hastened when gaps are introduced into the grafted tendon during an ACL reconstruction. Furthermore it can be deduced that the grafted tendon simply functions as a scaffold which supports the host mesenchymal cells that infiltrate and initiate the healing process.
Recently many researchers have reported improved tendon-to-bone integration utilizing various biomaterials as carriers for application of growth factors (19–21), and mesenchymal stem cells (MSCs) (22, 23). The advantage of this study is that histological improvement at the tendon bone interface was achieved via mechanical means alone. In addition, graft necrosis that transpires following ACL reconstruction was averted.
Clinically, inducing gaps within the tendon graft would be an easy step a surgeon could perform prior to graft placement. Furthermore the graft has the potential to be used as a scaffold and a carrier, and gaps could be injected with desired biomaterials to augment the interface between tendon and bone.
Future studies should focus on introducing biomaterials into the gaps created to further improve the healing of tendon-to-bone.
References
- 1.Walsh WR, Harrison JA, Van Sickle D. Patellar Tendon-to-Bone Healing Using High-Density Collagen Bone Anchor at 4 Years in a Sheep Model. The American Journal of Sports Medicine. [Feature Article] 2004;32(1):91–95. doi: 10.1177/0363546503260709. [DOI] [PubMed] [Google Scholar]
- 2.Rodeo S, Arnoczky S, Torzilli P, Hidaka C, Warren R. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Brophy RH, Kovacevic D, Imhauser CW, et al. Effect of Short-Duration Low-Magnitude Cyclic Loading Versus Immobilization on Tendon-Bone Healing After ACL Reconstruction in a Rat Model. J Bone Joint Surg Am. 2011;93(4):381–393. doi: 10.2106/JBJS.I.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh WR, Stephens P, Vizesi F, Bruce W, Huckle J, Yu Y. Effects of Low-Intensity Pulsed Ultrasound on Tendon-Bone Healing in an Intra-articular Sheep Knee Model. Arthroscopy. 2007;23(2):197–204. doi: 10.1016/j.arthro.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Watanabe N, Oshima Y. The Fate of Host and Graft Cells in Early Healing of Bone Tunnel After Tendon Graft. The American Journal of Sports Medicine. [Feature Article] 2005;33(12):1892–1897. doi: 10.1177/0363546505277140. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura S, Ying L, Kim H-J, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23(6):1425–1432. doi: 10.1016/j.orthres.2005.01.014.1100230627. [DOI] [PubMed] [Google Scholar]
- 7.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental Rotator Cuff Repair. A Preliminary Study. J Bone Joint Surg Am. 1999;81(9):1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter JF, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: A biomechanical and histologic analysis in an animal model. Journal of Shoulder and Elbow Surgery. 1998;7(6):599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 9.Thomopoulos S, Hattersley G, Rosen V, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: An in situ hybridization study. Journal of Orthopaedic Research. 2002;20(3):454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 10.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16(5, Supplement 1):S191–S197. doi: 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons—tendon ‘entheses’. Comparative Biochemistry and Physiology - Part A: Molecular & Integrative Physiology. 2002;133(4):931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 12.Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. Journal of Shoulder and Elbow Surgery. 2009;19(3):384–391. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Demirag B, Sarisozen B, Ozer O, Kaplan T, Ozturk C. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. Journal of Bone & Joint Surgery, American Volume. 2005;87(11):2401–2410. doi: 10.2106/JBJS.D.01952. [DOI] [PubMed] [Google Scholar]
- 14.Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft: an experimental study. J Bone Joint Surg Am. 1986;68(3):376–385. [PubMed] [Google Scholar]
- 15.Tomita F, Yasuda K, Mikami S, Sakai T, Yamazaki S, Tohyama H. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-Patellar tendon-Bone graft in anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2001;17(5):461–476. doi: 10.1053/jars.2001.24059. [DOI] [PubMed] [Google Scholar]
- 16.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for achilles tendon repair. Journal of Orthopaedic Research. 1998;16(4):406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 17.Kai T, Shao-qing G, Geng-ting D. In Vivo Evaluation of Bone Marrow Stromal-Derived Osteoblasts-Porous Calcium Phosphate Ceramic Composites as Bone Graft Substitute for Lumbar Intervertebral Spinal Fusion. Spine. 2003;28(15):1653–1658. doi: 10.1097/01.BRS.0000083168.37329.B4. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain G, Fox J, Ashton B, Middleton J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 19.Rodeo SA, Suzuki K, Deng X-h. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. [Feature Article] 1999;27(4):476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 20.Martinek V, Latterman C, Usas A, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: A histological and biomechanical study. Journal of Bone and Joint Surgery - Series A. 2002;84(7):1123–1131. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Chen C-H, Liu H-W, Tsai C-L, Yu C-M, Lin IH, Hsiue G-H. Photoencapsulation of Bone Morphogenetic Protein-2 and Periosteal Progenitor Cells Improve Tendon Graft Healing in a Bone Tunnel. The American Journal of Sports Medicine. 2008;36(3):461–473. doi: 10.1177/0363546507311098. [DOI] [PubMed] [Google Scholar]
- 22.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(9):899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Ouyang HW, Goh JCH, Lee EH. Use of Bone Marrow Stromal Cells for Tendon Graft-to-Bone Healing: Histological and Immunohistochemical Studies in a Rabbit Model. The American Journal of Sports Medicine. [Feature Article] 2004;32(2):321–327. doi: 10.1177/0095399703258682. [DOI] [PubMed] [Google Scholar]