Summary
We investigated the extent of mechanical interaction between rat flexor carpi ulnaris (FCU) and palmaris longus (PL) muscles following transfer of FCU to the distal tendons of extensor carpi radialis brevis and longus (ECRB/L) muscles. Five weeks after recovery from surgery, isometric forces exerted at the distal tendons of FCU and PL were quantified at various FCU lengths. PL was kept at a constant length. Changing the muscle-tendon complex length of transferred FCU (by maximally 3.5 mm) decreased PL force significantly (by 7%). A linear relationship was found between changes in FCU muscle belly length, being a measure of muscle relative positions, and PL force. These results indicate that despite transfer of FCU muscle to the extensor side of the forearm, changing FCU length still affects force transmission of its, now, antagonistic PL muscle. We conclude that a transferred muscle may still be mechanically linked to its former synergistic muscles.
Keywords: skeletal muscle, scar tissue, connective tissue, tendon transfer, myofascial, wrist
Introduction
In a recent study, we reported mechanical interactions between neighboring wrist flexors in the rat (1). Epimuscular myofascial force transmission is considered to be the responsible mechanism for such intermuscular interactions (2, 3). If the position of a muscle belly is changed relative to a neighboring muscle belly, connective tissue linkages between them will be strained and provide a pathway to transmit force. The most direct mechanical linkage exists between synergistic muscles adjacent within a muscular compartment and, accordingly, we found higher interaction between flexor carpi ulnaris (FCU) and palmaris longus (PL) muscles than between FCU and extensor carpi ulnaris (1).
In several surgical procedures, applied in for example tetraplegia and spastic cerebral palsy, the insertion of specific muscle-tendon complexes are transferred surgically to the tendon of an antagonistic muscle (4–6). The transferred muscle is considered to convert from its previous mechanical effect (e.g., joint flexion and adduction) to yielding a moment according to its new position with respect to the joint axis (e.g., joint extension and abduction). However, results contrary to that concept (i.e., no change in muscle function) have been reported (7, 8). As not only the distal tendon is transferred, but in most cases, also a substantial part of the muscle belly is rerouted, the characteristics of myofascial connections and, hence, the mechanical interactions with neighboring muscles is expected to change. Intermuscular connectivity may be affected also by the formation of scar tissue, occurring after such invasive surgical procedures (9, 10).
Transfer of FCU to the extensor carpi radialis brevis and/or longus (ECRB/L) tendons is commonly performed with the aim of restoring wrist extension or to correct spastic wrist flexion deformity (5, 11). In a recent study (12), we reported that, in the rat, even after transfer of FCU to a wrist extensor insertion site, force was transmitted from active FCU to the distal tendon of passive PL. In that study, FCU muscle was excited exclusively, so that effects of FCU on transmission of active forces generated by muscle fibers within PL were not studied. Pilot data suggest that also after tendon transfer, rat FCU and PL muscles are active simultaneously during the stance phase of walking (13).
Therefore, the purpose of the present study was to investigate the extent of mechanical interaction between active FCU and active PL after FCU tendon transfer. Effects of length changes of transferred FCU on forces exerted at the distal tendon of PL, kept at a constant muscle-tendon complex length, were assessed. We hypothesize that force transmission between FCU and PL muscles (both wrist flexors) to be decreased, but not to be completely absent after transfer of FCU to the ECRB/L tendons.
Materials and Methods
Animals
Data were obtained from 7 male Wistar rats. Surgical and experimental procedures were in strict agreement with the guidelines and regulations concerning animal welfare and experimentation set forth by Dutch law and approved by the Committee on Ethics of Animal Experimentation at the VU University.
All rats were housed in large cages (0.55 × 0.33 × 0.20 m, two rats per cage) with access to food and water ad libitum.
Surgical procedures for FCU-to-ECRBL tendon transfer
Tendon transfer surgeries were performed under aseptic conditions, while the rats (body mass at the time of surgery 182 ± 13 g) were deeply anesthetized (respiration of 1–3% isoflurane). At the same time, the rats were treated with a single dose (0.03 ml) of buprenorphin (Temgesic®, Schering-Plough, Maarssen, The Netherlands; 0.3 mg/ml solution) for pain relief. Body temperature was monitored and the anesthetic state was checked routinely by evaluating withdrawal reflexes. Surgical procedures have been described in detail previously (14). In brief, the distal tendon and distal half of the muscle belly of FCU was dissected free and transferred to the tenotomized distal tendons of extensor carpi radialis (ECRBL). After recovery from the surgery, the rats were kept in their cages for five weeks with access to food and water ad libitum.
Surgical procedures in preparation for assessing FCU-PL interaction
The rats were deeply anesthetized using intraperitoneally injected urethane (initial dose 1.2 ml/100 g body mass, 12.5% urethane solution), as judged by complete absence of withdrawal reflexes. If withdrawal reflexes could be elicited, supplemental doses (0.3–0.5 ml a time) were administered. To prevent hypothermia, the animals were placed on a heated water pad at approximately 37°C.
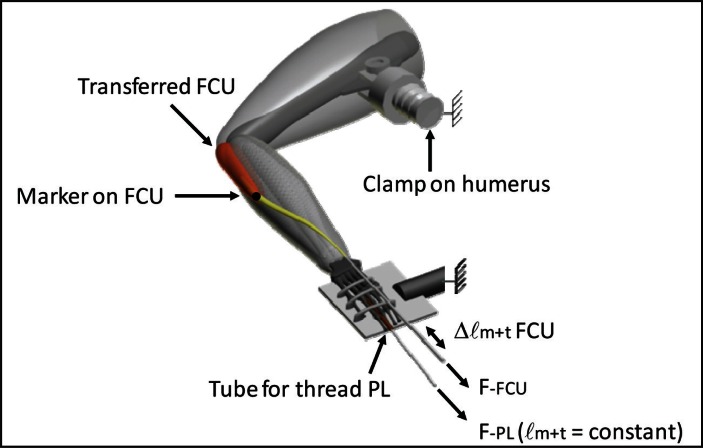
The right forelimb was shaved, and the skin was resected from the shoulder to the wrist. The distal tendons of PL and transferred FCU were identified. With the wrist in a neutral position (i.e., 180° flexion) and the elbow joint at approximately 90°, markers were placed on FCU and PL distal tendons, as well as on a fixed location on the forearm to indicate reference lengths, using 7-0 suture (Prolene, Ethicon). Subsequently, the distal tendons of FCU and PL were released from the skeleton and attached to Kevlar thread. Note that the distal tendons of transferred FCU were cut from the second metacarpal (tendon serving previously as ECR longus insertion) and from the third metacarpal (tendon serving previously as ECR brevis insertion). Further dissection was performed in the brachial compartment to secure a metal clamp to the humerus for later fixation to the experimental setup (Fig. 1). The connective tissues enveloping the muscle bellies in the antebrachial compartment were left intact.
Figure 1.
Schematic view of right forelimb in experimental setup
The rat was placed on a heated platform. The right forelimb was secured rigidly by clamping the humerus and by firmly tying the manus to a aluminum plate with 1-0 silk suture. The forearm was secured in horizontal position, with the dorsal side of the manus faced upwards, the wrist in neutral position (i.e., 180° flexion) and the elbow joint at approximately 90°. The distal tendons of m. flexor carpi ulnaris (FCU) and m. palmaris longus (PL) were connected to two separate force transducers, mounted on single axis micropositioners. PL was connected via a tube in between the plate and the palmar side of the manus. In the experiment, the muscle-tendon complex length of FCU was varied (Δ ℓm+t FCU). In contrast, muscle-tendon complex length of PL was kept constant. The connective tissues enveloping the muscle bellies were left intact. A marker (•) was placed at the most distal end of FCU muscle belly.
Within the brachial compartment, the ulnar and median nerves were identified and placed in a bipolar cuff electrode. The ulnar and median nerves innervate only palmar muscles of the antebrachium (‘wrist and digit flexors’) and, thus, the wrist and digit extensors were not excited, in all conditions of this experiment.
Experimental procedures
Body mass at the time of experiment was 354 ± 19 g. During the experiments, muscle temperature was controlled by an airflow (Holland Heating) around the muscle of 22 ± 0.5 °C and 80 ± 2 % humidity. Dehydration of forelimb was prevented by regularly irrigating the exposed tissues with isotonic saline. At the end of the experiments, the rats were euthanized without regaining consciousness with a lethal dose of pentobarbital sodium (200 mg, i.p.) and double-sided pneumothorax was performed.
The rat was placed on a heated platform. The right forelimb was secured rigidly by clamping the humerus and by firmly tying the manus to an aluminum plate with 1-0 silk suture (Fig. 1). Using the Kevlar threads connected in series with steel rods, the distal tendons of FCU and PL muscles were each connected to a force transducer (BLH Electronica, Canton, MA, USA; maximal output error <0.1%, compliance of 0.0162 mm/N). The force transducers were positioned in the line of pull for each muscle. The line of pull for PL runs as close as possible to the palmar side of the manus, which was obstructed by the forepaw plate. Therefore, we passed the Kevlar thread attached to PL tendon through a Teflon tube (inner diameter 0.8 mm) in between the plate and the palmar side of the manus (Fig. 1). Force loss in the tube due to friction was < 0.6% within the PL force range measured. For all experimental conditions, PL muscle was kept at a constant muscle-tendon complex length (i.e., the reference length). The ulnar and median nerves were stimulated supramaximally with the cuff electrode connected to a constant current source (0.3 mA, pulse width 100 μs). Stimulation of the ulnar and median nerves will excite FCU and PL muscles, as well as all palmar muscles of the antebrachium (“wrist and digit flexors”).
Isometric forces of PL and transferred FCU were measured at various muscle-tendon complex lengths (ℓm+t) of FCU. Starting from the lowest length at which active muscle force exerted at the tendon approaches zero, FCU muscle was lengthened distally using 0.5 mm increments to approximately 1 mm over the length corresponding maximal active force. Prior to excitation, FCU was brought to the target length. Two twitches were evoked, followed by a tetanic contraction of the muscles (stimulation frequency 100 Hz). After each contraction FCU was allowed to recover at low length for 2 minutes. Force signals were digitized at 1 kHz and stored on a PC using a data acquisition board (PCI-6221, National Instruments, Austin, TX, USA). Passive force was assessed by calculating the mean for the 50 ms time window just prior to the tetanic contraction. Total force was assessed by calculating the mean for the last 50 ms of the tetanic plateau.
MUSCLE BELLY LENGTH. To assess changes in muscle belly length (ℓm), a marker was placed on the most distal end of FCU muscle belly. Marker position at each lm+t was recorded during isometric contractions (ℓma) using a digital camera (Sony HC53E Digital Camcorder, 25 frames/s, 720 X 576 pixels, resolution 1 pixel ∼ 0.1 mm).
Treatment of data
Mathematical functions were fitted to the experimental data for further treatment and averaging.
In short, individual ℓm+t – passive force (Fp) data were least-squares fitted with an exponential function. Due to a different shape of the ℓm+t – Fp curve in four muscles, the exponential function did not yield an adequate fit and a polynomial function (see below) was used instead.
Active force (Fa) was defined as the added total force exerted at the force transducer upon supramaximal stimulation of the ulnar and median nerves. Fa was assessed by subtracting passive force from total force at equal ℓm+t.
The following characteristics were fitted using a polynomial: ℓm+t – Fa and ℓm+t – ℓma. The order of the polynomial most adequately describing the data was selected, using a stepwise polynomial regression procedure (i.e., the order of the polynomial was increased up to maximally a sixth order, as long as this yields a significant improvement to the description of the length-active force data, as determined by one-way ANOVA).
Fitted curves were used to (i) determine maximal active force and the corresponding muscle-tendon complex length, as well as the length corresponding to zero active force of FCU, (ii) to calculate mean data and standard deviations (SD).
For each individual muscle, optimal force is defined as the maximum of the fitted polynomial for Fa, and lo is defined as the corresponding muscle-tendon complex length. For both passive and active forces, muscle-tendon complex length was expressed as the deviation from the length corresponding maximal active force (Δ ℓm+t). Muscle belly length was expressed as the deviation from its lenght corresponding to zero force exerted of the tendon during muscle contraction.
Statistics
To test for effects of FCU length on PL and FCU forces, one-way ANOVAs for repeated measures were performed. P values < 0.05 were considered significant.
Results
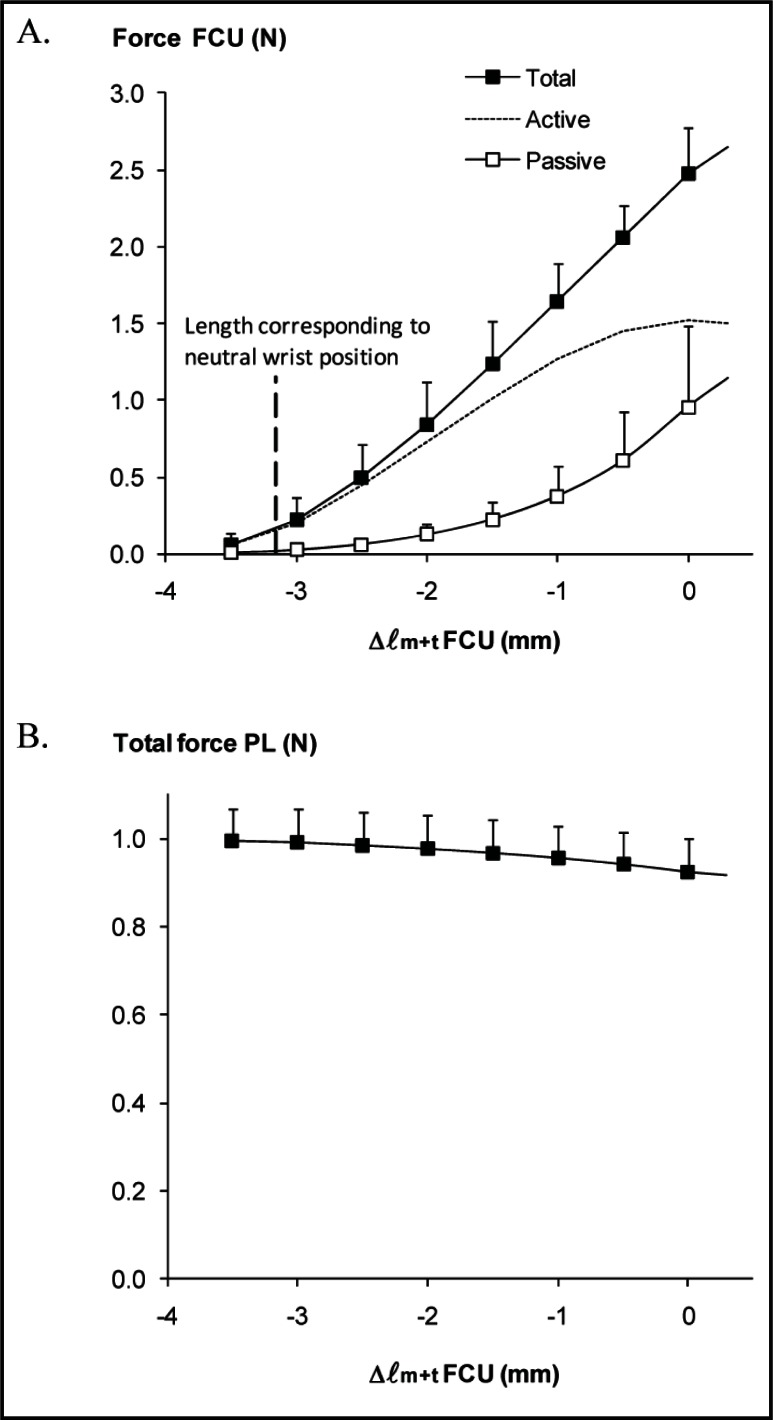
MUSCLE FORCES. FCU passive force as well as force exerted during the isometric contraction (total force) increased monotonically with lengthening FCU muscle-tendon complex (Fig. 2A). The calculated additional force as a result of muscle excitation (active force) attained a maximum of 1.52 ± 0.34 N and passive force at this length was 1.0 ± 0.5 N. Note the exertion of notable passive FCU forces also at low lengths (as low as Δ ℓm+t = −2.5 mm), typical for muscles acting within their connective tissue context. However, the ratio of passive force to active force (0.66 at Δ ℓm+t = 0 mm) is much higher in transferred FCU than commonly observed in physiological conditions.
Figure 2 A-B.
Effects of lengthening transferred FCU on distal forces of FCU and PL.
A. Forces exerted at the transferred FCU prior to (passive) and during tetanic contraction (total), as well as the calculated extra force as a result of muscle excitation (active). B. PL total force plotted as a function of FCU muscle-tendon complex length. Passive forces in PL muscle were negligible (not shown). Note that PL was kept at a constant muscle-tendon complex length.
Muscle-tendon complex length is expressed as the deviation from the length corresponding maximal active force (Δ ℓm+t).
Active muscle force was assessed by subtracting passive force from total muscle force at equal ℓm+t. Vertical dotted line in A indicates the mean reference length of FCU corresponding to the length at neutral wrist position (Δ ℓm+t = −3.2 ± 0.6 mm). Values are shown as means + SD (n = 7).
The length range between the lengths corresponding to zero and maximal active force was 3.9 ± 0.6 mm. At neutral wrist position, FCU attains a rather low muscle-tendon complex length (Δ ℓm+t = −3.2 ± 0.6 mm). This suggests that transferred FCU can exert fair forces (corresponding to a force higher than 50% of its optimal active force) at the new insertion exclusively in more flexed wrist positions (i.e., at higher lengths) combined with very limited or no forces at the new insertion in more extended wrist positions (i.e., at lower lengths).
ANOVA indicates that lengthening of transferred FCU distally yields significant force decreases at the distal tendon of PL (Fig. 2B). Note that PL was kept at a constant muscle-tendon complex length. PL force decreased from its initial value (1.00 ± 0.07 N at Δ ℓm+t = −3.5 mm) to 0.93 ± 0.08 N at Δ ℓm+t = 0 mm, i.e. a decrease of 7%. The mean slope of this decrease was −0.02 ± 0.01 N/mm.
These results indicate that despite transfer of FCU muscle to the extensor side of the forearm, changing FCU length still affects force transmission of its, now, antagonistic PL muscle.
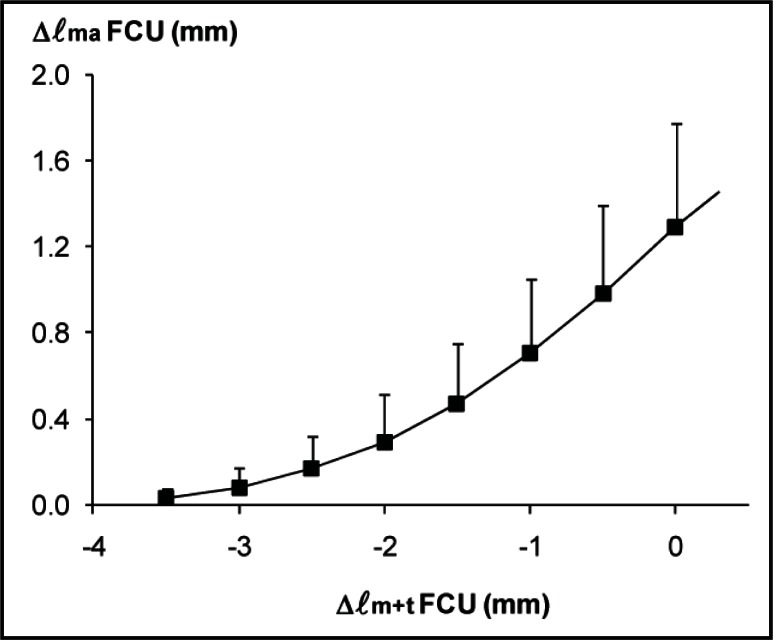
MUSCLE BELLY LENGTH. Length changes of FCU muscle belly were substantially smaller than the imposed changes in muscle-tendon complex length. After FCU muscle-tendon complex had been lengthened by 3.5 mm, its muscle belly length measured during contraction increased by 1.3 ± 0.5 mm (Fig. 3). In contrast to what one might expect for a maximally dissected FCU muscle, these results indicate that (part of) the distal tendon took up a major part of the FCU muscle-tendon complex length changes (i.e., 63%).
Figure 3.
Differences between FCU muscle-tendon complex length and muscle belly length changes.
Muscle belly length of transferred FCU during isometric contraction (Δ ℓma) plotted as a function of FCU muscle-tendon complex length (Δ ℓm+t). Muscle-tendon complex length is expressed as the deviation from the length corresponding maximal active force (Δ ℓm+t). Muscle belly length is expressed as the deviation from its length corresponding to zero force exerted at the tendon. Values are shown as means + SD (n = 6).
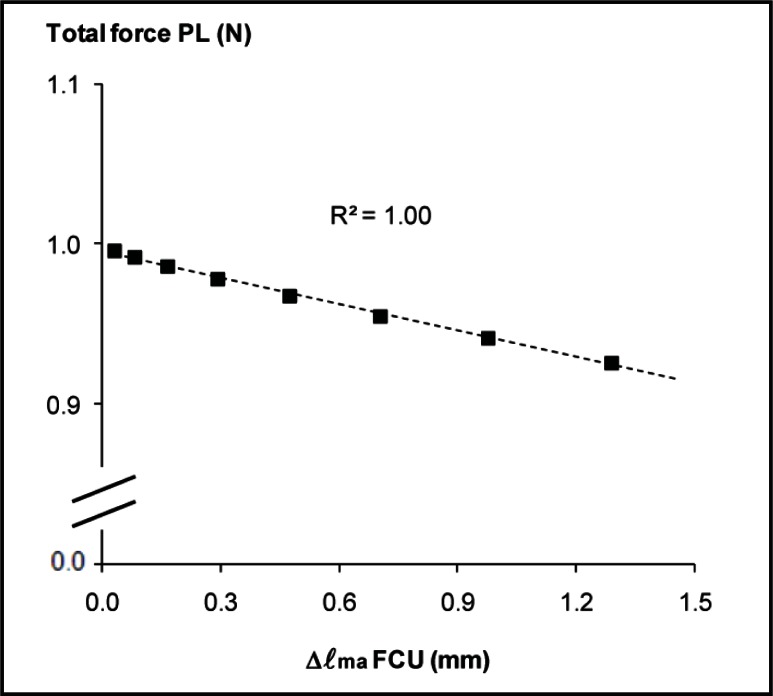
Changes in FCU muscle belly length (Δ ℓma) can be considered as a measure of changes in the position of FCU muscle belly relative to the muscle belly of PL. We found a linear relationship between Δ ℓma of FCU and force exerted at the distal tendon of PL muscle (Fig. 4). The slope of the regression line was −0.05 N/mm (r2 = 1.00). This indicates that the extent of mechanical interactions between FCU and PL is dependent on the relative position of their muscle bellies.
Figure 4.
Relationship between changes in FCU muscle belly length and distal PL force.
PL total force plotted as a function of muscle belly length of transferred FCU during isometric contraction (Δ ℓma). Note that PL was kept at a constant muscle-tendon complex length. The least-squares linear regression line and the value of r2 are shown.
Discussion
Mechanical interactions between non-transferred FCU and surrounding structures have been quantified previously in human patients and intact rats. Measurements performed during reconstructive surgery in patients with spastic cerebral palsy showed that tenotomized FCU muscle was still lengthened by passive wrist flexion (15). More recently, it was found that the mechanical effect of spastic FCU muscle at the wrist joint was not fully eliminated by tenotomy (16), which has also been reported for healthy cat soleus muscle (17). Surgical dissection of rat and human FCU muscle yields changes in length-force characteristics (18). In addition, we showed that length changes of FCU affect distal forces of length restrained PL in the healthy rat (1). All above results are manifestations of epimuscular myofascial force transmission (2, 3).
In agreement with our hypothesis, we found significant mechanical interactions between FCU and PL (two previously synergistic muscles) after transfer of FCU to ECRB/L (Fig. 2B). This indicates the presence of mechanical linkages between these muscles that are still providing pathways for force transmission, despite the fact that a substantial part of FCU muscle belly was rerouted to the extensor side of the forearm. As the tendon transfer yields a higher mean distance between FCU and PL muscle bellies, as well as a decreased interface area, we predicted smaller FCU-PL interaction. However, the slope of the decrements in PL force with FCU muscle-tendon complex lengthening in the present study (−0.020 N/mm) was similar to and not statistically different from the slope found (−0.025 N/mm) in healthy rats (1). This may be explained by (1) a higher stiffness of the linkages between FCU and PL at the proximal half of FCU muscle belly (i.e. the undissected part during tendon transfer surgery, see methods) or (2) the possibility that the decreased FCU-PL interface is compensated for by new stiff linkages (scar tissue), formed in response to the surgical intervention.
We found that changes of PL force were related linearly to changes in FCU muscle belly length (Fig. 4). In contrast, FCU muscle-tendon complex length - PL force data were best fitted with second or third order polynomials (see methods), being in agreement with several previous studies in which mechanical interactions between synergistic and antagonistic muscles were assessed (e.g., 19, 20).
The present study is actually the first one on epimuscular myofascial force transmission in which the relationship between muscle belly length and changes in force exerted by a constant after length adjacent muscle has been measured. In such conditions, changes in FCU muscle belly length are also a measure of changes in the position of FCU muscle belly relative to the muscle belly of PL. Therefore, this finding supports our previous findings that the extent of epimuscular myofascial force transmission is dependent on the relative position of muscle bellies (21, 22).
The magnitude of intermuscular mechanical interactions is, besides muscle relative positions, determined also by the stiffness of epimuscular myofascial connections. Scar tissue, formed as a response to invasive surgery, will affect intermuscular connectivity. In general, scar tissue is considered a factor limiting the successful outcome of tendon transfer surgery (10). During the experimental preparation for the mechanical measurements in this study, we observed more than normal amounts of connective tissues near the transferred FCU tendon. As we also found that lengthening FCU muscle-tendon complex resulted in relatively small changes in muscle belly length (Fig. 3), these results suggest that muscle belly movements of transferred FCU were limited, most likely by stiff scar tissue at the muscle-tendon boundaries. In a previous study, we showed that disruption of such scar tissue increased the ratio of muscle belly to muscle-tendon complex length changes (14).
FCU-to-ECRB/L tendon transfer is performed frequently by surgeons to provide wrist extension in paralysis or correct wrist flexion deformity in human patients with spastic pare-sis (e.g., 5, 11). However, the functional outcome of these surgeries varies substantially between patients (24–26) and do not always meet the initial expectations (8). For tendon transfer at the knee, it has even been reported that the mechanical effect of the muscle was unaltered following tendon transfer (7). It is commonly assumed that, after recovery from agonist-to-antagonist tendon transfer surgery, the transferred muscle will exert a joint moment according to its new muscle-tendon path and insertion. This will be true only if the transferred muscle is not linked to surrounding structures by either physiological connective tissues or newly formed scar tissues, or both. In the early 20th century, Biesalski and Mayer already recognized that low resistance gliding of the tendon within its surrounding sheath is important for muscle function after transfer (23).
The results of studies as the present one may be generalized to human clinical medicine only with great care (for a comprehensive discussion see 14), but ideas generated in such work may be taken by clinicians and considered for testing of surgical hypotheses. The essence of our data is that a transferred muscle may still be linked mechanically to (parts of) its former synergistic muscles, and thereby maintain some its former mechanical effects, that could interfere with the surgeons expectations. For clinical practice this suggests that it will be valuable to accentuate even more exploration of surgical approaches that minimize scar tissue formation or continue a search of additional interventions aimed at permanently minimizing physiological or pathological connective tissue linkages at the muscle and tendon boundaries.
Recently, it has been hypothesized that the characteristically flexed wrist in cerebral palsy patients is not the result of high forces generated within the spastic muscle itself, but instead caused by the transmission of force via epimuscular pathways from antagonistic muscles onto the spastic muscle (i.e., FCU) and transmitted from there across the wrist either via synergistic muscles or non muscular structures that cross the wrist joint (27). If that view is valid, just dissection of the spastic muscle possibly in combination with FCU tenotomy, may be as effective as transferring the FCU tendon to an extensor insertion site. However, this can only be true if the recovery of connective tissue linkages with adjacent muscles can be prevented. This example indicates how applying ideas regarding myofascial force transmission in clinical practice potentially may have consequences for surgical treatment.
Acknowledgments
The authors thank Oscar van Kooten and Dr. Marco Ritt for directions on the FCU-to-ECRBL tendon transfer surgery, and Guus Baan and Erwin van Gelderop for technical support.
The research leading to these results was in part supported by the European Community’s Seventh Framework Programme under grant agreement MIRG-CT-2007-203846. HM is currently supported by the Division for Earth and Life Sciences with financial aid from the Netherlands Organization for Scientific Research (Grant 864-10-011).
References
- 1.Maas H, Huijing PA. Synergistic and antagonistic interactions in the rat forelimb: acute effects of coactivation. J. Appl. Physiol. 2009;107:1453–1462. doi: 10.1152/japplphysiol.00328.2009. [DOI] [PubMed] [Google Scholar]
- 2.Maas H, Sandercock TG. Force Transmission between Synergistic Skeletal Muscles through Connective Tissue Linkages. J. Biomed Biotechnol. 2010 doi: 10.1155/2010/575672. Article ID 575672; doi:10.1155/2010/575672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huijing PA. Epimuscular myofascial force transmission: A historical review and implications for new research. International society of biomechanics Muybridge award lecture, Taipei, 2007. J. Biomech. 2009;42:9–21. doi: 10.1016/j.jbiomech.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 4.de Roode CP, James MA, Van Heest AE. Tendon transfers and releases for the forearm, wrist, and hand in spastic hemiplegic cerebral palsy. Tech Hand Up Extrem Surg. 2010;14:129–134. doi: 10.1097/BTH.0b013e3181e3d785. [DOI] [PubMed] [Google Scholar]
- 5.Friden J, Lieber RL. Evidence for muscle attachment at relatively long lengths in tendon transfer surgery. J Hand Surg - American Volume. 1998;23A:105–110. doi: 10.1016/S0363-5023(98)80097-X. [DOI] [PubMed] [Google Scholar]
- 6.Smeulders MJ, Kreulen M. Myofascial force transmission and tendon transfer for patients suffering from spastic paresis: a review and some new observations. J. Electromyogr. Kinesiol. 2007;17:644–656. doi: 10.1016/j.jelekin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Riewald SA, Delp SL. The action of the rectus femoris muscle following distal tendon transfer: does it generate knee flexion moment? Dev Med. Child Neurol. 1997;39:99–105. doi: 10.1111/j.1469-8749.1997.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Heest A, Stout J, Wervey R, Garcia L. Follow-Up Motion Laboratory Analysis for Patients With Spastic Hemiplegia Due to Cerebral Palsy: Analysis of the Flex-or Carpi Ulnaris Firing Pattern Before and After Tendon Transfer Surgery. J Hand Surg - American Volume. 2010;35A:284–290. doi: 10.1016/j.jhsa.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br. Med. Bull. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- 10.Strickland JW. Development of flexor tendon surgery: Twenty-five years of progress. J Hand Surg - American Volume. 2000;25A:214–235. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 11.Beach WR, Strecker WB, Coe J, Manske PR, Schoenecker PL, Dailey L. Use of the Green Transfer in Treatment of Patients with Spastic Cerebral-Palsy - 17-Year Experience. J Pediat Orthop. 1991;11:731–736. doi: 10.1097/01241398-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Maas H, Huijing PA. The mechanical effect of rat flexor carpi ulnaris muscle after tendon transfer: does it generate a wrist extension moment? J. Appl. Physiol. 2011;112:607–614. doi: 10.1152/japplphysiol.01275.2011. [DOI] [PubMed] [Google Scholar]
- 13.Maas H, van Alphen N, Huijing PA. Neuromuscular effects of FCU tendon transfer in the rat. The XVIII Congress of the International Society of Electrophysiology and Kinesiology 2010; Aalborg, Denmark. [Google Scholar]
- 14.Maas H, Huijing PA. Effects of tendon and muscle belly dissection on muscular force transmission following tendon transfer in the rat. J. Biomech. 2012;45:289–296. doi: 10.1016/j.jbiomech.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kreulen M, Smeulders MJC, Hage JJ, Huijing PA. Biomechanical aspects of progressive flexor carpi ulnaris muscle dissection. J. Bone Joint Surg. Br. 2003;85:856–859. [PubMed] [Google Scholar]
- 16.de Bruin M, Smeulders MJ, Kreulen M. Flexor carpi ulnaris tenotomy alone does not eliminate its contribution to wrist torque. Clin Biomech. 2011 doi: 10.1016/j.clinbiomech.2011.03.007. (Bristol, Avon) [DOI] [PubMed] [Google Scholar]
- 17.Maas H, Sandercock TG. Are skeletal muscles independent actuators? Force transmission from soleus muscle in the cat. J. Appl. Physiol. 2008;104:1557–1567. doi: 10.1152/japplphysiol.01208.2007. [DOI] [PubMed] [Google Scholar]
- 18.Smeulders MJ, Kreulen M, Hage JJ, Baan GC, Huijing PA. Progressive surgical dissection for tendon transposition affects length- force characteristics of rat flexor carpi ulnaris muscle. J. Orthop. Res. 2002;20:863–868. doi: 10.1016/S0736-0266(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 19.Maas H, Baan GC, Huijing PA. Intermuscular interaction via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J. Biomech. 2001;34:927–940. doi: 10.1016/s0021-9290(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 20.Huijing PA, van de Langenberg RW, Meesters JJ, Baan GC. Extramuscular myofascial force transmission also occurs between synergistic muscles and antagonistic muscles. J. Electromyogr. Kinesiol. 2007;17:680–689. doi: 10.1016/j.jelekin.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Maas H, Baan GC, Huijing PA. Muscle force is determined also by muscle relative position: isolated effects. J. Biomech. 2004;37:99–110. doi: 10.1016/s0021-9290(03)00235-5. [DOI] [PubMed] [Google Scholar]
- 22.Huijing PA, Baan GC. Myofascial force transmission: muscle relative position and length determine agonist and synergist muscle force. J. Appl. Physiol. 2003;94:1092–1107. doi: 10.1152/japplphysiol.00173.2002. [DOI] [PubMed] [Google Scholar]
- 23.Biesalski K, Mayer L. Die Physiologische Sehnenverpflanzung 1916. Julius Springer Verlag; Berlin: [Google Scholar]
- 24.Iannotti JP, Hennigan S, Herzog R, Kella S, Kelley M, Leggin B, Williams GR. Latissimus dorsi tendon transfer for irreparable posterosuperior rotator cuff tears. Factors affecting outcome. J. Bone Joint Surg. 2006;Am. 88:342–348. doi: 10.2106/JBJS.D.02996. [DOI] [PubMed] [Google Scholar]
- 25.Lo IKY, Turner R, Connolly S, Delaney G, Roth JH. The outcome of tendon transfers for C6-spared quadriplegics. J Hand Surg - British and European Volume. 1998;23B:156–161. doi: 10.1016/s0266-7681(98)80164-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoang PH, Mills C, Burke FD. Triceps to Biceps Transfer for Established Brachial-Plexus Palsy. J Bone Joint Surg - British Volume. 1989;71:268–271. doi: 10.1302/0301-620X.71B2.2647756. [DOI] [PubMed] [Google Scholar]
- 27.Huijing PA. Epimuscular myofascial force transmission between antagonistic and synergistic muscles can explain movement limitation in spastic paresis. J. Electromyogr. Kinesiol. 2007;17:708–724. doi: 10.1016/j.jelekin.2007.02.003. [DOI] [PubMed] [Google Scholar]