Summary
Meniscus is a difficult structure to repair and replace. An injured or degenerative meniscus promotes osteoarthritic joint changes that should be avoided. Research focused on promoting healing or replacement must cover three different working lines: biology, mechanics and surgical technique. Biology research line looks for specific factors able to develop a collagen tissue in a matrix with cells that joins the edges of the lesion and also looks for factors able to keep the elasticity and able to regenerate the damaged meniscus fibres. On the other side, scaffolds need the adequate viscoelasticity to allow the penetration of vessels and cells to avoid reabsorption.
Keywords: meniscus, growth factors, collagen, cartilage, angiogenesis
Introduction
The mechanisms of meniscal repair follow two patterns (1,2). The extrinsic pathway, which usually takes place in lesions of the vascular area where there is a net of capillaries, which supplied undifferentiated mesenchymal cells with nutrients to induce healing (Figs 1, 2). The intrinsic pathway is based on the self-repair capacity of the meniscal fibrocartilage and the synovial fluid (Fig. 3). The more central is the location of the meniscus injury, the lower the intrinsic responsiveness. In these cases, other factors are needed to provide a biological response (3) and synovial fluid surrounding is not an element that promotes the repair. Therefore, the intrinsic pathway is rejected and becomes essential to know the specific features of meniscal cells.
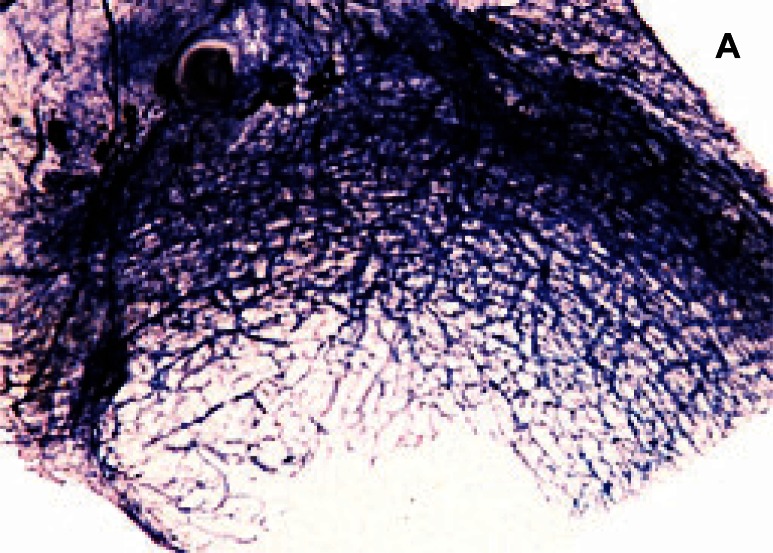
Figure 1.
Radial cut of sheep meniscus (Polarized light microscopy).
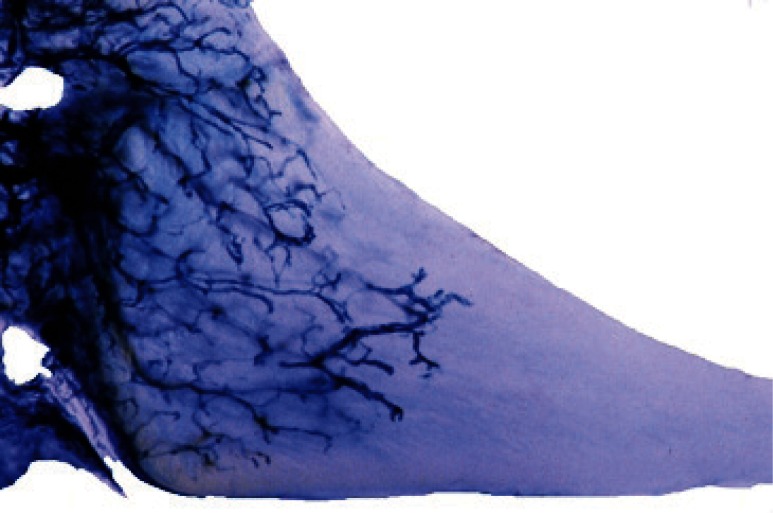
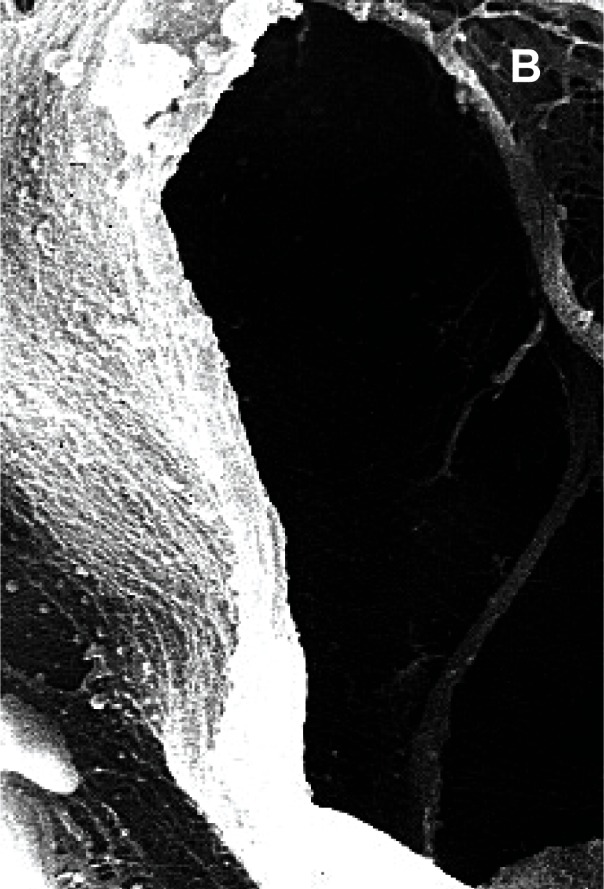
Figure 2 (a,b,c).
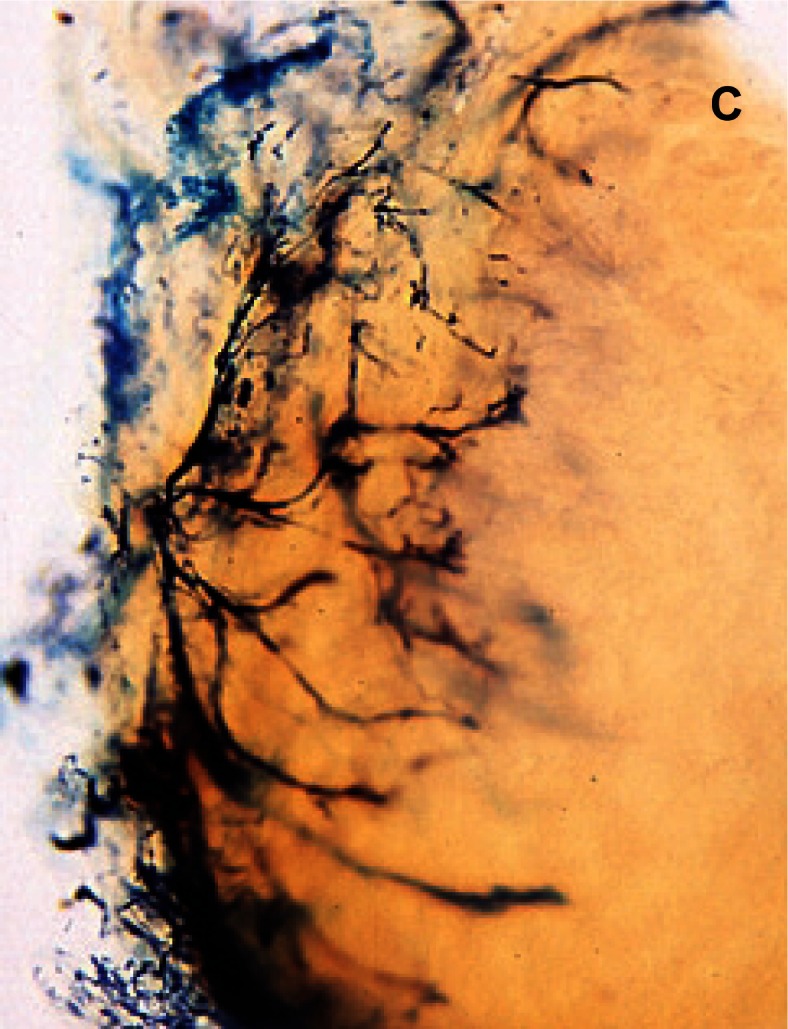
Sheep meniscus vascularization, A) 2 weeks old lamb; B) 1 month-old lamb; C) 5 months-old sheep.
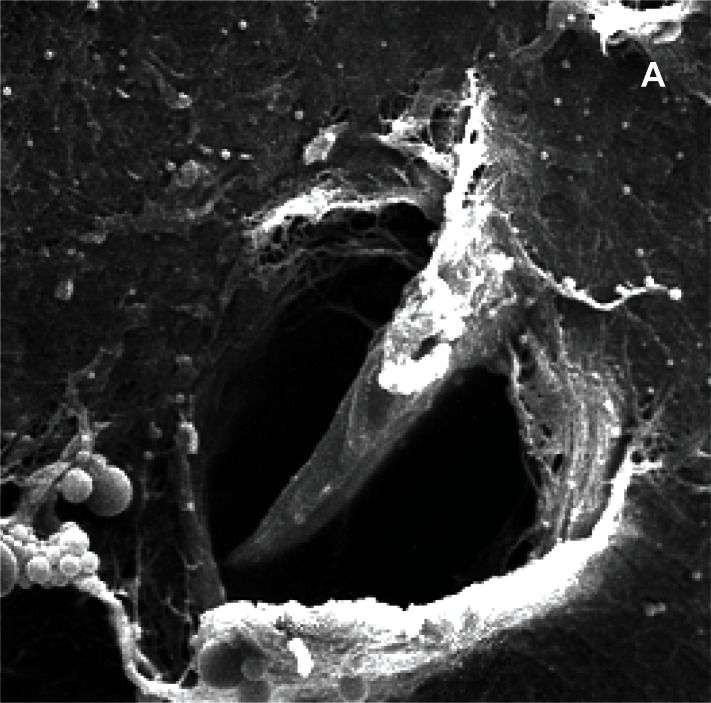
Figure 3 (a,b,c).
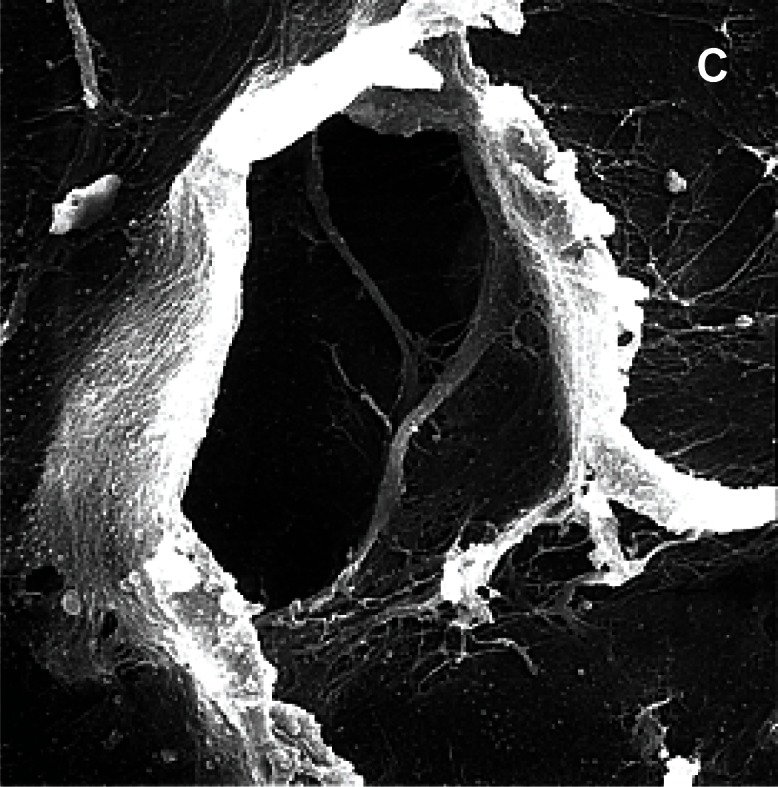
Pores in the avascular area of the meniscus, scanning microscopy, A) human meniscus (x3000); B) sheep meniscus (x1500); C) maccaca rhesus meniscus (x3.000).
The role of angiogenesis
Angiogenesis or new vessel formation from preexisting capillaries is essential for repairing the damaged tissues. There are signs that promote the expression of angiogenic growth factors such as metabolic parameters, inflamatory processes and genetic mutations. Inducers of angiogenesis are proteins such as fibroblastic growth factor (FGF), tissular growth factor (TGF), hepatocyte growth factor (HGF), tumoral necrosis factor (TNF), IL-8 and angiopeptin.
Angiogenesis is essential for tissue repair. From a molecular point of view, this process is based on a balance of stimulation and inhibition of different molecules. King and Vallee (4) placed angiogenin (vessel inducing protein) on a meniscal hole in 75 rabbits. They found revascularization of the defect in 52% of the animals compared with the 9% of the controls.
King (5), in 1936, studied the meniscus as a living tissue, integrated in the knee joint; in his classic work he noted that lesions in the menisci healed in the vascular area while did not in the white area. This work was later supported in different animal studies (1,6–8). Vascularization and repair were associated.
However it needs to be clarified that a meniscal injury has the potential to heal easily when is located in the vascular area, but just the vascular supply itself does not repair the meniscal defect. Cabaud et al. (9) and Heatley et al. (10) supported in dogs, monkeys and rabbits that lesions located in the periphery of the meniscus healed, due to the blood supply from the synovial.
From the initial studies of DeHaven et al. (3) who described the open meniscal suture, clinical experience has supported the good results of suturing peripheral meniscal lesions. Doubts rise when we find injuries further than the meniscal wall and disagreement in literature.
Ghadially et al. (11) performed an interesting animal trial with different species (rabbits, dogs, pigs and sheep). They produced a bucket handle lesion on the meniscus, away from the wall, and did not find any evidence of histological healing in neither of the specimens. The meniscal suture did not change the results and the bucket handle tear in the white area of the meniscus in the three different animal species not appear any healing sign at 6 months of follow up. In another group of animals, they performed “T” shape injuries that reached the meniscal periphery. Healing signs were found at 6 months of follow up.
Veth et al. (12,13) performed in rabbits, longitudinal lesions in the middle third and wedge lesions that reached the meniscal wall. Healing was significantly better in the wedge lesions of the meniscal wall where they found a fibrocartilage tissue similar to a control meniscus and even with higher cell count. From the 35 wedge lesions, 16 showed permanent signs of healing. In the group of the longitudinal lesions, 5 of 35 menisci showed evidence of healing. Therefore, location of the lesion is a relevant factor on the forecast of meniscal healing.
In an experimental study with sheep, Guisasola et al. (2) presented 2 cases of a longitudinal sutured lesion with healing signs. They found a new cellular infiltrate that came from the surface of the meniscus and from the channels of the suture in the gap.
Kobayashi et al. (14) developed a pilot study and showed that the power of meniscal healing was more increased in the periphery than in the avascular area. The stem cells that stimulate growth, support and tissue healing are located in the periphery of the meniscus. Other authors attribute this phenomenon to the presence of cytokines in the matrix (15 – 17).
The role of mechanical factors
The menisci are subjected to compression throughout the arc of motion of the knee. Rotation increases the stresses on the meniscus. Internal rotation of the knee increases twice the pressure on the lateral meniscus compared to neutral or external rotation. An injured meniscus is displaced and fragmented. In addition, the stress on the meniscus is also influenced by the decrease of synovial fluid and the tissue nutrition (18,19). Meniscal healing only happened in a 10% of all the meniscal ruptures reviewed by arthroscopy (20).
Kawai et al. (21) studied the healing process in dogs after performing a lesion in the vascular area of the meniscus. Subsequently to the meniscal suture, they did not apply any immobilization. A synovial pannus was confirmed in both sides of the lesions at 2 weeks. At 12 weeks, the maximum strength of the repaired tissue reached the 80% of the strength of a control meniscus. They concluded that immobilization and unloading were not relevant factors for meniscal healing in the vascular area. Years later this idea was also confirmed by Guisasola et al. (2). Conversely Zhongnan et al. (8) found better results in menisci of rabbits with immobilization. Dowdy et al. (22) pointed that the formation of collagen decreased from the 4th week of immobilization, and was even lower on the 10th week. From the 8th week of immobilization the loss of collagen was irrecoverable.
A good fixation seems to be more important than the immobilization (2,22,23). Port et al. (24) emphasize on the stability of the construct. They no find any statistical differences neither mechanical nor histological, between a group treated with two vertical sutures, another with fibrin clot plus suture, and a third one with fibrin clot plus bone marrow stem cells. The study was performed in goats. Values for tensile strength were less than 40% of the controls. Histological samples showed partial restoration with areas of giant cells and macrophages.
Molecular healing: growth factors and meniscal repair
Unlike the osseous tissue and despite immobilization, meniscus does not stimulate the cascade of the healing process. Causes of impairment would be the synovial fluid, mechanical stress, movement, loading. In our previous studies we found a vascular proliferation that did not result into a meniscal tissue formation (2).
Becker et al. (25) found an increased expression of vascular endothelial growth factor (VEGF) in menisci with lacerations on both areas, the peripheral and the free portion. The highest levels were on the 7th day after the injury and then decreased. VEGF levels were twice higher in defects located in the central area than in the periphery. This was attributable to a synergistic action of the cells of the central portion and the expression of VEGF. Endogenous anti-angiogenic factors such as troponin-1, angiostatin and endostatin are expressed to provide the lack of vessels in cartilage and in the avascular zone of the mensicus (4,6,25). The study of Becker et al. (25) showed a high expression of VEGF and mRNA in endothelial cells and fibro-chondrocytes of the vascular and avascular areas of the meniscal tissue. Despite the high concentrations of VEGF, lesions of the avascular portion did not heal successfully.
The growth factors released by the cells at the injury place together with the inflammatory infiltration of the scar tissue stimulate the meniscal cells to proliferation, migration, differentiation and matrix synthesis. The direct application of recombinant human proteins can stimulate meniscal repair but its application is limited by its short biological life and the need for repeated high doses of the growth factor.
Spindler et al. (26) showed that the avascular meniscal cells did not react to the presence of PDGF while Tumia and Johnstone (27) found that the IGF-1 stimulated the fibrochondrocytes of the avascular area. The activity of fibrochondrocytes is stimulated by the IGF-1 isolated or combined with 10% of fetal calf serum (FCS) by increasing the metabolism of thymidine and the DNA formation more in the inner zone than in the outer meniscus.
It has been shown (19) that synovial cells treated with hyaluronic acid (0.1 mg/mL) and Hylan® (Synvisc) (0.1 mg/mL) increased the expression of TGF-β1 and VEGF. However, the Hylan® decreased the connective tissue growth factor (CTGF) (0.66 times) and the VEGF (0.78 times) compared with the hyaluronic acid. Fortier et al. (28) proved the protective effects on synoviocytes of the tetracycline followed by the minocycline and compared with the doxycycline. They are all interesting products for the osteoarthritis management.
Therefore a growth factor carrier must meet the following conditions (29): (i) to release growth factors in time and with the adequate dosage. (ii) The presence of a substrate to stimulate the recruitment and cell adhesion, promoting chemotaxis in a space that allows cell migration and angiogenesis. (iii) To be biodegradable without causing immune, inflammatory or toxic reactions that would inhibit the repair process.
There have been reported four types of carriers for the growth factors: inorganic materials, synthetic polymers, natural polymers or “composites” and compounds made of the materials cited above. Among the most commonly used materials there are the type I collagen, hyaluronic acid gels, and polylactic and polyglycolic acid. The type I collagen is a carrier interesting for its fibrillar structure and for being the most abundant protein the extracellular matrix of bone and meniscus.
Other materials as acellular hydrogels have been implanted instead of a complete meniscus in rabbit and sheep (30,31). Degradable porous sponges have been developed (32,33). An attempt has been made to study natural materials as SIS (Small Intestinal Submucosa) or collagen and tissular implants (12,13,34–37). Many of these studies use animals and showed certain degree of chondral protection. Not all of them achieved a meniscal-like structure and its final function as protector of the cartilage (38). Baker et al. (15) have worked in a nanofibrous matrix of polycaprolactone (PCL) (E-caprolactone) combined with meniscal cells or mesenchymal cells. These group used cells derived from meniscal resection with good results; appropriate for their potential for autologous therapies without immune response and an appropriate phenotype.
The development of injectable carriers with growth factors is according to Seeherman et al. (39), one of the currently working lines.
Many of the synthetic polymers originate from the family of the polyesters that are degraded by gradual hydrolysis. The polymers are made from polyglycolic acid, polylactic and other copolymers. These polymers by changing its composition can adjust their mechanical properties and degradation time to simulate the repair tissue. The polymer matrix is made to allow the penetration of vascular structures to stimulate the cells to grow, develop and differentiate. Matrix can be supplemented with growth factors to induce the cellular development. Complex polymers are designed with different layers, to release different growth factors and improve the incorporation of the matrix and cell proliferation (35).
Most of the studies about meniscal repair confirm that the longitudinal lesions of the avascular area, the most common, are not able to heal properly and the meniscus do not reach its standard biomechanical conditions. There is evidence in experimental studies (2,40) that the meniscal strength after 2–3 months of the lesion does not reach the 30% of strength of a healthy meniscal tissue.
As we show, peripheral lesions located in the vascular area of the meniscus have better forecast. However results in literature are not homogeneous. The rate of success and failure is uncertain (41). It is difficult to assess giving the variety of lesions and the absence of registration. Any meniscal repair technique has to connect the edges of the lesion to protect it from the stresses of the joint. The meniscal repair process must prioritize the same principles as for other organic tissue repair: immobilization, an adequate fixation, rest, and delivery of healing substances.
To extend the indications of repairing in the avascular area, new techniques have been developed bringing blood supply from the capsule to the avascular area or directly repairing the injury.
Uysal et al. (42) analyzed 38 menisci from cadavers and surgical patients, aged below 40 years. There were three groups: non injured, with traumatic lesions and degenerative lesions. They studied the apoptotic cells in each group. The apoptotic cell count was statistically higher in the pathological groups than the health samples.
They did not find any differences between the pathological samples. Meniscal explants from the vascular and avascular areas showed an intrinsic healing response similar in vitro (43). This suggested that the meniscal repair process could be improved in vivo under appropriate intraarticular conditions.
External stimuli:
Haemarthrosis and fibrin clot
Arnoczky et al. (7) knowing the relevance of the hematoma in the initial healing phase of any injury applied a clot of fibrin in a meniscal defect in the avascular area of 12 dogs. The results showed a healed meniscal tissue with fibrocartilage. The origin of the repair cells was unknown giving the lack of vessels in the area of the defect. The clot provides a scaffold rich in platelet derived growth factors (PDGF) and fibronectine to enhance chemotaxis and mitogenic stimuli to the healing cells.
The fibrin clot seems to have the ideal features to guide the intrinsic meniscal response to heal, as a scaffold and as a source of stimulating factors. Spindle meniscal cells responded to chemotactic and mitogenic factors (43–46). Meniscal cells are able to multiply and develop an extracellular matrix in vitro when they are exposed to mitogenic and chemotactic factors existing in the hematoma (43–46). The origin of the repair cells is uncertain, but the early presence of fibroblasts in the meniscal injury suggests an important role of the superficial meniscal cells and the synovial cells as a source of stem cells in the joint. The control group did not show macroscopic healing, but in some cases there was a thin layer of tissue that filled the gap, probably due to a residual haemarthrosis of the surgery or to a mild proliferative response of the meniscal tissue. Webber et al. (44,46) looked on meniscal surgery with optimism. In vitro, fibrochondrocytes proliferated and synthesized extracellular matrix without blood supply whenever, they were surrounded by the appropriate media. For these authors the fibrin clot has the features to guide the intrinsic meniscal response, as a scaffold and as an input of growth factors to promote cell replication.
Van Trommel et al. (47) treated five complete radial external meniscal lesions, in the avascular popliteal portion, with a fibrin clot. Three years later, the arthroscopy control and the MRI testified that the lesions had healed. In the clinical experience, meniscal repair associated to the reconstruction of the ACL establishes a propitious environment. The haemarthrosis is full of growth factors that promote the meniscal repair (47). In an in vitro study, fibrochondrocytes exposed to the growth factors of a clot showed an increased proliferation rate and synthesis of a cartilage matrix (26,46–49).
Fibrin glue
The problem of the application of the fibrin clot is its poor adhesive property. It cannot stimulate healing if it doesn’t remain fixed and stable. Fibrin glue is a combination of coagulation factors (fibrinogen, thrombin, CaCl2 and Factor XIII) with aprotinin. The adhesive properties of the fibrin glue are superior to the clot but it lacks the biological properties. The fibrin glue is able to keep overlapping the edges of the injury without stimulating the repair process, therefore is only indicated in stable and small lesions (50). It has also been shown that the association of the fibrin glue and bone marrow stem cells promoted the meniscal healing, as well as the combination of fibrin glue and VEGF (51). To overcome the application problems of the fibrin clot, the preparation technique has been improved, increasing its consistency and the fibrin content. The fibrin glue can be used combined with a suture or be fragmented and applied with a syringe (52,53).
Synovial graft
The synovial holds the synovium-derived stem cells (SDSCs). They are a promising source of stem cells for the cartilage and meniscal repair, giving their higher condrogenic and osteogenic power than the MSC derived from the bone marrow, adipocytes or periosteum (18,19,28,54–57). Under adequate stimulation conditions they are able to migrate to the cartilage defect and produce a chondrogenic differentiation (58). They are also stable cells in vitro cultures from the step #3 to #10. These properties make them useful for transplantation to the avascular area of the meniscus and synovial cells play an important role in repopulation of the synovium grafts. They have a high differentiation rate and serve as a rich source of stem cells (54).
The synovial cells have an unknown function in the meniscal repair. In a study performed by this team showed that these cells were sensible to the stimuli of different growth factors. They modified the gene expression and stimulated the fibroblast derived growth factors. These factors had an important effect in expressing the type II collagen that simulates the meniscal healing and the MMP expression that are also involved in remodeling.
During the repair process, the cell lineage of the first 6 weeks is mostly fibroblastic. The origin of these cells is twofold: from the meniscal fibro-chondrocytes (44–46) and from the synovial cells. They reached the defect from an invagination of the meniscal surface, upper and lower surfaces, and from the channels performed by the suture. Collagen fibers were scarce and disorganized.
We believe that the use of substances to induce cell proliferation is a field of high interest in the meniscal tissue repair. The placement of a synovial flap over the sutured lesion serves as a stimulus for blood supply and for synovial stem cells. Kobuna et al. (57) have show conclusive results in dogs. By microangiography they demonstrated that vessels located on the femoral surface and the inner part of the meniscus reached the sutured area. At 6 weeks the lesions showed healing with a fibrovascular tissue. Cisa et al. (59) published good results in rabbits after using synovial flaps. Healing of the superficial layers is a consequence of the arrival of cellular elements from the synovial fluid and the fibro-chondrocytes. Healing of the deeper area is a consequence of the synovial autograft. However, the trigger factor of the healing process remains unknown.
Synovial cells have shown an over expression of agrecans, MMP-2 and bone morphogenetic protein-7 (BMP-7), when they were stimulated with fibroblastic growth factor (FGF), tissular growth factor (TGF), insulin growth factor (IGF) and BMP-7. We did not find an over expression of collagens or any effect when they were cultured with hyaluronic acid and a chondroprotective (60–62).
Following this idea, in longitudinal lesions in the medial meniscus of dogs, Shirakura et al. (63) analyzed the effectiveness of a free synovial autograft, a free muscular autograft on a Dacron® mesh and a control group of just sutured meniscus. Eleven of the 35 synovial autografts healed in 12 weeks with a fibrous tissue. They found new capillaries from the periphery without reaching the lesion. In none of the other groups the repair was achieved.
Jitsuiki et al. (64) advanced in the study of healing using synovial flaps. They tried to differentiate two concepts in the process of meniscal healing: the effect of vascularization and the effect of synovial tissue and synovial fluid. Meniscal lesions in rabbits healed by covering them with the synovial tissue. Fibro-chondrocytes had an intrinsic ability to repair, but they did not migrate and proliferate (44–46). Therefore, the reparative cells that appeared on the surface of the meniscus had to derive from cellular elements of the synovial fluid. These findings indicated that the healing process in the avascular zone of the meniscus was primarily due to synovial autograft together with the diffusion of synovial fluid and local factors.
The synovial autograft would simplify the surgical technique, avoiding the cutting of a flap, surgically more challenging. In vitro, the synovial graft showed a higher cell proliferation and collagen neoformation (65). On the 4th week the group of the synovial graft showed obvious signs of healing while in the control group there was an increase of cellular components on both sides of the lesion without filling. The cells of the fibrin clot covered the scaffold and the defect, but there was not collagen neoformation.
Periosteum
Parallel to the implementations o synovial grafts, the periosteum is a potential source of stem cells. From the periosteum arise chondrocytes and fibrochondrocytes that are involved in the meniscal repair process. In an animal study in dogs, the meniscal lesion of the avascular area was repaired with free periosteum graft and fibrin clot. After 16 weeks, the new tissue in the old defect was similar to the adjacent (52). The periosteum has been elected as a donor tissue in musculoskeletal bioengineering as a source of chondrogenic factors (58). The chondrogenesis of the periosteum has two different phases: a cellular proliferation followed by a cellular differentiation and the deposition of a matrix rich in proteoglycans (66). In vitro cartilage tissue can be obtained from periosteum and TGF-β1 stimulation (58).
PRP and MSC
Platelet-rich plasma (PRP) is a source of growth factors that induce healing response by stimulating the angiogenesis, chemotaxis, collagen matrix synthesis and cell proliferation (26). The role of the PRP in meniscal healing in literature is conflicting. Ishida et al. (67) reported good results in rabbits both in vitro and in vivo. In vitro, PRP stimulated the meniscal cell proliferation, the extracellular matrix synthesis, and the expression of fibrocartilage-related mRNA. In vivo, PRP together with Gelatin hydrogel showed better repair tissue in the meniscal defects than the control groups. These good results contrast with the study of Zellner et al. (68). They performed circular meniscal punch defects in the avascular zone of rabbit menisci. They were left empty of filled with different substances. Neither bone marrow nor PRP loaded in matrices induced improvement in meniscal healing.
Mesenchymal stem cells (MSC) are pluripotent cells that, thanks to their developmental plasticity are able to differentiate into specific therapeutic cell types (69). Studies have shown the production of abundant extracellular matrix around the cells in the avascular area and restoring a meniscal like tissue (17,68–71). The association of growth factors and MSC within scaffold implants demonstrated to increase proteoglycans and collagen synthesis (72). The combination of MSC and suturing, using or not fibrin glue, seems to be the most effective treatment (73).
It has been deeply studied the autologous chondrocyte implantation previously cultured in vitro, for cartilage defects (74,75). Peretti et al. (76,77) used the same idea and technology for meniscal defects in the avascular area, in rabbits. A meniscal allogenic scaffold with cultures cells is the most promising pattern to get meniscal repair (77). The same team (76) performed a longitudinal lesion in the avascular area of the menisci of 16 pigs. They divided the sample in 4 groups. In one of the groups they placed isolated chondrocytes over devitalized allogenic meniscal slices and maintained the structure with two sutures. The other three groups were managed as controls. One had an allogenic matrix without cells, another just a suture of the lesion without any external substances, and the last one was left untreated. The first group was the only one that showed meniscal repair signs. Dutton et al. (17) proved in pigs that the implant of MSC in meniscal lesions improved macroscopic and histological structure of the lesion but not their mechanical properties.
Cultured cells require a suitable medium, a biomaterial to growth and develop. Generally they consist in porous materials, with holes of 100–200 μm of diameter and a three dimensional arrangement. These materials have the advantages of being easily adaptable in the defect, and injectable, avoiding more aggressive insertion techniques (78,79). In a future, it could be of interest to obtain a viscous or liquid material that solidifies at body temperature, or to change other environmental conditions, as the pH.
Future outlines in meniscal repair
An important feature managing growth factors is their placement on suitable carrier materials or biomaterials. It is not easy to find the appropriate material for the meniscus. Bio-degradable porous substances are recommended to keep the concentration of growth factors in the target place (39). It is difficult to obtain a combination of a certain material that is able to retain the growth factor and which simultaneously is degraded to allow revascularization. Degradation rate has to be compatible with formation rate. The mechanical integrity of the repair process cannot commit the elimination process of the material. These characteristics must be commensurate with the type of treated tissue.
To study the possibilities of gene transfer in meniscal allografts, Martinek et al. (80) made a meniscal replacement using a meniscal allograft previously treated ex vivo with a retrovirus. They analyzed the expression of the gene marker: lacZ. In the superficial layers of the meniscus remained the gene expression. In the deeper layers of the meniscus they found transduced cells at the junction of the meniscus with the transplanted synovial.
As it has been shown, the meniscus is a difficult structure to repair and replace. An injured or degenerative meniscus promotes osteoarthritic joint changes that should be avoided. Research focused on promoting healing or replacement must cover three different working lines: biology, mechanics and technique.
Biology research line looks for specific factors able to develop a collagen tissue in a matrix with cells that joins the edges of the lesion. Over time, the scar tissue in the defect would increase its stiffness as similar as a healthy meniscus. The biology line, also looks for factors able to keep the elasticity and able to regenerate the damaged meniscus fibres.
Mechanics research line looks for creating scaffolds with the adequate features to allow the penetration of vessels and cells. Other important features are viscoelasticity, an excess of fragility to avoid reabsorption, and an excess of toughness to avoid the damage of cartilage.
Finally, the technical research line looks for managing meniscal injuries by arthroscopy avoiding more aggressive techniques in the joint. Currently, arthroscopically therapies are focused on meniscal transplantation, scaffold implantation and placing healing substances in the damaged meniscus.
The development of these three research lines presents a challenge to the orthopaedic surgeons and scientists to decrease the number of degenerative processes in the joint for the coming years.
References
- 1.Muñoz G, Álvarez E, Ripalda P, Forriol F. Nutrición de la zona avascular de los meniscos. Cuadernos de Artroscopia. 2001;18:19–25. [Google Scholar]
- 2.Guisasola I, Vaquero J, Forriol F. Knee immobilization on meniscal healing after suture: an experimental study in sheep. Clin Orthop Relat Res. 2002;395:227–233. doi: 10.1097/00003086-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 3.DeHaven K, Lohrer W, Lovelock J. Long term results of open meniscal repair. Am J Sports Med. 1995;23:524–530. doi: 10.1177/036354659502300502. [DOI] [PubMed] [Google Scholar]
- 4.King T, Vallee B. Neovascularisation of the meniscus with angiogenin. An experimental study in rabbits. J Bone Joint Surg (Br) 1991;73-B:587–590. doi: 10.1302/0301-620X.73B4.1712788. [DOI] [PubMed] [Google Scholar]
- 5.King D. The healing of semilunar cartilages. J Bone Joint Surg (Am) 1936;18-A:333–337. [Google Scholar]
- 6.Pufe T, Peterson W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44:1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 7.Arnoczky S, Warren R. The microvasculature of the meniscus and its response to injury: An experimental study in the dog. Am J Sports Med. 1983;11:131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 8.Zhongan Z, Kaiyuan T, Yinkan X, Wenming Z, Zhentian L, Shihuan O. Treatment of longitudinal injuries in avascular area of meniscus in dogs by trephination. Arthroscopy. 1988;4:151–156. doi: 10.1016/s0749-8063(88)80019-7. [DOI] [PubMed] [Google Scholar]
- 9.Cabaud H, Rodkey W, Fitzwater J. Medial meniscus repairs. An experimental and morphologic study. Am J Sports Med. 1981;9:129–134. doi: 10.1177/036354658100900301. [DOI] [PubMed] [Google Scholar]
- 10.Heatley FW. The meniscus, can it be repaired: an experimental study in rabbits. J Bone Joint Surg (Am) 1980;65-A:397–402. doi: 10.1302/0301-620X.62B3.6893331. [DOI] [PubMed] [Google Scholar]
- 11.Ghadially F, Wedge H, Lalonde J. Experimental methods of repairing injured menisci. J Bone Joint Surg (Br) 1986;63-B:106–110. doi: 10.1302/0301-620X.68B1.3753606. [DOI] [PubMed] [Google Scholar]
- 12.Veth R, denHeeten G, Jansen H, Nielsen H. Repair of the meniscus: an experimental study in rabbits. Clin Orthop Relat Res. 1983;175:258–262. [PubMed] [Google Scholar]
- 13.Veth R, Jansen H, Leenslang J, Pennings A. Experimental meniscal lesions reconstructed with a carbon fiber-polyurethane-poly (L-lactide) graft. Clin Orthop Relat Res. 1986;202:286–293. [PubMed] [Google Scholar]
- 14.Kobayashi K, Fujimoto E, Deie M, Sumen Y, Ikuta Y, Ochi M. Regional differences in the healing potential of the meniscus – an organ culture model to eliminate the influence of microvasculature and the synovium. Knee. 2004;11:271–278. doi: 10.1016/j.knee.2002.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Baker BM, Nathan AS, Russell Huffman G, Mauck RL. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis Cartilage. 2009;17:336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNulty A, Moutos F, Weinberg J, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum. 2007;56:3033–3042. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- 17.Dutton AQ, Choong PF, Goh JC-H, Lee EH, Hui JHP. Enhancement of meniscal repair in the avascular zone using mesenchymal stem cells in a porcine model. J Bone Joint Surg (Br) 2010;92-B:169–175. doi: 10.1302/0301-620X.92B1.22629. [DOI] [PubMed] [Google Scholar]
- 18.de Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y-T, Shao H-J, Wang J-H, Liu H-C, Hou S-M, Young T-H. Hyaluronic acid modulates gene expression of connective tissue growth factor (CTGF), Transforming growth factor-b1 (TGF-b1), and vascular endothelial growth factor (VEGF) in human fibroblast-like synovial cells from advanced-stage osteoarthritis in vitro. J Orthop Res. 2010;28:492–496. doi: 10.1002/jor.21029. [DOI] [PubMed] [Google Scholar]
- 20.Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med. 1992;20:104–111. doi: 10.1177/036354659202000202. [DOI] [PubMed] [Google Scholar]
- 21.Kawai Y, Fukubayashi T, Nishino J. Meniscal suture. An experimental study in the dog. Clin Orthop Relat Res. 1989;243:286–292. [PubMed] [Google Scholar]
- 22.Dowdy P, Miniaci A, Arnoczky S, Fowler P, Boughner D. The effect of cast immobilization on meniscal healing. An experimental study in the dog. Am J Sports Med. 1995;23:721–728. doi: 10.1177/036354659502300615. [DOI] [PubMed] [Google Scholar]
- 23.Bray RC, Smith JA, Eng MK, Leonard CA, Sutherland CA, Salo PT. Vascular response of the meniscus to injury: effects of immobilization. J Orthop Res. 2001;19:384–390. doi: 10.1016/S0736-0266(00)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Port J, Jackson D, Lee T, Simon T. Meniscal repair supplemented with exogenous fibrin clot and autogenous cultured marrow cells in the goat model. Am J Sports Med. 1996;24:547–555. doi: 10.1177/036354659602400422. [DOI] [PubMed] [Google Scholar]
- 25.Becker R, Pufe T, Kulow S, Giessmann N, Neumann W, Mentlein R, et al. Expression of vascular endothelial growth factor during healing of the meniscus in a rabbit model. J Bone Joint Surg (Br) 2004;86-B:1082–1087. doi: 10.1302/0301-620x.86b7.14349. [DOI] [PubMed] [Google Scholar]
- 26.Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB) J Orthop Res. 1995;13:201–207. doi: 10.1002/jor.1100130208. [DOI] [PubMed] [Google Scholar]
- 27.Tumia NS, Johnstone AJ. Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-I in vitro. J Bone Joint Surg (Br) 2004;86-B:1077–1081. doi: 10.1302/0301-620x.86b7.13747. [DOI] [PubMed] [Google Scholar]
- 28.Fortier LA, Motta T, Greenwald RA, Divers TJ, Mayr KG. Synoviocytes are more sensitive than cartilage to the effects of minocycline and doxycycline on IL-1a and MMP-13-induced catabolic gene responses. J Orthop Res. 2010;28:522–528. doi: 10.1002/jor.21006. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. J Bone Joint Surg (Am) 2002;84-A:103–144. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, et al. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22:2417–2424. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 31.Allemann F, Mizuno S, Eid K, Yates KE, Zaleske D, Glowacki J. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensional collagen scaffolds. J Biomed Mater Res. 2001;55:13–19. doi: 10.1002/1097-4636(200104)55:1<13::aid-jbm20>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Klompmaker J, Jansen H, Veth R, Nielsen H, de Groot J, Pennings A, et al. Meniscal repair by fibrocartilage? An experimental study in the dog. J Orthop Res. 1992;10:359–370. doi: 10.1002/jor.1100100308. [DOI] [PubMed] [Google Scholar]
- 33.Klompmaker J, Veth RP, Jansen HW, Nielsen HK, de Groot JH, Pennings AJ, et al. Meniscal repair by fibrocartilage in the dog: characterization of the repair tissue and the role of vascularity. Biomaterials. 1996;17:1685–1691. doi: 10.1016/0142-9612(96)87648-4. [DOI] [PubMed] [Google Scholar]
- 34.Bruns J, Kahrs J, Kampen J, Behrens P, Plitz W. Autologous perichondral tissue for meniscal replacement. J Bone Joint Surg (Br) 1998;80-B:918–923. doi: 10.1302/0301-620x.80b5.8023. [DOI] [PubMed] [Google Scholar]
- 35.Ibarra C, Koski JA, Warren RF. Tissue engineering meniscus: cells and matriz. Orthop Clin North Am. 2000;31:411–418. doi: 10.1016/s0030-5898(05)70160-7. [DOI] [PubMed] [Google Scholar]
- 36.Lantz GC, Badylak SF, Hiles MC, Coffey AC, Geddes LA, Kokini K, et al. Small intestinal submucosa as a vascular graft: a review. J Invest Surg. 1993;6:297–310. doi: 10.3109/08941939309141619. [DOI] [PubMed] [Google Scholar]
- 37.Wood D, Minns R, Strover A. Replacement of the rabbit medial meniscus with a polyester-carbon fibre bioprosthesis. Biomaterials. 1990;11:13–16. [PubMed] [Google Scholar]
- 38.Mora G, Álvarez E, Ripalda P, Forriol F. Articular cartilage degeneration after frozen meniscus and Achilles tendon allograft transplantation: experimental study in sheep. Arthroscopy. 2003;19:833–841. doi: 10.1016/s0749-8063(03)00731-x. [DOI] [PubMed] [Google Scholar]
- 39.Seeherman H, Wozney J, Li R. Bone morphogenetic protein delivery systems. Spine. 2002;27(suppl):16–23. doi: 10.1097/00007632-200208151-00005. [DOI] [PubMed] [Google Scholar]
- 40.Roeddecker K, Muennich U, Nagelschmidt M. Meniscal healing: a biomechanical study. J Surg Res. 1994;56:20–27. doi: 10.1006/jsre.1994.1004. [DOI] [PubMed] [Google Scholar]
- 41.Ahn JH, Wang JH, Yoo JC. Arthroscopic all-inside suture repair of medial meniscus lesion in anterior cruciate ligament-deficient knees: results of second-look arthroscopies in 39 cases. Arthroscopy. 2004;20:936–945. doi: 10.1016/j.arthro.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 42.Uysal M, Akpinar S, Bolat F, Cekin N, Cinar M, Cesur N. Apoptosis in the traumatic and degenerative tears of human meniscus. Knee Surg Sports Traumatol Arthrosc. 2008;16:666–669. doi: 10.1007/s00167-008-0536-8. [DOI] [PubMed] [Google Scholar]
- 43.Webber RJ. In vitro culture of rabbit meniscal tissue. Clin Orthop Relat Res. 1990;252:114–120. [PubMed] [Google Scholar]
- 44.Webber R, York J, Vanderschilden J, Hough A. An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am J Sports Med. 1989;17:393–400. doi: 10.1177/036354658901700314. [DOI] [PubMed] [Google Scholar]
- 45.Webber R, Zitaglio T, Hough A. Serum-free culture of rabbit meniscal fibrochondrocytes: proliferative response. J Orthop Res. 1988;6:13–23. doi: 10.1002/jor.1100060103. [DOI] [PubMed] [Google Scholar]
- 46.Webber RJ, Harris M, Hough AJ. Cell culture of rabbit meniscal fibrochondrocytes: proliferation and synthetic response to growth factors and ascorbate. J Orthop Res. 1985;3:36–42. doi: 10.1002/jor.1100030104. [DOI] [PubMed] [Google Scholar]
- 47.Van Trommel M, Simonian P, Potter H. Arthroscopic meniscal repair with fibrin clot of complete radial tears of the lateral meniscus in the avascular zone. Arthroscopy. 1998;14:360–365. doi: 10.1016/s0749-8063(98)70002-7. [DOI] [PubMed] [Google Scholar]
- 48.Mauck R, Martinez-Diaz G, Yuan X, Tuan R. Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec (Hoboken) 2007;290:48–58. doi: 10.1002/ar.20419. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H-N, Leng P, Wang Y-Z, Zhang J. Treating human meniscal fibrochondrocytes with hIGF-1 gene by liposome. Clin Orthop Relat Res. 2009;467:3175–3182. doi: 10.1007/s11999-009-0870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gershuni DH, Skyhar MJ, Danzing LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of the canine knee menisci. J Bone Joint Surg (Am) 1989;71-A:1363–1370. [PubMed] [Google Scholar]
- 51.Hashimoto J, Kurosaka M, Tyoshiya S, Hirohata K. Meniscal repair using fibrin sealant and endothelial cell growth factor. An experimental study in dogs. Am J Sports Med. 1992;20:537–541. doi: 10.1177/036354659202000509. [DOI] [PubMed] [Google Scholar]
- 52.Tsai C, Liu T, Liu C, Lim A. Meniscal repair with autogenous periosteum and fibrin adhesive system. Chung Hua I Hsueh Tsa Chih (Taipei) 1992;49:170–176. [PubMed] [Google Scholar]
- 53.Ishimura M, Ohgushi H, Habata T, Tamai S, Fujisawa Y. Arthroscopic meniscal repair using fibrin glue. Part I: Experimental study. Arthroscopy. 1997;13:551–557. doi: 10.1016/s0749-8063(97)90179-1. [DOI] [PubMed] [Google Scholar]
- 54.Potenza A, Herte M. The synovial cavity as a “tissue culture in situ”: science or nonsense. J Hand Surg. 1982;7:196–199. doi: 10.1016/s0363-5023(82)80088-9. [DOI] [PubMed] [Google Scholar]
- 55.Iwata H, Ono S, Sato K, Sato T, Kawamura M. Bone morphogenetic protein-induced muscle- and synovium-derived cartilage differentiation in vitro. Clin Orthop Relat Res. 1993;296:295–300. [PubMed] [Google Scholar]
- 56.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 57.Kobuna Y, Shirakura K, Niijima M. Meniscal repair using a flap of synovium. An experimental study in the dog. Am J Knee Surg. 1995;8:52–55. [PubMed] [Google Scholar]
- 58.Stevens MS, Marini RP, Martin I, Langer R, Shastri VP. FGF-2 enhances TGF-b1-induced periosteal chondrogenesis. J Orthop Res. 2004;22:1114–1119. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 59.Cisa J, Basora J, Madarnas P, Ghibely A, Navarro A. Meniscal repair by sinovial flap transfer: healing of the avascular zone in rabbits. Acta Orthop Scand. 1995;66:38–40. doi: 10.3109/17453679508994636. [DOI] [PubMed] [Google Scholar]
- 60.Merrihew Ch, Soeder S, Rueger DC, Kuettner KE, Chubinskaya S. Modulation of endogenous osteogenic protein-1 (OP-1®) by interleukin-1 in adult human articular cartilage. J Bone Joint Surg. 2003;85-A(Suppl 3):67–74. doi: 10.2106/00004623-200300003-00012. [DOI] [PubMed] [Google Scholar]
- 61.Hidaka Ch, Quitoriano M, Warren R, Cristal R. Enhanced matrix synthesis and in vitro formation of cartilage-like tissue by genetically modified chondrocyte expressing BMP-7. J Orthop Res. 2001;19:751–758. doi: 10.1016/S0736-0266(01)00019-5. [DOI] [PubMed] [Google Scholar]
- 62.Johnson RJ, Kettelkamp DB, Clark W, Leaverton P. Factors affecting late results after meniscectomy. J Bone Joint Surg (Am) 1974-A;56:719–729. [PubMed] [Google Scholar]
- 63.Shirakura K, Niijima M, Kobuna Y, Kizuki S. Free synovium promotes meniscal healing. Synovium, muscle and synthetic mesh compared in dogs. Acta Orthop Scand. 1997;68:51–54. doi: 10.3109/17453679709003975. [DOI] [PubMed] [Google Scholar]
- 64.Jitsuiki J, Ochi M, Ikuta Y. Meniscal repair by an interpositional free synovial autograft. An experimental study in rabbits. Arthroscopy. 1994;10:659–666. doi: 10.1016/s0749-8063(05)80065-9. [DOI] [PubMed] [Google Scholar]
- 65.Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg (Br) 2002;84-B:571–578. doi: 10.1302/0301-620x.84b4.11947. [DOI] [PubMed] [Google Scholar]
- 66.O'Driscoll S, Kelly FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion: An experimental investigation in rabbit. J Bone Joint Surg (Am) 1986;68-A:1017–1034. [PubMed] [Google Scholar]
- 67.Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tiss Eng. 2007;13:1103–1112. doi: 10.1089/ten.2006.0193. [DOI] [PubMed] [Google Scholar]
- 68.Zellner J, Mueller M, Berner A, Dienstknecht T, Kujat R, Nerlich M, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biom Mat Res-Part A. 2010;94:1150–1161. doi: 10.1002/jbm.a.32796. [DOI] [PubMed] [Google Scholar]
- 69.Oreffo ROC, Cooper C, Mason C, Clements M. Mesenchymal stem cells lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1:169–178. doi: 10.1385/SCR:1:2:169. [DOI] [PubMed] [Google Scholar]
- 70.Izuta Y, Ochi M, Adachi N, Deie M, Yamasaki T, Shinomiya R. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;12:217–223. doi: 10.1016/j.knee.2001.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, et al. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-β1 complementary deoxyribonucleic acid. Tiss Eng. 2007;13:2227–2237. doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 72.Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31:2583–2591. doi: 10.1016/j.biomaterials.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 73.Abdel-Hamid M, Hussein MR, Ahmad AF, Elgezawi EM. Enhancement of the repair of meniscal wounds in the red-white zone (middle third) by the injection of bone marrow cells in canine animal model. Int J Exp Pathol. 2005;86:117–123. doi: 10.1111/j.0959-9673.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brittberg M, Peterson L, Sjörgen-Larsson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. J Bone Joint Surg (Am) 2003;85-A(Suppl 3):109–115. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- 75.Peterson L, Brittberg M, Kiviranta I, Akelund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 76.Peretti GM, Gill TJ, Xu JW, Randolph MA, Morse KR, Zaleske DJ. Cell-based therapy for meniscal repair. A large animal study. Am J Sports Med. 2004;32:146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 77.Peretti GM, Caruso EM, Randolph MA, Zaleske DJ. Meniscal fracture repair using engineered tissue. J Orthop Res. 2001;19:278–285. doi: 10.1016/S0736-0266(00)90010-X. [DOI] [PubMed] [Google Scholar]
- 78.Van Tienen TG, Heijkants RGJC, Buma P, de Groot JH, Pennings AJ, Veth RPH. A porous polymer scaffold for meniscal lesion repair. A study in dogs. Biomaterials. 2003;24:2541–2548. doi: 10.1016/s0142-9612(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 79.De Groot JH, de Vrijer R, Pennings AJ, Klompmaker J, Veth RP, Jansen HW. Use of porous polyurethanes for meniscal reconstruction and meniscal prostheses. Biomaterials. 1996;17:163–173. doi: 10.1016/0142-9612(96)85761-9. [DOI] [PubMed] [Google Scholar]
- 80.Martinek V, Ueblacker P, Imhoff AB. Current concepts of gene therapy and cartilage repair. J Bone Joint Surg (Br) 2003;85-B:782–788. [PubMed] [Google Scholar]