Summary
Obesity is an important risk factor for Achilles tendinopathy, and running is usually carried out to reduce excess body weight. Aim of this study was to evaluate the prevalence of Achilles tendinopathy in young over-weight amateur runners.
Male runners and non runners were recruited and, in each category, divided in two groups: normal weight, and overweight. Data about Achilles tendon thickness, vascularisation and structural abnormalities were collected using a Power Doppler Ultrasonography device. Achilles tendon thickness was greater in both normal weight or overweight runners, but the difference was significant only in normal weight subjects. In non - runners, thickness was significantly higher only in over-weight subjects. Sonographic abnormalities were significantly prevalent in overweight runners.
Running is associated to a physiologic hypertrophy of Achilles tendon in normal weight subjects. Overweight runners may precociously develop tendon abnormalities, due to the increased stress and the unfavourable milieu of repair.
Keywords: Achilles tendon, obesity, running, tendinopathy, ultrasound
Introduction
Achilles tendinopathy (ATP) is very frequent among professional runners (1, 2). According to Kujala et al. (3), one out of every two professional runners will experience ATP before the age of 45, compared to one out of every ten persons in general population. This high prevalence can be explained by the increased and repetitive stress on lower limbs tendons, which predisposes to overuse changes (4). On the other hand, overweight and obesity (defined according to World Health Organization as Body Mass Index 25 – 29.9 and ≥ 30, respectively) are emerging risk factors for tendinopathies, which deserve attention and further investigations (5, 6).
Indeed, several observational studies have shown that tendinopathies are very frequent in overweight and obese subjects. Load - bearing tendons, such as Achilles and patellar tendons, and Plantar Fascia, are more frequently affected, but sonographic features of tendinopathy are also present in non load - bearing tendons, such as rotator cuff and elbow tendons (7–9).
Experimental research shows that genetically obese Zucker rats, compared with lean animals exhibit, at ultrastructural analysis, disorganized collagen fibril bundles and a reduced amount of non-collagen proteins and glycosaminoglycans. These organizational and structural modifications influence negatively the mechanical parameters, with a significant difference in maximum displacement and strain (10, 11).
Moreover, also muscle - tendon stiffness may be influenced by fat infiltration in leg skeletal muscles, as proved by Faria et al. (12) in post - menopausal women.
Obesity is a world epidemic, and one of the major public health problems in western countries; in this condition, physical activity is recommended to reduce excess body weight, prevent body weight regain, and decrease the subsequent risks of developing metabolic and orthopedic conditions (13).
Among different types of exercise, running is very popular, and considered an enjoyable form of leisure activity, and essential to prevent obesity in young age. Unfortunately, ATP prevalence in obese amateur runners, who largely differ from professional athletes for entity, duration and characteristics of engagement in running activities, is unknown.
Aim of the present paper is to evaluate, by means of Power Doppler Ultrasonography, the prevalence of AT abnormalities in young normal weight and overweight amateur runners, compared with sedentary subjects.
Material and methods
Male runners and non - runners were recruited. Subjects were classified as runners if they performed this activity regularly (at least twice weekly for more than 15 km/week). Number of years and kilometers per week spent in running were registered. For each subject, height and weight were measured and BMI was calculated. On the basis of BMI values, participants were divided in two subgroups: normal weight (BMI < 25) and overweight (BMI > 25).
Exclusion criteria were: positive history for ATP, foot trauma or surgery, rheumatic disorders, Kellgren - Lawrence grade III – IV ankle osteoarthritis, and familiar hypercholesterolemia.
Power Doppler Ultrasonography detection was performed by the same operator (AM), using a high - resolution, multi -frequency (10 – 14 MHz) linear array transducer (ProSound ALPHA 10, Aloka, Japan).
Achilles tendon (AT) thickness was measured, according to a standard protocol (14), at the midportion (maximum antero - posterior diameter), with the patients lying in prone position, their feet hanging over the edge of the table and ankles passively flexed at 90° (15). Both tendons were evaluated and the mean value was considered for analysis.
The presence of dishomogeneous hypo- or hyperechoic thicknening, diffuse or focal, the loss of the normal fibrillar pattern and/or the irregularity of the tendon margins, were considered as sonographic abnormalities (16).
Finally, intratendinous microvessels were evaluated by means of Power Doppler. To avoid artifacts, sensitivity was optimised for low flow, colour gain was set just below the noise level, and the pressure of the probe was kept to a minimun to avoid obliteration of small vessels.
The study was approved by the local Ethics Committee and participants signed an informed consent form prior of being enrolled in the study.
Statistical analysis
Data are reported as mean ± SD for continuous variables, whereas categorical and dichotomous variables are reported as frequencies and percentage. The significance level was determined at p < 0.05. The two - sample Student’s t - test was used to compare continuous variables, when the distribution of data was normal; the Wilcoxon’s rank sum test was used otherwise. The χ2 test was used to evaluate associations between categorical data. All analyses were done using SAS statistical software, release 8.1.
Results
Groups were well balanced for age and anthropometric measures (Tab. 1). Compared to normal weight subjects, overweight participants practiced running since longer time, but this difference was not significant (70 ± 47.8 vs 61.3 ± 26.2, p = ns).
Table 1.
Anthropometric data and running activity.
| Normal Weight | p | Overweight | p | |||
|---|---|---|---|---|---|---|
| Non Runners | Runners | Non Runners | Runners | |||
| Number | 16 | 21 | 19 | 25 | ||
| Age | 27.3 ± 4.4 | 29.2 ± 3.7 | ns | 28.9 ± 5.5 | 31.7 ± 6.9 | ns |
| Height | 175.5 ± 5.7 | 177.5 ± 6.4 | ns | 175.7 ± 5.9 | 176.2 ± 7.2 | ns |
| Weight | 69.5 ± 7.3 | 72.6 ± 6.5 | ns | 92.3 ± 7.8 | 93.1 ± 12.2 | ns |
| BMI | 22.5 ± 1.3 | 23 ± 1.5 | ns | 29.9 ± 1.7 | 29.9 ± 2.7 | ns |
| Running (months) | 61.3 ± 26.2 | 70 ± 47.8 | ns | |||
AT thickness was greater in runners, compared to sedentary subjects, but the difference was significant only in normal weight subjects (5.5 ± 1.1 vs 4.4 ± 0.6, p = 0.002) (Tab. 2). Overweight sedentary subjects showed AT thickness values significantly higher than normal weight sedentary subjects (5.2 ± 1.1 vs 4.4 ± 0.6, p = 0.02), while among runners no significant differences were found (5.9 ± 1.2 vs 5.5 ± 1.1, p = ns).
Table 2.
AT thickness and sonographic features.
| Normal weight | p | Overweight | p | |||
|---|---|---|---|---|---|---|
| Non runners | Runners | Non runners | Runners | |||
| AT thickness | 4.4 ± 0.6 | 5.5 ± 1.1 | 0.002 | 5.2 ± 1.1 | 5.9 ± 1.2 | ns |
| Sonographic abnormalities | 2 / 32 (6.2 %) | 4 / 42 (9.5 %) | ns | 4 / 38 (10.5 %) | 17 / 50 (34 %) | 0.01 |
| Power Doppler | 0 / 32 | 2 / 42 (4.5 %) | ns | 3 / 38 (7.8 %) | 12 / 50 (24 %) | 0.04 |
Sonographic abnormalities were more frequently observed in tendons of overweight participants (21/88 (23.8 %) vs 6/74 (8.1 %), p = 0.007) and, among them, were significantly prevalent in runners (17/50 vs 4/38, p = 0.01). Intratendinous microvessels were found more frequently in tendons of overweight participants (15/88 (17 %) vs 2/74 (2.7 %), p = 0.003), and, in this latter group, were significantly prevalent in runners (12/50 vs 3/38, p = 0.04) (Tab. 2).
Overweight participants with ultrasound alterations, compared to overweight free from abnormalities, were significantly older. Height and weight were also greater in this group, but the difference did not result statistically significant. Analogously, the number of months of running practice was higher, but not significantly, due to the large standard deviation (Tab. 3).
Table 3.
Anthropometric data and running activity in overweight subjects with / without sonographic abnormalities.
| Sonographic abnormalities | No sonographic abnormalities | p | |
|---|---|---|---|
| Number | 18* | 26 | |
| Age | 35.1 ± 5 | 28.5 ± 7 | 0.01 |
| Height | 178.8 ± 6.2 | 173.8 ± 7.5 | ns |
| Weight | 98 ± 13.1 | 88.6 ± 9.6 | ns |
| BMI | 30.5 ± 2.9 | 29.4 ± 2.2 | ns |
| Running (months) | 85.3 ± 52.4 | 55.9 ± 40 | ns |
3 subjects had bilateral sonographic abnormalities
Discussion
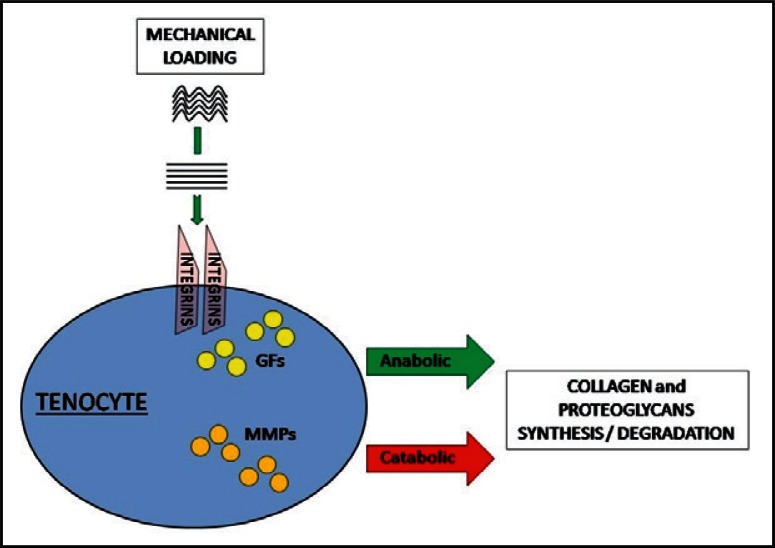
In young normal weight subjects, running has beneficial effects on tendon morphology and function (17). It is well known that mechanical loading is essential to maintain tendon homeostasis. As shown in Figure 1, when the collagen fibers are stretched, a signal is transmitted via integrins inside the tenocytes, growth factors are released, and the synthesis of proteoglycans and collagen is promoted (18). These effects are balanced by catabolism, due to metalloproteinases and aggrecanases expression. When the mechanical loading is repeated and intense, but still in the physiologic window, anabolism prevails on catabolism: new extracellular matrix and collagen fibers are formed, so that, after several months of sustained exercise, tendon cross sectional area increases and the biomechanical properties are improved (17). This is in agreement with our results which show that normal weight runners have an increased AT thickness with few sonographic abnormalities.
Figure 1.
In physiological conditions, mechanical loading enhances, via integrins, both GFs release, with extracellular matrix and neofibrils synthesis, and metalloproteinases secretion, with matrix degradation. GFs = Growth Factors; MMPs = Metalloproteinases.
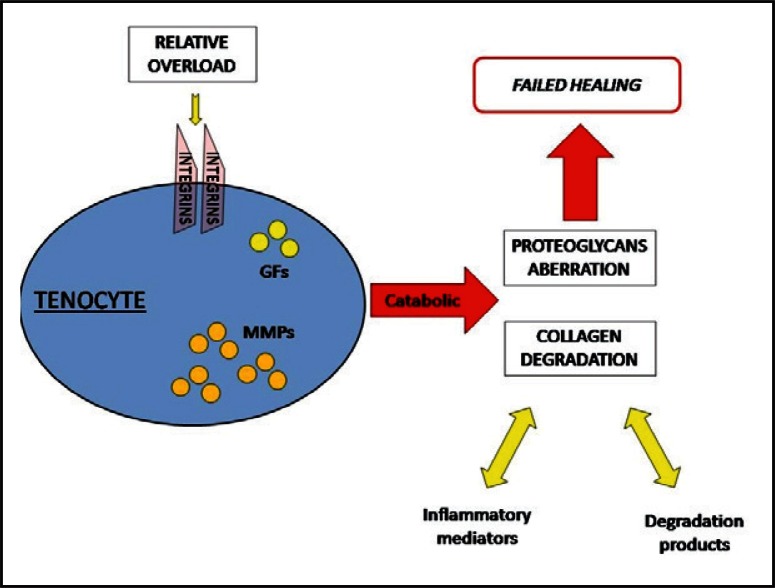
On the contrary, ultrasound alterations were observed in overweight subjects, and were significantly prevalent in runners. These findings may have several explanations. According to an accepted hypothesis (18), there is a threshold of loading frequency and magnitude that, once overcome, reverses tendon response from beneficial towards degenerative. An aberration in the proteoglycans metabolism is likely to drive the pathogenesis of tendon damage: indeed, their exceeding production leads to water retention and swelling, while, at the same time, the increased metalloproteinases expression favours the formation of degradation products (17). In addition, inflammatory molecules, such as Interleukin - 1 β, are released and may be implicated in the disease progression. After the failed healing process, a smoldering fibrogenesis can occur, with matrix turnover and cell activation without normal maturation (Fig. 2). Over time, such chronic processes can become symptomatic and lead to altered functioning of the affected tendon (18).
Figure 2.
When the stretching of collagen fibers overcomes tendon limits of adaptation (overload), catabolic processes prevail: several pathologic pathways are activated, and the final result is a failed healing response.
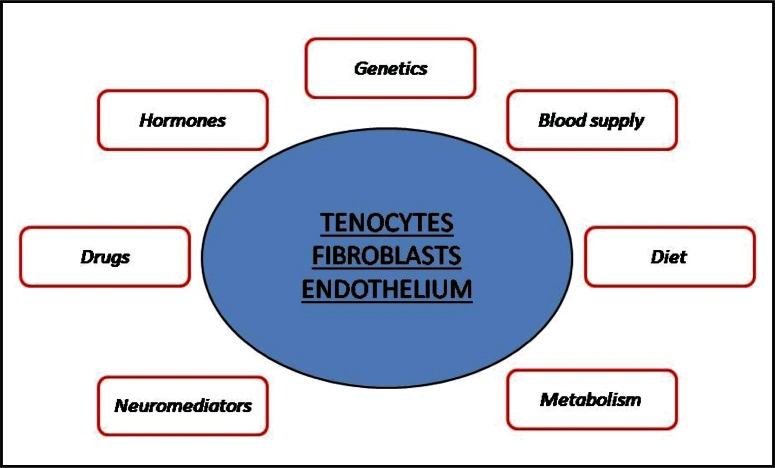
Tendon healing or healing failure can be influenced by several intrinsic and extrinsic factors. Actually, the disruption of tendon integrity occurs, at comparable high loads, only in some individuals, and can also occur when the tendon operates within a normal mechanical range (e.g. in a small subset of individuals exposed to environmental chemicals, such as fluoroquinolone antibiotics and statins) (18). In other words, tendon components (tenocytes, vessels, nerves, etc) react differently to mechanical loading, depending on the milieu within these components operate (Fig. 3).
Figure 3.
The metabolic activity of several cells is influenced by multiple factors, so that the capacity of tendon adaptation to mechanical loads is characterized by a large individual variability.
In obese subjects, bioactive peptides (adipokines, such as chemerin, lipocalin 2, serum amyloid A3, leptin and adiponectin) are released by adipose tissue (19). These peptides are provided of several activities on different mesenchimal cells (tenocytes, condrocytes and osteocytes), which may influence directly tendon structure. In particular, adipokines can induce type II nitric oxide synthase and are able to modulate cytokines, prostanoids and metalloproteinases production (19, 20).
Persistent raised levels of Prostaglandin E2 and Leukotriene B4, observed in obesity and situations of impaired insulin sensitivity, provides supplementary evidence that a systemic state of chronic low - grade inflammation is present in these conditions, and may act as a prolonged disruptor of tendon healing (21, 22).
Moreover, the migration of immune cells, such as macrophages and mastcells, into adipose tissue, is associated with a decrease in the circulating levels of these cells. As consequence, the release of profibrotic factors, as Transforming growth factor - β, is reduced, and this may have a detrimental effect on tendon healing, especially if the production of type I and III collagen is also reduced (22).
Two observations support the pathogenetic role of systemic factors. First, the association with adiposity is equally strong when non load - bearing tendons are compared with load -bearing tendons. Second, AT pathology is more frequently observed in subjects with central fat distribution, which, in turn, is closely related to insulin resistance and other metabolic abnormalities (23). Indeed, insulin - resistance leads to subclinical glucose intolerance and to increased formation of Advanced Glycosilation End Products, with subsequent cross - linking within collagen fibers (24). Besides that, Advanced Glycosilation End Products react with the cell surface specific receptors (RAGEs), which, in turn, triggers cell - specific signalling, resulting in enhanced generation of Reactive Oxygen Species, and in a sustained upregulation of pro - inflammatory mediators (24).
Beside the systemic influence of several metabolic factors and low grade chronic inflammation, in the case of over-weight runners, the increased stress on AT is, of course, an important pathogenetic factor. Indeed, it is well known that, during running, the load on AT can be as high as eight times body weight, so that modest increases in weight are amplified within the tendon (18). In this regard, it is worth noting that, in our study, overweight runners, who developed sonographic alterations, had a body weight higher than overweight runners without sonographic abnormalities, and were older. So, in our sample, age and the increased load on the tendon seem to play an important role.
Also the presence of intratendinous microvessels can be considered as a relevant finding because, according to a recent study (25), neovascularisation cannot be considered as part of an adaptation process but rather an indicator of tendinosis.
Some limitations of the study must be acknowledged. First, data on running were collected on a self - report basis. So, the evaluation of time spent in running could be inaccurate. Moreover, running speed, running surface (seashore, city road, country track), because the difficulty of evaluation and quantification, were not taken into account.
The inclusion of asymptomatic participants was a deliberate action designed to eliminate the confounding effect that pain could have in modifying the amount and regularity of physical activity behavior. So, the clinical meaning of our results must be clarified by further follow - up observation. Indeed, it is still unclear whether sonographic abnormalities are simply an adaptation process or indicative of a predisposition to clinically manifest tendinopathy. Recent studies point out that only some ultrasound abnormalities, such as neovessel formation and the spindle - shaped morphology of tendons, are predictors of future symptomatic tendinopathy in asymptomatic long - distance runners (25). In the present study, we found sonographic abnormalities and microvessels in some subjects; to enlarge the study sample and to start prospective observations are necessary actions to answer this important question.
In conclusion, this study highlights the prevalence of ultra-sound abnormalities in asymptomatic overweight subjects, who practice running to increase their energetic expenditure. These subjects should be frequently monitored for ultrasound abnormalities or should prefer other physical activity modalities to reduce the risks of this pathologic condition.
References
- 1.Tenforde AS, Sayres LC, McCurdy ML, Collado H, Sainani KL, Fredericson M. Overuse injuries in high school runners: lifetime prevalence and prevention strategies. PM R. 2011 Feb;3(2):125–131. doi: 10.1016/j.pmrj.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Hirschmüller A, Frey V, Deibert P, et al. Achilles tendon power Doppler sonography in 953 long distance runners - a cross sectional study. Ultraschall Med. 2010 Aug;31(4):387–393. doi: 10.1055/s-0029-1245189. [DOI] [PubMed] [Google Scholar]
- 3.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of Achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005 May;15(3):133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 4.Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010 Feb;3(1):29–32. doi: 10.1177/1938640009355191. [DOI] [PubMed] [Google Scholar]
- 5.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 6.Gaida JE, Cook JL, Bass SL. Adiposity and tendinopathy. Disabil Rehabil. 2008;30(20–22):1555–1562. doi: 10.1080/09638280701786864. [DOI] [PubMed] [Google Scholar]
- 7.Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007 Sep;28(9):996–999. doi: 10.3113/FAI.2007.0996. [DOI] [PubMed] [Google Scholar]
- 8.Jeswani T, Morlese J, McNally EG. Getting to the heel of the problem: plantar fascia lesions. Clin Radiol. 2009 Sep;64(9):931–939. doi: 10.1016/j.crad.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Holmes GB, Lin J. Etiologic factors associated with symptomatic achilles tendinopathy. Foot Ankle Int. 2006 Nov;27(11):952–959. doi: 10.1177/107110070602701115. [DOI] [PubMed] [Google Scholar]
- 10.Biancalana A, Veloso LA, Gomes L. Obesity affects collagen fibril diameter and mechanical properties of tendons in Zucker rats. Connect Tissue Res. 2010 Jun;51(3):171–178. doi: 10.3109/03008200903191312. [DOI] [PubMed] [Google Scholar]
- 11.Biancalana A, Velloso LA, Taboga SR, Gomes L. Implications of obesity for tendon structure, ultrastructure and biochemistry: A study on Zucker rats. Micron. 2012 Feb;43(2–3):463–469. doi: 10.1016/j.micron.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Faria A, Gabriel R, Abrantes J, Brás R, Moreira H. Tricepssurae musculotendinous stiffness: relative differences between obese and non-obese postmenopausal women. Clin Biomech (Bristol, Avon) 2009 Dec;24(10):866–871. doi: 10.1016/j.clinbiomech.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Nantel J, Mathieu ME, Prince F. Physical activity and obesity: biomechanical and physiological key concepts. J Obes. 2011;2011:650230. doi: 10.1155/2011/650230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinoli C, Bianchi S, Derchi LE. Tendon and nerve sonography. Radiol Clin North Am. 1999 Jul;37(4):691–711. viii. doi: 10.1016/s0033-8389(05)70124-x. [DOI] [PubMed] [Google Scholar]
- 15.Giacomozzi C, D'Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005 Jun;20(5):532–539. doi: 10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Salini V, Abate M. Percutaneous steroidal treatment in relapses of chronic tendinopathies: a pilot study. Int J Immunopathol Pharmacol. 2011 Jan-Mar;24(1):211–216. doi: 10.1177/039463201102400125. [DOI] [PubMed] [Google Scholar]
- 17.Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskelet Neuronal Interact. 2011 Jun;11(2):115–123. [PubMed] [Google Scholar]
- 18.Abate M, Silbernagel KG, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11(3):235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conde J, Gomez R, Bianco G, et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis. 2011 Mar;70(3):551–559. doi: 10.1136/ard.2010.132399. [DOI] [PubMed] [Google Scholar]
- 20.Berry PA, Jones SW, Cicuttini FM, Wluka AE, Maciewicz RA. Temporal relationship between serum adipokines, biomarkers of bone and cartilage turnover, and cartilage volume loss in a population with clinical knee osteoarthritis. Arthritis Rheum. 2011 Mar;63(3):700–707. doi: 10.1002/art.30182. [DOI] [PubMed] [Google Scholar]
- 21.Maffulli N, Longo UG, Loppini M, Denaro V. Current treatment options for tendinopathy. Expert Opin Pharmacother. 2010 Sep;11(13):2177–2186. doi: 10.1517/14656566.2010.495715. [DOI] [PubMed] [Google Scholar]
- 22.Battery L, Maffulli N. Inflammation in overuse tendon injuries. Sports Med Arthrosc. 2011 Sep;19(3):213–217. doi: 10.1097/JSA.0b013e31820e6a92. [DOI] [PubMed] [Google Scholar]
- 23.Gaida JE, Alfredson H, Kiss ZS, Bass SL, Cook JL. Asymptomatic Achilles tendon pathology is associated with a central fat distribution in men and a peripheral fat distribution in women: a cross sectional study of 298 individuals. BMC Musculoskelet Disord. 2010 Mar 2;11:41. doi: 10.1186/1471-2474-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility in diabetes and ageing: recent advances in pathogenesis and therapy. Int J Immunopathol Pharmacol. 2010 Oct-Dec;23(4):997–1003. doi: 10.1177/039463201002300404. [DOI] [PubMed] [Google Scholar]
- 25.Hirschmüller A, Frey V, Konstantinidis L, et al. Prognostic value of Achilles tendon Doppler sonography in asymptomatic runners. Med Sci Sports Exerc. 2012 Feb;44(2):199–205. doi: 10.1249/MSS.0b013e31822b7318. [DOI] [PubMed] [Google Scholar]