Summary
Objective: the purpose of this study was to introduce new procedure to determine the magnitude of functional recovery after knee surgery. Design: we compared the performance in the leg extension test and the response in the sEMG activity to vibration in the operated to the non-operated leg. Thirty-eight patients with knee operation and 14 healthy subjects participated in these experiments. Results: during leg extension test, the mechanical power of the operated leg showed a lower value (P<0.001) than the contralateral one, while no differences were noted in the sEMG activity. The sEMG activity during vibration treatment was higher in the operated compared to non-operated leg (P<0.001). It has been suggested that the reduced motility trigger functional adaptations that are exhibited via the vibration test. Conclusions: results of our study suggest that combination of vibration and sEMG recordings may detect the impairment as well as monitoring progress of the rehabilitation programs.
Keywords: sEMG, whole body vibration, leg extension test, knee surgery recovery
Introduction
Reparative knee joint surgery is usually followed, in a high percent of patients, by a long period of weakness of leg extensor muscles1, 2. It is likely that the strength modulation is the result of many factors such as increased pain sensitivity, the reduction in peripheral receptor input and the effects of disuse and immobility of the muscle tissue. Most rehabilitation programs, after knee surgery, usually involve isokinetic training, and most of the muscular evaluation assessments have been performed with constant speed dynamometers, even though such systems exhibit some limitations3– 5. The maximal speed allowed by the isokinetic apparatus is however lower than 25% of the maximal speed achieved during natural leg extension6. Consequently, patients were asked to develop a remarkably high muscle strength; however, a high level of muscle tension is strongly influenced by the patient’s pain threshold. Therefore, functional dynamic tests requiring very low muscular strength should be used more often in injury rehabilitation of the lower extremities. Pfeifer and Banzer7 have reported that sEMG different test conditions (stair descending, one-legged drop jump, one-legged cyclic hops) reveal different and persisting changes of the motor performance that cannot be detected by kinematics parameters alone. However, the assessment of neuromuscular function is far from sufficient for covering the large spectrum of biological changes, which occur with injures and after surgery. In this respect, a new technique consisting of monitoring the electromyographic activity of the leg muscles during a whole body vibration treatment is suggested. This method allows the possibility to detect and quantify proprioceptor function and altered neural strategy of motor pool recruitment after injury or surgery.
Therefore, the aim of the present investigation was to evaluate and quantify the magnitude of muscular power and sEMG activity developed during voluntary movement and proprioceptor functions induced by mechanical perturbation caused by vibration, following knee surgery. This procedure consist of two different tests; 1) the leg extension test measuring muscle power and monitor sEMG recordings and 2) the sEMG response to vertical sinusoidal whole body vibration (WBV).
Materials and Methods
Subjects
A group of thirty-eight male and female subjects (age 24.1 ± 3,9 yrs; weight 74.9 ± 10.8 kg; height 175.4 ± 10.2 cm) who previously underwent unilateral operation of the knee joint resulting from different types of injuries (e.g. anterior, and/or posterior ligament ruptures, meniscectomy, lateral and medial ligament surgery) volunteered to participate in this study. The Ethics Committee of the Italian Society for Movement Science approved the study and all subjects signed a written consent form before they were subjected to any testing. Patients were postoperatively tested from 6 to 270 weeks after surgery. The schedule consisted of three consecutive phases: 1) a period a warming up and stretching; 2) five trials of maximal leg extension; 3) two trials of 10 s training on the vertical sinusoidal whole body vibration (WBV). In addition, fourteen healthy subjects of similar age, height, and body weight, also participated in the experiment and were subjected to the same protocol.
Testing procedures
The testing session started with the anthropometric measurements of height and body weight followed by 10 min of warm up. The latter consisted of 10 min cycle ergometer pedaling at a speed of 21 km h−1 and at 50 watts (W) followed by 5 min stretching of the quadriceps and the triceps surae muscles.
Leg extension assessment for mechanical power calculation
All subjects, previously accustomed to exercises, performed a maximal dynamic leg extension exercise on the leg extensor rehabilitation machine without additional resistive load. The weight of the calf and the foot represents resistance of approximately 5% of the individual body weight and needed to be overcome by all patients. For each leg, five attempts were performed at 1 min inter trial interval. Since 2 or 3 trials were needed to reach a performance plateau, the last 2 trials of each set of measurements recorded (trial 4 and trial 5) were used for calculation of test re-test reliability2, 8 while best individual performance was used for statistical analysis. During the leg extension movement the displacement of the lower limb was monitored with a sensor (encoder) machine (Muscle Lab- Ergotest Technology A. S. Langensund, Norway), interfaced with a PC. When a subject moved a foot, a signal was transmitted by the sensor every 3 mm of foot displacement. From the obtained data, it was possible to calculate several variables, i.e. average velocity, acceleration, average force (F), and average power (P), corresponding to the load displacements. However, it has been shown that the P is the most sensitive variable among all the mechanical variables studied, therefore, only the P was considered for statistical analysis8.
sEMG analysis
The electrical activity from the vastus lateralis and vastus medialis muscles of each leg was recorded during the leg extension movements by bipolar surface electrodes (inter-electrode distance=1.2 cm) fixed longitudinally over the muscle belly and amplified (gain 600, input impedance 2 Giga-ohm, CMMR 100dB, band-pass filter 6–1500Hz). The sEMG raw signal was converted to a DC signal through the MuscleLab built in RMS converter (frequency response 450kHz, averaging constant 100ms, total error ± 0.5%). The resulting DC signal, representing the average power of the raw sEMG signal during the past 100ms, was then sampled at 100Hz simultaneously with other related biomechanical parameters. The sEMG was expressed as a function of time (mV).
The subjects wore a skin suit to prevent the sEMG cables from swinging and to reduce or eliminate any movement artifact. A personal computer (PC PentiumII 366MHz) was used to collect and store the data. The sEMG values of both vastus lateralis and vastus medialis muscles were averaged for statistical analysis, as suggested earlier9,10. The values of trial 1 and trial 2 were also averaged for statistical analysis.
Whole body vibration treatment procedure
Immediately after the leg extension assessment, all subjects were exposed to vertical sinusoidal whole body vibration (WBV) using the NEMES L-C (KB - Ergotest KY, Jyvaskyla, Finland) device as follows: vibration frequency=40Hz; vertical displacement=±4.0mm; acceleration=51.5m/s2; training duration:10s; number of trials =2; inter trial interval=120s. The vibratory stimuli were applied while the subjects were in standing position with the toes on the vibration platform; the knee angle was pre-set at a flexion of 100°. The sEMG activity was recorded from both vastus lateralis and vastus medialis muscles from the same place and with the same gain and amplification as during leg extension and in absence and in presence of vibratory stimuli.
Statistics
Standard statistical methods were employed, including mean ±SD. The Pearson Product Moment Correlation Coefficient (r) was used for test re-test measurement reliability. The CV of test re-test measurements was calculated using the following equation11:
Where x1 and x2 are the mean values of two successive measurements and ±SD is the standard deviation of the mean difference between test re-test measurements. Univariate and multivariate paired comparisons procedure with suitably defined contrasts; Wilcoxon and Friedman’s non-parametric tests were also used. The level of statistical significance was set at P<0.05.
Results
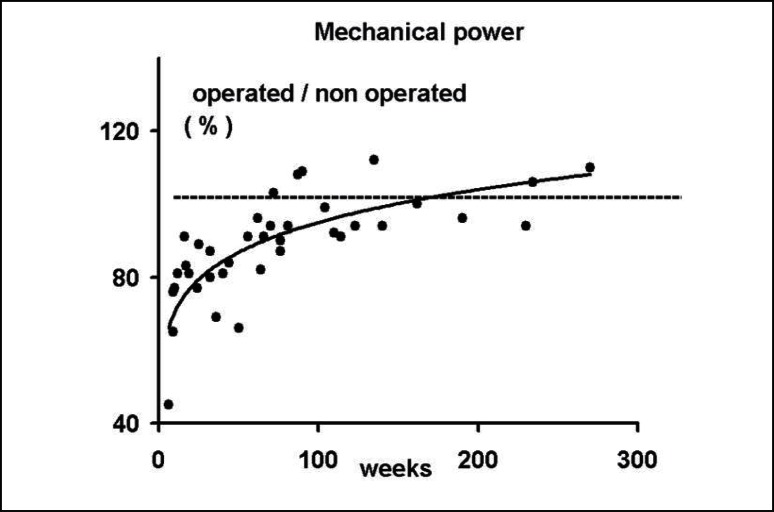
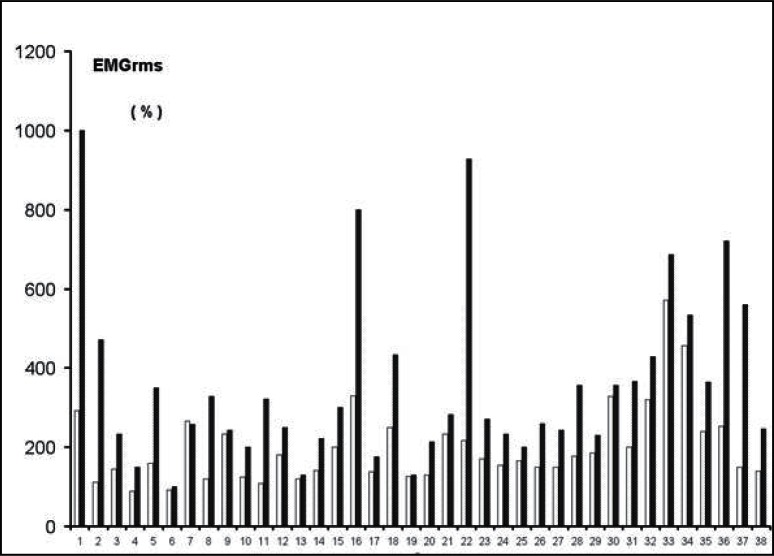
Table 1 shows the reliability of power and the sEMG values of the last two trials during the maximal leg extension, while Table 2 shows the reliability of sEMG values during the whole body vibration (WBV) in non-operated and the operated leg. During the leg extension test, the mechanical power of the operated leg showed a significantly lower value (P<0.001) than the controlateral leg (Tab. 3), while no differences were noted in the sEMG activity that was recorded simultaneously. The sEMG activity recorded during the WBV treatment was significantly higher (P<0.001) in the operated compared to non-operated leg (Tab. 3). The greater response in the operated side occurred at the beginning of the vibratory stimuli and lasted throughout the entire time of stimulation (Fig. 1). The sEMG differences during WBV between the two legs did not occur in either healthy subjects (Tab. 4, vastus lateralis and vastus medialis muscles) or in patients whose sEMG activity was recorded in the muscles below the knee (medial and lateral gastrocnemious muscles). The recovery of the mechanical function in the operated limb, represented by the ratio and expressed in percent (%) in operated/non-operated leg power, showed a positive relationship with the time elapsed since knee joint operation (Fig. 2). The same Figure 2 shows that in 10 patients the mechanical power developed in the operated leg was similar (within 5–7%) to that developed in the non-operated leg. The functional recovery of the operated leg showed a negative relationship with the sEMG activity induced by the WBV treatment (r = −0.45; P<0.006). When the sEMG activity was expressed as a percent of the baseline, no differences between the two legs were recorded in the leg extension test, while during WBV the operated limb demonstrated larger values (P<0.01) than the controlateral non-operated one (Fig. 3). In five patients, (6, 7, 9, 13, and 19 in Fig. 4) the sEMG activity during the WBV and the power developed during the leg extensor assessment was about the same (within 5–7%). These patients were tested between 70 to 135 weeks after surgery.
Table 1.
Reliability of two consecutive trials (trials 4 and 5). Values are mean ±SD of average mechanical power and the electromyogram mean square root (sEMG measured during leg extension performance of both operated and non-operated limbs. r = Pearson Product Moment Correlation Coefficient; CV = Coefficient of Variation for repeated measures; *P<0.001).
| Variables | Trial 4 | Trial 5 | r | CV |
|---|---|---|---|---|
| Operated leg | ||||
|
| ||||
| Power (W) | 71.2 ± 20.9 | 71.8 ± 22.6 | 0.97* | 5.7 |
| EMG (μV) | 206.1 ± 92.7 | 209.5 ± 112.7 | 0.97* | 4.5 |
|
| ||||
| Non-operated leg | ||||
| Power (W) | 80.9 ± 21.7 | 80.7 ± 22.7 | 0.92* | 15.3 |
| sEMG (μV) | 226.6 ± 115.0 | 219.3 ± 90.1 | 0.87* | 18.9 |
Values are mean ±SD.
significant at P<0.001
Table 2.
Test re-test reliability for operated subjects (N = 38) in basic conditions and during whole body vibration (WBV) treatment. The sEMG was collected for 10s in both conditions, from vastus lateralis and vastus medialis muscles of both legs. Values are mean ±SD.
| Variables | Treatment 1 | Treatment 2 | r | CV |
|---|---|---|---|---|
| Non-operated leg | 47.6 ± 16.9 | 48.9 ± 18.8 | 0.82 | 11.4 |
| Basic sEMG (μV) | ||||
| Operated leg | 42.1 ± 16.3 | 44.1 ± 17.3 | 0.83 | 13.1 |
|
| ||||
| Non-operated leg | 91.5 ± 48.5 | 94.2 ± 51.6 | 0.94 | 8.7 |
| WBV sEMG (μV) | ||||
| Operated leg | 158.7 ± 27.3 | 150.6 ±131.7 | 0.96 | 12.0 |
Values are mean ±SD.
Table 3.
The mean values (±SD) of sEMG recorded in microvolts (μV). Vastus lateralis and vastus medialis before (Basic) and during vibration treatment and during leg extension performances together with the mechanical power (Watts) are presented for the operated and non-operated legs of all subjects studied (N=38). The asterisks indicate statistical significance *P<0.05; **P<0.001. The statistical significance between basic and vibration treatment and basic and leg extension performance is also indicated § P<0.001.
| Variables | Leg | |
|---|---|---|
|
| ||
| Vibration | Operated | Non operated |
|
| ||
| Basic sEMG (μV) | 43.1 ± 16.7 | 48.2 ± 17.8* |
| Treatment sEMG (μV) | §154.6 ± 134.4 | § 92.7 ± 49.8** |
|
| ||
| Leg extension | ||
|
| ||
| Basic sEMG (μV) | 29.7 ± 15.5 | 30.0 ± 15.1 |
| Performance sEMG (μV) | § 211.9 ± 106.0 | § 230.4 ± 123.9 |
| Mechanical Power (W) | 73.8 ± 24.4 | 82.1 ± 24.1** |
Values are mean ±SD.
Significance
= P<0.05;
= P<0.001;
= P<0.001
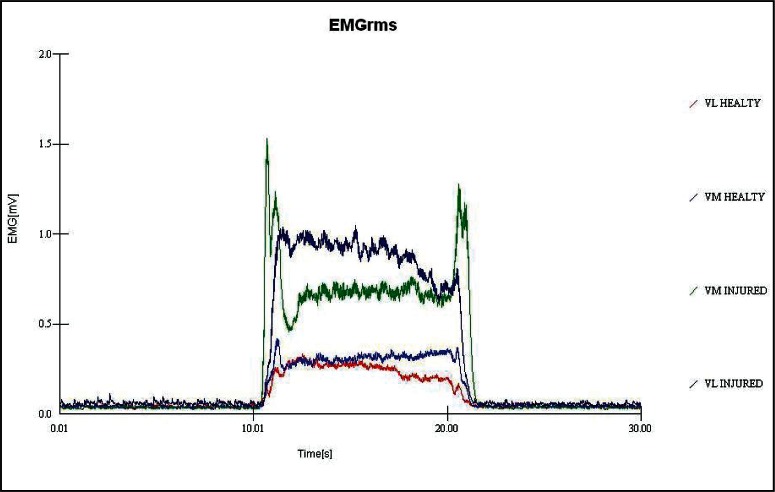
Figure 1.
Electromyographic recordings (sEMG) from vastus lateralis muscles in operated and non-operated legs. The arrow indicates the beginning of the vibration stimuli.
Table 4.
Test-retest for healthy subjects (N=14), in basic conditions and during whole body vibration (WBV) treatments. The sEMG was collected for 10s in both conditions, from vastus lateralis and vastus medialis muscles of both legs. Values are mean ±SD.
| Variables | Trial 1 | Trial 2 | r | CV |
|---|---|---|---|---|
| Dominant | 47.1 ± 9.9 | 49.6 ± 10.1 | 0.86 | 12.1 |
| Basic sEMG (μV) | ||||
| Non-dominant | 45.7 ± 11.4 | 48.9 ± 11.5 | 0.96 | 4.7 |
|
| ||||
| Dominant | 82.5 ± 24.7 | 88.2 ± 25.5 | 0.94 | 7.4 |
| WBV sEMG (μV) | ||||
| Non-dominant | 79.3 ± 27.3 | 84.3 ± 30.6 | 0.95 | 8.5 |
Values are mean ± SD.
Figure 2.
The mechanical power ratio (operated/non-operated x 100) developed during leg extension performance is presented as a function of time after surgery. Y = 71.0 + 0.337 x −0.0008 x 2 r= 0.62; P < 0.01; (N = 38).
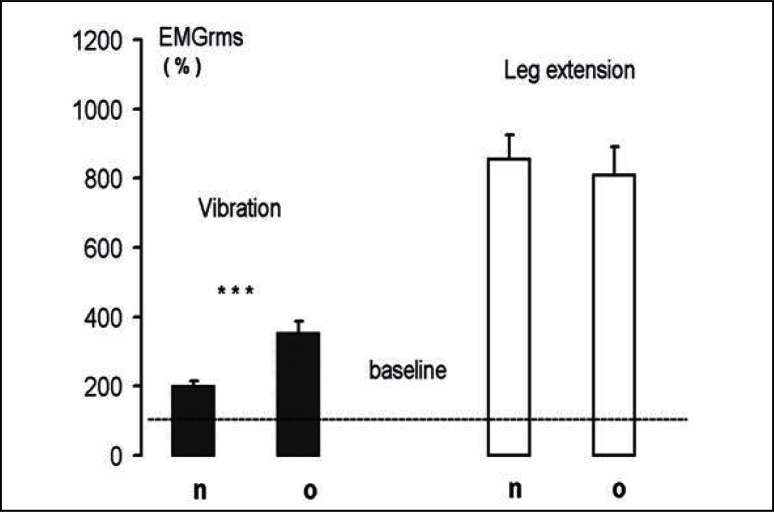
Figure 3.
The sEMG detected in the leg extensor muscles during WBV treatment is presented as a percent of baseline (100%). Statistically significant differences between mean ratios of non-operated (n) and operated (o) is shown (***p<0.001).
Figure 4.
The individual values of the sEMG recorded in the leg extensor muscles of the patients and expressed as percent of the baseline (100%), during WBV for non-operated (filled bars) and operated (empty bars) legs. Patients 6, 7, 9, 13, and 19 displayed almost the same sEMG activity (within 5–7%) in both legs in response to WBV.
Discussion
The results of our investigation suggest that during recovery from a knee joint surgery, a decreased muscular power during voluntary effort, in the operated leg was associated with an increase in sEMG response to total body vibration.
Before discussing this finding, it is important to address a few methodological issues. Our patients were familiar and accustomed with the leg extensor apparatus before the start of testing sessions. Moreover, in each patient the test re-test reliability showed an acceptable consistency in their performance with no signs of fatigue. Although, closed kinetic chain evaluation test have been promoted as more functionally appropriate and safer than open kinetic chain tests3– 5. With iso-inertial dynamometer used in the present study, it was possible to safely measure in the postoperative conditions the functions of muscular behavior during open chain performance. The leg extensor method allowed the use a very low external resistance, avoiding the development of high muscle tension, which for a post surgery patient represent a limitation of the neuromuscular behavior, since pain threshold sensibility may interfere with the voluntary effort. The leg extensor test was performed before the WBV and it has been reported that 10 min WBV was able to increase muscle force, power and movement velocity12, while repeated trials of WBV for 60 seconds were able to increase plasma concentrations of both testosterone and growth hormone13. To prevent possible effects in the sEMG response in the second WBV trial, vibratory stimuli were conducted for only 10s, and separated by two minutes inter-trial interval.
The sEMG activation by WBV was similar to that obtained by the tonic vibration reflex, a response elicited by mechanical vibration (10–200Hz) applied to a single muscle belly or to a tendon14. In the tonic vibration reflex the motor neuron excitation has been mainly attributed to activation of primary endings of muscle spindle receptors, although the amplitude and the frequency used are able to excite also other muscles, joints, tendons and ligament receptors15–17. Receptor activation may be related not only to direct effects of the mechanical stimuli but also, indirectly, to the muscle contraction that follows the motor neuron reflex excitation18. There is no doubt that both, the lesion that has preceded the operation, and the operation itself with the accompanying inflammatory reactions might have transiently affected the function of joint and ligament receptors.
During WBV the mechanical stimuli most likely elicit simultaneous and diffuse activation of spindle receptors innervating both agonistic and antagonist muscles acting on the same joint as well as vestibular receptors. The present experiments are not adequate to provide the mechanisms of the reduced mechanical power and of the increased sEMG response to WBV in the operated leg but they clearly indicate that there are sharp changes in both tests mainly in the early weeks after knee surgery. Besides muscle spindles, the role and the function of joint and ligament receptors on propioception and functional joint stability is highly debated. After knee joint surgery, either enhancement of the threshold for detection of passive motion19,20 and a reduced ability to actively reproduce a guided knee joint position in the ACL operated side with respect to healthy side21 or no difference between the two limbs22 has been reported. These different results might be due to the differences in reconstruction surgery and/or due to different types of rehabilitation programs. Our patients had been submitted to different types of knee joint surgery and to different rehabilitation programs and therefore their response to the WBV is more likely related to general mechanisms of adaptation such as response to pain, inflammation and immobility.
The sEMG activity deserves additional and further discussion. While in the operated side during the leg extensor test the mechanical power was lower than that in the controlateral side, no difference in the sEMG activity of both sides was recorded. On the contrary, in response to vibratory stimuli the sEMG activity was much higher in the operated side than in the non-operated one and only in the muscles acting in the proximal side of the knee joint: no differences between the two sides were recorded in the sEMG activity in the distal part (mm. medial and lateral gastrocnemious). We are aware that surface sEMG electrodes do not provide an exact recording of the underlying muscle fibers electrical activity. More in general, since the sEMG activity is the expression of motor neuron activity, it seems that, in a condition in which the strength of muscle fibers is decreased, the motor neuron activity is not modified in response to a central command for a maximal dynamic effort such as, the leg extension test but is greatly increased in response to a peripheral stimulus, such as the mechanical vibration.
The increased response could be due to a selectively modified response of the motor neuron to the peripheral input and/or to an increased excitability of the muscle spindles to the mechanical stimuli. The latter hypothesis is not supported by animal experiments23 that have shown that after long lasting limb immobilization of a healthy muscle at a short length there is a reduction in mean fiber area in all fiber types and a reduction in peak tetanic force. The reduction in peak force was greater than expected because of fiber atrophy in slower fiber units. As for muscle spindles and tendon organs, their responses in immobilized muscles reflected atrophy changes in extrafusal fibers with no substantial disturbance in receptor function. As for the first hypothesis, there is evidence that following training there are chronic adaptations in short and long latency stretch reflex responses, which facilitate greater force production for sport activities and quicker reaction times to unforeseen perturbations to planned movements24. In particular Wolpaw25 has developed a model in which primates can be trained to increase or decrease the amplitude of the spinal stretch reflex or H-reflex under conditions of a food reward and similar results have been reported in humans26. As for the present results, taking into account also other plastic changes, which occur following training27–30. It may be suggested that the increased response to WBV in the quadriceps muscle of the surgically operated knee, could represent the expression of adaptation mechanisms in the central nervous system to partial immobility and to reduced force in the corresponding muscle. In a motor neuron that is poorly involved in motor commands and therefore producing a reduced number of muscle contractions and stretches, the development of an increased responsiveness to muscle stretch in afferents represents a plastic mechanism that is able to improve the efficacy of the gamma loop during movement. Any peripheral or central stimulus, which excites fusi-motor neurons becomes particularly effective to increase the firing of the skeleto-motor neurons and therefore increases muscle strength. It is interesting to underline that this adaptive mechanism is reversible since it may subside when the muscle recovers the original strength.
Conclusion
The aim of the present study was to propose and assess new procedure to detect the impairment and the progress of a rehabilitation program. The leg extensor test displays a high reproducibility, uses very low external resistance and therefore may provide quantitative evaluation of muscle capacities a few weeks after surgery. A substantial limitation could be related to the subject motivation to perform a maximal contraction of an injured leg. The sEMG response to WBV has the advantage to record diffused muscle activation in which it is possible to compare contemporaneously the injured and the healthy leg reflex response to the same stimulus. The coupling of the two tests, we believe, offers the opportunity to assess the degree of recovery during a rehabilitation program and/or of a persistent deficit.
References
- 1.Engel A, Petschnig R, Baron R, et al. The effect of meniscectomy on the strength of the femoral quadriceps muscle after more than 3 years. Klin Wochenschr. 1990;22:663–666. [PubMed] [Google Scholar]
- 2.Tornvall G. Assessment of physical capabilities with special reference to the evaluation of maximal working capacity. Acta Physiol Scand. 1963;58:1–101. [Google Scholar]
- 3.Augustsson J, Esko A, Thomee R, et al. Weight training of the thigh muscles using closed vs. open kinetic chain exercises: a comparison of performance enhancement. J Orthop Sports Phys Ther. 1998;27(1):3–8. doi: 10.2519/jospt.1998.27.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Stiene HA, Brosky T, Reinking MF, et al. A comparison of closed kinetic chain and isokinetic joint isolation exercise in patients with patellofemoral dysfunction. J Orthop Sports Phys Ther. 1996;24(3):136–141. doi: 10.2519/jospt.1996.24.3.136. [DOI] [PubMed] [Google Scholar]
- 5.Toutoungi DE, Lu TW, Leardini A, et al. Cruciate ligament forces in the human knee during rehabilitation exercises. Clin Biomech. 2000;15(3):176–187. doi: 10.1016/s0268-0033(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 6.Bosco C, Viitsalo JH, Komi PV, et al. The combined effect of elastic energy and myoelectrical potentiation during stretch shortening cycle. Acta Physiol Scand. 1982;114:557–562. doi: 10.1111/j.1748-1716.1982.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer K, Banzer W. Motor performance in different dynamic tests in knee rehabilitation. Scand J Med Sci Sport. 1999;1:19–27. doi: 10.1111/j.1600-0838.1999.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 8.Bosco C, Belli A, Astrua M, et al. Dynamometer for evaluation of dynamic muscle work. Eur J Appl Physiol. 1995;70:379–386. doi: 10.1007/BF00618487. [DOI] [PubMed] [Google Scholar]
- 9.Bosco C, Viitasalo JT. Potentiation of myoelectrical activity of human muscles in vertical jump. Electromyogr Clin Neurophysiol. 1982;22:549–562. [PubMed] [Google Scholar]
- 10.Viitasalo JT, Bosco C. Electromechanical behavior of human skeletal muscles in vertical jumps. Eur J Appl Physiol. 1982;48:253–261. doi: 10.1007/BF00422986. [DOI] [PubMed] [Google Scholar]
- 11.Thorstensson A. Muscle strength, fiber types and enzyme activities in man. Acta Physiol Scand. 1976:1–45. [PubMed] [Google Scholar]
- 12.Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183–187. doi: 10.1046/j.1365-2281.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 13.Bosco C, Jacovelli M, Tsarpela O, et al. Hormonal responses to whole–body vibration in man. Eur J Appl Physiol. 2000;81:449–454. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- 14.Lebedev MA, Peliakov AV. Analysis of the interference electromyogram of human muscle after exposure to vibration. Neirofiziologia. 1991;1:57–65. 23. [PubMed] [Google Scholar]
- 15.Grigg P. Mechanical factors influencing response of joint afferent neurons from cat knee. J Neurophysiol. 1975;38:1473–1484. doi: 10.1152/jn.1975.38.6.1473. [DOI] [PubMed] [Google Scholar]
- 16.Grigg P, Greenspan BJ. Response of primary joint afferent neurones to mechanical stimulation of knee joint. J Neurophysiol. 1977;40:1–8. doi: 10.1152/jn.1977.40.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Kasai T, Kawanishi M, Yahagi S. The effects of wrist muscle vibration on human voluntary elbow flexion-extension movements. Exp Brain Res. 1992;90:217–220. doi: 10.1007/BF00229274. [DOI] [PubMed] [Google Scholar]
- 18.Hagbarth KE, Eklund G. Motor effects of vibratory stimuli in man. Muscular Afferents and Motor Control. In: Granit R, editor. Proceedings of the First Nobel Symposium. Stockholm: Almqvist and Wiksell; 1965. pp. 177–186. [Google Scholar]
- 19.Barrack RL, Skinner HB, Buckley SL. Proprioception in the anterior cruciate deficient knee. Am J Sport Med. 1989;17:1–6. doi: 10.1177/036354658901700101. [DOI] [PubMed] [Google Scholar]
- 20.Fedina L, Hultborn H. Facilitation from ipsolateral primary afferents of interneuronal transmission in the Ia inhibitory pathway to motoneurons. Acta Physiol Scand. 1972;86:59–81. doi: 10.1111/j.1748-1716.1972.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 21.Barrett DS. Proprioception and function after anterior cruciate reconstruction. J Bone Joint Surg Br. 1991;73:833–837. doi: 10.1302/0301-620X.73B5.1894677. [DOI] [PubMed] [Google Scholar]
- 22.Cho FH, Skinner HB, Cannon WB. Effect of reconstruction of the anterior cruciate ligament on proprioception of the knee and the hell strike transient. J Orthopaed Res. 1993;11:696–704. doi: 10.1002/jor.1100110512. [DOI] [PubMed] [Google Scholar]
- 23.Nordstrom MA, Enoka RM, Reinking RM, et al. Reduced motor unit activation of muscle spindles and tendon organs in the immobilized cat hindlimb. J Appl Physiol. 1995;78:901–913. doi: 10.1152/jappl.1995.78.3.901. [DOI] [PubMed] [Google Scholar]
- 24.Hutton RS, Atwater SW. Acute and chronic adaptations of muscle proprioceptors in response to increased use. Sport Med. 1992;14:406–421. doi: 10.2165/00007256-199214060-00007. [DOI] [PubMed] [Google Scholar]
- 25.Wolpaw JR. Adaptive plasticity in the spinal stretch reflex: an accessible substrate of memory. Cell Mol Neurobiol. 1997;5:147–165. doi: 10.1007/BF00711090. [DOI] [PubMed] [Google Scholar]
- 26.Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: preliminary studies. Neurosci Letters. 1989;105:353–355. doi: 10.1016/0304-3940(89)90646-0. [DOI] [PubMed] [Google Scholar]
- 27.Curl WW, Markey KL, Mitchell WA. Agility training following anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 1983;172:133–136. [PubMed] [Google Scholar]
- 28.Mardsen CD, Rothwell JC, Day BL. The use of peripheral feedback in the control of movement. Trend Neurosci. 1984;7:253–257. [Google Scholar]
- 29.Nyland J, Brosky T, Currier D, et al. Review of the afferent neural system of the knee and its contribution to motor learning. J Orthop Sport Phys Ther. 1994;19:2–11. doi: 10.2519/jospt.1994.19.1.2. [DOI] [PubMed] [Google Scholar]
- 30.Wall PD, Egger MD. Formations of new connections in adult rat brains after partial differentiation. Nature. 1971;232:542–545. doi: 10.1038/232542a0. [DOI] [PubMed] [Google Scholar]