Summary
Mesenchymal stem cells (MSCs) have the potential to replace or restore the function of damaged tissues and offer much promise in the successful application of tissue engineering and regenerative medicine strategies. Optimising culture conditions for the pre-differentiation of MSCs is a key goal for the research community, and this has included a number of different approaches, one of which is the use of mechanical stimuli. Mesenchymal tissues are subjected to mechanical stimuli in vivo and terminally differentiated cells from the mesenchymal lineage respond to mechanical stimulation in vivo and in vitro. MSCs have also been shown to be highly mechanosensitive and this may present an ideal method for controlling MSC differentiation. Here we present an overview of the response of MSCs to various mechanical stimuli, focusing on their differentiation towards the mesenchymal tissue lineages including bone, cartilage, tendon/ligament, muscle and adipose tissue. More research is needed to elucidate the complex interactions between biochemically and mechanically stimulated differentiation pathways.
Keywords: mechanical stimuli, mesenchymal stem cell, osteogenesis, tenogenesis
Introduction
Mesenchymal stem cells (MSCs) are a promising cell source for tissue engineering and regenerative medicine strategies and offer an alternative to fully differentiated cells that are often in limited supply due to tissue damage or disease. MSCs have multipotent differentiation potential, self-renewing ability, and apparent immunosuppressive properties1. Typically MSCs are isolated from the stroma of adult bone marrow (BMSCs), but cells with similar phenotypic characteristics and differentiation capabilities have also been isolated from a range of other mesenchymal tissues including adipose2 (ADMSCs), tendon, muscle and skin. MSCs cultured in vitro can be chemically-induced to differentiate into cell types of the mesoderm, including bone, cartilage, tendon/ligament and fat (for a recent review see Vater et al.3). Common biochemical agents and growth factors include: dexamethansone (dex) and bone morphogenetic proteins (BMPs) for osteogenesis, serum free medium and transforming growth factor ß (TGF-ß) for chondrogenesis, dex, insulin, and 3-isobutyl-1-methylxanthine (IBMX) for adipogenesis4(Fig. 1). It has also been claimed that MSCs are able to differentiate into other tissue types such as smooth muscle, endothelial and nervous tissue5.
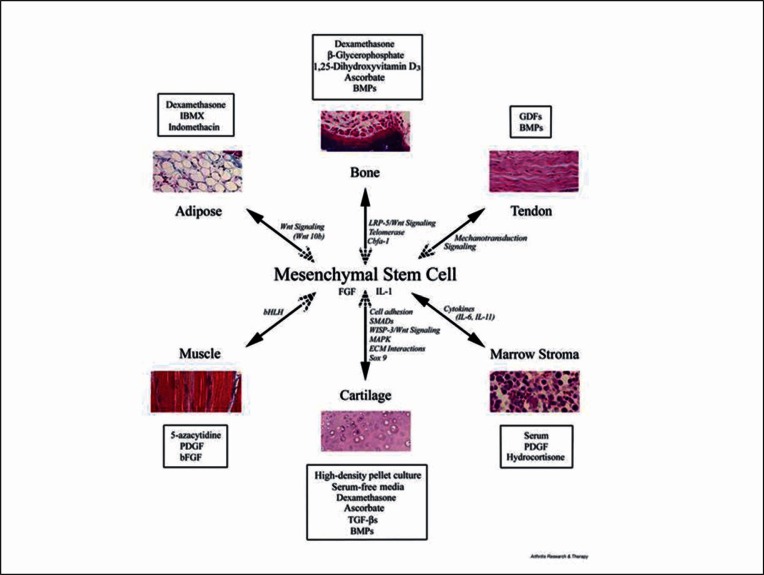
Figure 1.
Diagram summarising the lineage potential of adult human MSC. The figure depicts the in vitro culture conditions (boxed) used to promote the differentiation into the lineage indicated and some of the signalling pathways and transcription factors involved in the process (italics). Reprinted from Arthritis Research and Therapy (4), BioMed Central, with kind permission of Professor Tuan.
A key task for tissue engineers is to identify the appropriate culture conditions for development of a tissue engineered construct in vitro ready for implantation in vivo that forms the target tissue and reduces subsequent healing time. It is well known that biochemical cues, such as cytokines, growth factors and signalling events6, can control the function of stem cells, as well as environmental factors (e.g. surface chemistry and topography)7,8 but it is also becoming clear that mechanical forces can greatly influence stem cell behaviour.
Tissues and cells in the human body are exposed to a range of different external forces, which influence their development and maintenance9. For example, it is well documented that excercise increases bone and muscle mass10 and inadequate loading as occurs during space flight, prolonged periods of bed rest or spinal cord injury11 results in decreased muscle and bone mass. MSCs in vivo reside in the stem cell niche, which contains many biochemical factors that function to regulate their behaviour. In the bone marrow, mechanical forces in the form of tension, compression, and fluid-induced shear are all present12 but the nature of these forces is not well understood, neither is what effect they have on stem cell mobilization and function.
Many different cell types have been demonstrated to be highly mechanosensitive in vitro13 and recent TE strategies for MSC differentiation have included modifying intrinsic (via substrate stiffness8,14, and external stresses to simulate the physiologically relevant mechanical environment. Mechanical stimulation of MSCs in vitro has shown that tensile strain enhances osteogenesis and tenogenesis but inhibits adipogenesis15,16, hydrostatic pressure and compressive loading induces chondrogenesis17, and fluid flow induced shear stress upregulates genes associated with osteogenesis18,19. In this review we will describe some of the outcomes seen when these stimuli have been applied to MSCs. However, due to the wide variety of mechanical stimuli available, the enormous array of possible loading conditions, and the addition of different chemical stimulants, the optimum conditions for controlling lineage specific MSC differentiation remain unspecified.
Mechanical Regulation of MSCs
The most extensively studied differentiation pathways of MSCs are osteoblastic, chondrogenic, and adipogenic, other pathways such as tenogenesis and myogenesis have also been investigated to a lesser extent. The optimum durations, magnitudes and frequencies of mechanical loading for lineage specific differentiation of MSCs is not known due to the difficulty of undertaking multiple loading regimens within one set of experiments. The response of MSCs to loading are likely to be age-specific, may be specific to site of origin, and appear to depend on how differentiated the cells are at the time of loading, as well as whether loading is performed in conjunction with biochemical supplements20,21.
There are many ways in which researchers have stimulated cells with mechanical forces in vitro which can generally be categorized into the primary type of stress they induce22. These include stretching (tensile stress)23 hydrostatic pressure or platen abutment (compressive stress), fluid flow (shear stress)24,25, ultrasound26,27, high frequency, low magnitude displacement (vibration)28,29, and direct cell membrane magnetic stimuli30. For each stimulation mode the stimulus can be applied in 2D (monolayer culture) or 3D (multilayer culture) and differences between cells cultured in 2D and 3D have been observed in terms of cellular morphology and migration strategies, matrix adhesion, gene and protein expression and responses to fluid flow31.
It is important to note that in many loading systems there will be secondary effects along with the main mechanical stimulus. For example, in 3D tensile and compression loading systems, there will be fluid drawn in and out of the scaffold causing shear stress at the cell membrane and improved nutrient transfer to the cells32. Also, the cells will most likely be subjected to additional compressive or tensile forces caused by substrate bending or the Poisson’s effect. Scaffold architecture will also regulate how much of the applied force is received by the cells, for example in a cell-seeded gel the loading received will be relatively homogeneous throughout the scaffold whereas a porous scaffold will have an uneven strain transfer. Increased nutrient transfer from fluid flow can result in better cell infiltration and matrix distribution, as well as enhancement of cell differentiation due to mass transport effects33. Therefore, in order to optimise stimulation regimens it is important to identify the effects of individual stimuli.
Tensile Loading
Tenogenesis: when targeting tenogenesis, MSCs are often seeded on collagen-based or collagen coated scaffolds and then cultured in standard media as there is currently no defined medium for inducing tenogenesis of MSCs. Chen et al.34 subjected hBMSCs to 3% and 10% global strain and observed an increase in Collagen type I (Col I), Col III, and tenascin-C mRNA at 10%, whereas 3% strain favoured osteogenic differentiation. Farng et al.35 subjected mouse BMSC-seeded poly(caprolactone) scaffolds to 10% strain, which also increased tenogenic gene production of Col I, Col III, and the tendon transcription factor scleraxis. Zhang et al.36 observed the effects of varying the time period (3–36 h) rat BMSCs were subjected to 10% cyclic strain and saw that Col I, Col III, and tenascin-C mRNA were upregulated after 24 h. 10% strain at a low frequency (0.0167 Hz) on human and bovine BMSCs in collagen gels also resulted in an upregulation of Col I, Col III, and tenascin-C mRNA, but this took 14 days of culture37. Strains lower than 10% have also been used in an attempt to induce tenogenesis. 1% strain was applied to human BMSC-seeded collagen gels resulting in an upregulation of Col III and maintaining the level of scleraxis mRNA, whereas in static controls the expression reduced over time38. The effect of a 6.7N static strain was observed by van Eijk et al.39 on goat BMSC-seeded PLGA scaffolds. Initially, collagen content was highest in scaffolds strained during seeding, but after 21 days, unloaded scaffolds had the highest collagen content suggesting that a constant strain inhibits differentiation.
Myogenesis: the proportion of MSCs that have exhibit a myogenic phenotype is low using defined or conditioned culture medium40. Some studies have suggested that myogenic differentiation of MSCs can be influenced by mechanical tension. Increased gene and protein expression associated with myogenesis have been seen when bovine MSCs were subjected to stretching including the calcium binding protein calponin41, the calcponin related protein SM22α42 and α smooth muscle actin (SMA)43,44. However, Park et al.42 found that while uniaxial strain increased SM22α expression, equiaxial strain reduced its expression highlighting the different regulatory roles of these two stimuli. Ku et al.45 subjected human BMSCs to strains of 7–20% over 4 days and observed the highest collagen production at 14% strain and an increase in lysyl oxidase (Lox), however, there were no increases in Col II mRNA (the collagen found in cartilage) or Alkaline Phosphatase (ALP) activity. ALP is involved in bone mineralisation and high ALP activity is used to indicate osteogenic differentiation. Colazzo et al.46 subjected human BMSCs and ADMCSs to 14% strain for 3 days causing increases in collagen production and Col IV mRNA, and upregulation of Col III and elastin cross-linking in ADMSCs. Huang et al.40 tested a range a loading regimens and showed that 10% stretch at 1 Hz for 24 hours was optimal for inducing cardimyocyte gene upregulation in rat MSCs compared to lower or higher strains and frequencies, interestingly the stretch stimulus was much more effective than a 1 Pa unidirectional shear stress stimulus. In agreement, Maul et al.47 compared the effects of cyclic tension, compression, and fluid flow induced shear stress on the expression of smooth muscle related proteins and only stretch (1–10% at 1or 2.75 Hz) upregulated SMA and calponin.
Osteogenesis: application of cyclic tensile loading to MSCs has resulted in increased expression of early osteogenic markers as well as increased mineralised matrix deposition, both in the presence and absence of osteogenic media. Bone morphogenic protein 2 (BMP-2) expression was upregulated following cyclic loading of human BMSC-seeded gels48,49 and rat ADMSCs on 2D substrates50. Alkaline phosphatase activity (ALP) increased with 1 week of cyclic loading in human BMSCs. It appears that dexamethasone can have a synergistic effect or an inhibitory effect on mechanically induced osteogenesis, depending on the concentration used and the marker investigated. For example Jagodzinski et al.51 applied tensile strain to human BMSCs for 6 hours/day, on the first 3 days of culture, at two different strain rates (2% and 8%). Cyclic tensile strain upregulated COLI mRNA and ALP activity, but only at the 8% strain rate. Stretching alone was seen to be as effective as dex treatment alone and there was a synergistic effect of the combination of dex and cyclic tensile strain. Muaney et al.52 investigated the effect of the concentration of dex (0, 10 or 100nM) on the osteogenic enhancing potential of loading (bending). Without dex, loading alone was able to upregulate ALP activity and expression, but it had no effect on the bone matrix proteins osteopontin (OPN) and oscteoclacin (OCN). The addition of 10nM dex seemed to cause a synergistic response but at higher dex levels (100nM) the effect of loading was suppressed.
Summary of tensile stimuli: the literature agrees in general that osteogenesis of MSCs tends to occur at strain magnitudes lower than that for tenogenesis and that cardiomyogenesis is stimulated by using larger strains. In the absence of osteogenic media, upregulation of early (ALP activity) and late (mineralized matrix deposition) osteogenic markers have been observed53. Tenogenesis is also induced in the absence of any chemical inducers, but as for osteogenesis, it appears that static stretching or long term continuous loading has a negative effect on matrix production39,54. Stretching inhibits adipogenesis even in the presence of adipogenic media55 and stretching does not appear to be favourable for chondrogenesis, although tensile strains induced in a more biomimetic loading regimen, that of sliding contact loading does slightly enhance chondrogenesis of MSCs56. These findings indicate that intermittent, cyclic stretching of MSCs is beneficial for the osteogenic, tenogenenic, and myogenic lineages and the production of a fibrous matrix.
Compressive Loading
Compressive loading of MSCs has mainly been investigated for its potential in promoting chondrogeneic differentiation. The chondrogenic response of MSCs to loading is highly complex and the various loading regimens used and the effects that the time at which loading is applied to the cultures has on the outcome is reviewed in more detail elsewhere21.
Osteogenesis: a small number of studies have investigated the effect that global compressive loading20,57 or hydrostatic compression58 of cell-seeded scaffolds may have on osteogenic induction. Hydrostatic compression at loads lower than those usually used for chondrogenesis upregulated ALP activity and the bone transcription factor RUNX2/cbfa1 in MSCs58. Interestingly early markers of either osteogenesis or chondrogenesis can be induced by dynamic compression in the same batch of human MSCs under the same conditions, in an alginate gel-filled collagen sponge, just by varying the strain magnitude (10% strain induced osteogenic genes 15% induced both osteo and chondrogenic genes) but it is not clear what type of tissue would be formed by these cells59. In our studies20 scaffold compression of a polymer scaffold seeded with human MSCs upregulated genes associated with bone matrix formation (Fig. 2) and enhanced the formation of mineralised bone-like matrix. Continuous loading was not necessary to induce MSC differentiation in that study, just 2 hours of loading every 5 days upregulated calcium deposition by more than 50%. However as discussed previously in this type of porous scaffold the individual cells are unlikely to be subjected to compression but to secondary effects such as tension and bending of the scaffolds struts and fluid flow of media into and out of the scaffold20,21. Overall, true compressive loading of MSCs seems to be beneficial for the production of a non-fibrous, cartilage-like matrix, in contrast to tensile loading.
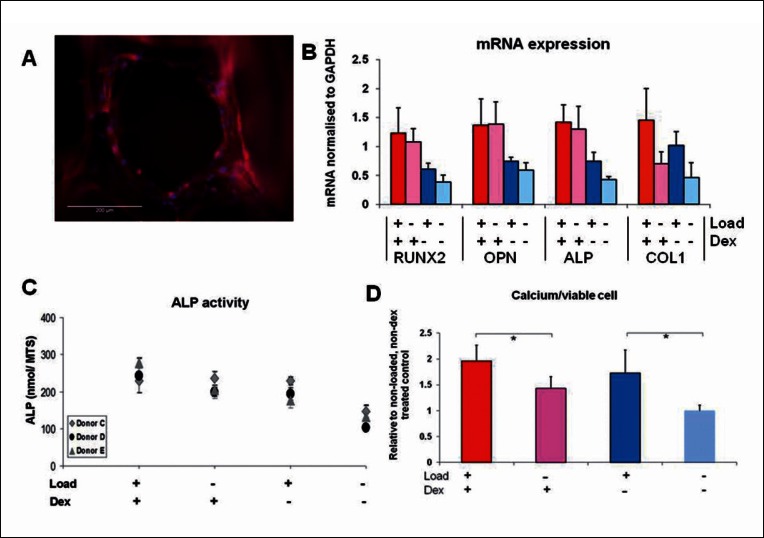
Figure 2.
Compression loading of human MSCs in polyurethane foam scaffolds. A: Fluorescent micrograph of a pore of the scaffold with MSCs attached (blue = cell nucleus stained with DAPI, red = cell cytoskeleton stained with TRITC-phaloidin). B: PCR analysis of mRNA for RUNX2, OPN and ALP showed that these genes were only slightly upregulated by the short (2 hour) loading period and not as much as by continuous dex treatment. However Col 1 was upregulated by loading and inhibited by dex which was also reflected in collagen analysis by Sirius red at a later time-point (data not shown). ALP activity was stimulated by loading to levels seen in dex treated cells as was calcium secretion which was highest with a combination of dex and loading. Adapted from (20) reproduced with kind permission from eCM journal (www.ecmjournal.org).
Fluid Flow Induced Shear Stress
The most commonly used method for inducing shear stresses over a cell monolayer is the parallel-plate flow chamber60,61 although simple lab equipment such as rockers and orbital shakers can also be used to apply a characterised, though less homogenous flow stimulus to cells62,64 (Brennan 2012). In 3D cultures flow is applied using perfusion bioreactors65,66 with a steady, pulsatile and unidirectional flow having all been investigated. The majority of studies have focused on osteogenic differentiation of MSCs. Given that osteoblasts (bone forming cells) and osteocytes (the terminally differentiated cell that reside in bone matrix) have been repeatedly shown to respond to fluid forces in vitro67–69, there is an natural assumption that fluid shear stress will influence osteogensis of MSCs. MSCs seeded on 2D substrates are usually subjected to levels of shear stress around (0.1–2 Pa) whereas the shear stresses experienced in 3D constructs are often much lower than in 2D experiments (0.1 mPa-0.03 Pa) as summarised in McCoy and O’Brien70.
Overall, fluid flow appears to either induce or enhance osteogenesis in MSCs. Osteogenic human MSCs were subjected to 1.2 Pa shear forces for either 30 or 90 mins and an increase in ALP activity was seen, with 30mins of stimulation showing the highest levels71. However, there was no increase in cbfa1expression and interestingly Col I gene expression was lower after flow. This enhancement of ALP activity was also seen in other 2D systems61 including our own62. In our laboratory only 1 hour per day of oscillating flow at 1Hz, beginning on day 5 of culture was sufficient to upregulate ALP, collagen and calcium production (Fig. 3). However, some studies have noticed an inhibition of ALP activity by flow72. Interestingly, Yourek et al.19 noted that although cellular ALP in human MSCs was lower after exposure to 24 hours of continuous fluid flow compared to static controls there was more ALP release into the media in the cells exposed to flow, which is not usually measured in most experimental set-ups, suggesting that flow causes ALP mobilisation, rather than inhibiting osteogenic differentiation. In those experiments there was little additional effect of fluid flow induced sear stress when dex was already present but shear stress upregulated the bone matrix proteins OPN and bone sialo-protein (BSP) and the growth factor BMP-2 to induce osteogenesis when dex was not present.
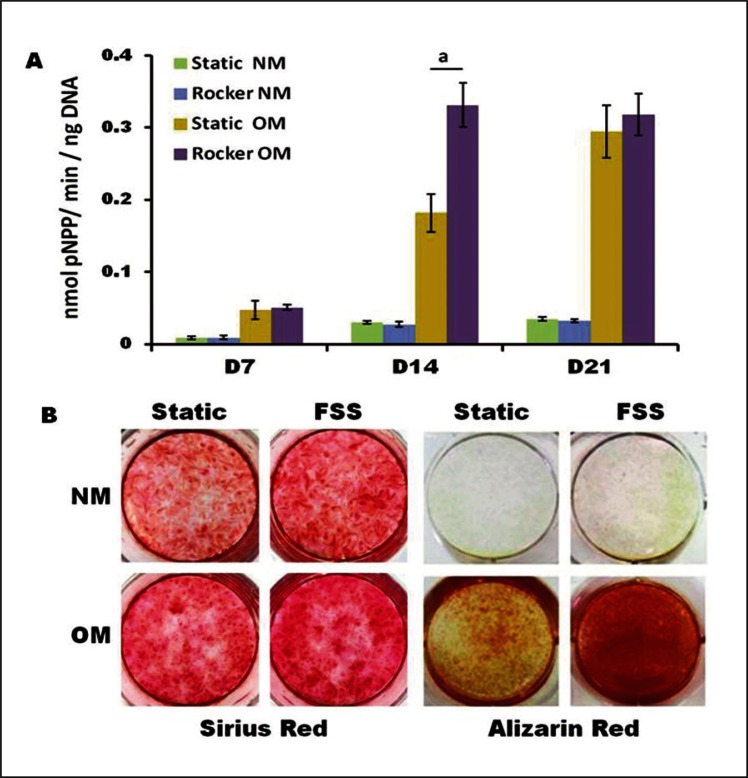
Figure 3.
Osteogenic progenitor cells of the hES-MP line were subjected to oscillatory fluid flow induced shear stress (FFSS) using a simple rocking platform. ALP activity (A) was significantly increased at day 14 with FSS for cells cultured in osteogenic media containing dex (OM). Matrix deposition at day 21 (B) was highest in both FFSS groups for Sirius Red (collagen) and in FSS + OM group for Alizarin Red (calcium). Adapted from (62) reproduced with kind permission from eCM journal (www.ecmjournal.org).
Steady perfusion of 3D cell-seeded constructs is almost always reported to stimulate ALP activity, with the greatest effects occurring at the earlier time points of 4–8 days65,66,73 and then often levelling off, although in some studies, significant increases have been seen up to 14–16 days of culture74,75. Increasing the flow rate can increase calcium production up to a point suggesting that increasing the fluid flow induced shear stress affects later stages of differentiation more than earlier stages or is more important for matrix formation than stimulation of differentiation66,74. The aim of continuous fluid flow through a scaffold is usually to improve nutrient perfusion with any mechanobiological effects of fluid flow induced shear stresses being a positive side-effect. We have shown that continuous perfusion is not necessary to upregulate ALP activity or mineralisation of human MSCs both of which can be upregulated by short bouts of oscillatory fluid flow in a variety of scaffold types including the polyurethane foam described in figure 2 and a non degradable borosilicate glass scaffold76,77.
Low Magnitude High Frequency Loading
Other methods of mechanically stimulating MSCs in vitro have included direct straining of cell-bound integrins by magnetic force for osteochondrogenesis78,79 and low-intensity pulsed ultrasound (LIPUS) for promoting osteogenesis80–82 or chondrogenesis81,83,84 (reviewed in 21). While these techniques have so far had limited use in the mechanical stimulation of MSCs, the studies performed suggest that they may be useful tools for non-invasive stimulation of MSC differentiation.
An intriguing recently advocated stimulus for MSC differentiation is low magnitude, high frequency (LMHF) loading (or vibration) for osteogenesis29,85. This is based on the findings that whole body vibration in animals enhances bone formation86 and decreases the formation of adipose tissue87,88. It was also shown by Luu et al.87 that more MSCs within a population showed commitment towards the osteogenic lineage compared to the adipogenic line-age after the animals were subjected to vibration. LMHF vibration could be a way to stimulate cells in 3D scaffolds without needing a specific bioreactor tailored to the shape of the construct and making it much easier to maintain sterile conditions. In our laboratory whole plate vibration (15–60 Hz) was performed on a human MSC cell line (hES-MP from Cellartis) resulting in increased ALP activity in the presence of dex at 60 Hz after 45 min of stimulation29. However, we found that the outcome was highly dependent on the precise combination of acceleration rate, frequency and even the serum lot that the cells were cultured in, ALP was not upregulated at any other frequency tested and we did not find effects on extracellular matrix deposition. Similarly Lau et al.89 found no effects of a 1h per day 60Hz LMHF loading regimen on rat MSCs. In contrast Sen et al.85 performed LMHF loading on a mouse MSC cell line in multipotential media and observed a down regulation in adiponectin and PPARα (adipogenic) gene expression, whereby the mRNA for the bone matrix protein osteocalcin was upregulated. Preliminary experiments in our laboratory subjecting MSCs to LMHF loading in a range of 3D scaffolds have also not yet provided any evidence of a positive effect of LMHF on osteogenesis. However Zhou et al.90 seeded MSC on demineralised bone scaffolds and found upregulation for the mRNA of a cbfa1 and range of osteogenic matrix proteins. As very little is understood about how cells sense these LMHF movements these different results could be explained by different orientation and arrangements of cells relative to the substrate movement causing the vibration to initiate very different mechanosensory effects.
Summary of interactions between loading and biochemical supplements
There are a number of studies that have shown mechanical loading alone is able to induce expression of osteogenic genes (Runx2, osteopontin, osteocalcin)19,61 and a few that report calcium deposition by MSCs can be stimulated as a result of mechanical stimulus alone91. In most cases, the addition of dex appears to enhance the sensitivity of MSCs to shear stress or strain including enabling mechanoregulated-increased calcium deposition62. However collagen production by MSCs can be inhibited by dex, including in our studies20,62 and tenogenesis in terms of improved Col1 production seems to occur best with no additional supplements36,92. Smooth muscle differentiation stimulated either by fluid flow induced shear stress or cyclic tensile strain was enhanced synergistically with the addition of 5-aza40,60. It may be that biochemical factors and mechanical forces need to work synergistically to stimulate specific pathways or that MSCs need to be at a certain level of maturity (along the specific differentiation pathway) before they sense the load.
Mechanical regulation of MSC proliferation and migration
When Li et al.72 subjected hMSCs to oscillatory fluid flow, they noticed an increase in intracellular calcium mobilization as well as cell proliferation and in another study by Riddle et al.93, fluid shear stresses were seen to enhance hMSC proliferation in part due to calcium signalling. Cyclic tensile strain can also increase MSC proliferation as demonstrated by Ghazanfari et al.94. In contrast, there are many studies that have shown mechanical stimulation to have no effect on cell proliferation95–97 as well as reducing MSC proliferation16,43,98. These mixed findings can most likely be explained by the diversity of conditions including mechanical stimulation, MSC species, and culture media used, as well as the wide range of loading parameters used. However, high strains and flow rates can be also detrimental to cell viability for instance Kearney et al.99 subjected rat MSCs to uniaxial cyclic strain and observed that 7.5% strain or greater lead to cell apoptosis.
In MSC-seeded tissue engineered constructs, flow perfusion, even for short bouts, appears to cause cells to spread evenly through the entirety of the construct, compared with poor spreading under static conditions, indicating that flow improves cell mobility100. However Ode et al.101 showed that MSCs in a fibrin clot subjected to high stains of 20% (aimed at mimicking a fracture healing environment) had a lower ability to migrate compared to non strained cells, an effect mediated by the surface proteins CD73 and integrin ß 1.
Mechanical loading has been shown to affect cell orientation and spreading, and in particular substrate strain can cause elongation and alignment of MSCs41,43,102. Differences in direction of alignment relative to the load direction have been observed with MSCs seeded on 2D substrates orientating perpendicular41,43 and MSCs seeded in 3D gels orientating parallel102. It is thought that on 2D substrates, cells re-orientate to minimize the stretch forces felt by the cell body42 while in 3D matrices, elongation of scaffold pores and struts caused by the strain may dictate cellular orientation. Fluid flow in a parallel plate flow chamber can also induce cell morphological changes and orientation usually in parallel to the flow direction, when it is unidirectional. Interestingly even when rat BM-SCs from the same batch, grown in the same bioreactor are subjected to fluid flow or tensile strain they align parallel to the flow direction but perpendicular to the tensile strain direction47.
Mechanotransduction mechanisms
Targeting the mechanisms responsible for the conversion of extracellular mechanical stimuli into biochemical signals will aid with future stem cell strategies. There have been a number of possible cell membrane mechanoreceptors identified including integrins (transmembrane proteins), stretch activated ion channels and g-protein coupled receptors, the pericellular glycocalyx, and the non-motile primary cilia.
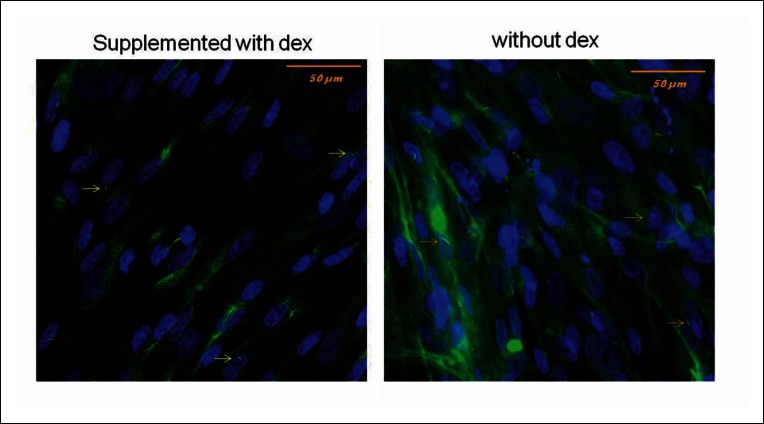
Integrins couple the cytoskeleton to the ECM and cluster at focal adhesion points on the cell surface forming an integrin-ligand bond with the ECM. Application of an external force pulls on the integrin-ligand bond, which transfers across the cell membrane and can result in cytoskeletal deformation. Another mechanism involves deformation of the plasma membrane causing ion flux into/out of the cell via stretch activated ion channels or g-protein coupled receptors103. The third proposed mechanism, the glycocalyx, is a pericelllular GAG-proteoglycan rich layer surrounding the cell membrane that creates a drag force when fluid flow passes over causing plasma membrane deformation104–106. More recently, the primary cilium, an immotile microtubule-based organelle, that protrudes like an antenna from the apical cell surface, has been implicated as a mechanosensor in a variety of cell types including MSCs107. Primary cilia have been shown to bend under fluid flow107, adjust their length in response to load in tendon cells108 and contain receptors that participate in numerous signalling events including stretch activated ion channels109 and integrins110. Recently it has been shown that the primary cilia of human MSCs are required for the modulation of osteogenic and adipogenic differentiation pathways in static conditions, opening up the possibility that they may also mediate mechanically activated differentiation pathways in these cells111. Mesenchymal progenitors from the cell line hES-MP that we have demonstrated is mechanosensitive to a range of fluid flow stimuli also exhibit primary cilia as visualised by acetylated α tubulin staining (Fig. 4).
Figure 4.
MSCs of the cell line hES-MP (embryonic derived mesenchymal progenitors) stained for acetylated alpha tubulin on day 7 of culture. The structures with a high aspect ratio that stain brightly are primary cilia (yellow arrows), the dispersed, background green staining is the cell microtubules. Nuclei are stained blue with DAPI.
Conclusions and Future Directions
Our understanding of the cues affecting MSC differentiation and development has advanced greatly over recent decades. Mechanical forces can greatly influence MSC differentiation and by harnessing its effects, it may be possible to improve pre-implantation culture methods of MSC-seeded constructs and also aid in the design of tissue stimulation/exercise regimens for a patient post-MSC implantation. External mechanical forces are able to induce or enhance MSC differentiation into a wide variety of tissue specific cells; however, precisely controlling the timing and outcome of MSC differentiation is still a large challenge.
Mechanical regulation of MSCs is a complex issue due to the wide range of external mechanical stimuli available (e.g. tension, compression, fluid shear) each accompanied by an almost unlimited choice of loading parameters. Further work is required to indentify which type of mechanical stimuli (or combinations) are the most appropriate as well as the load magnitude, duration and frequency, and when to initiate the loading during culture, in order to pinpoint the optimal strategies. Characterisation of the exact forces MSCs experience in loading systems is important to know what mechanisms are actually inducing the observed responses and to help simplify the design of future loading systems. While most bioreactor systems employ a common force type (e.g. tension or compression), there are also likely to be other mechanisms at work causing significant secondary effects which can cause a misinterpretation in the reason for the obtained results. Mathematical and computer modelling is important for characterising the forces that are being experienced by the cells and will subsequently provide a better understanding of what the cell is responding to112.
Acknowledgments
RMD-S is sponsored by the Engineering and Physical Sciences Research Council.
References
- 1.Sioud M, Mobergslien A, Boudabous A, Floisand Y. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011;38(2):385–390. doi: 10.3892/ijo.2010.869. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7(2):463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 4.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthrit Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augello A, De Bari C. The Regulation of Differentiation in Mesenchymal Stem Cells. Hum Gene Ther. 2010;21(10):1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 7.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature Materials. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 8.Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010;43(1):55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Vogel V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet N, Ferrari SL. Exercise and the Skeleton: How It Works and What It Really does. International Bone and Mineral Society. 2010;7:235–248. [Google Scholar]
- 11.Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: Review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev. 2008;45(2):283–296. doi: 10.1682/jrrd.2007.02.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurkan UA, Akkus O. The Mechanical Environment of Bone Marrow: A Review. Ann Biomed Eng. 2008;36(12):1978–1991. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- 13.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10(1):11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 14.De Santis G, Lennon AB, Boschetti F, Verhegghe B, Verdonck P, Prendergast PJ. How can cells sense the elasticity of a substrate? An analysis using a cell tensegrity model. European Cells and Materials. 2011;22:202–213. doi: 10.22203/ecm.v022a16. [DOI] [PubMed] [Google Scholar]
- 15.Sen B, Xie ZH, Case N, Ma MY, Rubin C, Rubin J. Mechanical Strain Inhibits Adipogenesis in Mesenchymal Stem Cells by Stimulating a Durable beta-Catenin Signal. Endocrinology. 2008;149(12):6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons CA, Matlis S, Thornton AJ, Chen SQ, Wang CY, Mooney DJ. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J Biomech. 2003;36(8):1087–1096. doi: 10.1016/s0021-9290(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 17.Luo ZJ, Seedhom BB. Light and low-frequency pulsatile hydrostatic pressure enhances extracellular matrix formation by bone marrow mesenchymal cells: an in-vitro study with special reference to cartilage repair. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine. 2007;221(H5):499–507. doi: 10.1243/09544119JEIM199. [DOI] [PubMed] [Google Scholar]
- 18.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics. J. Cell Sci. 2009;122(4):546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regenerative Medicine. 2010;5(5):713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sittichokechaiwut A, Edwards JH, Scutt AM, Reilly GC. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cells. European Cells & Materials. 2010;20:45–57. doi: 10.22203/ecm.v020a05. [DOI] [PubMed] [Google Scholar]
- 21.Delaine-Smith RM, Reilly GC. The effects of mechanical loading on mesenchymal stem cell differentiation and matrix production. Vitam Horm. 2011;87:417–480. doi: 10.1016/B978-0-12-386015-6.00039-1. [DOI] [PubMed] [Google Scholar]
- 22.Brown TD. Techniques for mechanical stimulation of cells in vitro: a review. J. Biomech. 2000;33(1):3–14. doi: 10.1016/s0021-9290(99)00177-3. [DOI] [PubMed] [Google Scholar]
- 23.Li S, Jia XL, Duance VC, Blain EJ. The effects of cyclic tensile strain on the organisation and expression of cytoskeletal elements in bovine intervertebral disc cells: an in vitro study. European Cells and Materials. 2011;21:508–522. doi: 10.22203/ecm.v021a38. [DOI] [PubMed] [Google Scholar]
- 24.El Haj AJ, Cartmell SH. Bioreactors for bone tissue engineering. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine. 2010;224(H12):1523–1532. doi: 10.1243/09544119JEIM802. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31(11):969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi Y, Sakai D, Iwashina T, Iwabuchi S, Mochida J. Low-intensity pulsed ultrasound stimulates cell proliferation, proteoglycans synthesis and expression of growth factor-related genes in a human nucleus pulposus cell line. European Cells & Materials. 2009;17:15–22. [PubMed] [Google Scholar]
- 27.Korstjens CM, van der Rijt RHH, Albers GHR, Semeins CM, Klein-Nulend J. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput. 2008;46(12):1263–1270. doi: 10.1007/s11517-008-0409-9. [DOI] [PubMed] [Google Scholar]
- 28.Dumas V, Ducharne B, Perrier A, Fournier C, Guignandon A, Thomas M, et al. Extracellular Matrix Produced by Osteoblasts Cultured Under Low-Magnitude, High-Frequency Stimulation is Favourable to Osteogenic Differentiation of Mesenchymal Stem Cells. Calcif. Tissue Int. 2010;87(4):351–364. doi: 10.1007/s00223-010-9394-8. [DOI] [PubMed] [Google Scholar]
- 29.Edwards JH, Reilly GC. Low magnitude, high frequency vibration modulates mesenchymal progenitor differentiation. Trans Annu Meet Orthop Res Soc. 2011;57 [Google Scholar]
- 30.Hughes S, El Haj AJ, Dobson J. Magnetic micro- and nanoparticle mediated activation of mechanosensitive ion channels. Med Eng Phys. 2005;27(9):754–762. doi: 10.1016/j.medengphy.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33(11):1469–1490. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka SM, Sun HB, Roeder RK, Burr DB, Turner CH, Yokota H. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcif Tissue Int. 2005;76:261–271. doi: 10.1007/s00223-004-0238-2. [DOI] [PubMed] [Google Scholar]
- 33.Donahue TLH, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36(9):1363–1371. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 34.Chen YJ, Huang CH, Lee IC, Lee YT, Chen MH, Young TH. Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connect Tissue Res. 2008;49(1):7–14. doi: 10.1080/03008200701818561. [DOI] [PubMed] [Google Scholar]
- 35.Farng E, Urdaneta AR, Barba D, Esmende S, McAllister DR. The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin Orthop. 2008;1937;466(8):1930. doi: 10.1007/s11999-008-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Tran N, Chen HQ, Kahn CJF, Marchal S, Groubatch F, et al. Time-related changes in expression of collagen types I and III and of tenascin-C in rat bone mesenchymal stem cells under co-culture with ligament fibroblasts or uniaxial stretching. Cell Tissue Res. 2008;332(1):101–109. doi: 10.1007/s00441-007-0564-6. [DOI] [PubMed] [Google Scholar]
- 37.Altman GH, Horan RL, Martin I, Farhadi J, Stark PRH, Volloch V, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 38.Kuo CK, Tuan RS. Mechanoactive Tenogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A. 2008;14(10):1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 39.Van Eijk F, Saris DBF, Creemers LB, Riesle J, Willems WJ, Van Blitterswijk CA, et al. The effect of timing of mechanical stimulation on proliferation and differentiation of goat bone marrow stem cells cultured on braided PLGA scaffolds. Tissue Eng Part A. 2008;14(8):1425–1433. doi: 10.1089/ten.tea.2007.0081. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Zheng L, Gong X, Jia X, Song W, Liu M, et al. Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells. PloS one. 2012;7(4):e34960. doi: 10.1371/journal.pone.0034960. Epub 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103(44):16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng. 2004;88:359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton DW, Maul TM, Vorp DA. Characterization of the response of bone marrow-derived progenitor cells to cyclic strain: implications for vascular tissue-engineering applications. Tissue Eng. 2004;10:361–369. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, et al. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32:1238–1245. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71(3):548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Colazzo F, Sarathchandra P, Smolenski RT, Chester AH, Tseng YT, Czernuszka JT, et al. Extracellular matrix production by adipose-derived stem cells: Implications for heart valve tissue engineering. Biomaterials. 2010;32(1):119–127. doi: 10.1016/j.biomaterials.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Maul TM, Chew DW, Nieponice A, Vorp DA. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech. Model. Mechanobiol. 2011;10(6):939–953. doi: 10.1007/s10237-010-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haudenschild AK, Hsieh AH, Kapila S, Lotz JC. Pressure and Distortion Regulate Human Mesenchymal Stem Cell Gene Expression. Ann Biomed Eng. 2009;37(3):492–502. doi: 10.1007/s10439-008-9629-2. [DOI] [PubMed] [Google Scholar]
- 49.Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: Effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12(12):3459–3465. doi: 10.1089/ten.2006.12.3459. [DOI] [PubMed] [Google Scholar]
- 50.Yang XM, Gong P, Lin YF, Zhang LR, Li XY, Yuan QA, et al. Cyclic tensile stretch modulates osteogenic differentiation of adipose-derived stem cells via the BMP-2 pathway. Archives of Medical Science. 2010;6(2):152–159. doi: 10.5114/aoms.2010.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jagodzinski M, Drescher M, Zeichen J, Hankemeier S, Krettek C, Bosch U, et al. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35–41. doi: 10.22203/ecm.v007a04. 2004. discussion 41. [DOI] [PubMed] [Google Scholar]
- 52.Mauney JR, Sjostorm S, Blumberg J, Horan R, O'Leary JP, Vunjak-Novakovic G, et al. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004 May;74(5):458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 53.Huang CH, Chen MH, Young TH, Jeng JH, Chen YJ. Interactive Effects of Mechanical Stretching and Extracellular Matrix Proteins on Initiating Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Cell Biochem. 2009;108(6):1263–1273. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- 54.Ngiam M, Liao S, Jie TOJ, Sui XD, Dong YX, Ramakrishna S, et al. Effects of mechanical stimulation in osteogenic differentiation of bone marrow-derived mesenchymal stem cells on aligned nanofibrous scaffolds. J Bioact Compatible Polym. 2011;26(1):56–70. [Google Scholar]
- 55.Lee JS, Ha L, Park JH, Lim JY. Mechanical stretch suppresses BMP4 induction of stem cell adipogenesis via upregulating ERK but not through downregulating Smad or p38. Biochem Biophys Res Commun. 418(2):278–283. doi: 10.1016/j.bbrc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Huang AH, Baker BM, Ateshian GA, Mauck RL. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. European cells & materials. 2012;24:29–45. doi: 10.22203/ecm.v024a03. [DOI] [PubMed] [Google Scholar]
- 57.Wagner DR, Lindsey DP, Li KW, Tummala P, Chandran SE, Smith RL, et al. Hydrostatic pressure enhances chondrogenic differentiation of human bone marrow stromal cells in osteochondrogenic medium. Ann Biomed Eng. 2008;36(5):813–820. doi: 10.1007/s10439-008-9448-5. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Zhao ZH, Li J, Zou L, Shuler C, Zou YW, et al. Hydrostatic Pressures Promote Initial Osteodifferentiation With ERK1/2 Not p38 MAPK Signaling Involved. J Cell Biochem. 2009;107(2):224–232. doi: 10.1002/jcb.22118. [DOI] [PubMed] [Google Scholar]
- 59.Michalopoulos E, Knight RL, Korossis S, Kearney JN, Fisher J, Ingham E. Development of Methods for Studying the Differentiation of Human Mesenchymal Stem Cells Under Cyclic Compressive Strain. Tissue Eng Part C-Methods. 18(4):252–262. doi: 10.1089/ten.tec.2011.0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang Y, Jia XL, Bai K, Gong XH, Fan YB. Effect of Fluid Shear Stress on Cardiomyogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells. Arch Med Res. 2010;41(7):497–505. doi: 10.1016/j.arcmed.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialo-protein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6):1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Delaine-Smith RM, Macneil S, Reilly GC. Matrix production and collagen structure are enhanced in two types of osteogenic progenitor cells by a simple fluid shear stress stimulus. European cells & materials. 2012;24:162–174. doi: 10.22203/ecm.v024a12. [DOI] [PubMed] [Google Scholar]
- 63.Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem. Biophys. Res Commun. 2011;412(1):182–187. doi: 10.1016/j.bbrc.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brennan MA. 2012. Close to the bone; investigations into bone tissue mineralisation and mechanobiology of osteoporosis. Thesis (Ph.D.) University of Southampton, Bioengineering Research Group. [Google Scholar]
- 65.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. Journal of Biomedical Materials Research Part A. 2005;72A(3):326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 66.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U. S. A. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein-Nulend J, Bacabac RG, Mullender MG. Mechanobiology of bone tissue. Pathol Biol (Paris) 2005;53(10):576–580. doi: 10.1016/j.patbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 69.You J, Reilly GC, Zhen XC, Yellowley CE, Chen Q, Donahue HJ, et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276(16):13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 70.McCoy RJ, O'Brien FJ. Influence of Shear Stress in Perfusion Bioreactor Cultures for the Development of Three-Dimensional Bone Tissue Constructs: A Review. Tissue Engineering Part B-Reviews. 2010;16(6):587–601. doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- 71.Grellier M, Bareille R, Bourget C, Amedee J. Responsiveness of human bone marrow stromal cells to shear stress. Journal of Tissue Engineering and Regenerative Medicine. 2009;3(4):302–309. doi: 10.1002/term.166. [DOI] [PubMed] [Google Scholar]
- 72.Li YJ, Batra NN, You LD, Meier SC, Coe IA, Yellow-ley CE, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22(6):1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 73.VanGordon SB, Voronov RS, Blue TB, Shambaugh RL, Papavassiliou DV, Sikavitsas VI. Effects of Scaffold Architecture on Preosteoblastic Cultures under Continuous Fluid Shear. Industrial & Engineering Chemistry Research. 2011;50(2):620–629. [Google Scholar]
- 74.Bancroft GN, Sikavitsast VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteloblasts in a dose-dependent manner. Proc Natl Acad Sci U. S. A. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomes ME, Sikavitsas VI, Behravesh E, Reis RL, Mikos AG. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. Journal of Biomedical Materials Research Part A. 2003;67A(1):87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- 76.Shaeri M, Phillips S, Athev D, Chong CK, Reilly GC. Effects of oscillatory and unidirectional flows on mesenchymal stem cells in 3D glass scaffold. J Biomech. 2012;45:S655. [Google Scholar]
- 77.Matsiko A, Edwards J, Reilly GC. Human mesenchymal stem cell responses to steady and oscillatory fluid flow in a porous scaffold. Regenerative Medicine. 2009;4:S159. [Google Scholar]
- 78.Kanczler JM, Sura HS, Magnay J, Green D, Oreffo ROC, Dobson JP, et al. Controlled Differentiation of Human Bone Marrow Stromal Cells Using Magnetic Nanoparticle Technology. Tissue Eng Part A. 2009;16(10):3241–3250. doi: 10.1089/ten.TEA.2009.0638. [DOI] [PubMed] [Google Scholar]
- 79.Kasten A, Muller P, Bulnheim U, Groll J, Bruellhoff K, Beck U, et al. Mechanical Integrin Stress and Magnetic Forces Induce Biological Responses in Mesenchymal Stem Cells Which Depend on Environmental Factors. J Cell Biochem. 2010;111(6):1586–1597. doi: 10.1002/jcb.22890. [DOI] [PubMed] [Google Scholar]
- 80.Angle SR, Sena K, Sumner DR, Virdi AS. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics. 2011;51(3):281–288. doi: 10.1016/j.ultras.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Ikeda K, Takayama T, Suzuki N, Shimada K, Otsuka K, Ito K. Effects of low-intensity pulsed ultrasound on the differentiation of C2C12 cells. Life Sci. 2006;79(20):1936–1943. doi: 10.1016/j.lfs.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 82.Marvel S, Okrasinski S, Bernacki SH, Loboa E, Dayton PA. The Development and Validation of a LIPUS System With Preliminary Observations of Ultrasonic Effects on Human Adult Stem Cells. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2010;57(9):1977–1984. doi: 10.1109/TUFFC.2010.1645. [DOI] [PubMed] [Google Scholar]
- 83.Lai CH, Chen SC, Chiu LH, Yang CB, Tsai YH, Zuo CS, et al. Effects of low-intensity pulsed ultrasound dexamethasone/TGF-beta1 and/or BMP-2 on the transcriptionsal expression of genes in human mesenchymal stem cells: chondrogenic vs. Osteogenic differentiation. Ultrasound Med Biol. 2010;36(6):1022–1033. doi: 10.1016/j.ultrasmedbio.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 84.Lee HJ, Choi BH, Min BH, Son YS, Park SR. Low-intensity ultrasound stimulation enhances chondrogenic differentiation in alginate culture of mesenchymal stem cells. Artif Organs. 2006;30(9):707–715. doi: 10.1111/j.1525-1594.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 85.Sen B, Xie ZH, Case N, Styner M, Rubin CT, Rubin J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44(4):593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ozcivici E, Luu YK, Rubin CT, Judex S. Low-Level Vibrations Retain Bone Marrow's Osteogenic Potential and Augment Recovery of Trabecular Bone during Reambulation. Plos One. 2010;5(6) doi: 10.1371/journal.pone.0011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, et al. Mechanical Stimulation of Mesenchymal Stem Cell Proliferation and Differentiation Promotes Osteogenesis While Preventing Dietary-Induced Obesity. J Bone Miner Res. 2009;24(1):50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U. S. A. 2007;104(45):17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lau E, Lee WD, Li J, Xiao A, Davies JE, Wu Q, et al. Effect of Low-Magnitude, High-Frequency Vibration on Osteogenic Differentiation of Rat Mesenchymal Stromal cells. J Orthop Res. 2011;29:1075–1080. doi: 10.1002/jor.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Y, Guan X, Zhu Z, Gao S, Zhang C, Li C, et al. Osteogenci differentiation of bone marrow-drived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of ERK1/2 activation. European cells & materials. 2011;22:12–25. doi: 10.22203/ecm.v022a02. [DOI] [PubMed] [Google Scholar]
- 91.Ward DF, Salasznyk RM, Klees RF, Backiel J, Agius P, Bennett K, et al. Mechanical strain enhances extracellular matrix-induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells and Development. 2007;16(3):467–479. doi: 10.1089/scd.2007.0034. [DOI] [PubMed] [Google Scholar]
- 92.Nirmalanandhan VS, Juncosa-Melvin N, Shearn JT, Boivin GP, Galloway MT, Gooch C, et al. Combined Effects of Scaffold Stiffening and Mechanical Preconditioning Cycles on Construct Biomechanics, Gene Expression, and Tendon Repair Biomechanics. Tissue Eng Part A. 2009;15(8):2103–2111. doi: 10.1089/ten.tea.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Riddle RC, Taylor AF, Genetos DC, Donahue HJ. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Physiol. 2006;290:C776–C84. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- 94.Ghazanfari S, Tafazzoli-Shadpour M, Shokrgozar MA. Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation into smooth muscle cells. Biochem. Biophys Res Commun. 2009;388:601–605. doi: 10.1016/j.bbrc.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 95.Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21(3):451–457. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 96.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25(11):2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 97.Thomas GP, el Haj AJ. Bone marrow stromal cells are load responsive in vitro. Calcif Tissue Int. 1996;58:101–108. doi: 10.1007/BF02529731. [DOI] [PubMed] [Google Scholar]
- 98.Zhao F, Chella R, Ma T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol. Bioeng. 2007;96(3):584–595. doi: 10.1002/bit.21184. [DOI] [PubMed] [Google Scholar]
- 99.Kearney EM, Prendergast PJ, Campbell VA. Mechanisms of strain-mediated mesenchymal stem cell apoptosis. J Biomech Eng. 2008;130:061004. doi: 10.1115/1.2979870. [DOI] [PubMed] [Google Scholar]
- 100.Bjerre L, Bunger CE, Kassem M, Mygind T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phospahte scaffolds. Biomaterials. 2008;229:2616–2627. doi: 10.1016/j.biomaterials.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Ode A, Kopf J, Kurtz A, Schmidt-Bleek K, Schrade P, Kolar P, et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. European cells & materials. 2011;22:26–42. doi: 10.22203/ecm.v022a03. [DOI] [PubMed] [Google Scholar]
- 102.Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp DA. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. Journal of Biomedical Materials Research Part A. 2007;81:523–530. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 103.Liedert A, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in osteoblastic and mesenchymal stem cells. Mechanosensitivity in Cells and Tissues. 2008:253–265. [Google Scholar]
- 104.Morris HL, Reed CI, Haycock JW, Reilly GC. Mechanisms of fluid-flow-induced matrix production in bone tissue engineering. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine. 2010;224(H12):1509–1521. doi: 10.1243/09544119JEIM751. [DOI] [PubMed] [Google Scholar]
- 105.Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE(2) release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology. 2003;40(6):591–603. [PubMed] [Google Scholar]
- 106.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu. Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 107.Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci. U. S. A. 2007;104(33):13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gardner K, Arnoczky SP, Lavagnino M. Effect of In vitro Stress-Deprevation and Cyclic Loading on the Length of Tendon Cell Cilia in situ. J Orthop Res. 2010;10:582–587. doi: 10.1002/jor.21271. [DOI] [PubMed] [Google Scholar]
- 109.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 110.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 111.Tummala P, Arnsdorf EJ, Jacobs CR. The role of the primary cilia in mesenchymal stem cell differentiation: A pivotal switch in guiding lineage commitment. Cell and Molecular Bioengineering. 2010;3:207–212. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson MS, Epari DR, Bieler F, Duda GN. In vitro models for bone mechanobiology: applications in bone regeneration and tissue engineering. Proceedings of the Institution of Mechanical Engineers Part H-Journal of Engineering in Medicine. 2010;224(H12):1533–1541. doi: 10.1243/09544119JEIM807. [DOI] [PubMed] [Google Scholar]