Summary
Tendon and tendon-bone junction injuries, while heal, have high re-tear rates. Mesenchymal stem cells (MSCs) have great appeal for the promotion of tendon and tendon-bone junction healing because of their high proliferation rate, multi-potency and relative ease of isolation from various tissues. Tendon stem cells have been identified recently and could be an alternative new cell source for tendon and tendon-bone junction repair. In this review, we summarized the in vitro characteristics of tendon stem cells. The evidence supporting the potential use of these cells for tendon and tendon-bone junction repair was presented. In order to therapeutically apply tendon stem cells in the clinical settings, standardization of tendon stem cell culture is essential. Issues relating to the sources, purity, efficacy, safety and delivery of tendon stem cells for tendon and tendon-bone junction repair were summarized and discussed. The direction for future research was suggested.
Keywords: tendon stem cells, tendon progenitors, tendon healing, tendon-bone junction healing, tissue engineering
Epidemiology of tendon and ligament injuries
Tendon/ligament injuries are common in both the work-place and sport; with more than 30 million injuries occurring annually worldwide1. Achilles tendon and anterior cruciate ligament (ACL) are the most common tendon and ligament sustaining acute injury, respectively. About 2 × 105 tendon and ligament repairs are performed annually in the United States2.
Current treatment
Tendon and ligament injuries, whether chronic or acute, are commonly managed by conservative treatments or surgery. The effects of the commonly-used conservative treatments are frequently symptomatic, ineffective and the treatment time is long. If the conservative treatments fail, surgery, with the use of autografts, allografts, xenografts and prosthetic devices, is frequently required for repairing the injured tendon and ligament (replacement if it is an ACL tear)3,4. As many surgical tendon/ligament reconstructions, such as ACL reconstruction and rotator cuff tendon repair, require passing a tendon graft through a bone tunnel, it makes tendon and ligament repair more challenging as it requires the healing of two inhomogenous tissues. Both tendon and tendon-bone junction tissues, while heal, have poor tissue quality and hence have high re-tear rates5–7. The treatment time is also long which prevents early return to sports.
Tissue engineering for tendon and ligament regeneration
Tissue engineering is an interdisciplinary field that applies the principles of biology and engineering to the development of functional substitutes that restore, maintain or improve tissue function. It is a promising approach for the promotion of tendon and tendon-bone junction repair by improving the quality of healing for full restoration of function per se and reducing the chance of re-injuries. Cells, scaffolds and suitable biochemical and/or physio-chemical factors are combined for the functional restoration or regeneration of biological tissues in tissue engineering.
Stem cell characteristics of tendon stem cells
Of the various cell types that are available for tendon and tendon-bone junction repair, mesenchymal stem cells (MSCs) are an attractive cell source as they have high proliferative potential and can differentiate into various cell types of the mesodermal lineage. MSCs were initially isolated in the bone marrow but later have also been isolated from many other tissues such as adipose tissue, umbilical cord, dental pulp, muscle and synovium. Recently, MSCs have also been identitied from tendon tissues of various species including human, mouse, rabbit, rat and horse, in vitro8–11. These tendon stem cells meet the MSC definition of the International Society for Cellular Therapy (ISCT)12. Previous studies showed that tendon stem cells were adherent to plastic, formed adherent colonies in cell culture and showed self-renewal potential8–10. They could differentiate into cells of osteogenic, chondrogenic and adipogenic lineages upon induction in vitro and could form tendon-like, bone-like, cartilage-like and tendon-bone junction-like tissues after subcutaneous transplantation in nude mouse or nude rat models8,9. Stem cells derived from the human patellar tendon proper expressed CD73, CD44, CD90 and CD105 but not CD34 and CD45 as shown by flow cytometry13. In another study, stem cells derived from rat patellar tendon showed null expression of CD11b and HLA-DR as well as very low level of CD79α(submitted unpublished results).
Evidences supporting tendon stem cells as a good alternative cell source for tendon and tendon-bone junction repair
Although MSCs isolated from various tissues demonstrated some common stem cell characteristics, their stem cell properties were not identical14. As stem cells isolated from tendon tissue, the use of tendon stem cells for tendon and tendon-bone junction repair might be advantageous considering the fact that the tendon milieu is an ideal and familiar environment, which might promote the engraftment and differentiation of the transplanted cells. Moreover, we showed that tendon-derived stem cells (TDSCs) exhibited higher colongenicity compared to BMSCs, indicating the ability to recruit more primitive stem cells in TDSC culture15. TDSCs also proliferated faster than BMSCs, which was beneficial for tissue engineering15. Similar finding was also reported by Bi et al.8. They found that human and mouse tendon stem/progenitor cells (TSPCs) proliferated faster than BMSCs isolated from the same person or animal8. The number of population doublings of mouse TSPCs was also higher than that of BMSCs, but this was not observed for human TSPCs8. Moreover, TDSCs expressed higher mRNA levels of tenogenic markers [scleraxis (Scx), tenomodulin (Tnmd)] and extracellular matrix components of tendon [Col1A1, Col1A1/Cl3A1 ratio, decorin (Dcn)] compared to BMSCs15. This finding was corroborated with the data of Bi et al.8 which showed that mouse TSPCs expressed higher mRNA level of Scx than mouse BMSCs whereas human TSPCs expressed higher mRNA level of TNMD than human BMSCs. The results suggested that tendon stem cells might be a good cell source for tendon repair. Recently, we showed that the transplantation of TDSCs histologically and biomechanically promoted tendon repair16 and the results were comparable to the use of BMSCs in a rat patellar tendon window injury model (unpublished results).
In addition to tendon repair, tendon stem cells might also be a good cell source for tendon-bone junction repair, which required the repair of bone and the interfacial fibrocartilage zone in addition to tendon. It has been reported that Scx regulated bone morphogenetic protein 4 (BMP4) in tendon cells at their insertion site during deltoid tuberosity formation17, suggesting that tendon might also contribute to fibrocartilage formation at the tendon-bone junction. Rat TDSCs also exhibited higher chondrogenic and osteogenic markers at basal state, as well as chondrogenesis and osteogenesis upon induction, compared to BMSCs15. It is known that BMPs accelerated tendon-bone junction healing in animal models18–20. TDSCs expressed higher levels of BMP receptors and were more sensitive to BMP-2-induced osteogenic differentiation21 compared to BMSCs. Higher osteogenic differentiation potential of mouse and human TSPCs compared to BMSCs was also observed in Bi et al.8’s study, but they did not compare the chondrogenic differentiation potential of TSPCs and BMSCs. Hence tendon stem cells might be suitable for tendon-bone junction repair with and without exogenous BMPs. It was likely that tendon stem cells were imprinted under the influence of local environmental niche so that they were more likely to produce tendon and junctional tissues. However, there has been no study to test the effect of tendon stem cells for tendon-bone junction repair. Further study is required to compare the effect of tendon stem cells and other MSCs for tendon-bone junction repair.
Below we discussed the practical issues pertaining to the therapeutic application of tendon stem cells for tendon and tendon-bone junction repair.
Issues relating to the therapeutic application of tendon stem cells
Sources of tendon stem cells
It is difficult to get autologous tendon stem cells without causing donor site morbidity. Therefore only the use of allogeneic tendon stem cells with large quantity and with no immuno-rejection will justify their therapeutic use for tendon and tendon-bone junction repair. Low immunogenicity and immunomodulatory effects of MSCs have been well-reported22–25. Recently, our research group showed that TDSCs lacked surface expression of MHCII, CD86 and CD80 (unpublished results). γ-interferon (γ-IFN) pretreatment did not increase MHCII and CD86 but slightly increased the expression of CD80. TDSCs did not induce proliferation and could escape the cytotoxic effect of lymphocytes isolated from the immunized animals. Incubation of TDSCs with the serum from the immunized animals also did not induce complement-dependent cell lysis and antibody recognition (unpublished results). There was no sign of inflammatory cells in the window wound after allogeneic TDSC transplantation16. Therefore, we believed that tendon stem cells were immune-privileged cells and might be used for allogeneic transplantation.
If an allogeneic source of tendon stem cells could be used for tissue engineering, then TDSCs could be easily isolated from the waste tendon tissue during tendon/ligament surgery, such as the residual tendon graft tissue in ACL reconstruction and the waste tendon tissue in total knee replacement. Our laboratory is currently collecting the discarded patellar and hamstring tendon tissues of ACL surgery for TDSC isolation13. A clinical-grade Good Manufacturing Practice – compliant (cGMP) allogeneic tendon stem cellbank for clinical application could be established. The use of allogeneic tendon stem cells for tendon and tendon-bone junction repair avoids delayed treatment to patients compared with the use of autologous cell source. Unlike bone marrow aspiration for the isolation of BMSCs, the harvest of residual tendon tissue does not require a separate surgery and does not impose additional pain to donors. The concentration of BMSCs obtained per milliliter decreased with increased volume of aspirated marrow for each puncture because of dilution of bone marrow sample with peripheral blood26. This would not be an issue for tendon stem cell isolation.
Purity and safety of tendon stem cell culture
Tendon stem cells, like other MSCs, are mainly isolated by an enrichment procedure. They are hencea heterogeneous cell population with variation in stem cell characteristics and might also be contaminated with undersirable cells types, such as tenocytes. The resulting variability limits standardization of tendon stem cell-based repair strategies and also impeds the comparison of treatment outcomes. The development of quality controls for the isolation of pure, safe and effective tendon stem cell populations is hence vital for their routine use in the clinical practice.
Although many different MSC markers have been reported, unique markers for the umambiguous identification of MSCs in vitro and in vivo are yet to be discovered. Commonly-used stem cell surface markers such as CD44, CD90, CD73, CD29 and CD105 were not specific to MSCs and were also expressed by fibroblasts27,28. Zhang and Wang9 characterized tendon stem cells and tenocytes in vitro and reported that tendon stem cells (TSCs), but not tenocytes, possessed multi-lineage differentiation potential. TSCs expressed stem cell marker proteins Oct-4, SSEA-4 and nucleostemin whereas tenocytes expressed none of these markers9. They therefore suggested using these markers to differentiate TSCs in situ. The discovery of surface markers that could specifically differentiate tendon stem cells from tenocytes and possibly other sources of MSCs would ensure the consistent isolation of a pure and effective tendon stem cell population for clinical applications. The genomic stability of tendon stem cells is essential for their safe application in tissue engineering. We (unpublished results) and others8,9 did not observe tumor formation after subcutaneous transplantation of tendon stem cells in nude animals. Still, the use of karyotoyping to demonstrate the stability of the genome of tendon stem cells during in vitro culture is essential, prior to their clinical use.
In vitro expansion of tendon stem cells
Tendon stem cells only made up 1–4% of the total nucleated cells in tendon8,10 and the number of TSPCs isolated from tendons was markedly reduced with aging29. The proliferation rate of aged TSPCs was also reported to be lower compared to young TSPCs29. Aged TSPCs expressed lower basal and TGF-β3-induced mRNA expression of Scx and Tnmd, but higher adipogenic differentiation potential following induction, compared to young cells29. Hence, in vitro culture and expansion of tendon stem cells are required for obtaining enough cells for tendon and tendon-bone junction repair. However, only the early passages of tendon stem cells might be useful for tendon and tendon-bone junction repair as long-term in vitro culture of rat TDSCs resulted in cellular senescence and loss of multi-lineage differentiation potential30. Therefore, strategies that can promote the expansion while maintain the stemness properties of tendon stem cells are essential. We showed that culturing TDSCs under a hypoxic environment (2%O2) enhanced clonogenicity and proliferation as well as reversibly suppressed the multi-lineage differentiation potential of TDSCs upon induction13. Culturing of TSCs on/in decellularized tendon matrix increased their proliferation, self-renewal capacity and multipotency compared to culturing TSCs on the plastic culture surface31. Implantation of TSCs in decellularized tendon matrix promoted tendon-like tissue formation while implantation of TSCs alone did not result in the formation of tendon-like tissue31. However, the fate of transplanted TSCs was not reported in this study, hence it was not clear if the transplanted cells without scaffold were still in place after surgery.
Tenogenic differentiation of tendon stem cells
Although BMSCs have been reported to promote tendon and tendon-bone junction healing in various animal models32–34, there were also reports of negative results34, risk of ectopic bone formation35 and tumor induction36 following transplantation of BMSCs. Therefore, strategies that promote the in vitro tenogenic differentiation of BMSCs prior to cell transplantation into tendon or tendon-bone injury sites were advocated37–41. Improvement in tendon and tendon-bone junction healing was reported after genetic modification or pretreatment of MSCs with these factors42–44 except in one study45. Similar to BMSCs, tendon stem cells theoretically could also induce formation of unwanted tissue after transplantation given their high multi-lineage differentiation potential8,15. We did not observe an increase in the risk of ectopic bone formation after transplantation of allogeneic TDSCs in a patellar tendon window injury rat model which showed ossification at the late stage of tendon healing in 3/6 of samples in histology at week 1246. Whether the use of tendon stem cells driven to the tenogenic lineage would further promote tendon healing and reduce the risk of ectopic bone formation in the patellar tendon window injury rat model requires further research.Various growth factors such as GDF-5, GDF-6 and GDF-7 as well as Scx over-expression have been reported to promote the tenogenic differentiation of BMSCs or adipose tissue-derived MSCs (AdMSCs)37–41. Whether these factors would have similar tenogenic effects on tendon stem cells were not clear. Zhang and Wang47 reported that platelet-rich plasma-clot releasate (PRCR) could promote the differentiation of TSCs into active tenocytes with high proliferation rate and collagen production capability.
Tendon stem cell delivery
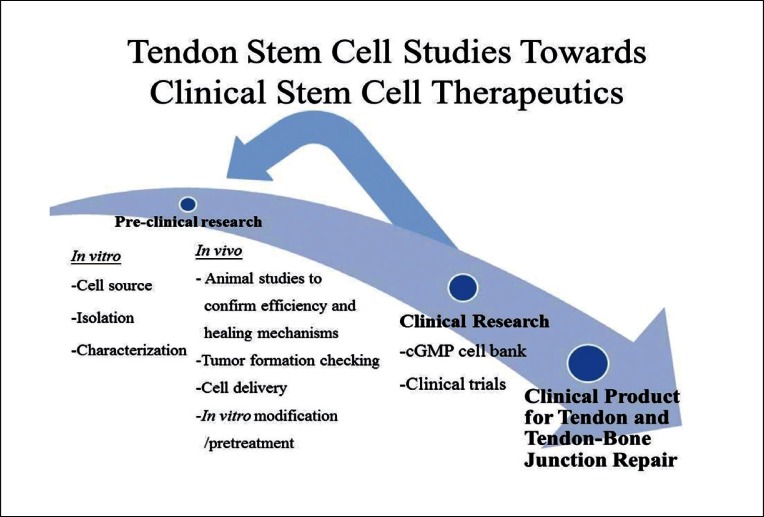
MSCs have homing ability to some tissues, particularly when the tissue is injured or under pathological conditions48. After tendon and tendon-bone junction injuries, MSCs could be delivered to the target site for repair either by systematic infusion or local delivery. Dudhia et al.49 compared the routes of administration of BMSCs for the repair of equine tendinopathies or desmopthies. They reported that intra-lesional injection of BMSCs retained the higher number of cells at the injury site, followed by regional perfusion. Intravenous injection of BMSCs resulted in the distribution of cells to the lungs, without detectable level of BMSCs in the tendon lesions. The best temporal therapeutic window for systematic administration of MSCs has to be identified as the recruitment and treatment effect of MSCs was reported to be time-dependent; and was effective only when MSCs were injected at the time when stromal cell-derived factor-1 (SDF-1) was expressed in the injured tissue at the early phase post-myocardial infarction50. There has been no study on the homing ability of systematically-injected tendon stem cell safter tendon and tendon-bone junction injuries. However, if surgery is required after injury, implantation of tendon stem cells in a bioscaffold seems to be a straight-forward method. Zhang et al.31 reported that acellular tendon matrix was a good biomaterial for the culture and delivery of tendon stem cells as it preferenatailly promoted tenogenic differentiation of TSCs in vitro and hence, tendon-like tissue formation, in vivo. Further research is needed to identify a good biomaterial for the delivery of tendon stem cells to the tendon or tendon-bone interface. Figure 1 shows e schematic diagram summarizing the studies required for traslating the application of tendon stem cell for tendon and tendo-bone junction repair.
Figure 1.
Schematic diagram showing the studies required for translating the application of tendon stem cells for tendon and tendon-bone junction repair.
Conclusion and direction of future research
In conclusion, we have summarized the in vitro characteristics of tendon stem cells. Given their favorable in vitro stem cell characteristics, tendon stem cells might be a good cell source for tendon and tendon-bone junction repair. The effect of tendon stem cells in tendon repair has been reported but there has been no study on their effects in tendon-bone junction repair. As tendon stem cells were not immunogenic, an allogeneic cell source could be used. We discussed the practical issues when applying tendon stem cells for tendon and tendon-bone junction repair, including the specific identification and genomic stability of tendon stem cells, both have not been solved and required further research. This information is essential for the future clinical application of tendon stem cells in tendon and tendon-bone junction repair. Some strategies for the in vitro expansion of tendon stem cells have been reported but new and better strategies to promote the expansion of tendon stem cells in vitro are needed. Whether tenogenic differentiated tendon stem cells would promote better tendon and tendon-bone junction healing in terms of rate and quality needed further research. The study on the biomaterials for the delivery of tendon stem cells is still in the infancy. Further research is needed to identify a good biomaterial for the delivery of tendon stem cells to the tendon or tendon-bone interface.
Acknowledgments
This work was supported by resources from the General Research Fund (project numbers: CUHK471411 and CUHK460170) from the Research Grants Council of the Hong Kong Special Administrative Region, China.
Footnotes
Conflict of Interest
We don’t have any conflict of interest.
References
- 1.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22(4):675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 2.Pennisi E. Tending tender tendons. Science. 2002;295(5557):1011. doi: 10.1126/science.295.5557.1011. [DOI] [PubMed] [Google Scholar]
- 3.Goh JCH, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(Suppl 1):S31–S44. doi: 10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- 4.Bagnaninchi PO, Yang Y, El Haj AJ, Maffulli N. Tissue engineering for tendon repair. Br J Sports Med. 2007;41(8):e10. doi: 10.1136/bjsm.2006.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982–989. [PubMed] [Google Scholar]
- 6.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Vergis A, Gillquist J. Graft failure in intra-articular anterior cruciate ligament reconstructions: a review of the literature. Arthroscopy. 1995;11(3):312–321. doi: 10.1016/0749-8063(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 8.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wang JHC. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculo skelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rui YF, Lui PPY, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multi-potent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16(5):1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 11.Lovati AB, Corradetti B, Lange Consiglio A, et al. Characterization and differentiation of equine tendon-derived progenitor cells. J Biol Regul Homeost Agents. 2011;25(2 Suppl):S75–S84. [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotentmesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Lee WYW, Lui PPY, Rui YF. Hypoxia mediated efficient expansion of human tendon-derived stem cells (hTDSCs) in vitro. Tissue Eng Part A. 2012;18(5–6):484–498. doi: 10.1089/ten.tea.2011.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 15.Tan Q, Lui PPY, Rui YF, Wong YM. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng Part A. 2012;18(7–8):840–851. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni M, Lui PPY, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30(4):613–619. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 17.Blitz E, Viukov S, Sharir A, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17(6):861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Nam HW, Hur CY, et al. The effect of platelet rich plasma from bone marrow aspirate with added bone morphogenetic protein-2 on the Achilles tendon-bone junction in rabbits. Clin Orthop Surg. 2011;3(4):325–331. doi: 10.4055/cios.2011.3.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coen MJ, Chen ST, Rundle CH, Wergedal JE, Lau KH. Lentiviral-based BMP-4 in vivo gene transfer strategy increases pull-out tensile strength without an improvement in the osteointegration of the tendon graft in a rat model of biceps tenodesis. J Gene Med. 2011;13(10):511–521. doi: 10.1002/jgm.1604. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Chang CH, Wang KC, et al. Enhancement of rotator cuff tendon-bone healing with injectable periosteum progenitor cells-BMP-2 hydrogel in vivo. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1597–1607. doi: 10.1007/s00167-010-1373-0. [DOI] [PubMed] [Google Scholar]
- 21.Rui YF, Lui PPY, Lee YW, Chan KM. Higher BMP receptors expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared to bone marrow-derived mesenchymal stem cells. Int Orthop. 2012;36(5):1099–1107. doi: 10.1007/s00264-011-1417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 23.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115(1):50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Yang Y, Yang D, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2008;126(2):220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Tang T, Shi Q, Fernandes JC, Dai K. The immunologic properties of undifferentiated and osteogenic differentiated mouse mesenchymal stem cells and its potential application in bone regeneration. Immunobiology. 2009;214(3):179–186. doi: 10.1016/j.imbio.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J Bone Joint Surg Am. 1997;79(11):1699–1709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20(1):53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 28.Alt E, Yan Y, Gehmert S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103(4):197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell. 2010;9(5):911–915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan Q, Lui PPY, Rui YF. Effect of in vitro passaging on the stem cell-related properties of tendon-derived stem cells (TDSCs) – Implication in tissue engineering. Stem Cells Dev. 2012;21(5):790–800. doi: 10.1089/scd.2011.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Li B, Wang JHC. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like formation in vivo. Biomaterials. 2011;32(29):6972–6981. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(9):899–910. doi: 10.1016/j.arthro.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Nourissat G, Diop A, Maurel N, et al. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS One. 2010;5(8):e12248. doi: 10.1371/journal.pone.0012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 35.Awad HA, Butler DL, Dressler MR, Smith F, Boivin GP, Young RG. Repair of patellar tendon injuries using mesenchymal stem cells and collagen scaffolds. J Orthop Res. 2003;21(3):420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 36.Tasso R, Augell A, Carida M, et al. Development of sacromas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30(1):150–157. doi: 10.1093/carcin/bgn234. [DOI] [PubMed] [Google Scholar]
- 37.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16(9):2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan SL, Ahmad RE, Ahmad TS, et al. Effect of growth differentiation factor 5 on the proliferation and tenogenic differentiation potential of human mesenchymal stem cells in vitro. Cells Tissues Organs. 2012 doi: 10.1159/000335693. [DOI] [PubMed] [Google Scholar]
- 39.Haddad-Weber M, Prager P, Kunz M, et al. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2012;12(4):505–513. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J BiosciBioeng. 2005;100(4):418–422. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- 41.Alberton P, Popov C, Pragert M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21(6):846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JY, Zhou Z, Taub PJ, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One. 2011;6(3):e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahab-Osterloh S, Witte F, Hoffmann A, et al. Mesenchymal stem cell-dependent formation of hetero-topic tendon-bone insertions (osteotendinous junctions) Stem Cells. 2010;28(9):1590–1601. doi: 10.1002/stem.487. [DOI] [PubMed] [Google Scholar]
- 44.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymalstem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(6):1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 45.Gulotta LV, Kovacevic D, Packer JD, Ehteshami JR, Rodeo SA. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39(1):180–187. doi: 10.1177/0363546510379339. [DOI] [PubMed] [Google Scholar]
- 46.Lui PP, Cheuk YC, Lee YW, Chan KM. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J Orthop Res. 2012;30(1):37–46. doi: 10.1002/jor.21495. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 48.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 49.Dudhia J, Becerra P, Valdes MA, et al. Tracking of mesenchymal stem cells in tendon injuries followingin vivo administration. Proceedings of the International Symposium on Ligaments & Tendons (ISL&T)-XII; 3rd Feb, 2012; San Francisco, California, USA. [Google Scholar]
- 50.Ma J, Ge J, Zhang S, et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol. 2005;100(3):217–223. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]