Abstract
Background
Patient-centeredness has gained importance over the last two decades. However, there is an absence of theoretical clarity regarding the term patient-centeredness. This results in inconsistent measurement of patient-centeredness, which leads to difficulties in comparing research results. To overcome these difficulties, the aims of this study are (1) to identify the dimensions of patient-centeredness and include them in an integrative model, (2) to select and assess the most relevant dimensions of the model, (3) to identify and assess measurement instruments and find evidence for the selected dimensions, and (4) to assess the relevance and applicability of the conceptualization and measurement of patient-centeredness in clinical practice and health services research.
Methods
This project is divided into four phases. First, a systematic review will be conducted to identify the constructs and dimensions of patient-centeredness. Second, experts (eg, researchers, clinicians, and patient representatives) will assess and prioritize the identified relevant dimensions using a Delphi survey. Third, the selected dimensions will be assessed regarding their operationalization, and the results will be summarized in a systematic review. Evidence of the measures will be assessed in a scoping review. Fourth, an expert workshop will be held. Experts will assess the results of the previous phases regarding the relevance and applicability of the conceptualization and measurement of patient-centeredness.
Discussion
This study will provide an integrative model of patient-centeredness based on the current literature. This model can be used to improve upon the comparability of research results. Through a detailed insight into the existence and evidence of measurement dimensions of patient-centeredness, existing gaps in this field will be shown. Finally, the expert assessment will show the relevance and feasibility of the concept and measures in clinical practice and health services research. Therefore, this study will contribute important knowledge to enhance future research on patient-centeredness and establish a foundation for its implementation.
Keywords: patient-centeredness, measurement, conceptual framework
Background
Over the past two decades, patient-centeredness has been a widely discussed topic in high-quality health care and modern medicine.1–4 Patient-centeredness accompanies an increased emphasis on the participation and self-determination of patients in their health care.5 Research has shown that patient-centeredness increases quality and efficiency and may reduce costs in health care.6–8 Furthermore, patient-centeredness has been found to be associated with multiple positive outcomes, eg, higher patient satisfaction, enhanced adherence, improved illness-related knowledge and health behavior, and decreased health care utilization.5,8,9 Moreover, the concept is increasingly demanded from governments across the world at the health policy level. In the UK, patient-centeredness is a core concept in professional medical guidance,10 and it has been described as one of the current “buzz phrases” in the British National Health Service (NHS).11 In the US, the Institute of Medicine (IOM) declared patient-centeredness to be one of six goals for improvement of the US health care system.12,13 This goal has been supported by the 2010 Patient Protection and Affordable Care Act.14 One of the Act’s outcomes was the foundation of the Patient-Centered Outcomes Research Institute (PCORI) (http://www.pcori.org), which has been established to foster better research for a deeper understanding of patient-centeredness. In 2003, Health Canada highlighted the essential meaning of patient-centeredness in health care by creating the Interdisciplinary Education for Collaborative Patient-Centered Practice (IECPCP) initiative.15 In Germany, the government launched a large research priority program on patient-centeredness and chronic diseases in 2007, including a total funding volume of over 20 million Euros allocated to 77 research projects (http://www.research-patientcenteredcare.org).
Although patient-centeredness has been the focus of research and health policy developments in previous decades, an absence of consensus remains regarding its definition and conceptualization. Whereas van Dulmen16 describes patient-centeredness as a “fuzzy concept,” Epstein et al17 outline it as a “multifaceted construct, like intelligence,” and Hobbs18 distinguishes patient-centeredness as a “poorly conceptualized phenomenon.” Additional results showing the ambiguity of the term patient-centeredness were found in a pilot study of this project.19 German researchers of 77 projects regarding patient-centeredness (http://www.research-patientcenteredcare.org) were interviewed on how they would define patient-centeredness, the dimensions they would include in their definition, and how they would operationalize the term. The responses showed a considerable diversity of definitions and dimensions. In particular, the discrepancy in responses on operationalization was high.19
Globally, a variety of models describing the components of patient-centeredness can be found. For instance, Mead and Bower20,21 postulate five key dimensions of patient-centeredness (biopsychosocial perspective, patient-as-person, sharing power and responsibility, therapeutic alliance, and doctor-as-person). In contrast, Stewart22 describe six elements of the patient-centered method (eg, exploring both the disease and the illness experience, finding common ground, enhancing the patient-doctor relationship, being realistic, etc). Furthermore, Gerteis et al23 named seven domains of patient-centered care (eg, respect for patients’ values, preferences, and needs; coordination and integration of care, transition, and continuity; etc). The dimensions of Gerteis et al have been adopted by the Picker Institute.24 Another concept, contributed by Epstein et al,17 refers to four domains of patient-centered communication (eg, eliciting and understanding the patient’s perspective and concerns, helping patients to share power and responsibility, etc).
Overall, there is not only a broad heterogeneity within the definitions of patient-centeredness but also a gap of conceptual explicitness of the concept. This leads to an ambiguity concerning relevant and adequate dimensions for the measurement of patient-centeredness.1,4,16 Consequently, outcomes regarding the effectiveness of patient-centeredness are related to its definitions used in respective studies,25 which limits the comparability of their results.26 However, consistent measurement instruments are indispensable2,8,26 for implementing the concept of patient-centeredness in routine clinical practice, which is demanded from a health policy level. Therefore, the following objectives will be addressed in this study:
Identify the different dimensions of patient-centeredness described in the literature and assemble them into an integrative model;
Assess the identified dimensions and select the most relevant dimensions of patient-centeredness using expert ratings;
Assess the selected dimensions regarding their operationalization in measurement instruments and respective evidence;
Assess the results of the previous phases regarding the relevance and applicability of conceptualization and measurements in health services research and clinical practice.
Materials and methods
Study design
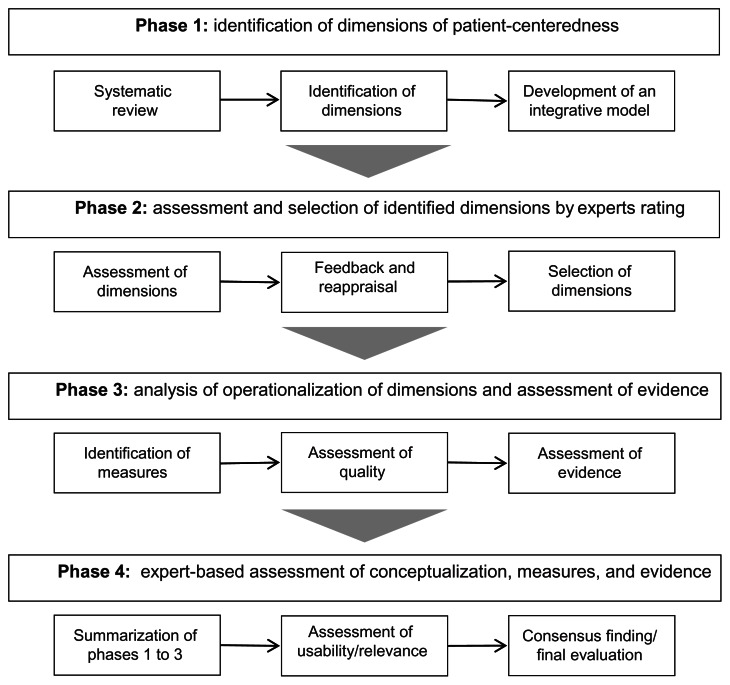
The present study is a 3-year research project divided into four distinct phases. A project overview is provided in Figure 1.
Figure 1.
Overview of the project phases.
All authors (JZ, IS, MH, JD) work for the faculty of medicine of the University Medical Center Hamburg-Eppendorf. The authors have a background in psychology and medicine. All authors are researchers focusing on health services research, particularly on patient-centeredness, patient involvement, and shared decision making. In addition all authors are enrolled in clinical work and medical education. All authors will participate in all phases of the project.
In the following, the four project phases and their respective methodologies are described in detail.
Phase 1: identification of the dimensions of patient-centeredness
The aim of the first phase is to identify the different dimensions of patient-centeredness described in the literature and to propose an integrative model of patient-centeredness based on these results. This aim will be addressed by conducting a protocol-driven systematic review.27
Identification of relevant work
The primary search strategy will consist of an electronic search of several databases (eg, Medline, EMBASE, Cochrane Library Database, PsychInfo, etc). Search strategies will be tailored to each database. The secondary search strategies will consist of reference and citation tracking. Furthermore, articles will be retrieved through personal knowledge, and international experts in the field will be contacted. Original articles, as well as theoretical and conceptual articles, book chapters, and books will be included in the review. The included articles are restricted to articles that clearly focus on patient-centeredness in the title.
Data extraction and assessment of quality
The search results will be imported into Endnote (Thomson Reuters, New York, NY, USA), and duplicates will be removed. A screening of titles and abstracts will be conducted independently by two members of the research team to exclude records that are obviously off-topic. Subsequently, full texts will be independently assessed for eligibility and quality by two members of the research team, ie, to determine whether they include a clear definition or model of patient-centeredness. Discrepancies will be resolved by discussions with a third member of the research team. No further assessment of quality and validity of the articles will be conducted, as the aim is to identify a broad range of models and definitions used in the literature about patient-centeredness.
Analysis
All articles that include a definition of patient-centeredness will be included in the review and imported into the MAXQDA software (VERBI GmbH, Berlin, Germany) for subsequent content analysis. In an initial step of this process, 50 articles will be randomly selected, and their definitions of patient-centeredness will be marked and divided into meaningful units. Coding rules and a coding sheet will be developed based on this preliminary review of the unitized definitions. Subsequently, all included articles will be independently reviewed and coded by two researchers. All disagreements will be resolved with a third team member. The coding sheet can be extended if new categories emerge during the analysis of further articles.
Summarization and interpretation
Finally, dimensions of patient-centeredness will be subsumed from the categories by two independent researchers of the team. These dimensions will be assembled into an integrative model integrating the different main models of patient-centeredness described in the literature as well as further definitions found in the review. Consensus will be sought in discussion with all four team members.
A timeframe of 12 months is scheduled for this initial phase.
Registration of this systematic review in the International Prospective Register of Systematic Reviews (PROSPERO) was not possible because it did not fulfill their inclusion criteria.
Phase 2: assessment and selection of the identified dimensions
The aim of the second phase is to assess the dimensions of the integrative model created in the first phase. The dimensions of patient-centeredness will be rated regarding their relevance. Further research in this study will focus on the most relevant dimensions. To achieve this aim an online Delphi survey will be performed.28,29
Delphi survey
The Delphi technique is a structured group discussion process, commonly used to reach a high group consensus.30
An online survey will be used because it has two main advantages, namely, faster data collection and higher flexibility for the participants, which enhances the response rate in comparison to a paper and pencil questionnaire.31 The participants of the survey will be asked to rate the relevance of the dimensions identified in the first phase. Furthermore, the participants will be asked to add and assess additional dimensions that have not been previously included. In the second round, the results of the first round will be reported to the participants, and they will be asked for a subsequent assessment of relevance. Therefore, their own assessment can be compared to the first-round assessment provided by other participants and corrected where necessary. This process will lead to a higher consensus among the participants. Subsequently, the most relevant dimensions will be selected based on these assessments.
Based on the results of the Delphi survey, further refinement of the integrative model developed in the first phase will be considered.
Sample
For the online Delphi survey, the target sample is N = 40 participants and will consist of German and international participants. The sample will include patient representatives, clinicians, and researchers. Demographic information (eg, gender, age, and professional background) of the participants will be gathered. Patient representatives will be recruited through patient organizations. Clinicians will be recruited within the University Medical Center Hamburg-Eppendorf and by contacting other national and international clinical centers and outpatient care practices. Researchers will be recruited by personal knowledge and through the identification of key authors in the field of patient-centeredness.
The selected dimensions will inform the third phase. The online Delphi survey will be conducted within a 6-month timeframe.
Phase 3: identification and assessment of measurement instruments and evidence of the dimensions
The aim of the third phase is to (1) identify and assess measurement instruments and (2) find existing evidence of the dimensions selected in the second phase. To achieve these aims, both systematic and scoping reviews will be conducted.
Identification and assessment of measures
A systematic review will be conducted to identify measurement instruments for each dimension rated as relevant in the Delphi survey in the second phase. Similar to the methodology of the first phase, the search strategy will consist of an electronic search of several databases (eg, Medline, EMBASE, Cochrane Library, PsychInfo, etc) and citation and reference tracking to identity relevant literature. The inclusion criteria will be that the article (1) describes an instrument designed to measure a dimension of patient-centeredness and (2) reports original information about a measure that had not already been reported in another article included in the review. All search results will be imported into Endnote and duplicates will be removed. All potentially relevant studies will be assessed by two members of the research team who will decide upon inclusion. Any differences of opinion will be resolved by consensus with a third member of the research team.
Subsequently relevant information (eg, sample or setting) from each article will be extracted consistently using a standard procedure. Two members of the team will extract the information from each article and any discrepancies in ratings will be discussed with a third member of the research team.
The quality of the studies will be evaluated from the assessment of the methodology and methods used. The methodological quality of the included measures will be assessed according to (1) whether a theoretical framework was used for scale development and (2) the adequacy of the measurement properties (eg, validity, reliability, responsiveness, etc).32,33 Measurement standards, eg, the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist,34 will be applied for the latter assessment. The quality of the studies will be assessed by two team members. Any disagreements will be clarified with a third reviewer of the research team.
Identification and assessment of evidence
In a second step, the existing evidence regarding the relationship between the dimensions of patient-centeredness on health-related outcomes will be assessed. Therefore scoping reviews will be conducted to summarize the range of evidence.35,36 The aim of this step is to determine what is known from the existing literature about the evidence of the dimensions of patient-centeredness. Therefore an electronic search and citation and reference tracking, similar to the previous step of this phase, will be initially conducted.
The inclusion criteria will be that the article (1) examines the relationship of a dimension of patient-centeredness and certain health outcomes, and (2) belongs to one of the following study types: meta-analyses, systematic reviews, or randomized, controlled studies. These study types have been found to have the highest evidence level according to the NICE guidelines.37
All articles will be imported into Endnote. Two researchers of the team will review the articles for inclusion using the NICE algorithm for the classification of study designs.37 When differences of opinion on article inclusion occur, consensus will be reached with a third reviewer. Second, two members of the team will independently extract data to determine whether the studies bear a minimum amount of evidence. The assessment of evidence will depend on whether for the dimensions of patient-centeredness, a relationship with health care outcomes can be found. Again any differences in opinion will be discussed with a third member of the research team.
Third, the results will be presented in a narrative form and will provide an overview of the volume and characteristics of existing evidence. The aim is to fulfill the goals of this phase within 12 months.
Phase 4: assessment of the conceptualization, measures, and evidence in health services research and clinical practice
The aim of the fourth phase is to assess the results of the three previous phases using experts. This aim will be addressed by conducting an expert workshop.
Expert workshop
The experts will be asked to assess the results concerning their relevance and applicability within evaluation and health services research and their usability in clinical practice. Prior to the workshop, the participants will receive an overview of the results of the previous three phases. During the workshop, the results will be presented in more detail. The experts’ assessments during the workshop will be based on various checklists used to assess endpoints and quality indicators in health care.38,39 These checklists will refer to relevance (eg, relevance for the health care system), scientific soundness (eg, psychometric characteristics), and practicability and feasibility (eg, comprehensibility, acceptance, costs, etc). The decision making process in the expert workshops will follow a procedure of structural consensus finding that is comparable to the development of guidelines in expert workshops.40
Sample
The group of experts in the workshop will be acquired from the 40 experts (patient representatives, clinicians, and researchers) that participated in the Delphi survey in the second phase. We aim to recruit a subsample of the expert group that participated in the second phase with an equal distribution of patient representatives, clinicians, and researchers. We aim for a final sample size of 15–20 participants. We will invite all experts that participated in the Delphi survey since, from our previous experience in conducting expert workshops, usually fewer experts are willing to participate in a workshop. If willingness to participate is higher than expected the workshop could be expanded.
Ethical considerations
The Ethics Committee of the State Chamber of Physicians in Hamburg (Germany) informed us that ethical approval is not necessary because no patients will be enrolled in the study. Nevertheless, ethical principles will be followed throughout the examination; the experts will be informed on the data collection and analysis. Participation will be on a voluntary basis, and data protection rules will be considered. All data produced will be archived for 5 years. For external quality control security, all data and study documents will be available for anonymous viewing by external reviewers.
Discussion
This study will respond to the necessity of a solid theoretical and conceptual foundation of patient-centeredness and the requirement of high-quality measures in this field. The results of the study can provide a basis for a sustainable implementation of patient-centeredness in routine clinical care, which is strongly demanded from a health policy level. This study will close some of the current gaps. First, the conceptual review will help identify essential dimensions of patient-centeredness, and an integrative conceptual model will be proposed. Second, the assessment of the dimensions of patient-centeredness by a broad range of experts will assure high practical relevance of the model. Third, by reviewing the measurement instruments for the selected dimensions, it will become apparent which dimensions can be assessed with existing instruments and which dimensions require revision or the development of new scales. Therefore, the current gaps regarding operationalization and evidence of the dimensions of patient-centeredness will be identified and can be used to guide further research. The developed integrative model of patient-centeredness will aid in future studies and intervention trials that propagate patient-centeredness. The comparison of research results will be increased by using a single integrative model and consensus standards for measurement scales.
Fourth, the final assessment of results by experts will be an initial step for developing measurement standards and quality indicators in this field to finally compare the current quality in care. The inclusion of experts throughout the study will improve the practical relevance and applicability of the results.
The results of this study will therefore eventually contribute to the international call for health care that truly places the patient at its center.
Acknowledgments
This project was funded by the German Ministry of Education and Research (project number: 01GX1043). We would like to thank Stephanie Pahlke and Marta Plonka for their work on the project as student research assistants.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
Author contributions
IS, JD, and MH conceived the study and sought funding. All authors conducted searches for relevant literature. JZ wrote the initial draft of the study protocol. All authors contributed to subsequent drafts of the manuscript and have read and approved the final version.
References
- 1.Epstein RM, Street RL., Jr The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. doi: 10.1370/afm.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence M, Kinn S. Defining and measuring patient-centred care: An example from a mixed-methods systematic review of the stroke literature. Health Expect. 2012;15(3):295–326. doi: 10.1111/j.1369-7625.2011.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewin S, Skea Z, Entwistle VA, Zwarenstein M, Dick J. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev. 2001;4:CD003267. doi: 10.1002/14651858.CD003267. [DOI] [PubMed] [Google Scholar]
- 4.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51(7):1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 5.Maizes V, Rakel D, Niemiec C. Integrative Medicine and Patient-Centered Care. Explore (NY) 2009;5(5):277–289. doi: 10.1016/j.explore.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Coulter A, Ellins J. QEI review: patient-focused interventions. A review of the evidence. London: The Health Foundation; 2006. [Accessed June 8, 2012]. Available from: http://www.health.org.uk/public/cms/75/76/313/526/Patient%20focused%20interventions.pdf?realName=juNCmR.pdf. [Google Scholar]
- 7.Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. 2007;335(7609):24–27. doi: 10.1136/bmj.39246.581169.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michie S, Miles J, Weinman J. Patient-centredness in chronic illness: What is it and does it matter? Patient Educ Couns. 2003;51(3):197–206. doi: 10.1016/s0738-3991(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600–607. doi: 10.1111/j.1745-7599.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- 10.Mansfield A, Nathanson V, Jayesinghe N, Foyle G. The Psychological and Social Needs of Patients. UK: British Medical Association; 2011. Available from: http://de.scribd.com/doc/94820870/BMA-psychologicalsocialneedsofpatients-tcm41-202964. [Google Scholar]
- 11.Davies PG. Patient centredness. J Epidemiol Community Health. 2007;61(1):39. [PMC free article] [PubMed] [Google Scholar]
- 12.Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood) 2002;21(3):80–90. doi: 10.1377/hlthaff.21.3.80. [DOI] [PubMed] [Google Scholar]
- 13.Committee on Quality of Health Care in America; Institute of Medicine. Crossing on Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 14.Representatives SaHo. The Patient Protection and Affordable Care Act. Washington: One Hundred Eleventh Congress of the United States of America; 2010. [Accessed June 6, 2012]. Available from: http://www.gpo.gov/fdsys/pkg/BILLS-111hr3590enr/pdf/BILLS-111hr3590enr.pdf. [Google Scholar]
- 15.Health Canada. Interdisciplinary Education for Collaborative, Patient-Centred Practice. Research and Findings Report. Ottawa: Health Canada; 2004. [Accessed June 6, 2012]. Available from: http://www.ferasi.umontreal.ca/eng/07_info/IECPCP_Final_Report.pdf. [Google Scholar]
- 16.van Dulmen S. Patient-centredness. Patient Educ Couns. 2003;51(3):195–196. doi: 10.1016/s0738-3991(02)00194-5. [DOI] [PubMed] [Google Scholar]
- 17.Epstein RM, Franks P, Fiscella K, et al. Measuring patient-centered communication in Patient-Physician consultations: Theoretical and practical issues. Soc Sci Med. 2005;61(7):1516–1528. doi: 10.1016/j.socscimed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs JL. A dimensional analysis of patient-centered care. Nurs Res. 2009;58(1):52–62. doi: 10.1097/NNR.0b013e31818c3e79. [DOI] [PubMed] [Google Scholar]
- 19.Scholl I, Härter M, Dirmaier J. The concept of patient-centeredness in health services research – an expert survey. Proceedings of the 13th Annual Meeting of the German Network for Evidence Based Medicine (DNEbM); March 15–17, 2012; Hamburg, Germany. Available from: http://www.egms.de/static/en/meetings/ebm2012/12ebm118.shtml. [Google Scholar]
- 20.Mead N, Bower P. Measuring patient-centredness: a comparison of three observation-based instruments. Patient Educ Couns. 2000;39(1):71–80. doi: 10.1016/s0738-3991(99)00092-0. [DOI] [PubMed] [Google Scholar]
- 21.Mead N, Bower P. Patient-centred consultations and outcomes in primary care: a review of the literature. Patient Educ Couns. 2002;48(1):51–61. doi: 10.1016/s0738-3991(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 22.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 23.Gerteis M, Edgman-Levitan S, Daley J, Delbanco TL. Through the Patient’s Eyes. San Francisco: Jossey-Bass; 1993. [Google Scholar]
- 24.The Institute for Alternative Futures. Patient-Centered Care 2015: Scenarios, Vision, Goals and Next Steps. Alexandria: The Institute for Alternative Futures; 2004. [Accessed June 5, 2012]. http://174.120.202.186/~pickerin/wp-content/uploads/2010/06/PCC-2015.pdf. [Google Scholar]
- 25.Laws MB, Epstein L, Lee Y, Rogers W, Beach MC, Wilson IB. The association of visit length and measures of patient-centered communication in HIV care: a mixed methods study. Patient Educ Couns. 2011;85(3):e183–e188. doi: 10.1016/j.pec.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith RC, Dwamena FC, Grover M, Coffey J, Frankel RM. Behaviorally defined patient-centered communication – A narrative review. J Gen Intern Med. 2011;26(2):185–191. doi: 10.1007/s11606-010-1496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White A, Schmidt K. Systematic literature reviews. Complement Ther Med. 2005;13(1):54–60. doi: 10.1016/j.ctim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–1015. [PubMed] [Google Scholar]
- 29.Khodyakov D, Hempel S, Rubenstein L, et al. Conducting online expert panels: a feasibility and experimental replicability study. BMC Med Res Methodol. 2011;11:174. doi: 10.1186/1471-2288-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476. doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Selm M, Jankowski NW. Conducting online surveys. Qual Quant. 2006;40:435–456. [Google Scholar]
- 32.Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess. 1998;2(14):i–iv. 1–74. [PubMed] [Google Scholar]
- 33.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Mokkink LB, Terwee CB, Stratford PW, et al. Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Qual Life Res. 2009;18(3):313–333. doi: 10.1007/s11136-009-9451-9. [DOI] [PubMed] [Google Scholar]
- 35.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 36.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute for Clinical Excellence. NICE: Guideline Development Methods. Reviewing and Grading the Evidence. London: National Institute for Clinical Excellence; 2005. [Accessed December 1, 2012]. Available from: http://www.nice.org.uk/niceMedia/pdf/GDM_Chapter7_0305.pdf. [Google Scholar]
- 38.Reiter A, Fischer B, Kötting J, Geraedts M, Jäckel WH, Döbler K. QUALIFY: Ein Instrument zur Bewertung von Qualitätsindikatoren. [QUALIFY – a Tool for Assessing Quality Indicators]. Z Evid Fortbild Qual Gesundhwes. 2008;101(10):683–688. doi: 10.1016/j.zgesun.2007.11.003. (Ger). [DOI] [PubMed] [Google Scholar]
- 39.Campbell SM, Braspenning J, Hutchinson A, Marshall M. Research methods used in developing and applying quality indicators in primary care. Qual Saf Health Care. 2002;11(4):358–364. doi: 10.1136/qhc.11.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeschke R, Guyatt GH, Dellinger P, et al. GRADE Working Group. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]