Abstract
The endoplasmic reticulum (ER) is a dynamic intracellular organelle with multiple functions essential for cellular homeostasis, development, and stress responsiveness. In response to cellular stress, a well-established signaling cascade, the unfolded protein response (UPR), is activated. This intricate mechanism is an important means of reestablishing cellular homeostasis and alleviating the inciting stress. Now, emerging evidence has demonstrated that the UPR influences cellular metabolism through diverse mechanisms, including calcium and lipid transfer, raising the prospect of involvement of these processes in the pathogenesis of disease, including neurodegeneration, cancer, diabetes mellitus and cardiovascular disease. Here, we review the distinct functions of the ER and UPR from a metabolic point of view, highlighting their association with prevalent pathologies.
1. INTRODUCTION
Our understanding of cellular reticulum began in 1945 when it was first described as a “lace-like” structure in the ground substance of cultured cells and examined in toto by electron microscopy. The term “endoplasmic reticulum” (ER) was coined in 1952 by Porter & Kallman to describe the observation of the preferential localization of vesicular elements in the perinuclear region of the cytoplasm, known as endoplasm (Palade, 1956). Early studies described two major types of membranous structures of ER, distinguishable by their biochemical and morphologic properties and their sedimentation features. One type corresponds to the tubular or “tadpole-like” structure recovered in the low-density fraction, and the other to the spherical vesicles present in the high-density fraction (Heuson-Stiennon et al., 1972).
The ER, although frequently associated with the cellular exo-endocytic pathway, is a complex organelle in terms of both its structure and function (Fig. 5.1). It plays critical roles in a wide range of processes, including (a) synthesis, folding, modification, and transport of proteins; (b) synthesis and distribution of phospholipids and steroids; (c) storage of calcium ions within its lumen and their regulated release into the cytoplasm (Schröder, 2008). Perturbations in any of these functions results in ER stress and aggregation of misfolded proteins. ER stress has been observed during physiological conditions, such as nutrient deprivation and the differentiation of type B lymphocytes into plasma cells, as well as in pathological conditions, such as viral infection, ischemia/reperfusion and cardiomyocyte hypertrophy.
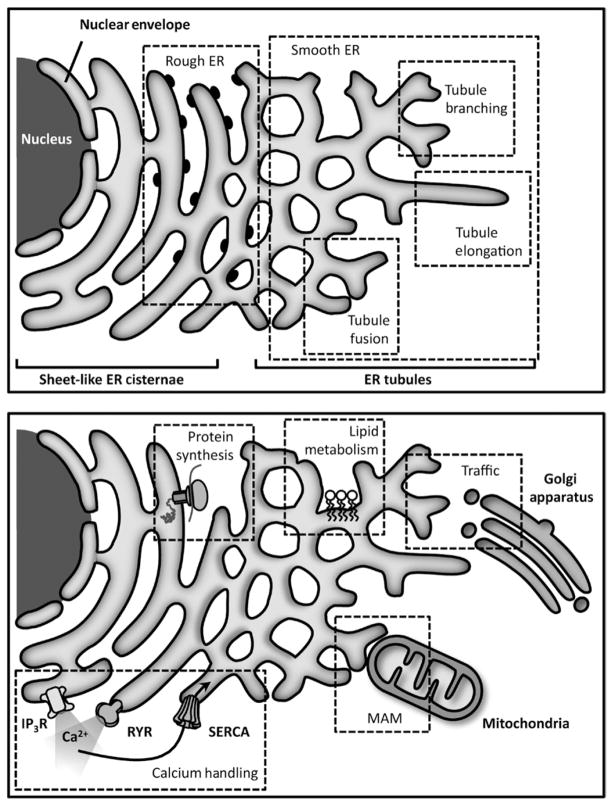
Fig 5.1. ER structure and general functions.
Upper panel: The ER can be subdivided into three well-defined domains, the sheet-like ER, the tubular ER and the nuclear envelope. The first one is characterized as being rich in ribosomes, for which it received the name rough ER. Since the tubular ER contains fewer ribosomes, it is commonly called smooth ER. Both the sheet-like and tubular ER are highly dynamic and interconvert between each other constantly. Most studies suggest that the tubular ER has the ability to fuse, elongate and branch dynamically inside the cell. Lower panel: The ER fulfills diverse functions in the cell, like calcium homeostasis through the use of a series of channels, pumps and buffer proteins. It is essential for lipid and protein synthesis, as well as the quality control and degradation of proteins. Together with the Golgi apparatus, it takes part in the process of cell trafficking, which is important for the export of products from reticulum toward the outside of the cell. Finally, the ER also regulates the function of other organelles, such as mitochondria through dynamic interaction zones called MAM.
2. STRUCTURE, FUNCTION AND DYNAMICS OF ENDOPLASMIC RETICULUM
2.1. General Structure of Endoplasmic Reticulum
2.1.1. Rough ER Sheets and Smooth ER Tubules
A number of approaches have established that the ER is a continuous compartment extending from nucleus to cytosol. Based on its structure, the ER is classically subdivided in the ribosome-studded rough endoplasmic reticulum (RER) and the ribosome-free smooth endoplasmic reticulum (SER) (English et al., 2009). Cells that secrete large amounts of protein are rich in RER, while steroid-synthesizing and muscle cells have abundant SER. In many cells, RER and SER do not occupy spatially segregated regions; however, in some cells such as hepatocytes and neurons, the smooth and rough portions of the ER occupy different cellular areas (Borgese et al., 2006). The SER morphology differs from that of RER by its typically more complex, tubular network and greater numbers of branch points. Xenobiotic-metabolizing enzymes are also preferentially located in the SER (Orrenius and Ericsson, 1966).
A more contemporaneous classification divides the ER in three domains: the nuclear envelope (NE), the sheet-like cisternae, and the polygonal array of tubules (Shibata et al., 2006). The NE controls the flow of information between the cytoplasm and the nucleoplasm, and consists of a double membrane enclosing a lumen. The NE surrounds the nucleus, with the inner and outer membranes connected only at the nuclear pores, the former serving as a scaffold for chromatin organization localized at the inner membrane (Dreier and Rapoport, 2000).
In yeast, there are more ribosomes in the cisternae than in the tubules, suggesting that either cisternae are better suited for ribosome binding and/ or ribosome binding stabilizes cisternal ER structure. In animal cells, the protein translocation machinery is also enriched in this structure. For example, the expression of p180, a yeast ribosome-binding protein anchored to the ER membrane, increases secretory activity. Moreover, ectopic expression of p180 in nonsecretory mammalian cells induces biogenesis of RER membrane, while upon p180 knockdown, THP-1 secretory cells decrease biogenesis in the area occupied by RER (Benyamini et al., 2009).
Reticular networks can be formed de novo in Xenopus egg extracts studied in vitro, in a cytoskeleton-independent manner but dependent on energy and protein elements which drive microsome fusion into tubule formation (Dreier and Rapoport, 2000). The integral membrane protein Reticulon 4a (Rtn4a), a member of an ER membrane-localized protein family, has been implicated in tubule formation. Consistent with this role, Rtn4a is absent in sheets and NE, but enriched in ER tubules (Voeltz et al., 2006). The yeast protein, Yop1p, and its mammalian ortholog, DP1, comprise another family of integral membrane proteins that form oligomers on the tubular ER and are fundamental for tubule formation (Shibata et al., 2008). Rtn4a expression generates more tubules, while Rtn4a depletion along with DP1/Yop1 converts the peripheral ER into sheets (Voeltz et al., 2006).
In muscle cells, a variant of the ER, termed sarcoplasmic reticulum (SR) is present, whose main function is to control calcium release for muscle contraction. In striated muscle, the plasma membrane forms long invaginations, called transverse tubules (T-tubules) that penetrate into the cytoplasm. These structures insert between two terminal SR cisternae, forming a triad. This highly organized T-tubule system is essential for rapid and precise excitation–contraction coupling. Different proteins collaborate in T-tubule biogenesis, and in the formation and maintenance of the triad in both cardiac and skeletal muscles: caveolae are cell surface structures that initiate membrane invaginations, while the invagination and tubulation processes are possible due to the participation of caveolin 3, amphiphysin 2 and dysferlin among others. In addition, a number of proteins play important roles in stabilizing the triad structure in skeletal muscle, including mitsugumins, junctophilins, myotubularin, ryanodine receptor (RyR), and the dihydropyridine receptor (Cav1.1 calcium channel) (Al-Qusairi and Laporte, 2011).
2.1.2. Nuclear Envelope
The NE is a highly specialized and selectively permeable double membrane that surrounds the genetic material of the cell. Electron microscopic analysis revealed early on that NE and ER form a continuous structure (Watson, 1955), sharing some common components, while others, like nuclear pore-forming nucleoporins (NUPs), are specific for the NE. Other important components are the transmembrane proteins of the NE, which interact with laminin, a protein localized in the internal surface of the NE, and with chromatin, thereby participating in anchorage of the genetic material and in gene regulation. On the other hand, KASH domain-containing proteins are small transmembrane proteins at the external face of the NE that associate with cytoskeletal proteins, thus determining the shape and positioning of the NE (Hetzer, 2010).
The NE is a highly dynamic structure, which is modulated during different stages of the cell cycle. In stage G2, the nucleus must duplicate its size by not only increasing its volume, but also by duplicating its NE proteins; during mitosis, specifically in prophase, the NE disintegrates. The processes that lead to dismantling of the NE are not fully understood, however, these events are preceded by loss of NUPs and transfer of the NE proteins to the mitotic ER. Following anaphase, NE restructuring occurs and terminates in complete morphological and functional restructuring of the nucleus. Our understanding of the NE restoration process is controversial, as there are two leading theories. The first suggests that the NE is fragmented into small vesicles that are not degraded completely during mitosis and subsequently merge to restructure the NE. The second theory proposes that the ER differentiates into the NE, which is supported by the mitotic ER structure and enriched in NE proteins (Hetzer, 2010).
2.1.3. Lipid-Raft-like Domains
Lipid rafts are cholesterol-rich plasma membrane domains which may contain caveolin. Lipid-raft-like domains are defined as caveolin-free plasma membrane regions enriched in cholesterol and members of the prohibitin domain-containing protein family, such as KE04p and C8orf2. These proteins are essential for the maintenance of lipid-raft-like structures. Although it is known that the ER has lower levels of cholesterol and glycosphingolip-ids than the plasma membrane and other organelles, two proteins homologous to KE04p and C8orf2, Erlin-1 and Erlin-2 (for ER lipid-raft protein), are found in the ER, suggesting the existence of lipid-raft-like domains in the ER (Browman et al., 2006).
Another protein involved in the maintenance of lipid-raft-like domains is the sigma-1 receptor chaperone (Sig-1R), a cholesterol-binding protein (Hayashi and Su, 2010). Sig-1R is also involved in mitochondria–ER binding through microdomains enriched in cholesterol and ceramides (Hayashi and Fujimoto, 2010), which form part of the mitochondria-associated ER membrane (MAM) structure.
2.2. Functions of Endoplasmic Reticulum
2.2.1. Protein Synthesis in Endoplasmic Reticulum
Proteins targeted to the ER and other organelles or destined for secretion must be incorporated into the ER. Nascent polypeptides possess a signal sequence in their N-termini that targets them to the ER. This sequence is not conserved and generally contains hydrophobic amino acids in the core region. This sequence is recognized by the signal recognition particle (SRP), consisting of six polypeptides bound to a small RNA, which cycles between the cytoplasm and the ER membrane. When the SRP recognizes and binds to the signal sequence of nascent polypeptides, a pause in translation occurs. Then, the SRP–ribosome complex translocates from the cytoplasm to the SER membrane and binds to the SRP Receptor (SR) (Corsi and Schekman, 1996). Once this process is complete, the SRP and SR are released, and the nascent polypeptide is translocated by the translocon complex (TC) formed by more than 25 polypeptides (Nikonov and Kreibich, 2003). The core of the TC is Sec61, a protein required for the translocation of both secretory and membrane proteins. In mammals, Sec61 is a complex formed by three subunits: Sec61α, Sec61β and Sec61γ. This complex possesses ribosomal binding sites, and its structure forms a pore through which the polypeptide traverses the membrane. The translocation-associated membrane protein (TRAM), another integral membrane protein component of the TC is required for the translocation of some precursors, like ppαF. The above-mentioned process is known as cotranslational translocation; however, targeting to the ER can also occur posttranslationally. This event occurs independently of SRP in both yeast and mammalian cells. In yeast, Sec62p, Sec63p, Sec71p and Sec72p form a tetrameric protein complex that may function as a receptor in this type of translocation process. Several chaperones are known to participate, including Sec61 (Wilkinson et al., 1997).
Another important part of protein synthesis is folding and proper assembly of the nascent polypeptide. To accomplish this, the ER contains a variety of proteins that assist in the process. Protein disulfide isomerase (PDI) and ERp57, for example, are thio-oxidoreductases that catalyze disulfide bond formation by means of the oxidative capacity provided by ER oxidoreduction 1 (ERO1). Glucose-regulated protein 78 (BiP or GRP78), a chaperone of the heat-shock protein family, recognizes and stabilizes unfolded proteins, and participates in posttranslational translocation (Ni and Lee, 2007). Another set of proteins is responsible for folding quality control, a process that will be described later in the text.
2.2.2. Endoplasmic Reticulum and Calcium Homeostasis
Calcium is recognized as one of the most important second messengers in the cell; it participates in a wide variety of cellular processes, including protein synthesis, muscle contraction, gene expression, secretion, cell cycle, metabolism and apoptosis (Coe and Michalak, 2009). Given its diverse functions, intracellular free calcium concentrations are tightly regulated. In this respect, a series of buffer proteins, pumps and carriers of calcium participate in this process, serving to diminish or increase calcium concentrations according to cell requirements.
The ER is the principal organelle involved in calcium homeostasis, and in turn, many of its diverse functions, like protein folding and glycosylation, are calcium-dependent by virtue of the fact that the enzymes involved are calcium-dependent (Kuznetsov et al., 1992). Cytoplasmic calcium regulation depends on the activity of a series of transporters in the ER membrane. Sarcoplasmic/endoplasmic reticulum calcium-ATPase (SERCA) is a calcium pump that imports calcium from the cytoplasm into the ER lumen, thereby maintaining low cytoplasmic calcium levels (Strehler and Treiman, 2004). On the other hand, the RyR and the inositol 1,4,5-trisphosphate receptor (IP3R) are calcium channels that release this ion back into the cytoplasm (Marks, 2001; Zalk et al., 2007; Taylor and Tovey, 2010).
2.2.2.1. Sarcoplasmic/Endoplasmic Reticulum Calcium-ATPase
SERCA is a type P ATPase pump that transports two calcium ions in exchange of the hydrolysis of one ATP molecule, functioning against a calcium gradient to restore luminal ER calcium levels (Guerrero-Hernandez et al., 2010). Small proteins, such as phospholamban and sarcolipin, modulate SERCA activity according to cellular requirements and/or extracellular signals (Periasamy and Kalyanasundaram, 2007; Brini and Carafoli, 2009; Guerrero-Hernandez et al., 2010).
Distinct genes code for the three different pumps, SERCA 1, 2 and 3, generating a total of 10 isoforms by alternative splicing. SERCA1 is expressed principally in skeletal muscle, with two known isoforms, SERCA1a (adult) and SERCA1b (fetal). SERCA2a is mainly expressed in cardiac and skeletal muscle, while SERCA2b and SERCA3 are present in nonmuscle cells, the latter being the most ubiquitous of the isoforms (Periasamy and Kalyanasundaram, 2007; Brini and Carafoli, 2009).
2.2.2.2. Ryanodine Receptor
RyR is an ER and SR calcium channel important for regulation of calcium transients in excitable cells. Three isoforms of this receptor are known to exist, RyR1, 2 and 3; RyR1 is mainly present in skeletal muscle, RyR2 in cardiac muscle, and RyR3 in nerve fibers and in nonexcitable cells (Marks, 2001; Zalk et al., 2007).
RyR conductance is governed by several proteins, such as voltage-gated channels (Cav 1.1/Cav 1.2 or dihydropyridine receptor), protein kinases (PKA and CaMKII), calcium-binding proteins (Calmodulin and Calsequestrin) and the FKBP-12 and 12.6 proteins (Lanner et al., 2010). Also, divalent cations, such as calcium and magnesium, and nucleotides, such as ATP, are other important regulators of RyR (Lanner et al., 2010). In addition, RyRs possess a series of sulfhydryl and cysteine groups sensitive to the redox state of the cell (Sun et al., 2008; Donoso et al., 2011).
2.2.2.3. IP3R
The IP3R is a tetrameric protein that transports calcium from the ER into the cytoplasm. It is responsible for calcium transients in nonexcitable cells, nuclear calcium regulation and calcium transfer between ER and mitochondria (Taylor and Tovey, 2010).
The IP3R requires elevated calcium levels to be activated by its agonist, IP3. IP3 binds to the IP3R at four sites in a cooperative manner. In parallel, calcium regulates IP3 function through calcium-binding sites, which are different from those used for ion transport. Depending on the calcium concentration, IP3Rs possess a two-phase response. Low amounts of calcium increase the IP3R response, independent of IP3 levels. On the contrary, high calcium concentrations have inhibitory effects on the channel (Taylor and Tovey, 2010). Furthermore, the IP3R is modulated by covalent modifications, such as phosphorylation of key residues by PKA, ERK and/or PKC (Patterson et al., 2004). Nucleotides, such as ATP and NADH, also play an important role in channel modulation, linking the energetic needs of the cell to IP3R activity (Patterson et al., 2004; Taylor and Tovey, 2010).
Finally, apoptotic proteins like caspase-3, calpains, and cytochrome c interact with and regulate IP3Rs, thereby contributing to life or death decisions of the cell (Patterson et al., 2004; Taylor et al., 2004).
2.2.2.4. Calcium Buffering
Calcium-binding chaperones, such as calreticulin and calnexin, also play an important role in ER luminal calcium regulation. By sequestering free calcium, chaperones work as ion buffers. Owing to the efficient actions of these proteins, the free calcium concentration in the ER is only 50–500 μM, whereas the actual luminal calcium concentration ranges around 2 mM (Coe and Michalak, 2009).
Calreticulin, the glucose-regulated protein 94 (GRP94) and BiP/ GRP78 are the most abundant calcium-binding chaperones in the ER. These proteins bind calcium with high affinity in their C-terminal domain where they possess several calcium-binding sites (Michalak et al., 2009). In the SR, the main chaperone responsible for calcium regulation is the calreticulin homolog, calsequestrin, which is of great importance in muscle contraction, not only because of its buffering capacity but also its ability to modulate RyR function (Michalak et al., 2009).
2.2.3. Lipid Synthesis at Endoplasmic Reticulum
Lipids fulfill several functions essential for cellular homeostasis. They are employed as a backup energy source, signaling molecules, and as membrane components, among other functions. Cellular lipids are quite heterogeneous, existing in the form of fatty acids, phospholipids, cholesterol, and sphingolipids (Laplante and Sabatini, 2009). The ER plays an essential role in lipid biogenesis, mainly in the synthesis of glycerophospholipids and sphingolipids, the major components of biological membranes. ER enzymes such as glycerol-3-phosphate acyltransferase-like and 1-acylglycerol-3-phosphate-O-acyltransferase transform glycerol and fatty acids into phospholipid precursors, such as triglycerides and diacylglycerol phosphate (DGP). In the ER lumen, DGP is dephosphorylated by phosphatidic acid phosphatases to form diacylglycerol (DG), which is converted to phosphatidylcholine and phosphatidylethanolamine. Phosphatidylinositol is synthesized in the ER by phosphatidylinositol synthase, and derivatives with different levels of phosphorylation are formed in the ER and play an important role in signaling and vesicle trafficking (Fagone and Jackowski, 2009).
De novo synthesis of sphingolipids and ceramides begins in the cytoplasm. Then, intermediates are generated in the ER and the process concludes in the Golgi apparatus and nucleus. The coordinated action of serine palmitoyltransferase, 3-ketodihydrosphingosine reductase and dihydroceramide synthase in the ER, convert serine and palmitoyl-CoA into cell membrane components (Gault et al., 2010).
Cholesterol synthesized at the ER is important for cell function due to this molecule’s structural role in membranes and as a precursor of various steroid hormones and bile salts. The 3-hydroxy-3-methyl-glutaryl CoA reductase, found in the membrane of the ER, participates in the first phase of cholesterol synthesis, which leads to the combination of three molecules of acetyl-CoA to form mevalonate. Mevalonate is then transformed into 3-isopentyl pyrophosphate. The condensation of six molecules of isopentyl pyrophosphate gives rise to squalene, owing to the action of a series of transferases and squalene synthase. Squalene is then cyclized by lanosterol cyclase, yielding lanosterol. After 19 reactions of bond reduction and methyl-group elimination, catalyzed by various enzymes, cholesterol is ultimately generated. Steroid hormones and bile acids are formed subsequently through the action of other pathways (Jo and Debose-Boyd, 2010; Maxfield and van Meer, 2010).
2.3. Endoplasmic Reticulum Dynamics
The tubular structure of the ER is highly dynamic and undergoes constant morphological remodeling. The cytoskeleton formed by microtubules (MTs), microfilaments and intermediate filaments plays a crucial role in the organization and structure of the ER. Treatment with depolymerizing agents reversibly and dramatically alters ER shape, causing slow retraction from the periphery to the center of the cell. MT and ER are highly interdependent structures. While the distribution of ER and MT are not identical, ER elongation and MT polymerization are tightly connected. The maintenance of the ER network requires the MT system (Terasaki et al., 1986). For example, the RER-specific membrane protein, CLIMP-63, mediates the interaction between this organelle and MTs through its cytosolic domain, and is responsible for the restraining mobility of the TC (Nikonov et al., 2007).
Two different mechanisms of ER tubule elongation along MT exist. The first, known as tip attachment complex (TAC), involves STIM1, a single-pass membrane protein mainly localized to the ER. This protein directly interacts with EB1, an MT-plus-end-binding protein (Grigoriev et al., 2008). In the TAC mechanism, the ER membrane selectively attaches to the growing end of an MT in regions where ER networks are dense and MT-plus ends are abundant. This mechanism allows ER tubule stretching dependent on MT growth. For the second, the Sliding mechanism, the tip of the ER tubules binds to an existing MT shaft, forming a sliding attachment that moves toward the plus end of a MT (Waterman-Storer and Salmon, 1998). This mechanism is faster and more prevalent than TAC, occurring mainly in MT harboring acetylated α-tubulin (Friedman et al., 2010).
ER tubules are capable of fusion and fission/branching processes, fundamental for the formation of the reticulated network (Anderson and Hetzer, 2007). It is known that ER fusion requires GTP and a family of proteins called atlastins, which possess GTPase activity and belong to the dynamin superfamily. In vertebrates, three isoforms are known, atlastin 1, 2, 3, and only one ortholog exists in invertebrates. Atlastins interact with different ER-shaping proteins, and these interactions are required for the formation of ER junctions (Barlowe, 2009; Farhan and Hauri, 2009; Hu et al., 2009). In Drosophila, depletion of atlastin produces ER fragmentation, while its overexpression favors ER fusion. In vitro, reconstitution of atlastin in liposomes promotes GTP-dependent fusion. Moreover, in humans, atlastins take part in the formation of the reticulated network and membrane remodeling (Hu et al., 2009; Muriel et al., 2009).
2.4. Contacts between ER and Other Organelles
Peripheral ER interacts with almost all cytoplasmic organelles. In this context, the ER forms physical contacts with mitochondria, with important functional implications. In yeast, this physical interaction is mediated by the ER–mitochondria encounter structure (ERMES), formed by four components: the mitochondrial outer-membrane proteins Mdm10 and Mdm34, the ER integral membrane protein Mmm1, and the cytosolic protein Mdm12 (Kornmann et al., 2009). In mammals, the contact sites are termed MAMs, and have a composition that differs from that of the membranes of other organelles. For one, the MAMs are enriched in cholesterol, which gives them a characteristic density and architecture (Hayashi and Fujimoto, 2010). Structural proteins that compose the MAM, include Mitofusin-2 (Mfn2), a GTPase protein present on the surface of both organelles that connects the two by forming dimers (de Brito and Scorrano, 2008). Other components, such as the sorting protein PACS2 or the GTPase Rab32, regulate MAM composition according to cellular requirements (Myhill et al., 2008; Bui et al., 2010).
Given that the ER and mitochondria represent the cell’s main source and sink of calcium, respectively (Berridge, 2002), it is not surprising that MAMs play a key role in calcium homeostasis (Hayashi et al., 2009). These interorganelle contacts allow the formation of microdomains of high calcium concentration at the surface of mitochondria, thereby facilitating rapid ion uptake (Rizzuto et al., 1998). Proteins known to be involved in calcium handling have been shown to be enriched in MAM. Examples here include IP3Rs (Mendes et al., 2005; Szabadkai et al., 2006), the RyR (García-Pérez et al., 2008; Kopach et al., 2008), the Sig-1R (Hayashi and Su, 2007), and perhaps even calnexin (Myhill et al., 2008), emphasizing the intimate relationship between ER and mitochondria in regulating calcium homeostasis (Mironov and Symonchuk, 2006). Conversely, calcium regulates relative positioning of ER and mitochondria via the autocrine motility factor receptor (AMFR) (Wang et al., 2000), apparently another MAM-enriched protein (Registre et al., 2004; Goetz and Nabi, 2006).
ER and mitochondrial dynamics are highly interrelated and their contacts are maintained in spite of this dynamism. Recent data obtained in yeast demonstrate that the contacts are conserved during mitochondrial fission. Moreover, fission itself can take place in the ER–mitochondria junctions (Friedman et al., 2011).
Contacts between plasma membrane and ER have been described in several cell types. The proteins STIM1 at the ER and ORAI1 at the plasma membrane regulate this interaction when calcium is depleted (Toulmay and Prinz, 2011). Another important group of proteins present in the ER–plasma membrane contacts is the Osh family. Osh3, for example, localizes to the contacts according to the level of PI4P. High PI4P levels recruit and activate Osh3 at contacts sites, leading to its interaction with VAP proteins in the ER and the activation of phosphatases, such as Sac1 (Stefan et al., 2011).
The ER and Golgi apparatus are functionally linked as constituents of the secretory pathway; however, direct physical contacts between the two organelles have been described, as well. Ceramides produced in the ER are transported to the trans-Golgi to be converted to sphingomyelin. In this case, appropriate trafficking requires the cytosolic protein CERT, which interacts with VAP on the ER surface (Kawano et al., 2006). Another example of a direct mechanism and contact between both organelles is the nonvesicular transport of phosphatidylinositol and diacylglycerol, which also depends on VAP, and, in the case of phosphatidylinositol, requires the Nir2 protein (Peretti et al., 2008).
As noted above, regulation of Ca2+ by the ER is a key component of cellular signaling, adaptation and survival. Additionally, the ER has been implicated in a complex communication system with other organelles, including the Golgi apparatus, the plasma membrane, the nucleus, and mitochondria. The next sections of this review will focus on these communication processes, particularly, on the ability of ER Ca2+ signals to modulate cellular bioenergetics, thereby influencing cellular metabolism during stress and survival.
3. ER STRESS AND UNFOLDED PROTEIN RESPONSE
3.1. General Aspects of the UPR
Conditions that alter ER homeostasis and proper ER functioning generate a state known as ER stress. Many different factors may lead to this state, which can be very detrimental to cell integrity, due to accumulation of toxic, unfolded proteins within the ER lumen. For this reason, restoring ER homeostasis is essential for cell survival. Cells subject to ER stress activate a series of processes, which, if insufficient to alleviate the stress, lead to cell death.
The cellular response to ER stress is known as the unfolded protein response (UPR). In principle, the UPR seeks to restore the normal functioning of the ER, using multiple strategies acting in parallel and in series. For example, expression of ER chaperone proteins increases to prevent protein aggregation and facilitate correct protein folding. Also, the amount of protein in transit through the ER is reduced by temporary inhibition of protein translation. Furthermore, ER volume increases by stimulating the synthesis of membrane lipids. Finally, degradation of unfolded proteins increases by activating the process of endoplasmic reticulum-associated protein degradation (ERAD).
During UPR, perturbations in ER homeostasis are sensed and transduced to the cytoplasm and nucleus causing a compensatory response. Several ER stress sensors are involved, all of which harbor luminal, transmembrane and cytoplasmic domains. Any increase in the concentration of misfolded proteins is detected by the luminal sensor domain and then transduced to the cytoplasm and nucleus, through different signals (Fig. 5.2).
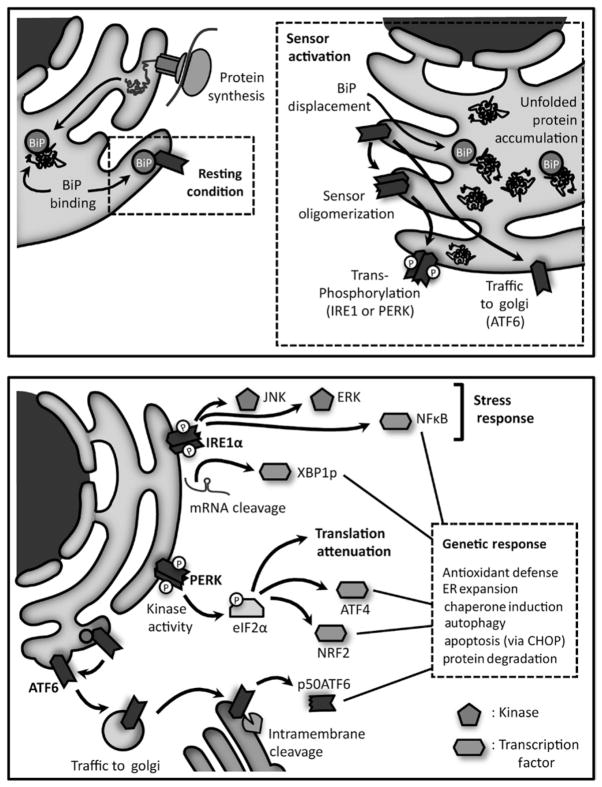
Fig 5.2. ER stress signaling.
Upper panel: In resting conditions, the stress sensors IRE1 and PERK and ATF6 interact with Bip/GRP78. Accumulation of misfolded proteins in the ER lumen separates the chaperone from each sensor. IRE1α and PERK activation involves oligomerization and transphosphorylation of their cytosolic effector region. ATF6 activation, on the other hand, requires its transport to the Golgi, where it is sequentially cleaved by S1P and S2P. A recent model for IRE1α activation, involves an initial dissociation of BiP/GRP78 that drives sensor oligomerization, while subsequent binding to unfolded proteins leads to activation. Lower panel: The signaling engaged by the three sensors activates several transcription factors (XBP1, ATF4, p50ATF6, NF-κ B, CHOP) and protein kinases (JNK, AKT), leading to the establishment of a genetic program termed unfolded protein response. This adaptive response involves chaperone induction, activation of degradation pathways like the proteasome and autophagy, ER expansion and increased antioxidant defense. Also, the PERK pathway inhibits general mRNA translation, through eIF2α phosphorylation. If the cell does not reduce the mis-folded protein overload, apoptosis is triggered.
Three branches of UPR have been described: PERK, IRE1α, and activating transcription factor (ATF6). In the rest of this chapter, we will review general aspects of the sensors and their relationship to various aspects of cell metabolism.
3.2. ER Stress Sensors
3.2.1. IRE1 Pathway
The inositol-requiring enzyme-1 (IRE1) is the most highly conserved branch of the UPR. It is an ER transmembrane protein that consists of an N-terminal luminal sensor domain, a single transmembrane domain and a C-terminal cytosolic effector region that manifests both kinase and endoribonuclease activity (Walter and Ron, 2011). The IRE1 pathway has been widely described by Peter Walter’s group in Saccharomyces cerevisiae, who observed that it increases the expression of ER chaperones, thereby enhancing cell viability during ER stress (Cox et al., 1993). IRE1 senses unfolded proteins in the ER lumen, which induces receptor oligomerization and transphosphorylation within the cytosolic effector region (Shamu and Walter, 1996). The cytosolic domain also exhibits site-specific endoribonuclease activity that cleaves a small stem–loop structure from splice junctions in the mRNA of the nuclear factor Hac1 (Sidrauski and Walter, 1997; Gonzalez et al., 1999). Hac1 is a basic-leucine zipper (bZIP) transcription factor, which binds to the promoter of UPR-regulated genes and is more resistant to ubiquitin-dependent degradation when the IRE1-mediated splicing replaces its C-terminal (Cox and Walter, 1996).
Mammalian cells have two paralogs of IRE1, IRE1α and IRE1β, sharing structural similarity but different functions. Under ER stress conditions, IRE1α catalyzes the splicing of X-box-binding protein 1 (XBP1) mRNA, the mammalian ortholog of Hac1, while IRE1β mediates the site-specific cleavage of 28S rRNA and translational attenuation (Tirasophon et al., 1998; Iwawaki et al., 2001). XBP1 is not the only target of IRE1α. Surprisingly, IRE1α controls its own expression by cleaving its own mRNA (Tirasophon et al., 2000); another 13 mRNA candidates of IRE1α cleavage have recently been identified (Oikawa et al., 2010).
The capacity of IRE1α to sense ER stress depends on its dissociation from BiP/GRP78 and on its direct interaction with unfolded proteins. These two observations are incorporated into the two-step model of IRE1α regulation, where initial dissociation of BiP/GRP78 from IRE1α drives oligomerization, while subsequent binding to unfolded proteins leads to IRE1α activation (Kimata et al., 2007; Pincus et al., 2010). A recent study suggests that IRE1α also senses changes in membrane composition. Mutant IRE1α incapable of sensing unfolded proteins is activated by depletion of the membrane lipid components and by deletion of genes involved in lipid homeostasis (Promlek et al., 2011). Indeed, IRE1 gene deletion confers auxotrophy for inositol, an important component of phospholipids in yeast cells (Nikawa and Yamashita, 1992).
Other proteins also control the activation of IRE1α. Indeed, the pro-apoptotic Bcl-2 family members Bax and Bak form a protein complex with the cytosolic domain of IRE1α, essential for its activation (Hetz et al., 2006). Bax inhibitor 1 (BI-1), an ER-resident protein, also forms a complex with IRE1α, decreasing its endoribonuclease activity (Lisbona et al., 2009; Madeo and Kroemer, 2009).
When IRE1α is activated, the luminal ER stress signal is transduced to the cytoplasm, activating various signaling pathways. One of these pathways is initiated by IRE1α-mediated splicing of XBP1 mRNA, which generates a protein that differs from its unspliced protein (XBP1u) in the C-terminal region (Yoshida et al., 2001). XBP1 is a potent transactivator that regulates genes involved in ER protein synthesis, folding, glycosylation, ERAD, redox metabolism, autophagy, lipid biogenesis and vesicular trafficking (Walter and Ron, 2011). XBP1u does not act directly as a transcription factor, but rather functions as negative feedback regulator of XBP1 by sequestering the protein from the nucleus and promoting its degradation by the proteasome (Yoshida et al., 2006). Moreover, a hydrophobic patch in the XBP1u nascent peptide chain recruits its mRNA (attached to the ribosome) to the ER membrane, where it is cleaved by IRE1α to produce pXBP1s (Yanagitani et al., 2009).
IRE1α also activates JNK by recruiting the TNF receptor-associated adapter protein TRAF2 (Urano et al., 2000). This activation by ER stress requires the presence of MAP3K, ASK1 and ASK1-interacting protein-1 (AIP1), a transducer in the ASK1-JNK signaling pathway (Nishitoh et al., 2002; Luo et al., 2008). JNK activation is associated with cell death (Kim et al., 2006), and also promotes survival through the activation of c-Jun (Zhao et al., 2008; Fuest et al., 2011), increased BiP/GRP78 and GRP94 expression (Shinkai et al., 2010), as well as activation of autophagy after ER stress (Ogata et al., 2006). Similar to JNK, ER stress induces activation of NF-κB, which is mediated by IRE1 and TRAF2 (Kaneko et al., 2003). This activation of NF-κB controls the expression of manganese superoxide dismutase (MnSOD), an antioxidant enzyme localized in the mitochondrial matrix (Kaneko et al., 2004).
Important modulators of cell metabolism also regulate the IRE1α pathway. It was recently described that both XBP1 splicing and JNK activation are controlled by the mTORC1 pathway, the major sensor of nutrient and energy availability in the cell (Ozcan et al., 2008; Pfaffenbach et al., 2010; Kato et al., 2012). IRE1α is phosphorylated by PKA, controlling glucagon-mediated expression of gluconeogenic genes (Mao et al., 2011). p85, a repressive regulatory subunit of PI3K, also interacts with XBP1, increasing its nuclear translocation and transcriptional activity (Park et al., 2010). IRE1α signaling is attenuated after prolonged ER stress and this process is characterized by IRE1α cluster dissolution, IRE1α dephosphorylation and a decline in endoribonuclease activity (Li et al., 2010; Rubio et al., 2011). Additionally, the E3 ubiquitin ligase synoviolin increases IRE1α ubiquitination and degradation in synovial fibroblasts (Gao et al., 2008).
3.2.2. PERK Pathway
The second ER stress transducer is PERK, first described in mammalian pancreatic islet cells by Shi et al. This protein has a PEK-like catalytic domain, which phosphorylates the α subunit of the eukaryotic translation initiation factor-2 (eIF2α) (Shi et al., 1998). PERK possesses a luminal domain similar to that of IRE1, and a cytoplasmic portion that manifests protein serine/threonine kinase activity. As such, PERK is a member of the eIF2α kinase subfamily together with PKR, GCN2 and HRI (Harding et al., 1999). PERK is conserved in all known metazoans, but is absent in the S. cerevisiae genome. In Drosophila melanogaster, its homolog is DPERK (Pomar et al., 2003).
As mentioned, PERK is a type I transmembrane protein with a typical protein kinase structure that includes a large C-terminal lobe and a smaller N-terminal lobe linked by a short hinge loop. The C-terminal lobe is formed by a large activation loop, seven α-helices and two short β-strands. The N-terminal lobe contains three α-helices and five β-strands. A phosphate moiety was found in the electron density map at the position Thr980, and phosphorylation at this site is thought to stabilize the activation loop and the eIF2α-binding site in the αG helix (Cui et al., 2011).
When the UPR has not been activated, BiP/GRP78 is bound to the PERK luminal domain. Upon unfolded protein accumulation, BiP/GRP78 dissociates from PERK, and the loss of this interaction correlates with the formation of high molecular mass complexes of activated PERK. BiP/ GRP78 overexpression attenuates this activation (Bertolotti et al., 2000).
ER stress leads to an increase in PERK’s protein kinase activity and eIF2α phosphorylation, which competitively binds to eIF2β, a guanine nucleotide-exchange factor. This results in eIF2α-GDP to eIF2α-GTP exchange inhibition, the latter being a key component in the formation of the active 43S translation-initiation complex (Dever, 2002). Therefore, the PERK pathway inhibits general mRNA translation, decreasing global protein synthesis and reducing the ER load. PERK also contributes to UPR transcriptional activation and in ER-stressed PERK knockout cells, a characteristic decrease in mRNA responsible for normal UPR is observed (Harding et al., 2003).
Gene expression in response to eIF2α phosphorylation is conserved among eukaryotes. The transcription factor ATF4 is translationally induced because it has an upstream open reading frame (ORF) in its 5′-untranslated region. This upstream ORF, which under normal conditions, prevents translation of the true ATF4, is bypassed only when eIF2α is phosphorylated, and therefore, ATF4 translation occurs (Harding et al., 2000). One favored gene during this process is gadd153, also known as chop, an ER stress-induced proapoptotic factor (Fawcett et al., 1999). Also, the Nrf2 transcription factor is a substrate of PERK. In unstressed cells, Nrf2 is maintained in the cytoplasm by its association with Keap1. PERK-mediated phosphorylation triggers dissociation of Nrf2/Keap1 complexes and inhibits their reassociation, consequently causing Nrf2 nuclear import (Cullinan et al., 2003).
The entire range of PERK-dependent gene expression relies on eIF2α phosphorylation in Ser51, which is blocked in the Ser51Ala eIF2α mutant (Lu et al., 2004). In addition to its role in the ER stress, PERK plays an important role in activation of autophagy as a survival mechanism during episodes of nutrient deprivation, hypoxia and radiation (Ogata et al., 2006; Rouschop et al., 2010; Rzymski et al., 2010). These two functions allow PERK to regulate growth and survival (Bi et al., 2005; Blais et al., 2006).
After restoring homeostasis, activated PERK is dephosphorylated (Bertolotti et al., 2000) by mechanisms that remain to be determined. Also, active eIF2α is dephosphorylated by two phosphatases that function independently, namely CReP, a constitutively expressed phosphatase (Jousse et al., 2003), and GADD34, whose expression is induced by phosphorylated eIF2α (Novoa et al., 2001). A HSP40 family member, P58(IPK), also regulates PERK by binding to the kinase domain of the sensor and decreasing eIF2α phosphorylation. This regulation affects the expression of its downstream targets, decreasing the translation of the UPR target proteins BiP and CHOP. Moreover, P58(IPK) has also been implicated in the inhibition of PERK autophosphorylation (Yan et al., 2002).
3.2.3. ATF6 Pathway and Novel-Related Sensors
There are several cAMP response element (CRE)/ATF transcriptional factors inserted in the ER membrane regulating multiples genes associated with the UPR. The best known of these proteins is ATF6, which, together with PERK and IRE1, is the third major branch of the UPR.
The ATF6 transcription factor was until fairly recently considered a member of the protein family associated with the regulation of genes with CRE sequences (Hai et al., 1989). This 90 kDa protein has a bZIP domain (Hai et al., 1989; Zhu et al., 1997) for DNA binding after homo- or heterodimerization (Parker et al., 2001). Almost 10 years after initial description, the transcriptional activity of ATF6 was linked for the first time to mammalian UPR, because it was shown to bind to ER stress response elements (ERSE) in GRP promoter regions (Yoshida et al., 1998). ATF6 is now known as a single-pass type 2 transmembrane ER protein that transmits stress signals directly from the ER to the nucleus and thereby induces compensatory responses (Haze et al., 1999, 2001).
ATF6 has three structural domains, a luminal C-terminal, a transmembrane and a cytoplasmic N-terminal domain. Two isoforms of ATF6 have been described, ATFα (90 kDa) and ATF6β/G13/CREB-RP (110 kDa). In the luminal domain, ATF6 has Golgi localization sequences (GLS), two in the case of the ATF6α isoform (GLS1 and GLS2) and one (GLS2) in the case of the ATF6β isoform (Shen et al., 2002). Under basal conditions, ATF6 is retained in the ER via interaction with the chaperone BiP/GRP78 and calreticulin (Breckenridge et al., 2003; Shen et al., 2005). During ER stress conditions, ATF6 is transported on vesicles toward the Golgi apparatus (Schindler and Schekman, 2009), where it is sequentially cleaved by the Site-1 and then Site-2 Proteases (S1P and S2P, respectively) (Shen and Prywes, 2004). These intramembrane proteases were initially implicated in cleavage of the transcription factor steroid regulatory element-binding protein (SREBP), involved in lipid metabolism (Ye et al., 2000).
The N-terminal fragment of ATF6, p50ATF6, then translocates to the nucleus where it recognizes its consensus sequences and promotes transcription of UPR genes, such as the chaperones BiP/GRP78 and GRP94 (Yamamoto et al., 2004; Schröder and Kaufman, 2005; Yamamoto et al., 2007), the transcription factors CHOP (Ma et al., 2002) and XBP1 (Yoshida et al., 2001), as well as other proteins such as p58IPK/DNAJC3 (van Huizen et al., 2003), Herp (Kokame et al., 2001) and SERCA (Thuerauf et al., 2001). After the cleavage of ATF6α/β by S1P and S2P, two fragments are generated from each isoform that translocate to the nucleus (p50ATF6α and p60ATF6β, respectively) (Thuerauf et al., 2004). ATF6 also plays a role in regulating ER volume increases in an XBP1-independent manner (Bommiasamy et al., 2009) and potentiates cellular adaptation to chronic ER stress (Wu et al., 2007). However, in ATF6α knockout mice, decreases in the basal expression of chaperones were not detected during either embryonic or postnatal development (Wu et al., 2007).
Interestingly, both ATF6 isoforms appear to play opposite roles in the UPR since ATF6β is a transcriptional repressor of the ATF6α signal and, therefore, a negative regulator of this branch of the UPR (Thuerauf et al., 2004). In contrast to ATF6β, ATF6α has a transactivation domain (TAD) in its N-terminal region with high homology to the section VN8 of the viral transcriptional factor VP16. This region was linked to an increase in the transcriptional activity of ATF6α and its degradation via the ubiquitin–proteasome system (Thuerauf et al., 2002). In vitro DNA interaction assays demonstrated that ATF6β binds to the consensus sequence in the BiP/GRP78 promoter region and blocks ATF6α binding (Thuerauf et al., 2007). Previous results have shown that ATF6β knockdown cells are more sensitive to tunicamycin-induced ER stress (Thuerauf et al., 2004). During the UPR, ATF6β levels regulate the intensity and the duration of responses to ATF6α, as well as susceptibility to cell death (Thuerauf et al., 2007).
Recently, it was determined that the ATF6β repressor effect depends on glycosylation. Nonglycosylated ATF6β is not cleaved and is retained in the ER membrane and, therefore, cannot function as a repressor (Guan et al., 2009). ATF6α is considered a very potent, but only transiently active transcription factor, as the increase in its transcriptional activity in response to unfolded proteins increases its own degradation by the proteasome (Thuerauf et al., 2002).
The subfamily of CREB3 transcription factors associate with ATF6 and function as sensors. These sensors are differentially expressed in different cell types and vary in their ability to initiate stress signals. At least five bZIP transcription factors related to ATF6 have been described. These are CREB3/ Luman, CREB3L1/OASIS, CREB3L2/BBF2H7, CREB3L3/CREBH and CREB3L4/CREB4/AIbZIP/Tisp40. For more detailed information, interested readers are referred to two excellent recently published reviews (Asada et al., 2011; Chan et al., 2011).
Briefly, CREB3/Luman is expressed in some cell types, such as monocytes and dendritic cells. The mechanism and physiological conditions that lead to activation of this sensor are not entirely clear, but it is known to participate in the expression of various genes involved in the ERAD, like Herp (Liang et al., 2006) and EDEM (DenBoer et al., 2005). Luman has been implicated in dendritic cell maturation, a process that is regulated by association with a complex containing the proteins DC-STAMP and OS9 (Eleveld-Trancikova et al., 2010). The transcriptional activity of Luman is regulated by differential interaction with the cofactor HCF-1 (herpes simplex virus-related host cell factor 1) or LRP (Luman recruitment factor), that favor or repress transcriptional activity, respectively (Audas et al., 2008).
On the other hand, OASIS/CREB3L1 is a transcription factor highly expressed in astrocytes and osteoblasts, and like the rest of subfamily members, is located in the ER membrane (Kondo et al., 2005). OASIS displays low homology with ATF6 in its luminal region but was described as a target for S1P/S2P proteases in response to ER stress (Murakami et al., 2006). OASIS-deficient mice suffer from severe osteopenia and spontaneous fractures generated by decreased ColIaI expression (Murakami et al., 2009). CREB3L2/BBF2H7 is a transcriptional factor analogous to OASIS that is highly expressed during the extension phase of long bones in the chondrocytes of the proliferating zone of cartilage. In addition, CREB3L2/ BBF2H7 is expressed in other tissues, such as the lung and nervous tissue (Kondo et al., 2007). Sec23a, a protein required for the recruitment of Sec13/31 and other components of the COPII vesicles, important for vesicle trafficking from ER to Golgi, is controlled by CREB3L2 (Saito et al., 2009). CREB3L3/CREBH is expressed in hepatocytes and is also cleaved by S1P/S2P proteases. This transcription factor is known to regulate the expression of the gluconeogenic enzyme PEPCK in response to cyclic AMP and protein kinase A (Asada et al., 2011). Finally, CREB3L4/ CREB4/AIbZIP/Tisp40 has been associated, among other things, with male germ cell development and the ER stress response during spermiogenesis (Adham et al., 2005).
3.3. Integral Response or Cross Talk between Different UPR Branches
ER stress sensors use different mechanisms and effectors to activate the UPR, but at some points, the three pathways communicate. One example is the close relationship between the IRE1α and ATF6 pathways. XBP1u not only functions as a negative regulator of XBP1 but also targets the active form of ATF6 to the proteasome (Yoshida et al., 2009), while ATF6, on the other hand, also controls the transcription of XBP1 (Yoshida et al., 2001). Additionally, ATF6 heterodimerizes with XBP1 to promote degradation of ERAD components (Yamamoto et al., 2007).
The PERK pathway is also linked to IRE1α and ATF6. Recently, a dominant negative form of PERK was shown to activate ATF6 and XBP1 (Yamaguchi et al., 2008). However, in another study, PERK was shown to facilitate both the synthesis and trafficking of ATF6 from the ER to the Golgi (Teske et al., 2011). All these studies underscore the notion that individual branches of the UPR are connected in ways that permit generating integrated responses to stress. Also, such connectivity explains the diverse defects associated with loss of any one of the sensors.
The integrated responses elicited by the three ER stress sensors contribute to either adaptation or death, but it remains unknown how each branch contributes to the final biological effect. A number of studies have attempted to resolve this issue. For instance, IRE1 and ATF6 activities are attenuated by persistent ER stress, while PERK signaling is not (Lin et al., 2007). Indeed, sustained PERK signaling impairs cell proliferation and promotes apoptosis. By contrast, signaling via IRE1α for an equivalent period of time enhances cell proliferation without promoting cell death (Lin et al., 2009), suggesting that the differential activation of PERK and IRE1α may determine cell fate following ER stress.
4. ENDOPLASMIC RETICULUM AND PROTEIN DEGRADATION
4.1. Endoplasmic Reticulum Quality Control
One of the main functions of the ER is the synthesis of proteins targeted to the secretory pathway. Proteins synthesized in the ER develop the appropriately folded native conformation following posttranslational modifications such as N-glycosylation and the formation of disulfide bonds. In order to export only correctly folded proteins, the ER is home to a variety of chaperones which facilitate protein folding. At the same time, they function as part of a quality-control system, which ensures that incompletely folded proteins are retained in the ER or targeted to degradation if appropriate folding cannot be achieved (Fig. 5.3).
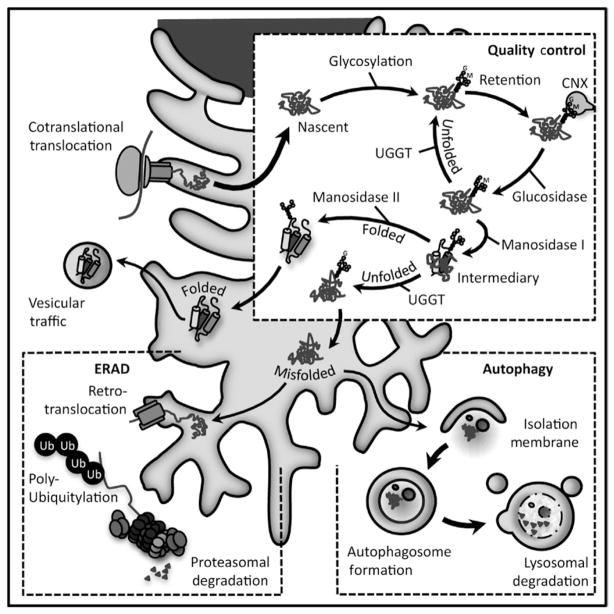
Fig 5.3. Proteins synthesized in the ER are subject to a quality control system.
The lectins, calnexin and calreticulin retain glycosylated proteins in the ER through re/deglucosylation cycles until they reach their native conformation. Folded proteins are exported through vesicle trafficking, while terminally misfolded proteins are degraded through retrotranslocation and the ubiquitin–proteasome system or autophagy.
The lectins calnexin and calreticulin play essential roles as chaperones in the ER quality-control system. As previously mentioned, most nascent polypeptide chains in the ER are N-glycosylated to facilitate the folding process (Schröder, 2008). Upon N-glycosylation, a branched oligosaccharide is attached to an asparagine residue on the target protein, thereby increasing the hydrophilicity of the nascent protein. Calnexin and calreticulin bind to glycosylated substrates containing a terminal glucose residue and aid in protein folding by inhibiting protein–protein aggregation. In addition, calnexin and calreticulin trap partially folded or unfolded proteins in the ER (Hebert et al., 2005). While correctly folded proteins are deglucosylated by glucosidase II, then released from calnexin and calreticulin and exported to the Golgi apparatus, misfolded proteins are reglucosylated by the glycoprotein glucosyltransferase and retained in the ER (Hebert and Molinari, 2007). Folding attempts continue with each cycle of re/deglucosylation up until demannosylation occurs via α-mannosidase I. Once demannosylated, glycoproteins become a weaker substrate for glucosidase II and glucosyltransferase, thus preventing them from entering new cycles of glucosylation (Hebert et al., 2005). Other lectins like EDEM work as acceptors for terminally misfolded proteins accelerating the demannosylation process (Olivari et al., 2006). Overexpression of EDEM greatly accelerates protein degradation while its downregulation prolongs folding attempts and delays protein degradation (Molinari et al., 2003). Altogether, the ER quality-control system ensures that correctly folded proteins leave the ER while incorrectly or terminally misfolded proteins are retained in the ER and degraded.
4.2. Misfolded Protein Degradation through ERAD and Autophagy
Terminally misfolded proteins can be degraded through two different pathways: The ubiquitin–proteasome pathway named ERAD and the lysosomal pathway termed macroautophagy (hereafter referred to as autophagy). The ERAD pathway consists in the retrotranslocation, polyubiquitination and proteasomal degradation of misfolded proteins from the ER (Meusser et al., 2005). Terminally misfolded proteins are translocated across the ER membrane into the cytoplasm where they are covalently bound to ubiquitin through a lysine residue. Attachment of multiple ubiquitins yields polyubiquitin chains, which are a signal for degradation inside a cylinder-shaped protein complex, termed the proteasome. Once inside the proteasome, the protein becomes a substrate for the multiple proteolytic activities present, which degrade the target protein and recycle the polyubiquitin side chain (Vembar and Brodsky, 2008). On the other hand, during autophagy, different molecular components such as proteins, lipids or even entire organelles are sequestered into double-membrane vesicles, which then fuse with lysosomes, to allow the degradation of vesicle contents (He and Klionsky, 2009).
Although ERAD has been described as the primary source for ER misfolded protein degradation, recent reports have shown that autophagy is involved in the removal of certain ER proteins such as α1-antitrypsin. These observations identified autophagy as an alternative cellular strategy to eliminate unfolded proteins (Yorimitsu et al., 2006;Yorimitsu and Klionsky, 2007).
4.2.1. ERAD
The exact mechanism by which ERAD substrates are retrotranslocated across the ER membrane is still a matter of debate. Several proteins have been identified that play an essential role in this process by creating a channel in the ER membrane that permits retrograde transport of misfolded proteins back to the cytoplasm (Meusser et al., 2005). The Sec61 complex has been proposed as a possible channel for protein retrotranslocation (Wiertz et al., 1996) although this complex was first described as a channel through which nascent polypeptides are imported into the ER (Mothes et al., 1994; Matlack et al., 1998). Another possible candidate retrotranslocation channel is Derlin-1, which forms a complex with the ubiquitin ligase Hrd-1 along with other adaptor proteins (Carvalho et al., 2010). Derlin-1 has the ability to form structures that span the ER membrane, suggesting that this protein may also function as a channel for ERAD retrotranslocation (Crawshaw et al., 2007).
Research in S. cerevisiae showed that protein substrates are recognized and degraded differently, depending on whether their misfolded domain is cytosolic (ERAD-C), transmembrane (ERAD-M) or luminal (ERAD-L) (Carvalho et al., 2006). While ERAD-C substrates are rapidly degraded through the ubiquitin ligase Doa10p (Swanson et al., 2001), ERAD-L and ERAD-M depend on the Hrd ubiquitin ligase complex for degradation (Bordallo et al., 1998; Schäfer and Wolf, 2009). Studies in yeast using different glycosylated and nonglycosylated degradation substrates identified the basic components of the Hrd ubiquitin ligase complex and its adapter protein functions. The basic structure of this complex is formed by the ubiquitin ligase Hrd1p, the substrate adapter protein Hrd3p, the luminal substrate adapter proteins Der1p and Usa1p, the ubiquitin conjugating enzyme Ubc7 and the glycan substrate adapter Yos9p (Carvalho et al., 2006; Mehnert et al., 2010). Different mammalian homologs of Hrd complex proteins have been identified by correlating with functions in yeast (Mehnert et al., 2010). Among these mammalian homologs, Herp, an ubiquitin-like ER membrane protein, has been recently found to regulate Hrd1-dependent ubiquitination (Kokame et al., 2000; Kny et al., 2011). Recent studies have shown that Herp is involved in regulating the degradation of multiple ER substrates such as immature nicastrin and α-1 antitrypsin (Kny et al., 2011; Marutani et al., 2011).
Retrotranslocation across the ER membrane of misfolded, polyubiquitinated proteins is an active process that requires ATP hydrolysis. The AAA-ATPase p97 and its adapter proteins Ufd and Npl4 are necessary to generate the driving force required for misfolded protein extraction (Ye et al., 2001). In mammalian cells, p97 binds Derlin-1 via VIMP and other cofactors, thereby permitting recruitment of misfolded proteins to the ER membrane, their export and proteasomal degradation in the cytoplasm (Ye et al., 2004).
4.2.2. Autophagy
Autophagy is induced by nutrient deprivation. The kinase AMPK responds to low energy levels of the cell, by sensing the declining ATP/AMP ratios. Once activated, AMPK phosphorylates mTOR, a master regulator of protein synthesis. mTOR normally functions as a Ser/Thr kinase, which hyperphosphorylates Atg13, thereby impeding the activation of autophagy. mTOR phosphorylation by AMPK reduces Atg13 phosphorylation, thus permitting its interaction with the kinase Atg1 and activation of a phosphorylation cascade that leads to the recruitment and activation of a series of Atg proteins indispensable for autophagy induction (He and Klionsky, 2009; Jung et al., 2010). Among the most important Atgs are LC3/Atg8 and Beclin-1/Atg6. Beclin-1 is part of a protein complex termed PI3K-III, which is important for the nucleation, recruitment and expansion of the double membrane that eventually sequesters the substrates destined for degradation. In normal conditions, Beclin-1 interacts with its inhibitor Bcl-2. Beclin-1 dissociation from Bcl-2 is indispensable for PI3K-III function and autophagy activation (Kang et al., 2011). During the autophagic pathway, LC3 is processed and attached to the autophagosome membrane by conjugation with phosphatidylethanolamine (Klionsky et al., 2007).
As indicated, recent work has shown that autophagy is induced under ER stress conditions to protect cells against death (Ogata et al., 2006; Pankiv et al., 2007). Work in yeast, utilizing classic ER stress inducers, such as DTT or tunicamycin, revealed that autophagy was induced, as assessed by LC3 processing and Atg1 kinase activity (Yorimitsu et al., 2006; Yorimitsu and Klionsky, 2007). This correlates with other work in which Hac1, an XBP1 yeast ortholog, induced important autophagy genes such as Atg5, Atg7 and Atg8 under ER stress conditions (Bernales et al., 2006). Other studies in mammalian cells showed that autophagy is a key component in the degradation of α1-antitrypsin retained inside the ER (Teckman and Perlmutter, 2000). ER-mediated regulation of autophagy has been linked to the PERK and IRE1 pathways. PERK phosphorylation of eIF2α is involved in Atg12 upregulation and LC3 conversion during polyglutamine aggregate-induced autophagy (Kouroku et al., 2007). PERK phosphorylation of eIF2α is also involved in the degradation of mutant dysferlin aggregates via LC3 conversion and autophagy (Fujita et al., 2007). Other studies have implicated the IRE1 pathway in ER stress-induced autophagy. IRE1 recruits the protein factor TRAF2, which activates JNK that phosphorylates Beclin-1, thereby allowing dissociation from its repressor Bcl-2 (Ogata et al., 2006). ER calcium release is also thought to participate in autophagy induction (Høyer-Hansen et al., 2007). CaMKKβ is activated by ER stress-induced calcium release, and in turn activates AMPK, leading to the inhibition of mTOR and induction of autophagy (Høyer-Hansen et al., 2007; Høyer-Hansen and Jäättelä, 2007).
4.2.3. Examples of ER Proteins Degraded by ERAD and/or Autophagy
Supporting the notion that autophagy is as important as ERAD for degradation of terminally misfolded ER proteins, recent studies have shown that dual degradation mechanisms exist for certain proteins, such as α1-antitrypsin and mutant fibrinogen. Both mutant proteins form aggregates in the ER, which, if not cleared, promote cell death. Recent studies have shown that high levels of α1-antitrypsin or mutant fibrinogen can saturate the ERAD pathway, leading to the accumulation of protein aggregates that must be cleared through the autophagy pathway (Kruse et al., 2006a, 2006b). Another example where dual degradation pathways are observed is dysferlin. While wild-type dysferlin is degraded through the ERAD pathway, mutant dysferlin forms protein aggregates that impair ERAD and are degraded through autophagy (Fujita et al., 2007). ERAD and autophagy also play a role in serpin and procollagen degradation. ERAD seems to have a selective role in mutant neuroserpin degradation, while autophagy degrades all forms of neuroserpins. Thus, autophagy becomes an important clinical target since induction of this degradation pathway could help to overcome the accumulation of mutant protein aggregates (Kroeger et al., 2009). Autophagy degrades misfolded procollagen that accumulates as trimers, while the misfolded procollagen monomers are degraded through the ERAD pathway (Ishida and Nagata, 2009; Ishida et al., 2009). Interestingly, EDEM1, a protein involved in quality control and ERAD, is also degraded through autophagy, suggesting that the ERAD pathway may be regulated by autophagy (Le Fourn et al., 2009).
4.3. ER-phagy
ER-phagy, as well as mitophagy and peroxiphagy, are organelle-specific autophagic processes that degrade ER, mitochondria or peroxisomes, respectively. ER-phagy is a specific process, which uses several autophagy genes induced by ER stress. Normally, ER stress increases the volume of the ER in order to inhibit protein–protein aggregation. ER-phagy is the sequestration of ER into double-membrane vesicles. Interestingly, both the sequestered content and the vesicle-forming membranes are of ER origin, which suggests that the ER engulfs itself in order for ER-phagy to proceed. Even though autophagy is a degradation process in which the autophagosome fuses with the lysosome to permit degradation of the autophagosome content, ER-phagy is not a degradation process since mutants lacking vacuolar proteases are able to sequester the ER. Thus, the function of ER-phagy seems to involve sequestering damaged portions of the ER, misfolded protein aggregates that cannot be degraded by other means, as well as reducing the volume of the ER once ER stress is over (Bernales et al., 2006, 2007).
In summary, secretory proteins and most integral membrane proteins enter into ER for proper folding and covalent modifications to assemble into complexes of higher order. ER-resident chaperones and other modifying enzymes assist proteins to achieve their active and final, three-dimensional conformation. Only properly folded and assembled proteins are allowed to exit the ER, thereby providing an exquisite quality-control mechanism that maintains the fidelity of protein synthesis. The process is regulated at multiple levels ensuring that ER folding capacity is not overwhelmed, thus maintaining ER homeostasis. At the center of this regulation is the phylogenetically conserved process of the UPR, a key signaling pathway that triggers comprehensive remodeling of the ER and the biosynthetic pathway according to the different cellular needs. In parallel, autophagy is another important degradation pathway normally induced by nutrient deprivation and more recently, under ER stress conditions, to protect cells from death. Two reports (Bernales et al., 2006, 2007) that describe the new process of ER-phagy provide direct evidence that the ER can serve as a membrane source for autophagosome formation. Further, they indicate that this process entails engulfment of the ER by itself, targeting the ER as the major organelle involved in the regulation of cellular component degradation. As such, it plays an important role in protein- and organelle-degradation functionality of the ER critical to homeostatic cellular control.
5. UPR AND CELL METABOLISM
The main goal of the UPR is to restore the equilibrium between protein load and folding capacity of the ER. From a metabolic point of view, protein folding in the ER is a demanding process, due to its high-energy requirements (Fig. 5.4). For example, saccharides are needed for the N-glycosylation of client proteins, reductive equivalents are consumed in the formation of disulphide bonds and an appropriate supply of ATP is essential for calcium accumulation in the ER and chaperone activity.
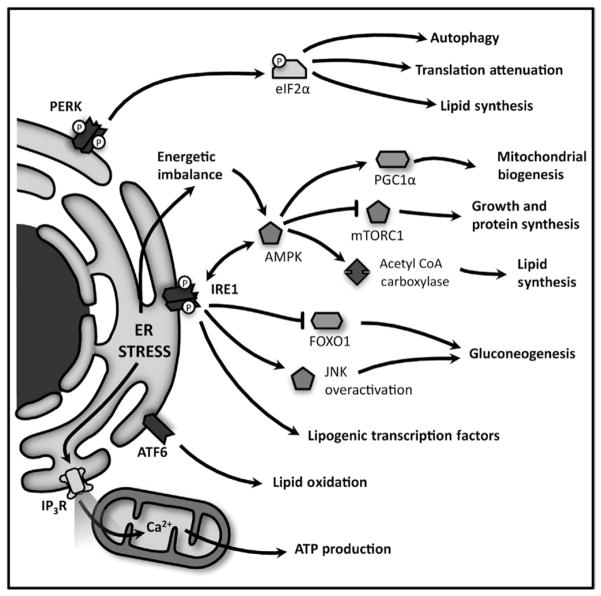
Fig 5.4. The ER modulates important metabolic pathways during ER stress.
PERK induces global attenuation in protein anabolism, and also favors lipid synthesis, both effects via eIF2α phosphorylation. IRE1 cooperates with these responses and stimulates mitochondrial biogenesis through the regulation of master metabolic switches, such as PGC1α, mTOR, AMPK and FOXO1. ATF6, on the other hand, stimulates lipid utilization. Calcium released by IP3R increases mitochondrial activity during ER stress, in order to revert energy imbalance.
5.1. UPR-Mediated Regulation of Master Metabolic Switches
The best-known effect of UPR on cellular metabolism is the attenuation of general protein translation via eIF2α phosphorylation. Such negative regulation not only reduces protein load in the ER but also increases ATP availability for processes such as protein folding and degradation.
As already mentioned, conditions of low nutrient supply are known to induce the UPR as well as autophagy. The relationship between these two responses becomes clear considering that nutrient deprivation increases protein misfolding, which is a potent trigger of both processes. However, cross talk between the UPR and master metabolic regulators has received little attention until recently. AMPK has been shown to require the endoribonuclease activity of IRE1 for nitric oxide-induced signaling (Meares et al., 2011). Moreover, stimulation of the IRE1 endoribonuclease activity with the flavonoid quercetin also engages IRE1-dependent AMPK activation. This signaling pathway is important for maintaining cellular metabolism because it regulates the activity of critical proteins, such as acetyl-CoA carboxylase, PGC-1α and mTORC1. This cooperative effect is of particular interest in tissues with high metabolic activity, such as the heart. The AMPK activators AICAR or metformin relieve hypoxia/reoxygenation injury, a process known to trigger apoptosis in cardiomyocytes, by generating ER stress (Yeh et al., 2010). This response is achieved by the concomitant decrease in proapoptotic branches of the UPR (CHOP and caspase-12) while retaining an adaptive increase in BiP/GRP78 protein levels. Taken together, this evidence suggests that AMPK signaling may cooperate with, or even be part of the adaptive steps of UPR, by diminishing death-inducing irreparable damage. However, many details of this complex cross talk process still need to be unraveled.
When ER stress is induced by increased protein load, instead of decreased nutrients, the role of mTOR has been shown to be important. Such is the case for plasma cells, which secrete large amounts of immunoglobulins when they are stimulated with LPS. These cells, upon induction of ER stress, attenuate the mTOR pathway, leading to reduced protein synthesis. This reduction is important for cellular adaptation as B cells with elevated mTOR activity are prone to undergo apoptosis upon LPS stimulation (Goldfinger et al., 2011). A second example that shows the preponderance of mTOR during ER stress has been established in a rat model of minimal-change disease, which is characterized by podocyte ER stress-dependent proteinuria. In this model, mTORC1 activation was shown to precede UPR, leading to a decrease in the ATP/ADP ratio and activation of AMPK, presumably due to an increase in energy-consuming processes, such as cell growth and proliferation. Pretreatment with the mTORC1 inhibitor, everolimus, prevented the reduction in ATP levels, AMPK activation and UPR, thus inhibiting proteinuria (Ito et al., 2011). Studies in tumor cells have shown that constitutive activation of mTOR induces ER stress, which is part of a negative feedback loop emerging from growth factor receptors upstream of mTOR (Ozcan et al., 2008). On the other hand, during ER stress, mTORC1 triggers apoptosis by suppressing Akt activity, and selectively inducing the IRE1-JNK pathway (Kato et al., 2011). These observations highlight the delicate balance that exists between cellular energy sensing and ER stress.
5.2. UPR Regulation of Lipid Metabolism
ER stress is known to stimulate lipogenesis through the UPR, thus providing lipids for ER expansion, a hallmark of cellular adaptation. ER stress activates, for example, SREBP1 (Wang et al., 2005), as well as SREBP2 (Colgan et al., 2007), two master transcriptional regulators of fatty acid and cholesterol biosynthesis. Relief of ER stress by overexpression of BiP/GRP78 inhibits SREBP1 activation in the liver of ob/ob mice, highlighting the importance of the UPR in the control of lipid metabolism (Kammoun et al., 2009).
As mentioned in previous sections, the IRE1/XBP1 pathway was first shown to be important for the differentiation of highly secretory cells, such as antibody-secreting B cells. Likewise, XBP1-deficient cells are reportedly deficient in adipogenic differentiation (Sha et al., 2009). XBP1 was shown to be directly downstream the essential adipogenic factor C/EBPβ, and splicing of this factor is indispensible for the development of the adipose phenotype. One mechanism by which XBP1 promotes lipogenesis is through activation of enzymes of the CDP-choline pathway, thus leading to increased phosphatidylcholine biosynthesis and ER biogenesis (Sriburi et al., 2004, 2007). In the liver, the IRE1/XBP1 pathway directly controls genes involved in fatty acid synthesis in response to carbohydrate diet, while hepatic deletion of XBP1 causes hypocholesterolemia and hypotriglyceridemia (Lee et al., 2008). Furthermore, hepatocyte-specific IRE1 deletion predisposes mice to ER stress-induced hepatosteatosis, suggesting that this UPR sensor is also important for intracellular lipid secretion and accumulation (Zhang et al., 2011).
PERK has also been demonstrated to be important for lipogenic tissue development since knockout mice have impaired mammary gland lipogenesis during pregnancy, which results in reduced free fatty acid content of the milk (Bobrovnikova-Marjon et al., 2008). Loss of PERK also resulted in a decrease in key lipogenic enzymes, such as stearyl-CoA desaturase-1, fatty acid synthase and ATP citrate lyase, due to excessive activation of SREBP1, the master regulator of lipid homeostasis within the cell. The PERK pathway is also important for development of hepatosteatosis in mice fed a high-fat diet. Reduction of eIF2α phosphorylation results in a lower expression of PPARγ and C/EBP, key transcriptional regulators of lipid synthesis (Oyadomari et al., 2008).
On the other hand, ATF6 has also been implicated in lipid metabolism. ATF6 knockout mice treated with tunicamycin develop hepatosteatosis to a higher degree than wild-type mice. In these animals, a more pronounced decrease in the expression of genes related to lipid handling, such as PGC1α, PPARα and SREBP1, is observed, which results in increased lipid accumulation in the liver. However, unlike the other UPR pathways, ATF6 seems to be more important for fatty acid oxidation than lipid biosynthesis (Rutkowski et al., 2008).
5.3. UPR Regulation of Carbohydrate Homeostasis
In rat liver, it was recently shown that physiological postprandial increases in glucose and lipids cause ER stress (Boden et al., 2011), suggesting that the UPR may participate in carbohydrate metabolism. FOXO1, a transcription factor that promotes hepatic gluconeogenesis and inhibits glucose utilization (Zhang et al., 2006), is regulated by XBP1 in an ER stress response-independent manner (Zhou et al., 2011). Spliced XBP1 interacts with FOXO1 to enhance proteasome-mediated degradation. This effect is observed even at low levels of XBP1 that are unable to induce UPR-related genes. This evidence shows that UPR sensors control carbohydrate metabolism and diminish glucose production. Alternatively, another line of evidence links ER stress-induced JNK hyperactivation to increased FOXO1 activity and increased hepatic gluconeogenesis (Lim et al., 2009). This mechanism may be important for regulation of blood sugar during long periods of hypoglycemia since this condition has been shown to induce ER stress in the liver, thereby linking the UPR and glucose production (Gonzales et al., 2008). These contradictory results suggest that the connection between UPR and hepatic glucose handling is complex and further studies are required to fully dissect the details.
The pathological implications of UPR for glucose metabolism in different organs have been extensively documented in recent years, due to its importance in understanding type 2 diabetes. This issue will be covered in the next section.
5.4. ER Stress-Modulated Mitochondrial Metabolism
While the role of the ER as a physiologically relevant calcium store is well established, a similar role for mitochondria has been long debated. Calcium ions are known to be taken up by mitochondria and to accumulate in the mitochondrial matrix (Deluca and Engstrom, 1961; Vasington and Murphy, 1962), but the relevance of this process remained unclear. Moreover, evidence suggesting the existence of a low-affinity calcium uptake system questioned the physiological relevance of mitochondria in calcium handling.
For many years, calcium accumulation and protection of the cell against calcium overload was considered to be the only function of mitochondria in the control of intracellular calcium metabolism. However, this view was challenged by observations showing that the activity of pyruvate, isocitrate and α-ketoglutarate dehydrogenases in permeabilized or homogenized mitochondria was enhanced by calcium in a direct or an indirect manner (McCormack et al., 1990; Carafoli, 2010). Interestingly, agonist-induced calcium release can lead to improved mitochondrial function as evidenced by increased ATP production after restoring normal mitochondrial calcium levels (Jouaville et al., 1999). Additionally, also many other mitochondrial processes, such as fatty acid oxidation, amino acid catabolism, F1-ATPase manganese superoxide dismutase activity, aspartate and glutamate carrier, as well as the adenine-nucleotide translocase activity, are regulated by mitochondrial calcium (McCormack et al., 1990; Hayashi et al., 2009). Moreover, Cárdenas et al. (2010) recently showed that basal IP3R activity was required to control mitochondrial bioenergetics. Absence of this calcium transfer results in enhanced phosphorylation of pyruvate dehydrogenase and AMPK activation, which in turn activates autophagy as a prosurvival response. Thus, constitutive IP3R-mediated calcium release to mitochondria is required for efficient mitochondrial respiration and maintenance of normal cellular bioenergetics.
Work from our group performed in HeLa cells demonstrated that early stages of ER stress increase physical coupling and calcium transfer from ER to mitochondria, leading to augmented mitochondrial bioenergetics as a means of adaptive ATP production (Bravo et al., 2011b). Physical coupling between ER and mitochondria is observed upon treatment with different ER stressors (tunicamycin, brefeldin A and thapsigargin). However, it remains unknown to what extent the UPR is involved in these events.
Additionally, a recent study performed in skeletal muscle showed that adaptation of muscle fibers to acute exercise is mediated by the UPR. ATF6 is required for the recovery process, involving the coactivation of PGC1α (Wu et al., 2011). Such cross talk between the UPR and PGC1α is a potential mechanism that may explain how ER stress-mediated control of mitochondrial metabolism is achieved, given the importance of PGC1α in mitochondrial biogenesis and fatty acid oxidation (Koves et al., 2005; Safdar et al., 2011). Despite such insights, the field of ER-modulated mitochondrial metabolism is still rather nascent, and further studies are required to determine their relevance to the development of human diseases. Some of these pathological implications will be discussed in the next and final sections.
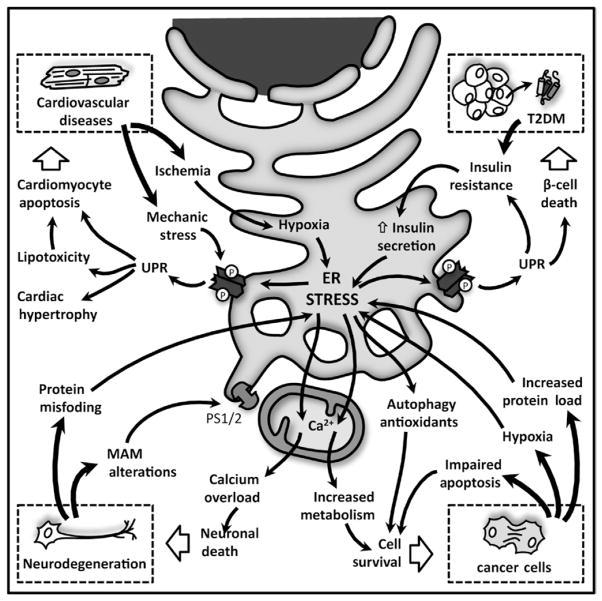
6. UPR AND PATHOLOGIES
Pathophysiological alterations in cellular calcium along with ER stress signaling are hallmarks of a broad variety of pathologies such as type-2 diabetes mellitus (T2DM), insulin resistance, cardiovascular diseases and Alzheimer disease (AD) (Kelley et al., 2002; Lim et al., 2009). These pathologies are also characterized by a marked decrease in cellular metabolism; however, the molecular mechanisms responsible for this dysfunction are not fully understood. As discussed previously, alterations in the contacts between ER and mitochondria, and more importantly, in calcium transfer between these two organelles, could lead to mitochondrial dysfunction and the metabolic imbalance observed in these diseases (Fig. 5.5). Moreover, recent studies have also linked ER–mitochondria communication to cancer growth and progression. The influence of both organelle interplay and UPR signaling on human pathologies will be discussed subsequently.
Fig 5.5. ER and pathologies.
ER stress and alterations in cellular calcium characterize diverse pathologies such as type-2 diabetes mellitus, insulin resistance, and some cardiovascular and neurodegenerative diseases. These pathologies are also characterized by marked decrease in cellular metabolism, which is accompanied with protein misfolding and aggregation, and some characteristic hallmarks of UPR. However, the molecular mechanisms linking all these processes are not yet fully understood.
6.1. UPR and Diabetes
Diabetes mellitus (DM) is considered a multifactorial pathology and is associated with obesity, dyslipidemia, endothelial dysfunction, inflammation and hypertension (Petersen and Shulman, 2006). T2DM is one of the most common diseases in the developed world and it is now considered as a global health burden (Zimmet et al., 2001). Peripheral insulin resistance, deregulated hepatic glucose production and inadequate insulin secretion characterize T2DM. Furthermore, this disease involves defects in insulin signaling due to reduced insulin receptor function and downstream phosphorylation events (Lee and White, 2004). Recent reports using genetically obese (ob/ ob) or high-fat diet (HFD)-induced obese mice identified an increase in UPR markers in the liver and adipose tissues (Ozcan et al., 2004). Moreover, elevated ER stress markers, such as GRP78, XBP1s, phosphorylated eIF2a and JNK, are detected in the liver and adipose tissue of obese insulin-resistant individuals (Boden et al., 2008). Additionally, JNK activation via UPR is associated with insulin resistance via phosphorylation of IRS-1. Hotamisligil’s group described a causal link between ER stress and the development of metabolic diseases like insulin resistance and T2DM. Using different cell culture and mouse models, they showed that obesity causes ER stress. Moreover, mice deficient in the XBP-1 protein develop insulin resistance, demonstrating that ER stress is a central feature of peripheral insulin resistance and T2DM at the molecular and cellular level (Ozcan et al., 2004). In another more recent study, XBP-1 was shown to interact with the transcription factor FOXO1, a key regulator of gluconeogenesis, and promote its proteasomal degradation. Moreover, hepatic overexpression of XBP-1 results in significantly reduced blood glucose levels and increased glucose tolerance in mouse models of insulin resistance and type 1 and 2 diabetes. All these changes are accompanied by a reduction in hepatic FoxO1, revealing that XBP-1 regulates glucose homeostasis in response to ER stress (Zhou et al., 2011).
Enhanced gluconeogenesis is another important component of insulin resistance in T2DM. Interestingly, the induction of acute ER stress results in reduced hepatic glucose output mediated by ATF6 interaction with the CREB-regulated transcription coactivator 2 (CRTC2) that releases CREB, which regulates fasting gluconeogenesis (Wang et al., 2009c). Moreover, in CRTC2-knockout animals, decreased fasting gluconeogenesis and improved insulin sensitivity are observed upon diet-induced obesity (Wang et al., 2010). Additionally, chronic ER stress also increases gluconeogenesis. Obese mice have lower levels of hepatic ATF6 and XBP-1, which reduces the interaction of ATF6 with CRTC2, thereby favoring upregulation of the gluconeogenesis program (Wang et al., 2009c). Furthermore, XBP-1 reduction contributes to enhanced JNK activation that triggers insulin resistance (Ozcan et al., 2004).
PERK, the third branch of the UPR, acutely and chronically phosphorylates eIEF2α during hepatic ER stress (Ozcan et al., 2004; Lin et al., 2007), increasing gluconeogenesis via the translational activation of C/EBP. Additionally, the dephosphorylation of hepatic eIEF2α improves insulin sensitivity in HFD mice (Oyadomari et al., 2008). eIF2α is also implicated in hepatic lipogenesis. Thus, ATF6 knockout and chronic dephosphorylation of eIF2α in a mouse model decrease C/EBP expression and downstream lipogenic transcription factor activity (Oyadomari et al., 2008; Rutkowski et al., 2008). Finally, ER stress contributes to hepatic fat accumulation through changes in the nuclear localization of SREBP1c by an insulin-independent pathway (Kammoun et al., 2009).
During the development of T2DM, insulin production by β-cells is increased to compensate for insulin resistance. This increase in insulin demand, together with increased fatty acid levels and hyperglycemia, activates UPR in pancreatic cells (Cnop et al., 2005). Different kinds of mutations are associated with diabetes. Missense mutations that interrupt disulphide bridge formation and correct proinsulin folding cause neonatal diabetes (Støy et al., 2007; Colombo et al., 2008). Moreover, mutations in several UPR genes, like eif 2ak3 (which codes for PERK) and wsf1, are considered risk factors for the development of diabetes, in the Wolcott–Rallison and Wolfram syndromes, respectively (Inoue et al., 1998) (Delépine et al., 2000). Prolonged T2DM can activate the UPR by hyperglycemia. In contrast, free fatty acids (FFA) may trigger ER stress early in diabetes. Several groups have demonstrated that chronically high glucose levels cause ER stress (Wang et al., 2005; Lipson et al., 2006; Elouil et al., 2007). For example, cultured rat pancreatic islets exposed to high glucose (30 mM) increase the mRNA levels of Grp78, Grp94, Edem and Chop (Elouil et al., 2007). However, a study in MIN6 insulinoma cells exposed to low or high glucose failed to induce the UPR response (Webb et al., 2000). Besides, high glucose levels are also associated with an increased level of ROS and a reduction in β-cell function (Robertson, 2004; Robertson et al., 2004). Although ROS can induce ER stress (Malhotra and Kaufman, 2007), they also cause numerous other cellular effects.
As for hyperglycemia, elevated levels of FFA are also linked to obesity and contribute to the development of T2DM (Robertson et al., 2004). Exposure of human islets to palmitate induces ER stress and an NF-kB-mediated inflammatory response (Igoillo-Esteve et al., 2010). Furthermore, the treatment of β-cells with palmitate causes ER distention and activation of the three branches of the UPR. This activation may contribute to FFA-induced β-cell death through CHOP upregulation and the activation of JNK as well as the caspase-12 cascade (Kharroubi et al., 2004), which rapidly degrades carboxypeptidase E, an enzyme involved in the insulin processing. FFA also induces the depletion of ER calcium and impedes protein trafficking by ceramide synthesis (Boslem et al., 2011). All these events contribute to ER stress by the accumulation of unprocessed proinsulin molecules in the secretory pathway (Jeffrey et al., 2008).
Finally, obesity and occidental diet are associated with elevated plasma levels of glucose and FFA. Both factors contribute to the progression of T2DM by promoting pancreatic β-cell death (Cunha et al., 2008, 2009). While CHOP has a proapoptotic effect in β-cells, PERK antagonizes the apoptosis antagonizing transcription factor (AATF), which regulates Akt1 (Ishigaki et al., 2010). Additionally, the upregulation of C/EBP sensitizes cells to apoptosis (Matsuda et al., 2010). Although our understanding of how chronic ER stress contributes to the development of diabetes is improving, further studies are needed to fully appreciate the existing connections between UPR and the metabolic nodes regulated by mTOR and AMPK, as well as how the drugs metformin and resveratrol affect ER stress.
6.2. UPR and Cardiovascular Diseases
6.2.1. Ischemia/Reperfusion
All three branches of the UPR are activated in ischemic heart disease (Glembotski, 2007). In an in vitro model using HL-1 cells, exposure to 8 h of hypoxia triggered phosphorylation of PERK and expression of CHOP (Perman et al., 2011). Also, in rat neonatal cardiomyocytes, ischemia stimulated significant upregulation of BiP/GRP78 and ATF6, as well as an increase in XBP1 splicing (Thuerauf et al., 2006). The majority of these and other in vitro findings have been replicated in animal models and clinical studies under various conditions (Thuerauf et al., 2006; Severino et al., 2007; Toko et al., 2010; Perman et al., 2011). Studies in wild-type animals showed that ATF6 transcription was increased 6 h after ischemia (Perman et al., 2011). Alternatively, Thuerauf et al. (2006) found that BiP/GRP78 levels were elevated even 4 days after ischemic injury in cardiomyocytes.
Under ischemic conditions, the lack of oxygen and nutrients poses a serious threat to cardiomyocyte viability. At the mechanistic level, insufficient ATP impairs disulfide bond formation and fosters inappropriate calcium handling, both processes that have been suggested to trigger UPR in heart. Disulfide bond formation is critical for folding of secreted proteins, and the ER-resident chaperones involved, Ero1, PDI and ERp57, require oxygen as a final electron acceptor for the serial redox reactions required for this process (Anelli and Sitia, 2008). Correct glycosylation of membrane proteins is required for both trafficking and proper biological function. Recent studies suggest that insufficient ATP availability affects UDP-sugar formation and triggers ER stress by interfering with glycosylation. The sudden drop in nutrient flux during ischemic heart disease leads rapidly to energy and ATP shortage. Additionally, high calcium levels in the ER are required for chaperones such as BiP/GRP78 to aid in protein folding. The pathological changes in ER calcium levels during ischemia due to decreased SERCA2 activity can effectively stimulate ER stress (Liu et al., 2011).
Although activation of the UPR in ischemic heart disease is well established, the precise biological role of the UPR in this process remains unclear, since available evidence indicates that activation of the UPR can be either beneficial or detrimental. Most studies have attempted to address this question by evaluating activity of proapoptotic branches of the UPR following genetic or pharmacological manipulation. For instance, studies with VLDL-deficient rats established that protection of the heart from ischemic insult correlated with a decrease in caspase activity and apoptotic cell death (Perman et al., 2011). Likewise, this correlation was evident in a rat ischemia model where BiP/GRP78, CHOP and caspase-12 were evaluated (Song et al., 2011). These studies suggest that ER stress during ischemia stimulates cell death, and inhibition of ER stress may ameliorate cardiomyopathy. However, evidence for a beneficial role of the UPR in this process also exists (Thuerauf et al., 2006), since in vitro and in vivo approaches using gain- and loss-of function approaches showed that the UPR protects cardiomyocytes from cell death. While a detailed understanding of the role of the UPR in ischemic heart disease remains to be established, an emerging concept is that initial low levels of UPR appear to be beneficial and favor adaptive responses, whereas persistent ER stress is detrimental for cell survival.
Reperfusion is the only means to terminate continuous lethal consequences of an ischemic insult. This process, however, comes at a price as reperfusion has been shown to trigger additional damage. Recently, the UPR has been implicated in ischemia reperfusion (IR) (Groenendyk et al., 2010). All available evidence suggests the UPR is strongly activated during IR (Minamino et al., 2010). In cultured cardiomyocytes exposed to conditions simulating IR, significant activation of BiP/GRP78, CHOP, ATF6 and Xbp1s is observed (Ngoh et al., 2009; Yeh et al., 2010). These in vitro observations have been corroborated in vivo in mouse and rat models (Miyazaki et al., 2011; Tao et al., 2011).
Mechanistically, increased ROS levels observed upon reestablishing of oxygen and nutrient supply, as well as rebooting mitochondrial energy production, are likely to stimulate the UPR. Indeed, suppression of ROS production effectively eliminates IR injury and UPR markers are dramatically decreased. Recent evidence suggested that ROS are an integral component in some types of the UPR and contribute there to activating proapoptotic pathways (Santos et al., 2009). UPR inducers can effectively increase ROS formation and knockdown of the proapoptotic branches of the UPR is associated with a drop in ROS levels.
As for the role of the UPR in IR, most studies to date propose models based on the correlation observed using genetic and pharmacological strategies to improve cardiac recovery following IR. While these data provide substantial support for the involvement of the UPR in IR, conclusive results are hard to obtain. The increase in O-GlcNAc modifications can inhibit IR-provoked cardiomyocyte death, which is associated with a decrease in the UPR signaling (Ngoh et al., 2009). Similar results have been obtained using AMPK activators and Apelin (Yeh et al., 2010; Tao et al., 2011). Moreover, recent studies found that IR injury is significantly decreased in mice lacking CHOP (Miyazaki et al., 2011). While these data point toward a detrimental role for UPR in IR injury, other findings lead to alternative conclusions. Natarajan et al. (2009) found that prolyl hydroxylase inhibition can attenuate IR injury, which may be mediated by UPR induction. Acute induction of the UPR by brief exposure to a UPR inducer effectively protects the heart from IR insult (Petrovski et al., 2011). Studies using an inducible ATF6 overexpression model discovered that UPR induction favors cardiomyocyte survival during IR, by reducing necrosis and apoptosis (Martindale et al., 2006). More studies are warranted to define the biological role of the UPR in IR of the heart.
In summary, despite all the genetic and pharmacological data that seek to establish a direct correlation between IR, ER stress and UPR, a definitive conclusion is still lacking, mainly due to recent results that point to the UPR as an adaptive response under these conditions and not as a cell death pathway per se. Moreover, given the importance of the metabolic changes observed during the development of IR injury, future studies should not only focus on clarifying the metabolic regulation exerted by the ER, but also recognize the importance of this organelle in the control of the cellular homeostasis and how this affects cellular survival.
6.2.2. Cardiac Hypertrophy and Heart Failure
It has been widely reported that both ER stress and UPR are activated in models where pressure or volume overload or mechanical stress are used to induce hypertrophy and heart failure (Okada et al., 2004; Groenendyk et al., 2010; Minamino et al., 2010; Sari et al., 2011). Initially, cardiac hypertrophy is characterized as an adaptive response of the heart to overload. In general terms, if the stress persists, contractile dysfunction ensues and the process is termed pathological hypertrophy. However, if contractile function is not affected, the alterations observed are considered as physiological hypertrophy. At the cellular level, hypertrophy is characterized by an increase in the number and organization of sarcomeres, increased protein synthesis and reexpression of genes typically observed in embryonic stages. In addition, all these changes are accompanied by alterations in both calcium homeostasis and metabolism, which, together with increased protein synthesis, can induce ER stress and consequently trigger the UPR (Berridge, 2006; Abel and Doenst, 2011; Dickhout et al., 2011; Ni et al., 2011).
Some studies report that during hypertrophy induced by pressure overload, there is an increase in BiP/GRP78 synthesis and in the activation of the XBP1, IRE1α/TRAF2 pathways (Dickhout et al., 2011; Sari et al., 2011). Alternatively, activation of the fetal gene expression program characteristic of hypertrophy can be altered by ATF6 and XBP-1 by modulating the activation of key transcription factors characteristic of this disease, such as MEF2C, NFAT and GATA (Groenendyk et al., 2010).
Moreover, considering that ATP is required not only for chaperone function but also IRE1 activation, changes in cellular energy metabolism may influence the development and maintenance of the UPR (Burkart et al., 2011). During the onset of cardiac hypertrophy, it is not clear whether changes in fatty acid metabolism and ATP levels occur in cardiomyocytes. However, while studies of physiological hypertrophy suggest an increase in fatty acid metabolism (Burelle et al., 2004; Strøm et al., 2005), this contrasts with the decrease observed during pathological hypertrophy (van Bilsen et al., 2009; Doenst et al., 2010; Abel and Doenst, 2011). However, these changes are still controversial since other studies suggest that during pathological hypertrophy, there are no metabolic changes in cardiac cells (Degens et al., 2006).
Thus, while at the onset cellular metabolism may remain unchanged and UPR may represent part of a cellular adaptation process, persistent stress along with other unknown factors, such as changes in energy levels, could induce a switch from protective ER stress signaling to cell death.
Thus, along with the activation of ER stress, both apoptosis and loss of cardiac function can be observed in the progression from hypertrophy to heart failure. Indeed, ER stress is involved in cardiotoxicity and progression of heart disease to heart failure (Groenendyk et al., 2010; Minamino et al., 2010). Furthermore, cardiac dysfunction is generally associated with a decrease in the cellular energy (Groenendyk et al., 2010; Schulz et al., 2010; Dickhout et al., 2011; Ni et al., 2011).
While various studies report on activation of the UPR in both hypertrophied and failing hearts, activation of proapoptotic CHOP-induced ER stress has only been reported for heart failure or during transition from hypertrophy to failure. Also, for CHOP knockout mice, decreased degrees of hypertrophy and cardiac dysfunction are observed after transverse aortic constriction in comparison to wild-type animals (Okada et al., 2004; Minamino et al., 2010). On the other hand, during pressure overload-induced ER stress, SREBP1c is activated, which in turn induces the synthesis of triglycerides, while the activation of XBP-1 stimulates the synthesis of phosphatidylcholine. These observations, in conjunction with reduced fatty acid oxidation that induces triglyceride accumulation in cardiomyocytes, may contribute to lipotoxicity that has been described in the heart failure (Okada et al., 2004; Groenendyk et al., 2010).
Clearly, further studies are required to clarify the relationship between induction of ER stress in the heart, development of cardiac hypertrophy and progression to heart failure. Similarly, the relationship between UPR induction and cardiac metabolism is an issue that requires more attention to understand how contractile dysfunction and cell death ensue.
6.3. UPR and Neurodegeneration
In neurons and in several models of neurodegenerative diseases, calcium plays a critical role as a second messenger, controlling synaptic function and cell viability. In basal cellular conditions, ER calcium concentrations are close to 300 μM, whereas cytosolic calcium concentrations usually range between 5 and 50 nM (Pozzan et al., 1994). Several key ER chaperones require optimal calcium concentrations for their protein folding activity, pointing to ER-calcium depletion as an important target in the folding and maturation of proteins and as a key element in the development of some neurodegenerative diseases where the folding capacity of the neurons is challenged (Corbett et al., 1999; Ashby and Tepikin, 2001). Additionally, a sustained increase in the cytosolic calcium may also induce mitochondria-mediated apoptosis via the activation of proteins like calcineurin or by an overload of mitochondrial calcium (Wang et al., 1999). Moreover, recent findings correlate ER stress with the control of mitochondrial function and metabolism through mechanisms involving calcium transfer from the ER to mitochondria (Cárdenas et al., 2010; Troncoso et al., 2011; Bravo et al., 2011a, 2011b). For all these reasons, regulation of the calcium concentration in the cytosol and ER are critical for maintaining normal neuronal function and homeostasis.
One critical example is AD, one of the most common forms of dementia in developed countries, where defects in calcium signaling due to altered ER (specifically MAM) function could explain the well-known disturbances in calcium homeostasis and mitochondrial deficits observed in this disease (Smith et al., 2005; Small, 2009; Yu et al., 2009), including the enhanced IP3-mediated release of calcium in fibroblasts from patients with the sporadic (SAD) (114) or the familial (FAD) form of the Alzheimer disease (Ito et al., 1994; Leissring et al., 1999a, 1999b; Nelson et al., 2007). The majority of FAD cases are caused by point mutations in the genes of two homologous proteins, presenilin 1 (PS1) and presenilin 2 (PS2), essential components of the γ-secretase complex responsible for the production of the amyloid β peptides (Aβ) (Goedert and Spillantini, 2006) that mainly localize to at the MAM surface (Schon and Area-Gomez, 2010). Accumulating evidence suggests that FAD is linked to an imbalance of cellular calcium homeostasis (Bojarski et al., 2008; Mattson, 2010) and, in particular, the presenilins appear to play a key role in the control of calcium concentration within the ER. In this way, it has been reported that several FAD-linked presenilin mutants altered the expression or sensitivity of the RyR and the IP3R in different models, including cell lines, neurons and brain microsomes (Mattson, 2010). In parallel, it has also been proposed that the SERCA could be a target for presenilins, although opposite regulatory effects have been reported in this respect (Green et al., 2008; Brunello et al., 2009). Finally, Tu et al. reported that WT presenilins form low-conductance calcium leak channels in the ER membrane (Tu et al., 2006; Nelson et al., 2007). These results favor the “calcium overload” hypothesis for FAD, which proposes that the reduced ER calcium leak caused by FAD-linked presenilin mutants results in increased ER-calcium concentrations and exaggerated calcium release upon cell stimulation (LaFerla, 2002; Thinakaran and Sisodia, 2006). However, among all these observations, the most important evidence for a role of ER—and more specifically for MAM—in calcium dysregulation in AD is the finding that PS1 and PS2 are able to directly interact with the IP3R, and that FAD-linked mutants not only dramatically enhance the gating of the channel but also stimulate the production of Aβ (Cheung et al., 2008, 2010). More recently and reinforcing the same concept, Zampese et al. (2011), by directly measuring mitochondrial calcium dynamics, showed that PS2, favors calcium transfer between ER and mitochondria, an effect that was reduced by PS2 downregulation and enhanced by the expression of PS2 mutants. However, how this transfer may modulate mitochondrial function and neuronal metabolism is still a matter of debate.
Since mitochondria need to be strategically positioned at sites where metabolic demand is high (Zenisek and Matthews, 2000; Li et al., 2004), a problem with calcium handling not only would affect the control of the mitochondrial functioning (through an imbalance in the control of the Krebs cycle enzymes) but also would provoke alterations in the calcium-mediated mitochondrial movement along axons, triggering highly deleterious effects on neuronal function in general, and on synaptic transmission in particular (as reported by Stokin et al., 2005; Q. Wang et al., 2008, 2009a; 2009b). According to these data, it has been proposed that a model of altered MAM function could provide a unifying hypothesis to explain many of the features of the AD, including the elevated ratios of Aβ42/Aβ40 (via changes in the content of cholesterol), hyperphosphorylation of tau (via changes in the phospholipid metabolism) and aberrant mitochondrial dynamics, with retention of large numbers of fragmented mitochondria in the perinuclear region of cells (via abnormal calcium transport between ER and mitochondria) (Schon and Area-Gomez, 2010). Notably all these metabolic and morphological features have been found in both FAD and SAD (Wang et al., 2009b), pointing to the MAM hypothesis as a unifying explanation able to relate previously unconnected phenotypes.
Another interesting example is the pathogenesis of prion disease (PrD) where several groups have reported the occurrence of ER stress responses, including the activation of UPR transcription factor XBP1 splicing (Hetz et al., 2008) as well as JNK and ERK (Steele et al., 2007; Hetz et al., 2008). In Creutzfeldt–Jakob disease (CJD) patients and in some mouse models, upregulation of several chaperones and foldases such as BiP/GRP78, GRP94 and GRP58/ERp57 is observed, which suggests abnormal ER homeostasis (Yoo et al., 2002; Hetz et al., 2003, 2005; Brown et al., 2005; Rane et al., 2008). These kinds of perturbations lead to the generation of intermediary mis-folded forms of the cellular prion protein (PrPc) increasing its susceptibility to convert into the PrP protease resistant form (PrPRES) in vitro (Apodaca et al., 2006; Orsi et al., 2006). Moreover, recently, Hetz’s group described the contribution of calcium as another element in the development of infectious and familial PrD (Torres et al., 2010). Using purified PrPRES from the brain of scrapie-infected mice, they evaluated its impact on ER stress responses and calcium homeostasis. They found that the acute exposure of PrPRES to Neuro2a cells induces release of calcium from the ER and ER stress, associated with the upregulation of several chaperones and foldases including ERp57, BiP/GRP78 and GRP94. Consistent with these results, cells chronically infected with scrapie prions also display altered ER calcium levels. Additionally, scrapie-infected cells are more susceptible to undergo ER stress-mediated cell death, associated with stronger UPR activation after exposure to different ER stress-inducing agents (Torres et al., 2010). The exact mechanisms involved in calcium disturbance and how this directly affects neuronal metabolism remain to be determined.
As a last example, in the fatal neurodegenerative disease amyotrophic lateral sclerosis (ALS), motor neurons degenerate with signs of organelle fragmentation, free radical damage, mitochondrial calcium overload, impaired axonal transport and accumulation of proteins in intracellular inclusion bodies (Grosskreutz et al., 2010). In ALS, chronic excitotoxicity mediated by calcium-permeable AMPA-type glutamate receptors seems to initiate a self-perpetuating process of intracellular calcium dysregulation with consecutive ER calcium depletion and mitochondrial calcium overload. In this context, Berridge et al. had postulated a model based on the chronic shift of calcium from the ER compartment to mitochondria, inducing a long-term accumulation of misfolded proteins in the ER due to a chronically low intraluminal calcium concentration (Berridge, 2002). Calcium seems to be mobilized between the ER and mitochondria in a process called the “ER–mitochondria calcium cycle” (ERMCC), which is driven by the synaptic excitatory input and electrical activity in neurons rather than occurring spontaneously as in nonexcitable cells. Given the hypothetical nature of this model, it can be speculated that reducing ER calcium release may restore balance to the ERMCC, concomitant with a reduction in ER protein throughput and increasing protein-folding fidelity. A different strategy could be to block the mitochondrial uniporter and thereby prevent mitochondrial calcium overload but the effect of this on overall cellular metabolism needs to be revisited.
As we can see from the previous examples, prolonged ER stress and mitochondrial dysfunction is a key feature of a broad range of neurodegenerative diseases, suggesting that disruption of the ER–mitochondrial axis may be responsible for the metabolic alterations detected and associated with these diseases. Future studies should focus in the establishment of a clear relationship between the metabolic regulation exerted by the ER on mitochondria and how this connection might be related to other features of these diseases.
6.4. UPR and Cancer
Elevated proliferation rates of cancer cells observed during tumor development require increased membrane and protein synthesis. These changes pose major challenges to the molecular machinery within and associated with the ER. In this context, steps of adaptation involving an increase in the ability to sustain adequate protein folding are essential. Cell proliferation associated with tumor growth often takes place in hypoxia and conditions of limited substrate availability (“starvation”). As one possibility, tumor cells adapt to this “stressful” environment by activating the UPR and, in this scenario, an adaptive UPR response favors tumor growth. Alternatively, however, a sizable body of literature is also available indicating that enhancing the UPR may represent a mechanism to preclude tumor progression. Both these facets must be taken into account in developing a better understanding of how the UPR participates in oncogenesis and particularly how such insights might be exploited in novel cancer therapies. In this section, we will focus the discussion on such aspects.
6.4.1. UPR in Tumor Cell Survival
The ability to upregulate the UPR appears to be essential for tumor survival (Bi et al., 2005). Indeed, the UPR has been implicated in inflammation-induced oncogenesis through heat-shock proteins (Goldstein and Li, 2009). Both the ATF6 and IRE1 branches induce BiP/ GRP78 expression in conditions of ER stress and this chaperone promotes ER homeostasis and resistance to apoptosis by interacting with BIK and caspase-7 (Rao et al., 2002; Reddy et al., 2003; Fu et al., 2007). Conditional ablation of BiP/GRP78 in prostate abolishes tumorigenesis in a PTEN-null mouse, where the chaperone is critical for neovascularization during tumor growth and metastasis. Also, in a transgene-induced breast cancer model, BiP/GRP78 was shown to favor tumor proliferation and angiogenesis (Fu et al., 2008; Dong et al., 2011). The participation of BiP/GRP78 is also suggested from other studies. In particular, a correlation between BiP/GRP78 expression and cancer progression is observed in a number of other types of cancers, including gastric carcinomas, melanoma, hepatocellular carcinoma and breast cancer (Shuda et al., 2003; Lee et al., 2006; Daneshmand et al., 2007; Dong et al., 2008; Zheng et al., 2008; Zhuang et al., 2009). Such elevated levels of BiP/GRP78 in tumor cells suggest that basal UPR levels are increased and may contribute to decreased apoptosis. Taken together, currently available data indicate that enhancing the UPR and BiP/GRP78 expression favors adaptation to the hypoxic environment and tumor growth under these restrictive conditions. Moreover, essentially as a corollary, recent preclinical studies have reported on the use of BiP/GRP78 inhibitors for cancer treatment (Backer et al., 2009).
6.4.1.1. IRE1-XBP1 Branch
Several lines of evidence suggest that the IRE1-XBP1 branch of the UPR is essential for tumor cell survival under hypoxic conditions and maintenance of malignancy (Romero-Ramirez et al., 2004). For instance, in XBP1-deficient cells, increased apoptosis and decreased clonogenic survival are observed under hypoxic conditions. Upon subcutaneous injection of immunodeficient mice with XBP1 knockout cells, decreased tumor growth and higher sensitivity to hypoxia was observed in comparison to wild-type controls (Romero-Ramirez et al., 2004). Furthermore, downregulation of XBP1 using specific shRNA diminished blood vessel formation in tumors from mouse embryonic fibroblast and fibrosarcoma cells and expression of the spliced form of XBP1 restored angiogenesis in cells expressing dominant negative IRE1α (Romero-Ramirez et al., 2009). The latter observations provide evidence linking successful angiogenesis to the IRE1-XBP1 branch in tumor cells. Moreover, in transgenic mice, expressing an XBP1-luciferase construct as a reporter for ER stress and downstream responses relevant to the tumor microenvironment, a positive correlation between XBP1-luciferase activity and tumor growth rates was observed for several primary mammary tumor cells (Spiotto et al., 2010). This branch is also implicated in transcriptional regulation of VEGF, an angiogenic factor highly expressed in tumors (Plate et al., 1992) that protects them against ischemic injury and plays a prominent role in sustaining tumor growth (Shweiki et al., 1992). VEGF mRNA increases under conditions of ER stress, but when IRE1 is silenced by shRNA, VEGF induction is decreased, resulting in the loss of blood vessels. These authors also demonstrated that the two other branches (PERK, ATF6) independently regulate VEGF expression and that inactivation of any one of these three pathways impairs VEGF upregulation as a consequence of ER stress, suggesting that coordinated activation of all three pathways is essential for increased VEGF expression (Ghosh et al., 2010).
6.4.1.2. PERK Branch
Activation of the PERK branch is considered an essential part of the responses triggered in tumor cells required for survival in hypoxia. In tumors, PERK is activated, phosphorylates eIF2α and thereby favors activation of ATF4 and NRF2, transcription factors which promote cell survival by increasing autophagy and activating antioxidant mechanisms. Initial evidence from a study in MEF cells expressing an eIF2α mutant (S51A) that cannot be phosphorylated by PERK indicated that survival of such cells under prolonged hypoxia was reduced (Bi et al., 2005). PERK ablation decreased tumor growth rates in nude mice and in PERK knockout mice, reduced insulinoma formation and vascularization were observed (Bi et al., 2005; Gupta et al., 2009). The ATF4 pathway is also implicated in tumor adaptation in the response to hypoxia (Ye et al., 2010). One of these tumor adaptation responses is related to enhanced expression of VEGF, whereby synthesis and processing in the ER for secretion (Ferrara and Davis-Smyth, 1997) are increased in hypoxia by mechanisms involving the ATF4 pathway (Roybal et al., 2004). On the other hand, secretion of de novo synthesized VEGF is controlled by a heat-shock protein 70 family molecular chaperone localized in the ER (ORP150), which is upregulated under hypoxia (Ozawa et al., 2001). In addition, PERK participates in the survival of dormant tumor cells found in small, chemotherapy resistant and asymptomatic tumors, via mechanisms involving augmented expression of BiP/ GRP78, reduced protein synthesis and exit from the cell cycle in the G1 phase (Ranganathan et al., 2006). Alternatively, PERK facilitates survival of extracellular matrix (ECM)-detached cells by promoting autophagy, ATP production and an antioxidant response in human mammary epithelial cells. This mechanism may potentially be exploited by ECM-detached tumor cells in breast ductal carcinoma in situ lesions (Avivar-Valderas et al., 2011). However, paradoxically, PERK ablation was recently also shown to promote mammary tumorigenesis in aged mice through increased ROS and DNA damage. In doing so, PERK ablation favors DNA mutation and neoplastic growth (Bobrovnikova-Marjon et al., 2010).
6.4.1.3. ATF6 Branch
The role of the ATF6 branch in cancer has been poorly explored to date, although indirect evidence exists indicating that ATF6α is an important modulator of chronic ER stress. For instance, a complex between XBP1 and ATF6α that upregulates ERAD components is detected in MEF cells (Yamamoto et al., 2007). This process must be carefully regulated as cells degrade proteins whose synthesis consumes high levels of ATP. Moreover, the ERAD pathway per se also requires large amounts of ATP. A second piece of evidence implicating ATF6α as a key regulator in persistent ER stress was obtained using cells from ATF6α knockout mice. There, reduced survival following long-term ER stress correlated with lower levels of chaperones, such as BiP and GRP94 (Wu et al., 2007). Indirect evidence implicating ATF6 is provided by studies identifying p50ATF6 within the nucleus in moderately or poorly differentiated hepatocellular carcinomas (Shuda et al., 2003). On the other hand, ATF6 activation is crucial to long-term survival of tumors by augmenting resistance to chemotherapy, nutrient starvation and stress in quiescent squamous carcinoma cells, where ATF6 increases Rheb expression, which in turn activates mTOR activity in an Akt-independent manner (Schewe and Aguirre-Ghiso, 2008). Finally, activation of the UPR, through PERK, IRE1α and ATF6, promotes VEGF expression and subsequent initiation of the angiogenic program (Ghosh et al., 2010).
6.4.2. UPR as an Adaptive State in Cancer
Generally, normal, nonsecretory cells are not subject to permanent ER stress and the UPR pathways exist in a quiescent state. In contrast, tumor cells must adapt to hypoxia and a stressful environment, and do so in ways, which require developing an adaptive UPR state. Although the precise molecular signals that induce this state in tumor cells remain poorly defined, high ROS levels and protein synthesis rates, which are prevalent in cancer cells, are likely to be involved. In normal mammalian cells, considerable evidence is available indicating that excessive ER stress induces the apoptotic program through CHOP and JNK activation (Zinszner et al., 1998; Hatai et al., 2000). Additionally, mitochondrial calcium overload promotes apoptosis mediated by ERO1α and BIM expression (Tabas and Ron, 2011). However, unlike normal cells, tumor cells often become resistant to apoptosis either by increasing the expression of antiapoptotic proteins or by reducing the function of proapoptotic factors. In an analogous manner, tumor cells also modulate their UPR so as to favor proliferation, antioxidant responses, metabolic plasticity and survival in conditions of hypoxia and nutrient starvation. Taken together, these data suggest that during tumor development, cancer cells undergo an adaptive response that facilitates their survival by downregulating components of the UPR response that are utilized to provoke death in normal cells.
6.4.3. ER–Mitochondria Connection
In addition to these ER-localized stimuli, mitochondrial stress induced by calcium, nitric oxide or oligomycin is also known to activate PERK (Lu et al., 2009; Silva et al., 2009; Bollo et al., 2010). Additionally, nitric oxide induces mitochondrial calcium release and activation of calcium-dependent serine protease, which cleaves p90ATF6 to yield p50ATF6. The later translocates to the nucleus and promotes transcription of cytoprotective GRP78 (Xu et al., 2004). Thus, both mitochondrial and ER stress can induce UPR activation. Moreover, it is important to bear in mind that calcium shuttling between ER and mitochondria plays an important role in regulating metabolism, autophagy and apoptosis. Available evidence indicates that as an early response to ER stress, ER–mitochondria coupling, mitochondrial OXPHOS activation and enhanced ATP synthesis are observed (Bravo et al., 2011a, 2011b). Several studies now show that MAMs are involved in the transfer of calcium from ER to mitochondria and proteins specifically localized in this compartment regulate this function. Moreover, loss-of-function mutations of some proteins localized in MAMs promote cell proliferation and desensitize cells to apoptotic stimuli (Chen et al., 2004; Simmen et al., 2005; Hayashi and Su, 2007). Based on such observations, it has been proposed that in tumor cells, the interaction between ER and mitochondria may be affected in a manner that alters the UPR response.
PACS-2 is a protein involved in protein sorting that localizes to MAMs (Simmen et al., 2005). Reduction or depletion of PACS-2 expression has been reported in cancer tissues (Aslan et al., 2009). PACS-2 knockdown induces BiP/GRP78 expression and reduces the capacity to undergo apoptosis in response to staurosporine, thapsigargin or anti-FAS antibodies (Simmen et al., 2005). Sig-1R is a chaperone protein that is highly expressed in several tumor cell lines (Aydar et al., 2006). Pharmacological agonists of Sig-1R function promote resistance to apoptosis (Spruce et al., 2004). This protein is found in MAMs, where it interacts with BiP/GRP78 (Hayashi and Su, 2007). Upon ER stress, Sig-1R dissociates from BiP/GRP78 and promotes mitochondrial calcium uptake via the inositol triphosphate receptor. Overexpression of Sig-1R counteracts ER stress-induced apoptosis (Hayashi and Su, 2007). This protein may also play an important role in cancer progression. In physiological conditions, Sig-1R induces mitochondrial calcium uptake, while its overexpression results in resistance to apoptotic stimuli (Hayashi and Su, 2007). Mitofusin 2 (Mfn2) is involved in mitochondrial dynamics and fusion of the outer mitochondrial membrane. Mfn2 is localized in MAMs, where it participates in the transfer of calcium between ER and the mitochondria. Mfn2 loss-of-function increases ER and mitochondria calcium levels but decreases mitochondrial calcium uptake (de Brito and Scorrano, 2008). On the other hand, decreased levels of Mfn2 enhance proliferation while its overexpression induces cell cycle arrest in rat vascular smooth muscle cells (Chen et al., 2004).
Available data suggest that ER–mitochondria coupling is essential to regulate proliferation or apoptosis. In addition, proteins involved in ER morphology may also impact ER–mitochondria coupling. A recent study revealed an interesting link between ER stress and mitochondrial adaptation in an animal model of pulmonary arterial hypertension (PAH), a pathology characterized by proliferation and resistance of smooth muscle cells to death signals (Sutendra et al., 2011). These authors further demonstrated that NOGO-B, a reticulon-4 isoform that participates in the formation and maintenance of ER shape, regulates mitochondrial activity in hypoxia. NOGO-B protein levels are increased in smooth muscle cells isolated from PAH patients subjected to hypoxia. NOGO-B upregulation under those conditions is dependent upon ATF6 activity. In addition, NOGO-B upregulation leads to the disruption of ER–mitochondria coupling. This loss causes a decrease in lipid and calcium transfer from the ER to the mitochondria, which impairs mitochondrial OXPHOS, and decreases Krebs cycle intermediates. Under these conditions, HIF-1α is activated and, subsequently, the glycolytic and antiapoptotic programs. This report suggests that ER–mitochondria uncoupling promotes an adaptive metabolic response, similar to the Warburg effect in proliferating cells. Based on this, one may hypothesize that alterations in the ER–mitochondria coupling are important for adaptive ER responses to stress in cells exposed to hypoxia or starvation, such as those found in tumor cells.
6.4.4. Therapeutic Strategies Based on Suppressing the UPR
Most of the time, ER stress responses are not observed in normal cells, but in tumor cells, the opposite seems to be the case. This difference between normal and tumor cells can be exploited to develop drugs that target the UPR as anticancer agents. In this respect, two approaches have been described: namely either stimulation of accumulation of misfolded proteins in the ER to overcome UPR, or inhibition of adaptive and antiapoptotic pathways (Li et al., 2011). An example within the first group is bortezomib, a proteasome inhibitor that has efficacy in multiple myeloma (Obeng et al., 2006), as well as cytotoxic effects in breast, colorectal, ovarian, pancreatic, lung and oral cancer cells (Sterz et al., 2008). Other chemical agents that cause accumulation of misfolded proteins are the ERAD inhibitors. For instance, Eeyarestatin I, an inhibitor of the ER-associated p97 ATPase required for ERAD (X. Wang et al., 2008), has been shown to be cytotoxic for hepatocellular carcinoma (HepG2) cells (Cross et al., 2009). Given the importance of chaperones in the UPR, the use of agents that target ER chaperones as anticancer drugs, emerges as an interesting possibility. Indeed, the HSP90 inhibitor 17AAG (17-allyamino-17-demethoxygeldanamycin) and radicicol activate all three branches of UPR in human myeloma cells, inhibiting proliferation and increasing the expression of BiP and GRP94 chaperones, thereby promoting caspase-dependent cell death (Davenport et al., 2007). For versipelostatin, a BiP/GRP78 inhibitor, cytotoxicity toward glucose-deprived tumor cells was observed, as well as inhibition of UPR in cell lines derived from solid tumors, including human colon cancer HT29, fibrosarcoma HT1080, and stomach cancer MKN47 cells (Park et al., 2004). Also, inhibiting the adaptive IRE1α/XBP1 pathway has been shown to result in antitumor activity. For example, irestatin an inhibitor of XBP1s transcriptional activity reduces growth and survival of hypoxia-exposed myeloma cells in vitro and in subcutaneous xenografts (Li et al., 2011).
6.4.5. UPR in Tumor Cell Death
Thus far, we have reviewed aspects of the UPR that favor the survival of tumor cells. However, persistent or excessive ER stress is known to trigger cell death, mainly apoptosis, by both mitochondria-dependent and -independent pathways (Rao et al., 2002; Breckenridge et al., 2003).
6.4.5.1. IRE1-XBP1 Branch
The IRE1-XBP1 branch has been suggested to connect to apoptosis by recruiting TRAF2, activating procaspase-4, and subsequently caspase-9 and caspase-3, thereby triggering mitochondria-independent apoptosis (Wu and Kaufman, 2006). Paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride), a widely used herbicide, induces UPR, activating the IRE1/ASK1/JNK axis, leading to apoptosis in neuroblastoma SH-SY5Y cells (Yang et al., 2009).
6.4.5.2. PERK Branch
The PERK branch can be activated during loss of adhesion and regulate cellular fate. Inhibition of PERK signaling by the expression of two dominant negative PERK mutants results in hyperproliferative MCF10A mammary epithelial cells. In the same study, introduction of mutated PERK converted MCF10A cells into tumorigenic cells after implantation in denuded mouse mammary fat pads (Sequeira et al., 2007). Moreover, the PERK branch also regulates angiogenesis in response to hypoxia. Using microarray analysis in K-ras transformed PERK−/− cells, Blais et al. (2006) demonstrated that under hypoxic conditions, several proangiogenesis transcripts are less efficiently translated, resulting in increased apoptosis, nonfunctional, irregularly structures blood vessels and reduced microvessel density. The induction of UPR can sensitize tumor cells to cisplatin-induced death. Cisplatin is widely used to treat solid tumors and it is believed to kill cells by binding to DNA and interfering with repair, thereby activating cell cycle arrest and apoptosis (Pascoe and Roberts, 1974). However, cisplatin also efficiently induces UPR and activates caspase-12 in a nucleus-independent manner in human melanoma and colon cancer (HCY116) cytoplasts. In summary, the proapoptotic effects of cisplatin not related to its ability to bind to DNA but may also be linked to effects on protein folding (Mandic et al., 2003). Additionally, a correlation between UPR and sensitization to DNA-crosslinking agents, such as carboplatin (Yamada et al., 1999), melphalan and BCNU (Chatterjee et al., 1997), has been observed. The mechanisms underlying these observations are not clear but it is tempting to speculate that the protein complexes required for DNA repair might be adversely affected by changes in protein synthesis and degradation when UPR is triggered.
6.4.5.3. ATF6 Branch
During metastasis, tumor cells must detach from the ECM, which frequently leads to anoikis. CHOP, a downstream effector in both the ATF6 and PERK branches, is widely attributed proapoptotic functions (Zinszner et al., 1998). For instance, CHOP is suggested to upregulate proapoptotic BCL2 family members, like BAK/BAD, while downregulating antiapoptotic BCL2 and increasing reactive oxygen species (ROS), which together favor cytochrome c release from mitochondria and trigger apoptosis (Ma and Hendershot, 2004). ER stress conditions also promote calcium release from the ER to the cytosol, thereby favoring activation of calpains, which then cleave and activate caspase-12 (Nakagawa and Yuan, 2000). Then, caspase-12 in turn cleaves and activates cytosolic caspase-9, triggering apoptosis in a manner independent of cytochrome c release from mitochondria.
In summary, UPR is crucial to malignant cell survival in the cytotoxic environment within a tumor. These conditions activate UPR as an adaptive response that allows tumor cells to persist. For this reason, the possibility of developing chemical agents that target some key UPR components is an interesting therapeutic option. On the another hand, if ER stress conditions prevail and cellular homeostasis cannot be reinstated, UPR can trigger apoptosis. Indeed, as we have detailed here, UPR can sensitize tumor cells to certain anticancer agents. However, given that in tumor cells protein expression and signaling are frequently altered in ways that allow these cells to avoid apoptosis, we suspect that the balance is tilted toward UPR as a prosurvival response. In any case, an integrated vision of the UPR in tumor cells is essential to understand how this response contributes to the cancer growth.
7. CONCLUSION AND PERSPECTIVES
The ER is a fascinating organelle that plays a central role within the cell. This extremely complex and heterogeneous cellular compartment spreads throughout the cytoplasm forming various subdomains with diverse morphology and function. As such, the ER is a highly dynamic structure that modulates organization according to cellular needs and participates in protein synthesis, lipid metabolism, calcium handling, metabolic regulation and signaling. All of the latter processes are essential to the cell, making the ER susceptible to a variety of perturbations. The degree of complexity associated with optimal ER function positions this organelle as the largest stress sensor of the cell and, therefore, a major contributing factor to disease.
Under stress conditions, the ER induces the UPR, a well-known signaling cascade that acts as an important regulator of cellular homeostasis. The UPR generates an integrated genetic response that serves to re-establish homeostasis in the always-varying intracellular or extracellular milieu. Failure to regain homeostasis causes the UPR to activate cell death pathways. This dual role of the UPR has become an important target of study since failure in its activation has been implicated in the onset of multiple pathologies.
One emerging area of study is the ER’s ability to interact with other cellular organelles. Contacts between cellular structures and compartments are important in cellular regulation, especially of metabolism and apoptosis. The dynamic nature of various organelles makes their interface susceptible to be remodeled depending on cellular homeostasis; however, this is a topic that still requires more attention.
The properties of the ER determine the physiology of different organs, allowing them to fulfill their normal functions. Sensing of nutrient levels, continuous calcium signaling, protein aggregation and adaptation to ever-changing environments are processes in which the ER plays a vital role. Despite the advances accomplished in the treatment of diabetes, cardiovascular pathologies, neurodegeneration and cancer, many strategies based on ER function are not yet fully understood and remain to be developed. Additional studies should address the enormous therapeutic opportunities the ER holds.
Acknowledgments
We thank the support of Comision Nacional de Ciencia y Tecnologia (CONICYT), Chile: FONDAP 15010006 (S.L., A.Q. and E.J.), Anillo de Investigación de Ciencia y Tecnología ACT1111 (S.L., A.Q. and E.J.), FONDECYT 1120212 (S.L.), FONDECYT 1090071 (A.F.G.Q.). R.B., V.P., D.G., A.E.R. and F.P. hold fellowships from CONICYT, Chile.
References
- Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res. 2011;90:234–242. doi: 10.1093/cvr/cvr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adham IM, Eck TJ, Mierau K, Müller N, Sallam MA, Paprotta I, Schubert S, Hoyer-Fender S, Engel W. Reduction of spermatogenesis but not fertility in Creb3l4-deficient mice. Mol Cell Biol. 2005;25:7657–7664. doi: 10.1128/MCB.25.17.7657-7664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qusairi L, Laporte J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet Muscle. 2011;1:26. doi: 10.1186/2044-5040-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9:1160–1166. doi: 10.1038/ncb1636. [DOI] [PubMed] [Google Scholar]
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca J, Kim I, Rao H. Cellular tolerance of prion protein PrP in yeast involves proteolysis and the unfolded protein response. Biochem Biophys Res Commun. 2006;347:319–326. doi: 10.1016/j.bbrc.2006.06.078. [DOI] [PubMed] [Google Scholar]
- Asada R, Kanemoto S, Kondo S, Saito A, Imaizumi K. The signalling from endoplasmic reticulum-resident bZIP transcription factors involved in diverse cellular physiology. J Biochem. 2011;149:507–518. doi: 10.1093/jb/mvr041. [DOI] [PubMed] [Google Scholar]
- Ashby MC, Tepikin AV. ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol. 2001;12:11–17. doi: 10.1006/scdb.2000.0212. [DOI] [PubMed] [Google Scholar]
- Aslan JE, You H, Williamson DM, Endig J, Youker RT, Thomas L, Shu H, Du Y, Milewski RL, Brush MH, et al. Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis. Mol Cell. 2009;34:497–509. doi: 10.1016/j.molcel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audas TE, Li Y, Liang G, Lu R. A novel protein, Luman/CREB3 recruitment factor, inhibits Luman activation of the unfolded protein response. Mol Cell Biol. 2008;28:3952–3966. doi: 10.1128/MCB.01439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E, Onganer P, Perrett R, Djamgoz MB, Palmer CP. The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 2006;242:245–257. doi: 10.1016/j.canlet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Backer JM, Krivoshein AV, Hamby CV, Pizzonia J, Gilbert KS, Ray YS, Brand H, Paton AW, Paton JC, Backer MV. Chaperone-targeting cytotoxin and endoplasmic reticulum stress-inducing drug synergize to kill cancer cells. Neoplasia. 2009;11:1165–1173. doi: 10.1593/neo.09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. Atlasin GTPases shape up ER networks. Dev Cell. 2009;17:157–158. doi: 10.1016/j.devcel.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Benyamini P, Webster P, Meyer DI. Knockdown of p180 eliminates the terminal differentiation of a secretory cell line. Mol Biol Cell. 2009;20:732–744. doi: 10.1091/mbc.E08-07-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. Plos Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Remodelling Ca2+ signalling systems and cardiac hypertrophy. Biochem Soc Trans. 2006;34:228–231. doi: 10.1042/BST20060228. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, Cavener D, Diehl JA. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29:3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci USA. 2008;105:16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Song W, Duan X, Cheung P, Kresge K, Barrero C, Merali S. Infusion of glucose and lipids at physiological rates causes acute endoplasmic reticulum stress in rat liver. Obesity (Silver Spring) 2011;19:1366–1373. doi: 10.1038/oby.2011.71. [DOI] [PubMed] [Google Scholar]
- Bojarski L, Herms J, Kuznicki J. Calcium dysregulation in Alzheimer’s disease. Neurochem Int. 2008;52:621–633. doi: 10.1016/j.neuint.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Bollo M, Paredes RM, Holstein D, Zheleznova N, Camacho P, Lechleiter JD. Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS One. 2010;5:e11925. doi: 10.1371/journal.pone.0011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordallo J, Plemper RK, Finger A, Wolf DH. Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell. 1998;9:209–222. doi: 10.1091/mbc.9.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, Francolini M, Snapp E. Endoplasmic reticulum architecture: structures in flux. Curr Opin Cell Biol. 2006;18:358–364. doi: 10.1016/j.ceb.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boslem E, MacIntosh G, Preston AM, Bartley C, Busch AK, Fuller M, Laybutt DR, Meikle PJ, Biden TJ. A lipidomic screen of palmitate-treated MIN6 β-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. 2011;435:267–276. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AFG, Rothermel BA, et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol. 2011a;44:16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011b;124:2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci. 2006;119:3149–3160. doi: 10.1242/jcs.03060. [DOI] [PubMed] [Google Scholar]
- Brown AR, Rebus S, McKimmie CS, Robertson K, Williams A, Fazakerley JK. Gene expression profiling of the preclinical scrapie-infected hippocampus. Biochem Biophys Res Commun. 2005;334:86–95. doi: 10.1016/j.bbrc.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Brunello L, Zampese E, Florean C, Pozzan T, Pizzo P, Fasolato C. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. J Cell Mol Med. 2009;13:3358–3369. doi: 10.1111/j.1582-4934.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui M, Gilady SY, Fitzsimmons REB, Benson MD, Lynes EM, Gesson K, Alto NM, Strack S, Scott JD, Simmen T. Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem. 2010;285:31590–31602. doi: 10.1074/jbc.M110.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burelle Y, Wambolt RB, Grist M, Parsons HL, Chow JCF, Antler C, Bonen A, Keller A, Dunaway GA, Popov KM, et al. Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2004;287:H1055–H1063. doi: 10.1152/ajpheart.00925.2003. [DOI] [PubMed] [Google Scholar]
- Burkart A, Shi X, Chouinard M, Corvera S. Adenylate kinase 2 links mitochondrial energy metabolism to the induction of the unfolded protein response. J Biol Chem. 2011;286:4081–4089. doi: 10.1074/jbc.M110.134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. The fateful encounter of mitochondria with calcium: how did it happen? Biochim Biophys Acta. 2010;1797:595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Carvalho P, Goder V, Rapoport TA. Distinct ubiquitin-ligase complexes define convergent pathways for the degradation of ER proteins. Cell. 2006;126:361–373. doi: 10.1016/j.cell.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Carvalho P, Stanley AM, Rapoport TA. Retrotranslocation of a misfolded luminal ER protein by the ubiquitin-ligase Hrd1p. Cell. 2010;143:579–591. doi: 10.1016/j.cell.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CP, Kok KH, Jin DY. CREB3 subfamily transcription factors are not created equal: recent insights from global analyses and animal models. Cell Biosci. 2011;1:6. doi: 10.1186/2045-3701-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Hirota H, Belfi CA, Berger SJ, Berger NA. Hypersensitivity to DNA cross-linking agents associated with up-regulation of glucose-regulated stress protein GRP78. Cancer Res. 1997;57:5112–5116. [PubMed] [Google Scholar]
- Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- Cheung K-H, Mei L, Mak D-OD, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VMY, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 (Suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Cross BC, McKibbin C, Callan AC, Roboti P, Piacenti M, Rabu C, Wilson CM, Whitehead R. Eeyarestatin I inhibits Sec61-mediated protein translocation at the endoplasmic reticulum. J Cell Sci. 2009;122:4393–4400. doi: 10.1242/jcs.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe H, Michalak M. Calcium binding chaperones of the endoplasmic reticulum. Gen Physiol Biophys. 2009;28:F96–F103. (pec No Focus) [PubMed] [Google Scholar]
- Colgan SM, Tang D, Werstuck GH, Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biochem. 2007;39:1843–1851. doi: 10.1016/j.biocel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, et al. Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. 2008;118:2148–2156. doi: 10.1172/JCI33777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EF, Oikawa K, Francois P, Tessier DC, Kay C, Bergeron JJ, Thomas DY, Krause KH, Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J Biol Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
- Corsi AK, Schekman R. Mechanism of polypeptide translocation into the endoplasmic reticulum. J Biol Chem. 1996;271:30299–30302. doi: 10.1074/jbc.271.48.30299. [DOI] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Crawshaw SG, Cross BCS, Wilson CM, High S. The oligomeric state of Derlin-1 is modulated by endoplasmic reticulum stress. Mol Membr Biol. 2007;24:113–120. doi: 10.1080/09687860600988727. [DOI] [PubMed] [Google Scholar]
- Cui W, Li J, Ron D, Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr D Biol Crystallogr. 2011;67:423–428. doi: 10.1107/S0907444911006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf 2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha DA, Ladriere L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, Marchetti P, Eizirik DL, Cnop M. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58:2851–2862. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, Lee AS, Pinski J. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, Davies FE. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Degens H, de Brouwer KFJ, Gilde AJ, Lindhout M, Willemsen PHM, Janssen BJ, van der Vusse GJ, van Bilsen M. Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol. 2006;101:17–26. doi: 10.1007/s00395-005-0549-0. [DOI] [PubMed] [Google Scholar]
- Delépine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- Deluca HF, Engstrom GW. Calcium uptake by rat kidney mitochondria. Proc Natl Acad Sci USA. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DenBoer LM, Hardy-Smith PW, Hogan MR, Cockram GP, Audas TE, Lu R. Luman is capable of binding and activating transcription from the unfolded protein response element. Biochem Biophys Res Commun. 2005;331:113–119. doi: 10.1016/j.bbrc.2005.03.141. [DOI] [PubMed] [Google Scholar]
- Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- Doenst T, Pytel G, Schrepper A, Amorim P, Färber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res. 2010;86:461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y, Jhaveri N, Zhu G, Ye R, Liu Z, et al. A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res. 2011;71:2848–2857. doi: 10.1158/0008-5472.CAN-10-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso P, Sanchez G, Bull R, Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci. 2011;16:553–567. doi: 10.2741/3705. [DOI] [PubMed] [Google Scholar]
- Dreier L, Rapoport TA. In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol. 2000;148:883–898. doi: 10.1083/jcb.148.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleveld-Trancikova D, Sanecka A, van Hout-Kuijer MA, Looman MWG, Hendriks IAM, Jansen BJH, Adema GJ. DC-STAMP interacts with ER-resident transcription factor LUMAN which becomes activated during DC maturation. Mol Immunol. 2010;47:1963–1973. doi: 10.1016/j.molimm.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SMA, Schuit FC, Jonas JC. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia. 2007;50:1442–1452. doi: 10.1007/s00125-007-0674-4. [DOI] [PubMed] [Google Scholar]
- English AR, Zurek N, Voeltz GK. Peripheral ER structure and function. Curr Opin Cell Biol. 2009;21:596–602. doi: 10.1016/j.ceb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50 (Suppl):S311–S316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan H, Hauri HP. Membrane biogenesis: networking at the ER with atlastin. Curr Biol. 2009;19:R906–R908. doi: 10.1016/j.cub.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339 (Pt 1):135–141. [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuest M, Willim K, Macnelly S, Fellner N, Resch GP, Blum HE, Hasselblatt P. The transcription factor c-Jun protects against sustained hepatic endoplasmic reticulum stress thereby promoting hepatocyte survival. Hepatology. 2011;55:408–418. doi: 10.1002/hep.24699. [DOI] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- Gao B, Lee SM, Chen A, Zhang J, Zhang DD, Kannan K, Ortmann RA, Fang D. Synoviolin promotes IRE1 ubiquitination and degradation in synovial fibroblasts from mice with collagen-induced arthritis. EMBO Rep. 2008;9:480–485. doi: 10.1038/embor.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez C, Hajnóczky G, Csordás G. Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J Biol Chem. 2008;283:32771–32780. doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101:975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Goetz JG, Nabi IR. Interaction of the smooth endoplasmic reticulum and mitochondria. Biochem Soc Trans. 2006;34:370–373. doi: 10.1042/BST0340370. [DOI] [PubMed] [Google Scholar]
- Goldfinger M, Shmuel M, Benhamron S, Tirosh B. Protein synthesis in plasma cells is regulated by crosstalk between endoplasmic reticulum stress and mTOR signaling. Eur J Immunol. 2011;41:491–502. doi: 10.1002/eji.201040677. [DOI] [PubMed] [Google Scholar]
- Goldstein MG, Li Z. Heat-shock proteins in infection-mediated inflammation-induced tumorigenesis. J Hematol Oncol. 2009;30 (2):5. doi: 10.1186/1756-8722-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales JC, Gentile CL, Pfaffenbach KT, Wei Y, Wang D, Pagliassotti MJ. Chemical induction of the unfolded protein response in the liver increases glucose production and is activated during insulin-induced hypoglycaemia in rats. Diabetologia. 2008;51:1920–1929. doi: 10.1007/s00125-008-1094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez TN, Sidrauski C, Dörfler S, Walter P. Mechanism of non-spliceosomal mRNA splicing in the unfolded protein response pathway. EMBO J. 1999;18:3119–3132. doi: 10.1093/emboj/18.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J Cell Biol. 2008;181:1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Hoogenraad CC, et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Guan D, Wang H, Li VE, Xu Y, Yang M, Shen Z. N-glycosylation of ATF6beta is essential for its proteolytic cleavage and transcriptional repressor function to ATF6alpha. J Cell Biochem. 2009;108:825–831. doi: 10.1002/jcb.22310. [DOI] [PubMed] [Google Scholar]
- Guerrero-Hernandez A, Dagnino-Acosta A, Verkhratsky A. An intelligent sarco-endoplasmic reticulum Ca2+ store: release and leak channels have differential access to a concealed Ca2+ pool. Cell Calcium. 2010;48:143–149. doi: 10.1016/j.ceca.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Gupta S, McGrath B, Cavener DR. PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PLoS One. 2009;4:e8008. doi: 10.1371/journal.pone.0008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai TW, Liu F, Coukos WJ, Green MR. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yone-hara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol. 2010;77:517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Subcell Biochem. 2010;51:381–398. doi: 10.1007/978-90-481-8622-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnóczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Okada T, Yoshida H, Yanagi H, Yura T, Negishi M, Mori K. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev. 2007;87:1377–1408. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- Hebert DN, Garman SC, Molinari M. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol. 2005;15:364–370. doi: 10.1016/j.tcb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci USA. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Russelakis-Carneiro M, Wälchli S, Carboni S, Vial-Knecht E, Maundrell K, Castilla J, Soto C. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J Neurosci. 2005;25:2793–2802. doi: 10.1523/JNEUROSCI.4090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000539. doi: 10.1101/cshperspect.a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuson-Stiennon JA, Wanson JC, Drochmans P. Isolation and characterization of the sarcoplasmic reticulum of skeletal muscle. J Cell Biol. 1972;55:471–488. doi: 10.1083/jcb.55.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–561. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer-Hansen M, Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, et al. Control of macro-autophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Igoillo-Esteve M, Marselli L, Cunha DA, Ladrière L, Ortis F, Grieco FA, Dotta F, Weir GC, Marchetti P, Eizirik DL, et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010;53:1395–1405. doi: 10.1007/s00125-010-1707-y. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, Marshall H, Donis-Keller H, Crock P, et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome) Nat Genet. 1998;20:143–148. doi: 10.1038/2441. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Nagata K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy. 2009;5:1217–1219. doi: 10.4161/auto.5.8.10168. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Yamamoto A, Kitamura A, Lamandé SR, Yoshimori T, Bateman JF, Kubota H, Nagata K. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell. 2009;20:2744–2754. doi: 10.1091/mbc.E08-11-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki S, Fonseca SG, Oslowski CM, Jurczyk A, Shearstone JR, Zhu LJ, Permutt MA, Greiner DL, Bortell R, Urano F. AATF mediates an antiapoptotic effect of the unfolded protein response through transcriptional regulation of AKT1. Cell Death Differ. 2010;17:774–786. doi: 10.1038/cdd.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Nishibori Y, Ito Y, Takagi H, Akimoto Y, Kudo A, Asanuma K, Sai Y, Miyamoto KI, Takenaka H, et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab Invest. 2011;91:1584–1595. doi: 10.1038/labinvest.2011.135. [DOI] [PubMed] [Google Scholar]
- Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, Tsuru A, Kohno K. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3:158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- Jeffrey KD, Alejandro EU, Luciani DS, Kalynyak TB, Hu X, Li H, Lin Y, Townsend RR, Polonsky KS, Johnson JD. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci USA. 2008;105:8452–8457. doi: 10.1073/pnas.0711232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y, Debose-Boyd RA. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit Rev Biochem Mol Biol. 2010;45:185–198. doi: 10.3109/10409238.2010.485605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Takahashi T, Niinuma Y, Nomura Y. Manganese superoxide dismutase is induced by endoplasmic reticulum stress through IRE1-mediated nuclear factor (NF)-kappaB and AP-1 activation. Biol Pharm Bull. 2004;27:1202–1206. doi: 10.1248/bpb.27.1202. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ. 2012;2:310–320. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Kumagai K, Nishijima M, Hanada K. Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem. 2006;281:30279–30288. doi: 10.1074/jbc.M605032200. [DOI] [PubMed] [Google Scholar]
- Kelley D, He J, Menshikova E. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- Kimata Y, Ishiwata-Kimata Y, Ito T, Hirata A, Suzuki T, Oikawa D, Takeuchi M, Kohno K. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol. 2007;179:75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3:181–206. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- Kny M, Standera S, Hartmann-Petersen R, Kloetzel PM, Seeger M. Herp regulates Hrd1-mediated ubiquitylation in a ubiquitin-like domain-dependent manner. J Biol Chem. 2011;286:5151–5156. doi: 10.1074/jbc.M110.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K, Agarwala KL, Kato H, Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–32853. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- Kokame K, Kato H, Miyata T. Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem. 2001;276:9199–9205. doi: 10.1074/jbc.M010486200. [DOI] [PubMed] [Google Scholar]
- Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, Iseki K, Wanaka A, Imaizumi K. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005;7:186–194. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- Kondo S, Saito A, Hino SI, Murakami T, Ogata M, Kanemoto S, Nara S, Yamashita A, Yoshinaga K, Hara H, et al. BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol. 2007;27:1716–1729. doi: 10.1128/MCB.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopach O, Kruglikov I, Pivneva T, Voitenko N, Fedirko N. Functional coupling between ryanodine receptors, mitochondria and Ca(2+) ATPases in rat submandibular acinar cells. Cell Calcium. 2008;43:469–481. doi: 10.1016/j.ceca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER–mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Kroeger H, Miranda E, MacLeod I, Pérez J, Crowther DC, Marciniak SJ, Lomas DA. Endoplasmic reticulum-associated degradation (ERAD) and autophagy cooperate to degrade polymerogenic mutant serpins. J Biol Chem. 2009;284:22793–22802. doi: 10.1074/jbc.M109.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse KB, Brodsky JL, McCracken AA. Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human alpha-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Mol Biol Cell. 2006a;17:203–212. doi: 10.1091/mbc.E04-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse KB, Dear A, Kaltenbrun ER, Crum BE, George PM, Brennan SO, McCracken AA. Mutant fibrinogen cleared from the endoplasmic reticulum via endoplasmic reticulum-associated protein degradation and autophagy: an explanation for liver disease. Am J Pathol. 2006b;168:1299–1308. doi: 10.2353/ajpath.2006.051097. (uiz 1404–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov G, Brostrom MA, Brostrom CO. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J Biol Chem. 1992;267:3932–3939. [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fourn V, Gaplovska-Kysela K, Guhl B, Santimaria R, Zuber C, Roth J. Basal autophagy is involved in the degradation of the ERAD component EDEM1. CMLS Cell Mol Life Sci. 2009;66:1434–1445. doi: 10.1007/s00018-009-9038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- Lee YH, White MF. Insulin receptor substrate proteins and diabetes. Arch Pharm Res. 2004;27:361–370. doi: 10.1007/BF02980074. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Parker I, LaFerla FM. Presenilin-2 mutations modulate amplitude and kinetics of inositol 1, 4,5-trisphosphate-mediated calcium signals. J Biol Chem. 1999a;274:32535–32538. doi: 10.1074/jbc.274.46.32535. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer’s pre-senilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J Neurochem. 1999b;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- Li H, Korennykh AV, Behrman SL, Walter P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci USA. 2010;107:16113–16118. doi: 10.1073/pnas.1010580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician’s perspective. J Hematol Oncol. 2011;23 (4):8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto KI, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26:7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009;21:169–177. doi: 10.1016/j.cellsig.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Zhang ZY, Andersson KB, Husberg C, Enger UH, Ræder MG, Christensen G, Louch WE. Cardiomyocyte-specific disruption of Serca2 in adult mice causes sarco(endo)plasmic reticulum stress and apoptosis. Cell Calcium. 2011;49:201–207. doi: 10.1016/j.ceca.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Laszlo CF, Miao Z, Chen H, Wu S. The role of nitric-oxide synthase in the regulation of UVB light-induced phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Biol Chem. 2009;284:24281–24288. doi: 10.1074/jbc.M109.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, He Y, Zhang H, Yu L, Chen H, Xu Z, Tang S, Urano F, Min W. AIP1 is critical in transducing IRE1-mediated endoplasmic reticulum stress response. J Biol Chem. 2008;283:11905–11912. doi: 10.1074/jbc.M710557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- Madeo F, Kroemer G. Intricate links between ER stress and apoptosis. Mol Cell. 2009;33:669–670. doi: 10.1016/j.molcel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278:9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- Mao T, Shao M, Qiu Y, Huang J, Zhang Y, Song B, Wang Q, Jiang L, Liu Y, Han JDJ, et al. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc Natl Acad Sci USA. 2011;108:15852–15857. doi: 10.1073/pnas.1107394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR. Ryanodine receptors/calcium release channels in heart failure and sudden cardiac death. J Mol Cell Cardiol. 2001;33:615–624. doi: 10.1006/jmcc.2000.1343. [DOI] [PubMed] [Google Scholar]
- Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- Marutani T, Maeda T, Tanabe C, Zou K, Araki W, Kokame K, Michikawa M, Komano H. ER-stress-inducible Herp, facilitates the degradation of immature nicastrin. Biochim Biophys Acta. 2011;1810:790–798. doi: 10.1016/j.bbagen.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Matlack KE, Mothes W, Rapoport TA. Protein translocation: tunnel vision. Cell. 1998;92:381–390. doi: 10.1016/s0092-8674(00)80930-7. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Kido Y, Asahara SI, Kaisho T, Tanaka T, Hashimoto N, Shigeyama Y, Takeda A, Inoue T, Shibutani Y, et al. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest. 2010;120:115–126. doi: 10.1172/JCI39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. ER calcium and Alzheimer’s disease: in a state of flux. Sci Signal. 2010;3:pe10. doi: 10.1126/scisignal.3114pe10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, van Meer G. Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol. 2010;22:422–429. doi: 10.1016/j.ceb.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Meares GP, Hughes KJ, Naatz A, Papa FR, Urano F, Hansen PA, Benveniste EN, Corbett JA. IRE1-dependent activation of AMPK in response to nitric oxide. Mol Cell Biol. 2011;31:4286–4297. doi: 10.1128/MCB.05668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert M, Sommer T, Jarosch E. ERAD ubiquitin ligases: multifunctional tools for protein quality control and waste disposal in the endoplasmic reticulum. Bioessays. 2010;32:905–913. doi: 10.1002/bies.201000046. [DOI] [PubMed] [Google Scholar]
- Mendes CCP, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multiprocess calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Symonchuk N. ER vesicles and mitochondria move and communicate at synapses. J Cell Sci. 2006;119:4926–4934. doi: 10.1242/jcs.03254. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- Molinari M, Calanca V, Galli C, Lucca P, Paganetti P. Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science. 2003;299:1397–1400. doi: 10.1126/science.1079474. [DOI] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994;13:3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Kondo S, Ogata M, Kanemoto S, Saito A, Wanaka A, Imaizumi K. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem. 2006;96:1090–1100. doi: 10.1111/j.1471-4159.2005.03596.x. [DOI] [PubMed] [Google Scholar]
- Murakami T, Saito A, Hino SI, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- Muriel MP, Dauphin A, Namekawa M, Gervais A, Brice A, Ruberg M. Atlastin-1, the dynamin-like GTPase responsible for spastic paraplegia SPG3A, remodels lipid membranes and may form tubules and vesicles in the endoplasmic reticulum. J Neurochem. 2009;110:1607–1616. doi: 10.1111/j.1471-4159.2009.06258.x. [DOI] [PubMed] [Google Scholar]
- Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell. 2008;19:2777–2788. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Salloum FN, Fisher BJ, Smithson L, Almenara J, Fowler AA. Prolyl hydroxylase inhibition attenuates post-ischemic cardiac injury via induction of endoplasmic reticulum stress genes. Vascul Pharmacol. 2009;51:110–118. doi: 10.1016/j.vph.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Nelson O, Tu H, Lei T, Bentahir M, De Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297:H1711–H1719. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Zhou C, Duan Q, Lv J, Fu X, Xia Y, Wang DW. β-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS One. 2011;6:e27294. doi: 10.1371/journal.pone.0027294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J, Yamashita S. IRE1 encodes a putative protein kinase containing a membrane-spanning domain and is required for inositol phototrophy in Saccharomyces cerevisiae. Mol Microbiol. 1992;6:1441–1446. doi: 10.1111/j.1365-2958.1992.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Nikonov AV, Kreibich G. Organization of translocon complexes in ER membranes. Biochem Soc Trans. 2003;31:1253–1256. doi: 10.1042/bst0311253. [DOI] [PubMed] [Google Scholar]
- Nikonov AV, Hauri HP, Lauring B, Kreibich G. Climp-63-mediated binding of microtubules to the ER affects the lateral mobility of translocon complexes. J Cell Sci. 2007;120:2248–2258. doi: 10.1242/jcs.008979. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino SI, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa D, Tokuda M, Hosoda A, Iwawaki T. Identification of a consensus element recognized and cleaved by IRE1 alpha. Nucleic Acids Res. 2010;38:6265–6273. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada KI, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- Olivari S, Cali T, Salo KEH, Paganetti P, Ruddock LW, Molinari M. EDEM1 regulates ER-associated degradation by accelerating de-mannosylation of folding-defective polypeptides and by inhibiting their covalent aggregation. Biochem Biophys Res Commun. 2006;349:1278–1284. doi: 10.1016/j.bbrc.2006.08.186. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Ericsson JL. Enzyme-membrane relationship in phenobarbital induction of synthesis of drug-metabolizing enzyme system and proliferation of endoplasmic membranes. J Cell Biol. 1966;28:181–198. doi: 10.1083/jcb.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi A, Fioriti L, Chiesa R, Sitia R. Conditions of endoplasmic reticulum stress favor the accumulation of cytosolic prion protein. J Biol Chem. 2006;281:30431–30438. doi: 10.1074/jbc.M605320200. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Tsukamoto Y, Hori O, Kitao Y, Yanagi H, Stern DM, Ogawa S. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Res. 2001;61:4206–4213. [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. The endoplasmic reticulum. J Biophys Biochem Cytol. 1956;2:85–98. doi: 10.1083/jcb.2.4.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Park HR, Tomida A, Sato S, Tsukumo Y, Yun J, Yamori T, Hayakawa Y, Tsuruo T, Shinya K. Effect on tumor cells of blocking survival response to glucose deprivation. J Natl Cancer Inst. 2004;96:1300–1310. doi: 10.1093/jnci/djh243. [DOI] [PubMed] [Google Scholar]
- Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, Phan T, Baumeister P, Roy B, Cheriyath V, Roy AL, Lee AS. Identification of TFII-I as the endoplasmic reticulum stress response element binding factor ERSF: its autoregulation by stress and interaction with ATF6. Mol Cell Biol. 2001;21:3220–3233. doi: 10.1128/MCB.21.9.3220-3233.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe JM, Roberts JJ. Interactions between mammalian cell DNA and inorganic platinum compounds. II Interstrand cross-linking of isolated and cellular DNA by platinum(IV) compounds. Biochem Pharmacol. 1974;23:1345–1357. doi: 10.1016/0006-2952(74)90354-2. [DOI] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–3884. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Perman JC, Boström P, Lindbom M, Lidberg U, StÅhlman M, Hägg D, Lindskog H, Scharin Täng M, Omerovic E, Mattsson Hultén L, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovski G, Das S, Juhasz B, Kertesz A, Tosaki A, Das DK. Cardioprotection by endoplasmic reticulum stress-induced autophagy. Antioxid Redox Signal. 2011;14:2191–2200. doi: 10.1089/ars.2010.3486. [DOI] [PubMed] [Google Scholar]
- Pfaffenbach KT, Nivala AM, Reese L, Ellis F, Wang D, Wei Y, Pagliassotti MJ. Rapamycin inhibits postprandial-mediated X-box-binding protein-1 splicing in rat liver. J Nutr. 2010;140:879–884. doi: 10.3945/jn.109.119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus D, Chevalier MW, Aragón T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Pomar N, Berlanga JJ, Campuzano S, Hernandez G, Elias M, de Haro C. Functional characterization of Drosophila melanogaster PERK eukaryotic initiation factor 2alpha (eIF2alpha) kinase. Eur J Biochem. 2003;270:293–306. doi: 10.1046/j.1432-1033.2003.03383.x. [DOI] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Promlek T, Ishiwata-Kimata Y, Shido M, Sakuramoto M, Kohno K, Kimata Y. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol Biol Cell. 2011;22:3520–3532. doi: 10.1091/mbc.E11-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane NS, Kang SW, Chakrabarti O, Feigenbaum L, Hegde RS. Reduced translocation of nascent prion protein during ER stress contributes to neurodegeneration. Dev Cell. 2008;15:359–370. doi: 10.1016/j.devcel.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- Registre M, Goetz JG, St Pierre P, Pang H, Lagacé M, Bouvier M, Le PU, Nabi IR. The gene product of the gp78/AMFR ubiquitin E3 ligase cDNA is selectively recognized by the 3F3A antibody within a subdomain of the endoplasmic reticulum. Biochem Biophys Res Commun. 2004;320:1316–1322. doi: 10.1016/j.bbrc.2004.06.089. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran POT, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53 (Suppl 1):S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, Le QT, Koong AC. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouschop KMA, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer SF. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- Rubio C, Pincus D, Korennykh A, Schuck S, El-Samad H, Walter P. Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J Cell Biol. 2011;193:171–184. doi: 10.1083/jcb.201007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene. 2010;29:4424–4435. doi: 10.1038/onc.2010.191. [DOI] [PubMed] [Google Scholar]
- Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Saito A, Hino SI, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, et al. Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated Sec23a pathway is essential for chondrogenesis. Nat Cell Biol. 2009;11:1197–1204. doi: 10.1038/ncb1962. [DOI] [PubMed] [Google Scholar]
- Santos CXC, Tanaka LY, Wosniak J, Laurindo FRM. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Sari FR, Widyantoro B, Thandavarayan RA, Harima M, Lakshmanan AP, Zhang S, Muslin AJ, Suzuki K, Kodama M, Watanabe K. Attenuation of CHOP-mediated myocardial apoptosis in pressure-overloaded dominant negative p38α mitogen-activated protein kinase mice. Cell Physiol Biochem. 2011;27:487–496. doi: 10.1159/000329970. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Wolf DH. Sec61p is part of the endoplasmic reticulum-associated degradation machinery. EMBO J. 2009;28:2874–2884. doi: 10.1038/emboj.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schewe DM, Aguirre-Ghiso JA. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci USA. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, Area-Gomez E. Is Alzheimer’s disease a disorder of mitochondria-associated membranes? J Alzheimers Dis. 2010;20 (Suppl 2):S281–S292. doi: 10.3233/JAD-2010-100495. [DOI] [PubMed] [Google Scholar]
- Schröder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Westermann D, Isken F, Voigt A, Laube B, Thierbach R, Kuhlow D, Zarse K, Schomburg L, Pfeiffer AFH, et al. Activation of mitochondrial energy metabolism protects against cardiac failure. Aging (Albany NY) 2010;2:843–853. doi: 10.18632/aging.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PLoS One. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino A, Campioni M, Straino S, Salloum FN, Schmidt N, Herbrand U, Frede S, Toietta G, Di Rocco G, Bussani R, et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol. 2007;50:1029–1037. doi: 10.1016/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Sha H, He Y, Chen H, Wang C, Zenno A, Shi H, Yang X, Zhang X, Qi L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- Shen J, Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem. 2004;279:43046–43051. doi: 10.1074/jbc.M408466200. [DOI] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Shen J, Snapp EL, Lippincott-Schwartz J, Prywes R. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol Cell Biol. 2005;25:921–932. doi: 10.1128/MCB.25.3.921-932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126:435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, Voeltz GK. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. J Biol Chem. 2008;283:18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Yamamoto C, Kaji T. Lead induces the expression of endoplasmic reticulum chaperones GRP78 and GRP94 in vascular endothelial cells via the JNK-AP-1 pathway. Toxicol Sci. 2010;114:378–386. doi: 10.1093/toxsci/kfq008. [DOI] [PubMed] [Google Scholar]
- Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Silva JM, Wong A, Carelli V, Cortopassi GA. Inhibition of mitochondrial function induces an integrated stress response in oligodendroglia. Neurobiol Dis. 2009;34:357–365. doi: 10.1016/j.nbd.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DH. Dysregulation of calcium homeostasis in Alzheimer’s disease. Neurochem Res. 2009;34:1824–1829. doi: 10.1007/s11064-009-9960-5. [DOI] [PubMed] [Google Scholar]
- Smith IF, Green KN, LaFerla FM. Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium. 2005;38:427–437. doi: 10.1016/j.ceca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Song XJ, Yang CY, Liu B, Wei Q, Korkor MT, Liu JY, Yang P. Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int J Med Sci. 2011;8:564–572. doi: 10.7150/ijms.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiotto MT, Banh A, Papandreou I, Cao H, Galvez MG, Gurtner GC, Denko NC, Le QT, Koong AC. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Res. 2010;70:78–88. doi: 10.1158/0008-5472.CAN-09-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce BA, Campbell LA, McTavish N, Cooper MA, Appleyard MV, O’Neill M, Howie J, Samson J, Watt S, Murray K, et al. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004;64:4875–4886. doi: 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem. 2007;282:7024–7034. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Hetz C, Yi CH, Jackson WS, Borkowski AW, Yuan J, Wollmann RH, Lindquist S. Prion pathogenesis is independent of caspase-12. Prion. 2007;1:243–247. doi: 10.4161/pri.1.4.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Sterz J, von Metzler I, Hahne JC, Lamottke B, Rademacher J, Heider U, Terpos E, Sezer O. The potential of proteasome inhibitors in cancer therapy. Expert Opin Investig Drugs. 2008;17:879–895. doi: 10.1517/13543784.17.6.879. [DOI] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004;4:323–335. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- Strøm CC, Aplin M, Ploug T, Christoffersen TEH, Langfort J, Viese M, Galbo H, Haunsø S, Sheikh SP. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 2005;272:2684–2695. doi: 10.1111/j.1742-4658.2005.04684.x. [DOI] [PubMed] [Google Scholar]
- Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci USA. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Yamaguchi N, Xu L, Eu JP, Stamler JS, Meissner G. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry. 2008;47:13985–13990. doi: 10.1021/bi8012627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, et al. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med. 2011;3:88ra55. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Locher M, Hochstrasser M. A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 2001;15:2660–2674. doi: 10.1101/gad.933301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Zhu W, Li Y, Xin P, Li J, Liu M, Li J, Redington AN, Wei M. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol. 2011;301:H1471–H1486. doi: 10.1152/ajpheart.00097.2011. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, da Fonseca PCA, Morris EP. IP(3) receptors: the search for structure. Trends Biochem Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Teckman JH, Perlmutter DH. Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol. 2000;279:G961–G974. doi: 10.1152/ajpgi.2000.279.5.G961. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Chen LB, Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J Cell Biol. 1986;103:1557–1568. doi: 10.1083/jcb.103.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, Wek RC. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Sisodia SS. Presenilins and Alzheimer disease: the calcium conspiracy. Nat Neurosci. 2006;9:1354–1355. doi: 10.1038/nn1106-1354. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Hoover H, Meller J, Hernandez J, Su L, Andrews C, Dillmann WH, McDonough PM, Glembotski CC. Sarco/endoplasmic reticulum calcium ATPase-2 expression is regulated by ATF6 during the endoplasmic reticulum stress response: intracellular signaling of calcium stress in a cardiac myocyte model system. J Biol Chem. 2001;276:48309–48317. doi: 10.1074/jbc.M107146200. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC. Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. J Biol Chem. 2007;282:22865–22878. doi: 10.1074/jbc.M701213200. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Morrison L, Glembotski CC. Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J Biol Chem. 2004;279:21078–21084. doi: 10.1074/jbc.M400713200. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Morrison LE, Hoover H, Glembotski CC. Coordination of ATF6-mediated transcription and ATF6 degradation by a domain that is shared with the viral transcription factor, VP16. J Biol Chem. 2002;277:20734–20739. doi: 10.1074/jbc.M201749200. [DOI] [PubMed] [Google Scholar]
- Tirasophon W, Lee K, Callaghan B, Welihinda A, Kaufman RJ. The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 2000;14:2725–2736. doi: 10.1101/gad.839400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/ endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toko H, Takahashi H, Kayama Y, Okada S, Minamino T, Terasaki F, Kitaura Y, Komuro I. ATF6 is important under both pathological and physiological states in the heart. J Mol Cell Cardiol. 2010;49:113–120. doi: 10.1016/j.yjmcc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Torres M, Castillo K, Armisén R, Stutzin A, Soto C, Hetz C. Prion protein mis-folding affects calcium homeostasis and sensitizes cells to endoplasmic reticulum stress. PLoS One. 2010;5:e15658. doi: 10.1371/journal.pone.0015658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso R, Vicencio JM, Parra V, Nemchenko A, Kawashima Y, del Campo A, Toro B, Battiprolu PK, Aranguiz P, Chiong M, et al. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc Res. 2011;93:320–329. doi: 10.1093/cvr/cvr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–428. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2alpha signaling. J Biol Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- Vasington FD, Murphy JV. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J Biol Chem. 1962;237:2670–2677. [PubMed] [Google Scholar]
- Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci. 2005;118:3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Guay G, Pogan L, Sauvé R, Nabi IR. Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J Cell Biol. 2000;150:1489–1498. doi: 10.1083/jcb.150.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li L, Ye Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J Biol Chem. 2008;283:7445–7454. doi: 10.1074/jbc.M708347200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009a;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009b;109 (Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y, Hedrick S, Vera L, Montminy M. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009c;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr Biol. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- Watson ML. The nuclear envelope; its structure and relation to cytoplasmic membranes. J Biophys Biochem Cytol. 1955;1:257–270. doi: 10.1083/jcb.1.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb GC, Akbar MS, Zhao C, Steiner DF. Expression profiling of pancreatic beta cells: glucose regulation of secretory and metabolic pathway genes. Proc Natl Acad Sci USA. 2000;97:5773–5778. doi: 10.1073/pnas.100126597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson BM, Regnacq M, Stirling CJ. Protein translocation across the membrane of the endoplasmic reticulum. J Membr Biol. 1997;155:189–197. doi: 10.1007/s002329900171. [DOI] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Boström P, Tyra HM, Crawford RW, Campbell KP, et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GDY, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu L, Charles IG, Moncada S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nat Cell Biol. 2004;6:1129–1134. doi: 10.1038/ncb1188. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tomida A, Yun J, Cai B, Yoshikawa H, Taketani Y, Tsuruo T. Cellular sensitization to cisplatin and carboplatin with decreased removal of platinum-DNA adduct by glucose-regulated stress. Cancer Chemother Pharmacol. 1999;44:59–64. doi: 10.1007/s002800050945. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Larkin D, Lara-Lemus R, Ramos-Castañeda J, Liu M, Arvan P. Endoplasmic reticulum (ER) chaperone regulation and survival of cells compensating for deficiency in the ER stress response kinase, PERK. J Biol Chem. 2008;283:17020–17029. doi: 10.1074/jbc.M802466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136:343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- Yan W, Frank CL, Korth MJ, Sopher BL, Novoa I, Ron D, Katze MG. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Tiffany-Castiglioni E, Koh HC, Son IH. Paraquat activates the IRE1/ ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol Lett. 2009;191:203–210. doi: 10.1016/j.toxlet.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Yanagitani K, Imagawa Y, Iwawaki T, Hosoda A, Saito M, Kimata Y, Kohno K. Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol Cell. 2009;34:191–200. doi: 10.1016/j.molcel.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW. AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediators Inflamm. 2010;2010:130636. doi: 10.1155/2010/130636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo BC, Krapfenbauer K, Cairns N, Belay G, Bajo M, Lubec G. Overexpressed protein disulfide isomerase in brains of patients with sporadic Creutzfeldt-Jakob disease. Neurosci Lett. 2002;334:196–200. doi: 10.1016/s0304-3940(02)01071-6. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy. 2007;3:160–162. doi: 10.4161/auto.3653. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Oku M, Suzuki M, Mori K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol. 2006;172:565–575. doi: 10.1083/jcb.200508145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Uemura A, Mori K. pXBP1(U), a negative regulator of the unfolded protein response activator pXBP1(S), targets ATF6 but not ATF4 in proteasome-mediated degradation. Cell Struct Funct. 2009;34:1–10. doi: 10.1247/csf.06028. [DOI] [PubMed] [Google Scholar]
- Yu JT, Chang RCC, Tan L. Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol. 2009;89:240–255. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem. 2007;76:367–385. doi: 10.1146/annurev.biochem.76.053105.094237. [DOI] [PubMed] [Google Scholar]
- Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc Natl Acad Sci USA. 2011;108:2777–2782. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang S, Malhotra J, Hassler JR, Back SH, Wang G, Chang L, Xu W, Miao H, Leonardi R, et al. The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J. 2011;30:1357–1375. doi: 10.1038/emboj.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- Zhao P, Xiao X, Kim AS, Leite MF, Xu J, Zhu X, Ren J, Li J. c-Jun inhibits thapsigargin-induced ER stress through up-regulation of DSCR1/Adapt78. Exp Biol Med (Maywood) 2008;233:1289–1300. doi: 10.3181/0803-RM-84. [DOI] [PubMed] [Google Scholar]
- Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y. Over-expression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, Lee J, Fisher SJ, White MF, Biddinger SB, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Johansen FE, Prywes R. Interaction of ATF6 and serum response factor. Mol Cell Biol. 1997;17:4957–4966. doi: 10.1128/mcb.17.9.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, Thompson JF, Hersey P. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]