Abstract
Rationale
Pressure-induced arterial depolarization and constriction (the myogenic response), is a smooth muscle cell (myocyte)-specific mechanism that controls regional organ blood flow and systemic blood pressure. Several different non-selective cation channels contribute to pressure-induced depolarization, but signaling mechanisms involved are unclear. Similarly uncertain is the contribution of anion channels to the myogenic response and physiological functions and mechanisms of regulation of recently discovered transmembrane 16A (TMEM16A) chloride (Cl−) channels in arterial myocytes.
Objective
Investigate the hypothesis that myocyte TMEM16A channels control membrane potential and contractility and contribute to the myogenic response in cerebral arteries.
Methods and Results
Cell swelling induced by hyposmotic bath solution stimulated Cl− currents in arterial myocytes that were blocked by TMEM16A channel inhibitory antibodies, RNAi-mediated selective TMEM16A channel knockdown, removal of extracellular calcium (Ca2+), replacement of intracellular EGTA with BAPTA, a fast Ca2+ chelator, and Gd3+ and SKF-96365, non-selective cation channel blockers. In contrast, nimodipine, a voltage-dependent Ca2+ channel inhibitor, or thapsigargin, which depletes intracellular Ca2+ stores, did not alter swelling-activated TMEM16A currents. Pressure (−40 mmHg)-induced membrane stretch activated ion channels in arterial myocyte cell-attached patches that were inhibited by TMEM16A antibodies and were of similar amplitude to recombinant TMEM16A channels. TMEM16A knockdown reduced intravascular pressure-induced depolarization and vasoconstriction, but did not alter depolarization (60 mmol/L K+)-induced vasoconstriction.
Conclusions
Membrane stretch activates arterial myocyte TMEM16A channels, leading to membrane depolarization and vasoconstriction. Data also provide a mechanism by which a local Ca2+ signal generated by non-selective cation channels stimulates TMEM16A channels to induce myogenic constriction.
Keywords: Arterial smooth muscle, ClCa channel, TMEM16A, ANO1, myogenic tone, contractility, smooth muscle cells
INTRODUCTION
Resistance-size cerebral arteries control brain regional blood flow and maintain perfusion during changes in arterial pressure. One important functional stimulus that controls cerebral artery contractility is intravascular pressure. An elevation in intravascular pressure stimulates depolarization, leading to the activation of smooth muscle cell voltage-dependent calcium (Ca2+) channels, an intracellular calcium concentration ([Ca2+]i) elevation and vasoconstriction. 1 This “myogenic response” regulates regional brain blood flow, maintains perfusion over a range of intravascular pressures, and provides a baseline diameter from which other stimuli can either dilate or constrict. Several pathologies, including hypertension, are associated with altered myogenic responsiveness. 2 Therefore, defining mechanisms that control the myogenic response is critical to a better understanding of vascular diseases.
Arterial smooth muscle cell cation channels, including CaV1.2, several K+ and non-selective transient receptor potential (TRP) channels control vascular contractility. 1, 2 Multiple TRP channels also contribute to pressure-induced depolarization, leading to vasoconstriction, although mechanisms involved are unclear. 2 In contrast, vascular contractility regulation by arterial smooth muscle cell anion channels is poorly understood. Chloride (Cl−) is the most abundant intracellular anion in vascular smooth muscle cells, with intracellular [Cl−] ~50 mmol/L 3. The estimated reversal potential (Erev) for Cl− in smooth muscle cells is between −30 and −20 mV 4. The entire working range of rat cerebral arteries from fully dilated to fully constricted, occurs between membrane potentials of ~−60 and −20 mV, which elevates global arterial wall [Ca2+]i from ~ 100 to 350 nmol/L. 5 With physiological ionic gradients, Cl− channel activation would result in Cl− efflux and arterial myocyte depolarization and vasoconstriction. 1. This is in contrast to some other cell types, including adult neurons where Cl− Erev is ~ −75 mV, a voltage near resting potential. 6 The concept that Cl− channels contribute to myogenic constriction has previously been suggested from experiments that used highly non-specific pharmacological Cl− channel modulators. 1, 3, 7, 8 Indeed, poor selectivity of pharmacological Cl− channel modulators and uncertain molecular identity of the protein(s) involved has hindered progress in defining functions of Cl− channels in contractile arterial smooth muscle cell and their involvement in the regulation of vascular contractility.
Transmembrane protein 16A (TMEM16A) channels are recently discovered Ca2+-activated Cl− (ClCa) channels 9–11. Our group and others recently demonstrated that TMEM16A channels are expressed in arterial smooth muscle cells and generate ClCa currents. 12–15 TMEM16A has recently been described as a negative regulator of arterial smooth muscle cell proliferation. 15 However, regulation of contractility by arterial smooth muscle cell TMEM16A channels is unclear. Here, we demonstrate that cell swelling and pressure-induced membrane stretch stimulate TMEM16A channels in arterial smooth muscle cells, leading to depolarization and vasoconstriction. Data also suggest that membrane distention activates non-selective cation channels that stimulate TMEM16A channels through local Ca2+ signaling. Thus, we show that arterial smooth muscle cell TMEM16A channels are one component of a mechanosensitive mechanism that contributes to the myogenic response.
METHODS
Tissue and cell preparation
Animal protocols were reviewed and approved by the AnimalCare and Use Committee of the University of Tennessee HealthScience Center. Male Sprague-Dawley rats (6–8 weeks) were euthanized by intraperitoneal injection of sodium pentobarbital (150 mg/kg). The brain was removed and placed into physiological saline solution (PSS) of composition: (in mmol/L) 112 NaCl, 4.8 KCl, 24 NaHCO3, 1.8 CaCl2, 1.2 MgSO4,1.2 KH2PO4, and 10 glucose, which was gassed with 21% O2-5%CO2-74% N2 to pH 7.4. Resistance-size (~200 μm diameter) posterior cerebral, cerebellar, and middle cerebral arteries were dissected from the brain, pooled and used for experimentation, unless specified.
Patch clamp electrophysiology
Smooth muscle cells were isolated from cerebral arteries as previously described 16. Patch-clamp electrophysiology was performed using isolated cerebral artery smooth muscle cells or human embryonic kidney 293 (HEK293) cells expressing recombinant TMEM16A channels. For whole-cell current measurements, the pipette solution contained (in mmol/L): 126 CsCl, 10 HEPES, 10 D-Glucose, 1 EGTA or 1 BAPTA, 1 MgATP, 0.2 GTP·Na, and 40 sucrose and pH adjusted to 7.2 with CsOH. Total MgCl2 was adjusted to give final free Mg2+ of 1 mmol/L. For whole-cell current experiments on arterial smooth muscle cells, pipette free Ca2+ was 200 nM. For whole-cell current measurements in HEK293 cells expressing recombinant TMEM16A channels, pipette free Ca2+ was 1 μM. Free Mg2+ and Ca2+ were calculated using WebmaxC Standard (http://www.stanford.edu/~cpatton/webmaxcS.htm) and confirmed using a Ca2+-sensitive and reference electrode (Corning; Acton, MA). Bath solutions used for whole-cell recordings are described in Online Table I. For cell-attached patch measurements, the pipette solution contained (in mmol/L): 134 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 1 TEA+ and 5 4-aminopyridine (pH 7.4, NaOH). For cell-attached patch measurements, the bath solution contained (in mmol/L): 140 KCl, 10 glucose, 10 HEPES, 2 CaCl2, 1 MgCl2 (pH 7.4, KOH). To study anion permeability, Cl− was replaced with equimolar aspartate or I−. The osmolarity of solutions was measured using a Wescor 5500 Vapor Pressure Osmometer (Logan, UT). To minimize junction potential, the reference Ag/AgCl electrode was immersed in a solution of 3 mmol/L KCl continuous with an agar bridge (4% agar in 3 mmol/L KCl). Whole-cell Cl− currents were measured by applying 1.5 s voltage steps between −80 mV and +80 mV in 20 mV increments using an interpulse holding potential of −40 mV. Currents were normalized to membrane capacitance. Cell-attached currents were measured at a steady membrane potential of −80 mV. Pharmacological agents and rabbit monoclonal anti-TMEM16A antibody (Abcam) were introduced directly into the experimental chamber. Boiled (15 min at 98 °C) denatured TMEM16A antibody served as a control for active antibody. Pressure-induced stretch was applied to the plasma membrane contained within the patch pipette using a ez-gSEAL 100B controller (Neo Biosystems). Membrane currents were recorded using an Axopatch 200B amplifier equipped with a CV 203BU headstage, Digidata 1332A, and Clampex 8 or 9 (Molecular Devices). Whole cell currents were filtered at 1 kHz using a low pass Bessel filter and digitized at 4 kHz. Single channel currents were filtered at 2 kHz and digitized at 8 kHz. The relative anion permeability ratio of I− to Cl− (PI/PCl) or aspartate (Asp) to Cl− (PAsp/PCl) was calculated using the shift in reversal potential (Erev) and the constant field equation:
Western blotting
Cerebral arteries were homogenized using Laemmli sample buffer (2.5% SDS, 10% glycerol, 0.01% bromphenol blue, and 5% β-mercaptoethanol in 100 mmol/L Tris·HCl, pH 6.8) and centrifuged at 6,000× g for 10 min to remove cellular debris. Proteins (40 μg/lane) were separated on a 7.5% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. Blots were physically cut at 75 kDa to permit probing for TMEM16A, TRPC6, TRPM4 or TRPP2 at the higher molecular weight and for actin at the lower molecular weight. Membranes were incubated with rabbit monoclonal anti-TMEM16A (1:100, Abcam), rabbit anti-TRPC6 (1:250, Sigma), rabbit anti-TRPM4 (1:500, Thermo Scientific), rabbit anti-TRPP2 (1:1000, Johns Hopkins Polycystic Kidney Disease Research and Clinical Core Center or 1:100, Santa Cruz) and mouse monoclonal anti-actin (1:5,000 dilution, Chemicon International) primary antibodies overnight at 4°C in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) and 5% nonfat dry milk. Proteins were visualized using horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution; Pierce) and a chemiluminescent detection kit (Pierce). Band intensity was quantified by digital densitometry using Quantity One software (Bio-Rad). Protein band intensity was normalized to actin.
TMEM16A channel knockdown
Three small interference RNA (siRNA) sequences targeting TMEM16A or negative control siRNA (Invitrogen), as used previously 12, were inserted intracellularly into cerebral arteries using either reverse permeabilization, as described 17–21 or a Bex CUY21Vivo-SQ electroporator. Arteries were then maintained in serum-free DMEM F12 media supplemented with 1% penicillin-streptomycin (Sigma) for 4 days following reverse permeabilization or 3 days after electroporation at 37°C in a sterile incubator (21% O2, 5% CO2). Western blotting was used to compare the effect of TMEM16A siRNA with control siRNA on protein expression. Band intensity of proteins from arteries treated with either TMEM16A siRNA or control siRNA were compared on the same membranes. Reverse permeabilization and electroporation similarly reduced TMEM16A protein (reverse permeabilization, 62±5% of control siRNA, n=7; electroporator, 56±1% of control siRNA, n=3) in arteries (P>0.05).
Cell culture and transfection
HEK293 (HEK293) cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin under standard tissue culture conditions (21% O2-5% CO2; 37°C). HEK293 cells were transiently transfected with pcDNA3 encoding full-length recombinant TMEM16A (2 μg), a kind gift from Dr. Luis Galietta, Istituto Giannina Gaslini, Italy. Transfection was done using Effectene (Qiagen). Cells were used between 36 and 72 h post-transfection.
Pressurized artery membrane potential and diameter measurement
Experiments were performed using endothelium-denuded middle cerebral arteries. Arteries were maintained in PSS containing (in mmol/L): 112 NaCl, 4.8 KCl, 26 NaHCO3, 1.8 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 10 glucose, gassed with 74% N2, 21% O2, 5% CO2 (pH 7.4). Artery segments 1–2 mm in length were cannulated at each end in a temperature-controlled perfusion chamber (Living Systems Instrumentation; Burlington, VT). Intravascular pressure was altered using a reservoir and monitored using a pressure transducer.
Arterial wall diameter was measured at 1 Hz using a CCD camera attached to a Nikon TS100-F microscope and the automatic edge detection function of Ion Wizard software (Ionoptix, Milton, MA). Luminal flow was absent during experiments. Myogenic tone (%) was calculated as 100×(1−active diameter/passive diameter). Endothelial denudation was confirmed using methods previously described 18.
Membrane potential measurements were obtained in arteries at 10 or 60 mmHg that had developed steady-state myogenic tone. This was done by maintaining arteries at steady pressure for at least 2 hours and confirmed using edge detection. Membrane potential was measured by inserting glass microelectrodes filled with 3 M KCl (50–90 mΩ) into the adventitial side of pressurized arteries. Membrane potential was recorded using a WPI FD223 amplifier and digitized using pClamp 9.2 software (Axon Instruments) and a personal computer. Criteria for successful intracellular impalements were 1) a sharp negative change in potential upon insertion; 2) stable voltage for at least 1 min after entry; 3) a sharp positive voltage deflection upon exit from the recorded cell; and 4) a <10% change in tip resistance after the impalement.
Statistical analysis
OriginLab and GraphPad InStat software were used for statistical analyses. Values are expressed as mean±SEM. Student’s t-test was used for comparing paired and unpaired data from two populations, and ANOVA with Student–Newman–Keuls post-hoc test used for multiple group comparisons. P<0.05 was considered significant. Power analysis was performed on all data where P>0.05 to verify that sample size was sufficient to give a power value of >0.8. All points histograms were fit with a multi-peak Gaussian function using Microcal Origin.
Expanded Materials and Methods are provided as Supplemental Documentation.
RESULTS
Cell swelling activates TMEM16A currents in arterial smooth muscle cells
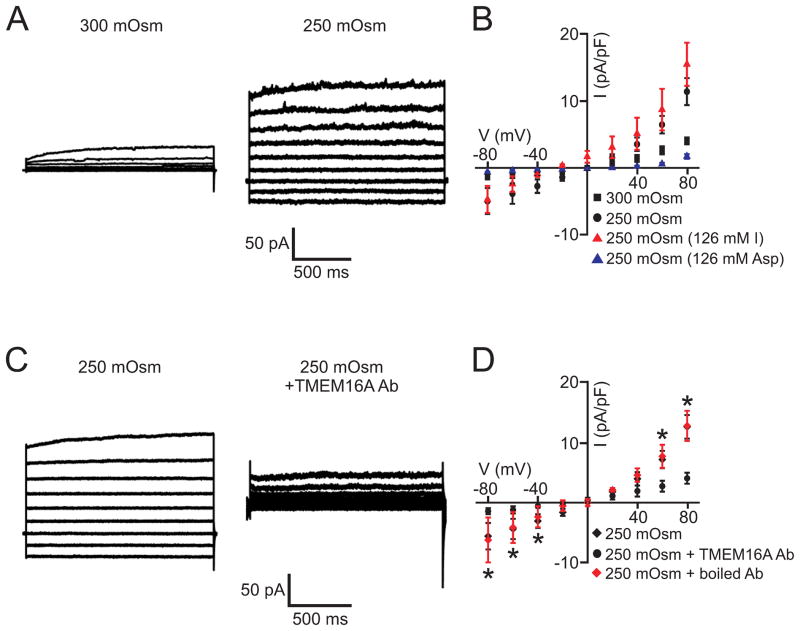
To study the mechanosensitivity of Cl− currents in arterial smooth muscle cells, we measured responses to hyposmotic bath solution, a commonly used method to induce cell swelling. 8, 22–24 Experiments were performed using solutions that abolish Na+ and K+ currents (Online Table I). In isosmotic solution (300 mOsm/L), smooth muscle cells generated outwardly-rectifying currents (Fig. 1A,B). Switching to hyposmotic bath solution (250 mOsm/L) increased currents from 4.0±0.5 to 11.3±2.0 pA/pF or 2.8-fold (at +80 mV) and linearized the IV relationship, reducing the rectification index (I80/I−80) from ~3.1 to 2.3 (Fig. 1A,B). The swelling-activated current peaked in ~90 seconds (Online Fig. I). Equimolar replacement of 126 mmol/L external Cl− with I− caused a −26.1±1.7 mV hyperpolarizing shift in Erev, indicating a PI/PCl of 2.9 (Fig. 1B). In contrast, equimolar replacement of 126 mM external Cl− with aspartate shifted Erev by +13.2±4.2 mV, indicating a PAsp/PCl of 0.6 (Fig. 1B). These data indicate that cell swelling activates a Cl− current (ICl) in arterial smooth muscle cells.
Figure 1. Cell swelling activates TMEM16A currents in arterial smooth muscle cells.
A, Reducing osmolarity from 300 to 250 mOsm/L stimulated a Cl− current. B, Mean data: 300 mOsm/L NMDG-Cl, n=6; 250 mOsm/L NMDG-Cl, n=6; 250 mOsm/L NMDG-Asp, n=4; 250 mOsm/L NMDG-I, n=7. C, TMEM16A antibody (1:100) inhibits swelling-activated Cl− current. D, Mean data: 250 mOsm/L NMDG-Cl, n=6; 250 mOsm/L NMDG-Cl+TMEM16A Ab (1:100), n=4; 250 mOsm/L NMDG-Cl+boiled TMEM16A Ab (1:100), n=5.
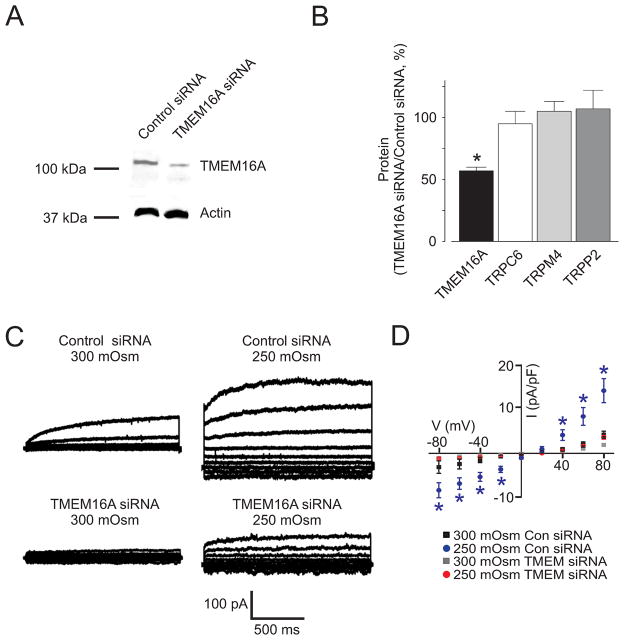
Next, we examined the molecular identity of channels that generate swelling-activated Cl−currents in arterial smooth muscle cells. A TMEM16A antibody that inhibits currents generated by recombinant TMEM16A channels 12 reduced mean swelling-activated Cl− currents in smooth muscle cells from 12.5±1.9 to 4.0±0.9 pA/pF, or by ~71% (at +80 mV, Fig. 1C,D). In contrast, boiled TMEM16A antibody did not alter swelling-activated Cl− currents (Fig. 1D). RNAi was used as a complimentary approach. Western blotting indicated that TMEM16A siRNA reduced arterial TMEM16A protein by 43±3%, when compared with control siRNA (Fig. 2A). In contrast, TMEM16A siRNA did not alter TRPC6, TRPM4, or TRPP2 expression (Fig. 2B, Online Fig. II). Hyposmotic (250 mOsm/L) bath solution increased Cl− currents from 4.2±0.8 to 14.1±2.7 pA/pF (at +80 mV), or ~3.3-fold in control siRNA-treated smooth muscle cells (Fig. 2C,D). In contrast, hyposmotic bath solution increased Cl− current density only 1.9-fold (at +80 mV) in smooth muscle cells in which TMEM16A was knocked down. In hyposmotic bath solution, mean Cl− current density in TMEM siRNA-treated cells was ~30 % of that in control siRNA-treated cells (Fig. 2D). These results indicate that cell swelling activates TMEM16A currents in arterial smooth muscle cells.
Figure 2. TMEM16A knockdown reduces swelling-activated Cl− currents in arterial smooth muscle cells.
A, Representative Western blot illustrating that TMEM16A siRNA reduced TMEM16A expression in cerebral arteries. B, Mean data of the effect of TMEM16A siRNA on TMEM16A (n=9), TRPC6 (n=4), TRPM4 (n=5), and TRPP2 (n=9) protein. * indicates P<0.05 when compared to 250 mOsm TMEM16A siRNA. C, Exemplary recordings illustrating that TMEM16A knockdown attenuates swelling-activated Cl− currents in smooth muscle cells. D, Mean data. Control siRNA: 300 mOsm/L, n=8; 250 mOsm/L, n=8. TMEM16A siRNA: 300 mOsm/L, n=9; 250 mOsm/L, n=10.
Non-selective cation channels stimulate TMEM16A currents through local intracellular Ca2+ signaling
Mechanisms by which cell swelling activates TMEM16A currents in arterial smooth muscle cells were investigated. Extracellular Ca2+ removal abolished swelling-activated TMEM16A currents, whereas nimodipine, a voltage-dependent Ca2+ channel inhibitor, and thapsigargin, a SR Ca2+-ATPase inhibitor that depletes SR Ca2+ load, did not alter swelling-activated TMEM16A currents (Fig. 3A,B). These data suggest that swelling activates TMEM16A currents by inducing Ca2+ influx through a pathway that is independent of voltage-dependent Ca2+ channels. Therefore, we tested the hypothesis that swelling activates one or more non-selective cation channels, leading to Ca2+ influx that activates TMEM16A currents. SKF-96365 and Gd3+, non-selective cation channel blockers, inhibited swelling-induced TMEM16A currents in smooth muscle cells. In contrast, SKF-96365 and Gd3+ did not alter currents generated by recombinant TMEM16A channels expressed in HEK293 cells (Online Fig. III). Next, we studied whether Ca2+ influx activates TMEM16A channels via a local signaling mechanism. This hypothesis has merit as the Ca2+-sensitivity of recombinant TMEM16A channels is lower than the physiological global [Ca2+]i range in arterial smooth muscle cells 5, 10. Consistent with our hypothesis, equimolar replacement of pipette solution EGTA for BAPTA, a fast Ca2+ chelator, abolished swelling-activated TMEM16A currents. These data indicate that swelling activates non-selective cation channels, which generate a local intracellular Ca2+ signal that stimulates TMEM16A currents, in arterial smooth muscle cells.
Figure 3. Cell swelling stimulates non-selective cation channels that activate TMEM16A currents through local Ca2+ signaling.
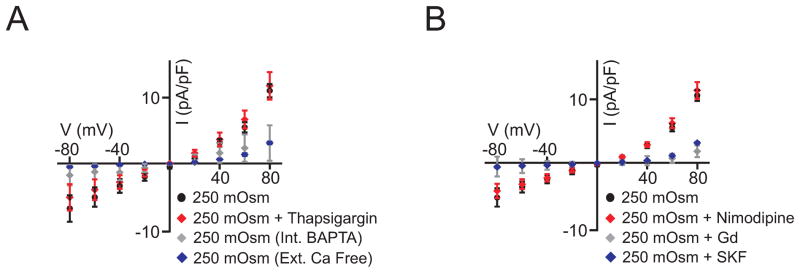
A, Ca2+-free bath (n=6) and pipette BAPTA (n=5) abolish, whereas thapsigargin (100 nmol/L, n=5) did not alter, swelling-activated TMEM16A currents (control, n=7). Data for BAPTA and Ca2+ free are significantly different (P<0.05) from 250 mOsm at −80, −60, +60, and +80 mV B, SKF96365 (n=5) and Gd3+ (n=6) abolish, whereas nimodipine (100 nmol/L, n=5) did not alter, swelling-activated TMEM16A currents (control, n=5).
Pressure-induced membrane stretch stimulates TMEM16A channels in arterial smooth muscle cells
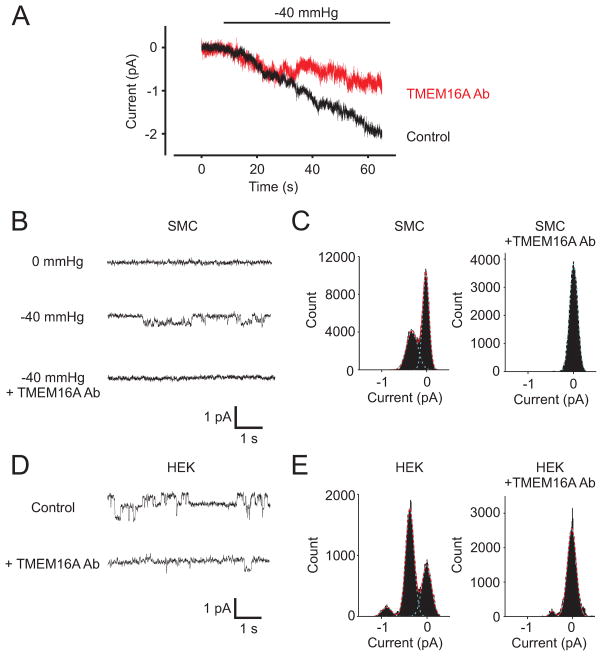
As an alternative approach to investigate ICl activation by osmolarity, negative pressure was applied to cell-attached patches to induce membrane stretch. These experiments were performed using a pipette solution containing physiological 140 mmol/L NaCl and 2 mmol/L Ca2+. Experiments were performed at −80 mV with the pipette solution containing 1 mmol/L TEA+ and 5 mmol/L 4-aminopyridine to block large conductance Ca2+-activated K+ (BKCa) and voltage-dependent K+ (Kv) channels, respectively. The bath solution contained 140 mmol/L KCl to depolarize smooth muscle cells and permit efficient voltage-clamp of the plasma membrane patch within the pipette. Application of −40 mmHg pipette pressure reversibly activated channels in membrane patches (Fig. 4A–E). In 29/37 patches, −40 mmHg pressure stimulated multiple simultaneously gating ion channels (Fig. 4A). Averaging these recordings indicated that pressure stimulated a mean current of ~2.0±0.3 pA (Fig. 4A). To determine the contribution of TMEM16A channels to these currents, experiments were repeated in the presence of the TMEM16A inhibitory antibody. The TMEM16A antibody reduced pressure-induced membrane currents by ~50 % (P<0.05, Fig. 4A). In 8/37 patches, −40 mmHg pressure activated single channels that when analyzed using all points histograms had a mean amplitude of 0.36±0.01 pA (Fig. 4B,C). Inclusion of the TMEM16A antibody in the pipette solution inhibited these single channels (Fig. 4B,C). Next, we compared the properties of these stretch-activated single channels to recombinant TMEM16A channels expressed in HEK293 cells. At −80 mV, the amplitude of single recombinant TMEM16A channels was 0.37±0.02 pA (Fig. 4D,E). This amplitude is almost identical to that of the stretch-activated, TMEM16A antibody-inhibited channels in smooth muscle cells. (Fig. 4B,C). The TMEM16A antibody also blocked recombinant TMEM16A channels (Fig. 4D,E). These data indicate that pressure-induced membrane stretch activates TMEM16A channels in arterial smooth muscle cells.
Figure 4. Pressure-induced membrane stretch activates TMEM16A channels in arterial smooth muscle cells.
A, A reduction in pipette pressure from 0 to −40 mmHg stimulates inward currents that are partially inhibited by the TMEM16A inhibitory antibody. Traces represent the average of current recordings of 29 control and 18 TMEM16A antibody-exposed patches at −80 mV. B, Exemplary recordings of single channels activated by −40 mmHg pressure and inhibited by the TMEM16A inhibitory antibody. C, All points histograms of arterial smooth muscle cell patches fit with a Gaussian function for −40 mmHg pressure in the absence and presence of the TMEM16A antibody. D, Original cell-attached recordings of recombinant TMEM16A channels expressed in HEK293 cells in the absence and presence of the TMEM16A inhibitory antibody. No patch presure was applied during experiments on HEK293 cells. E, All points histograms of recombinant TMEM16A channels fit with a Gaussian function in the absence and presence of the TMEM16A antibody.
TMEM16A channels contribute to pressure-induced arterial depolarization and constriction
To examine physiological functions of smooth muscle cell TMEM16A channels, membrane potential regulation by intravascular pressure was measured in endothelium-denuded arteries. At 10 mmHg, TMEM16A knockdown did not alter mean arterial smooth muscle cell membrane potential (Fig. 5A,B). An intravascular pressure elevation from 10 to 60 mmHg depolarized control siRNA-treated arteries from −66.8±2.3 to −35.5±1.5mV, or by ~31.3 mV (Fig. 5A,B). In contrast, the same pressure elevation depolarized TMEM16A siRNA-treated arteries by ~15.6 mV, or by ~50% of that in control arteries (Fig. 5A,B).
Figure 5. TMEM16A channels contribute to pressure-induced arterial smooth muscle cell depolarization.
A, Original membrane potential recordings at 10 and 60 mmHg in arteries treated with control siRNA and TMEM16A siRNA. Traces show intracellular microelectrode impalement followed by removal. B, Mean data (10 mmHg: control siRNA, n=5, TMEM16A siRNA, n=5. 60 mmHg: control siRNA, n=6; TMEM16A siRNA, n=6). # P<0.05 compared to control at 60 mmHg.
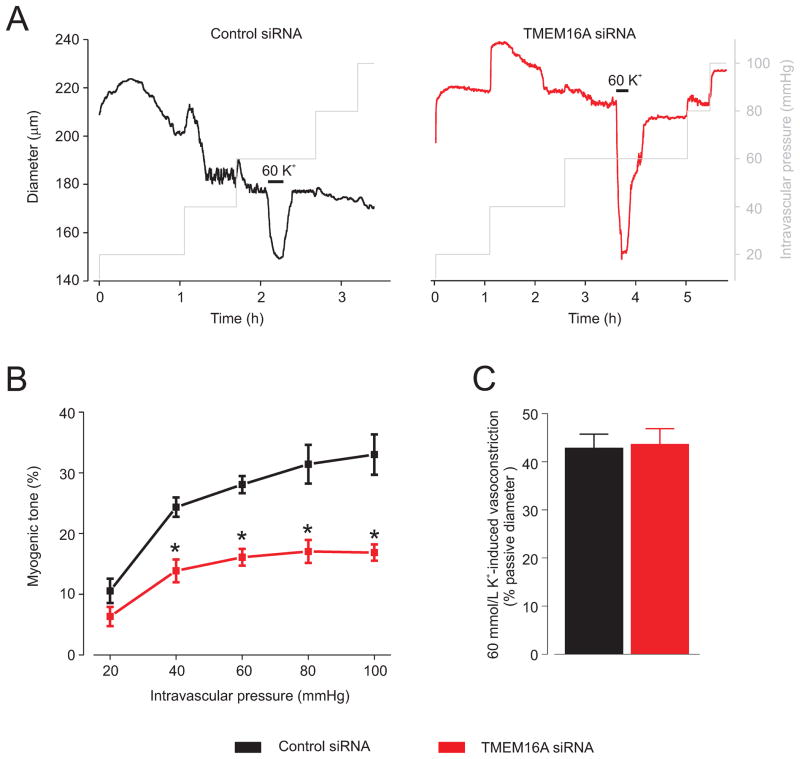
Myogenic tone was measured at intravascular pressures between 20 and 100 mmHg in endothelium-denuded arteries. Elevating intravascular pressure induced a graded elevation in myogenic tone in control siRNA-treated arteries (Fig. 6A,B). TMEM16A knockdown reduced myogenic tone at pressures between 40 and 100 mmHg by between 43 and 49% (Fig. 6A,B). In contrast, TMEM16A knockdown did not alter membrane depolarization-induced (60 mmol/L K+) vasoconstriction (Fig. 6C). TMEM16A knockdown also did not alter passive arterial diameter (control siRNA, 243.1±11.6 μm, n=7; TMEM16A siRNA, 238.4 ± 11.3 μm, n=7; P>0.05). These data indicate that intravascular pressure-induced smooth muscle cell TMEM16A channel activation contributes to arterial depolarization and thus, vasoconstriction.
Figure 6. TMEM16A channels contribute to pressure-induced vasoconstriction.
A, Exemplary traces illustrating diameter responses to increasing pressure steps and 60 mmol/L K+ at 60 mmHg in arteries treated with control siRNA and TMEM16A siRNA. B, Mean data: control siRNA, n=7, TMEM16A siRNA, n=7. C, TMEM16A knockdown did not alter 60 mmol/L K+-induced constriction. Control siRNA, n=7, TMEM16A siRNA, n=7.
DISCUSSION
The regulation of vascular contractility by anion channels is poorly understood. Similarly unclear are physiological functions and mechanisms of regulation of TMEM16A channels in contractile arterial smooth muscle cells. Here, we show that TMEM16A channels control smooth muscle cell membrane potential and contractility and contribute to the myogenic response in cerebral arteries. We show that cell swelling activates TMEM16A currents and pressure-induced membrane stretch activates single TMEM16A channels in arterial smooth muscle cells. Our data also indicate that non-selective cation channels generate a local intracellular Ca2+ signal that activates TMEM16A currents. These data provide a mechanism by which pressure-induced activation of arterial myocyte non-selective cation channels stimulates TMEM16A currents, leading to arterial smooth muscle cell depolarization and vasoconstriction.
Two distinct types of ClCa currents are present in vascular myocytes: “classic” ClCa and cGMP-dependent ClCa currents 25, 26. ClCa currents have been characterized in myocytes of several vascular beds as outwardly-rectifying Cl− currents that are activated by [Ca2+]i 3, 27. Outward rectification of classic ClCa currents at nanomolar [Ca2+]i is linearized by an elevation in [Ca2+]i 27. Cell swelling activates Cl− currents in cerebral, pulmonary and renal artery and portal vein smooth muscle cells. 8, 23, 24 A reduction in extracellular Cl− elevated myogenic tone and non-selective Cl− channel blockers hyperpolarized and dilated pressurized cerebral arteries. 7 Cl− efflux, measured using self-referencing ion-selective electrodes, also correlated with the myogenic response. 28 Our data obtained using extracellular ionic replacement, inhibitory antibodies, RNAi, and comparison of single channel properties to recombinant TMEM16A channels indicate that cell swelling, pressure-induced membrane stretch and intravascular pressure activate TMEM16A channels in arterial smooth muscle cells. We show that linearization of the I-V relationship by cell swelling and the relative permeability of swelling-activated currents to I− and Cl− is also similar to that of recombinant TMEM16A channels.9 We also demonstrate that selective TMEM16A knockdown attenuates intravascular pressure-induced arterial depolarization and vasoconstriction. These data indicate that smooth muscle cell TMEM16A channels contribute to myogenic constriction in cerebral arteries. Recent studies demonstrated that TMEM16A channels are expressed in smooth muscle cells of rat small cerebral and mouse conduit arteries, cultured rat pulmonary artery smooth muscle cells and interstitial cells of Cajal. 12–15, 29 Another recent study demonstrated that T16Ainh-A01, a TMEM16A current inhibitor with unclear selectivity, reduced a chronic hypoxia-induced elevation in serotonin contraction in rat pulmonary arteries. 30 These findings suggest that smooth muscle cell TMEM16A channels may regulate contractility not only in cerebral arteries, but in anatomically diverse vasculature and other smooth muscle cell types.
We show that removal of extracellular Ca2+ and replacement of intracellular EGTA with BAPTA abolished swelling-activated TMEM16A currents. These data are similar to those from a previous study that measured Cl− current regulation by cell swelling in smooth muscle cells of basilar artery, a large cerebral vessel. 24 In contrast, swelling-induced TMEM16A currents were not altered by thapsigargin or nimodipine, arguing against the functional involvement of SR Ca2+ release and voltage-dependent Ca2+ channels. A previous report described that swelling activated non-selective cation currents, but did not stimulate Cl− currents, in cerebral artery smooth muscle cells. 22 In this earlier study, intracellular and extracellular solutions were Ca2+-free. Therefore, these data are consistent with ours that swelling-induced Ca2+ influx activates TMEM16A currents. To determine the mechanism by which cell swelling activates TMEM16A channels, we tested the hypothesis that non-selective cation channels, which have been previously implicated in mediating myogenic constriction, were involved. This approach also permitted us to test the associated hypothesis that TMEM16A channels may be mechanosensitive. Our data show that Gd3+ and SKF96365 blocked swelling-activated TMEM16A currents, but did not alter currents generated by recombinant TMEM16A channels in HEK293 cells. These data indicate that swelling activates non-selective cation channels, leading to Ca2+ influx that stimulates TMEM16A channels. Consistent with our data, Gd3+ blocked both swelling- and pressure-induced depolarization in cerebral artery smooth muscle cells. 22 In contrast, swelling-activated Cl− currents dissimilar to classic ClCa were not inhibited by BAPTA in portal vein smooth muscle cells. 23 Depolarization-induced Cl− currents attributed to TMEM16A have been described in interstitial cells of Cajal.29 Based in part on their activation latency following stimulation, these Cl− currents have been suggested to be activated by Ca2+-induced Ca2+ release. 29 Collectively, these studies suggest that diverse mechanisms of ClCa current activation may exist in smooth muscle cells of different tissues. Future studies will determine if these different activation mechanisms also apply to regulation of TMEM16A channels. Our data indicate that a mechansosensitive mechanism stimulates non-selective cation channels that activate TMEM16A via local Ca2+ signaling in cerebral artery smooth muscle cells. These data also suggest that arterial smooth muscle cell TMEM16A currents are not directly activated by cell swelling.
The molecular identity of non-selective cation channels that stimulate TMEM16A currents was not determined here. We show that membrane stretch induced by negative pipette pressure stimulates single TMEM16A channels in arterial smooth muscle cells. In a majority (~80 %) of membrane patches, pressure activated currents to which multiple simultaneously gating channels contributed. The TMEM16A inhibitory antibody reduced stretch-activated currents by ~50 %, indicating that TMEM16A channels contribute almost half of the current. In a minority of patches (~20 %), single ion channels identical to recombinant TMEM16A channels were activated. These data suggest that TMEM16A channels may cluster in the plasma membrane with other stretch-activated channels, consistent with other evidence in this study that closely localized non-selective cation channels activate TMEM16A following membrane stretch. Several non-selective cation channels expressed in arterial smooth muscle cells are Ca2+-permeant, including multiple TRPC and TRPM subfamily members, TRPP1/2, TRPV2, and TRPV4. 2 Conceivably, one or more of these channels, including TRP heteromultimers, may control TMEM16A channel activity. Previous studies have indicated that TRPC6, TRPM4, and TRPP1/2 activation contributes to the myogenic response. 2, 31 Under physiological conditions, TRPC6 and TRPP1/2 activation would lead to both Na+ and Ca2+ influx. 32 In contrast, TRPM4 channels are primarily Na+-permeant. 32 Our data support the concept that both local Ca2+ signaling and Na+ influx mediated by non-selective cation channels contribute to pressure-induced depolarization and vasoconstriction. 2 Data indicate that cell swelling did not stimulate TMEM16A currents via activation of voltage-dependent Ca2+ channels or intracellular Ca2+ release. This finding is consistent with evidence that voltage-dependent Ca2+ channel activation does not contribute to pressure-induced depolarization, but produces the depolarization-induced global [Ca2+]i elevation, and that acute Ca2+ store depletion inhibits Ca2+ sparks, leading to vasoconstriction 1. Given the large number of potential candidates, that currently unidentified channels may be involved, more than one channel may be engaged, and heteromultimeric proteins may be involved, it was beyond the scope of this study to determine the molecular identity of non-selective cation channels that activate TMEM16A channels. Future studies should be designed to identify Ca2+-permeant ion channels that control TMEM16A channels in arterial smooth muscle cells.
The contribution of TMEM16A channels to myogenic vasoconstriction was studied between 20 and 100 mmHg, a range that encompasses physiological intravascular pressures in the cerebral circulation. Physiological cerebral artery membrane potential over this range of pressures is ~−65 to −36 mV. 5, 33 The predicted Erev for Cl− is ~−30 mV, indicating that TMEM16A channel-mediated Cl− efflux would contribute to membrane depolarization and myogenic constriction over the range of pressures studied. Elevating pressure above 100 mmHg does not further depolarize cerebral arteries with a plateau at ~−30 mV, a potential similar to the predicted Cl− Erev. 5, 33 Data here indicate that the RNAi-mediated reduction in TMEM16A protein (~43 %) and myogenic response (43–49 %) were similar over the entire pressure range. These data suggest that TMEM16A channels contribute equally to myogenic constriction over this pressure range. These observations could be interpreted as indicating that TMEM16A channels are the major contributor to pressure-induced depolarization and that the Cl− reversal potential determines maximal depolarization. However, multiple mechanisms can contribute to the pressure-induced depolarization plateau. At voltages more positive than ~−30 mV, Cl− efflux will switch polarity to influx that will oppose depolarization mediated by non-selective cation current. Pressure-induced depolarization is also opposed through the activation of K+ channels, including Kv and BKCa. 1 Although the RNAi-mediated reduction in TMEM16A protein and myogenic response were similar, the reduction in swelling-activated TMEM16A currents was larger. Explanations for this result include that a threshold level of TMEM16A protein may be required for the formation of functional ion channels in arterial smooth muscle cells. In addition, multiple processes contribute to pressure-induced depolarization and myogenic constriction, with some of these mechanisms interacting, as we show here. 2 Therefore, the partial loss of one signaling component may lead to amplification of functional effects. Future studies should examine the relative contribution of non-selective cation and TMEM16A channels to the myogenic vasoconstriction both in vitro and in vivo. This determination would require the molecular identification of the non-selective cation channels that communicate with TMEM16A.
In summary, data indicate that membrane stretch activates TMEM16A channels in arterial smooth muscle cells. TMEM16A channels regulate arterial smooth muscle cell membrane potential and contractility and contribute to the myogenic response. Data also suggest that non-selective cation channels activate TMEM16A channels through local Ca2+ signaling, leading to pressure-induced depolarization and vasoconstriction.
Supplementary Material
Novelty and Significance.
What Is Known?
Intravascular pressure stimulates arterial smooth muscle cell membrane depolarization, leading to voltage-dependent calcium (Ca2+) channel activation, an elevation in intracellular Ca2+ concentration, and vasoconstriction (the myogenic response).
Smooth muscle cell non selective cation channels, including transient receptor potential (TRP) channels, contribute to pressure-induced membrane depolarization.
Arterial smooth muscle cell chloride (Cl−) channels may also regulate vascular contractility and contribute to the myogenic response, but defining physiological functions of these channels in the vasculature has been hindered by uncertain identity of protein(s) involved and a lack of selective modulators.
What New Information Does This Article Contribute?
Cell swelling and pressure-induced membrane stretch activate TMEM16A, a Ca2+-activated Cl− channel, in cerebral artery smooth muscle cells.
Membrane stretch activates TMEM16A currents through a mechanism that involves upstream induction of non-selective cation channels that generate a local intracellular Ca2+ signal to stimulate TMEM16A.
Intravascular pressure stimulates smooth muscle cell TMEM16A channels, leading to membrane depolarization and vasoconstriction.
The myogenic response is a physiological smooth muscle-specific mechanism that controls systemic blood pressure and regional organ blood flow. Cardiovascular diseases, including hypertension, are associated with an augmented myogenic response, which elevates blood pressure and can induce end organ damage. Intravascular pressure has been proposed to activate several different non-selective cation channels, including members of the TRP family, to induce myogenic vasoconstriction. The concept that Cl− channels regulate vascular contractility has been suggested from experiments that used Cl− channel modulators with low, or uncertain, specificity. However, the molecular identity of Cl− channels that control vascular contractility and contribute to the myogenic response was unclear. Here, we used a combination of molecular, electrophysiological, and functional approaches to show that membrane stretch activates TMEM16A channels in cerebral artery smooth muscle cells. Our data suggest that a stretch-induced local intracellular Ca2+ signal generated by non-selective cation channels stimulates TMEM16A channels. Intravascular pressure-induced TMEM16A channel activation contributes to membrane depolarization and vasoconstriction. These data indicate that TMEM16A channels are one component of a mechanosensitive mechanism that contributes to the myogenic response. These results also identify a new approach to modulate the myogenic response through the manipulation of TMEM16A channel activity.
Acknowledgments
We thank Dr. Damodaran Narayanan for reading the manuscript.
SOURCES OF FUNDING
This study was supported by NHLBI/NIH grants HL67061, HL110347, and HL094378 to J.H. Jaggar.
Non-standard Abbreviations
- TMEM16A
Transmembrane protein 16A
- TRP
Transient receptor potential
- RNAi
RNA interference
- siRNA
small interfering RNA
- ClCa
Ca2+-activated chloride
- [Ca2+]I
intracellular Ca2+ concentration
- Ca2+
calcium
- K+
potassium
- Cl−
chloride
- IV
current-voltage
Footnotes
DISCLOSURES
None.
References
- 1.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91:973–83. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 2.Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 2010;119:19–36. doi: 10.1042/CS20090641. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol. 2005;83:541–56. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura K, Yamazaki J. Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn J Pharmacol. 2001;85:351–7. doi: 10.1254/jjp.85.351. [DOI] [PubMed] [Google Scholar]
- 5.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 7.Nelson MT, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. J Physiol. 1997;502:259–64. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. J Physiol. 1998;507:729–36. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–29. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 11.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–4. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 12.Thomas-Gatewood C, Neeb ZP, Bulley S, Adebiyi A, Bannister JP, Leo MD, Jaggar JH. TMEM16A channels generate Ca2+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2011;301:1819–27. doi: 10.1152/ajpheart.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis AJ, Forrest AS, Jepps TA, Valencik ML, Wiwchar M, Singer CA, Sones WR, Greenwood IA, Leblanc N. Expression profile and protein translation of TMEM16A in murine smooth muscle. Am J Physiol Cell Physiol. 2010;299:C948–C959. doi: 10.1152/ajpcell.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manoury B, Tamuleviciute A, Tammaro P. TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J Physiol. 2010;588:2305–14. doi: 10.1113/jphysiol.2010.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, Du YH, Lv XF, Liu J, Zhou JG, Guan YY. Downregulation of TMEM16A calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]
- 16.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 17.Adebiyi A, Zhao G, Narayanan D, Thomas CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–12. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell a2d-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res. 2009;105:948–55. doi: 10.1161/CIRCRESAHA.109.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannister JP, Thomas-Gatewood CM, Neeb ZP, Adebiyi A, Cheng X, Jaggar JH. CaV1.2 channel N-terminal splice variants modulate functional surface expression in resistance size artery smooth muscle cells. J Biol Chem. 2011;286:15058–66. doi: 10.1074/jbc.M110.182816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesh RE, Somlyo AP, Owens GK, Somlyo AV. Reversible permeabilization. A novel technique for the intracellular introduction of antisense oligodeoxynucleotides into intact smooth muscle. Circ Res. 1995;77:220–30. doi: 10.1161/01.res.77.2.220. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh DG, Nelson MT, Eckman DM, Brayden JE. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J Physiol. 2000;527:139–48. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood IA, Large WA. Properties of a Cl− current activated by cell swelling in rabbit portal vein vascular smooth muscle cells. Am J Physiol. 1998;275:H1524–H1532. doi: 10.1152/ajpheart.1998.275.5.H1524. [DOI] [PubMed] [Google Scholar]
- 24.Yano S, Ishikawa T, Tsuda H, Obara K, Nakayama K. Ionic mechanism for contractile response to hyposmotic challenge in canine basilar arteries. Am J Physiol Cell Physiol. 2005;288:C702–C709. doi: 10.1152/ajpcell.00367.2003. [DOI] [PubMed] [Google Scholar]
- 25.Matchkov VV, Larsen P, Bouzinova EV, Rojek A, Boedtkjer DM, Golubinskaya V, Pedersen FS, Aalkjaer C, Nilsson H. Bestrophin-3 (vitelliform macular dystrophy 2-like 3 protein) is essential for the cGMP-dependent calcium-activated chloride conductance in vascular smooth muscle cells. Circ Res. 2008;103:864–72. doi: 10.1161/CIRCRESAHA.108.178517. [DOI] [PubMed] [Google Scholar]
- 26.Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R. Bestrophin and TMEM16-Ca2+ activated Cl− channels with different functions. Cell Calcium. 2009;46:233–41. doi: 10.1016/j.ceca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- 28.Doughty JM, Langton PD. Measurement of chloride flux associated with the myogenic response in rat cerebral arteries. J Physiol. 2001;534:753–61. doi: 10.1111/j.1469-7793.2001.t01-1-00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–18. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Xia Y, Paudel O, Yang XR, Sham JS. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol. 2012 doi: 10.1113/jphysiol.2012.232520. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell. 2009;139:587–96. doi: 10.1016/j.cell.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 32.Clapham DE, Julius D, Montell C, Schultz G International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–50. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 33.Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behavior. Am J Physiol. 2002;283:H2260–H2267. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.