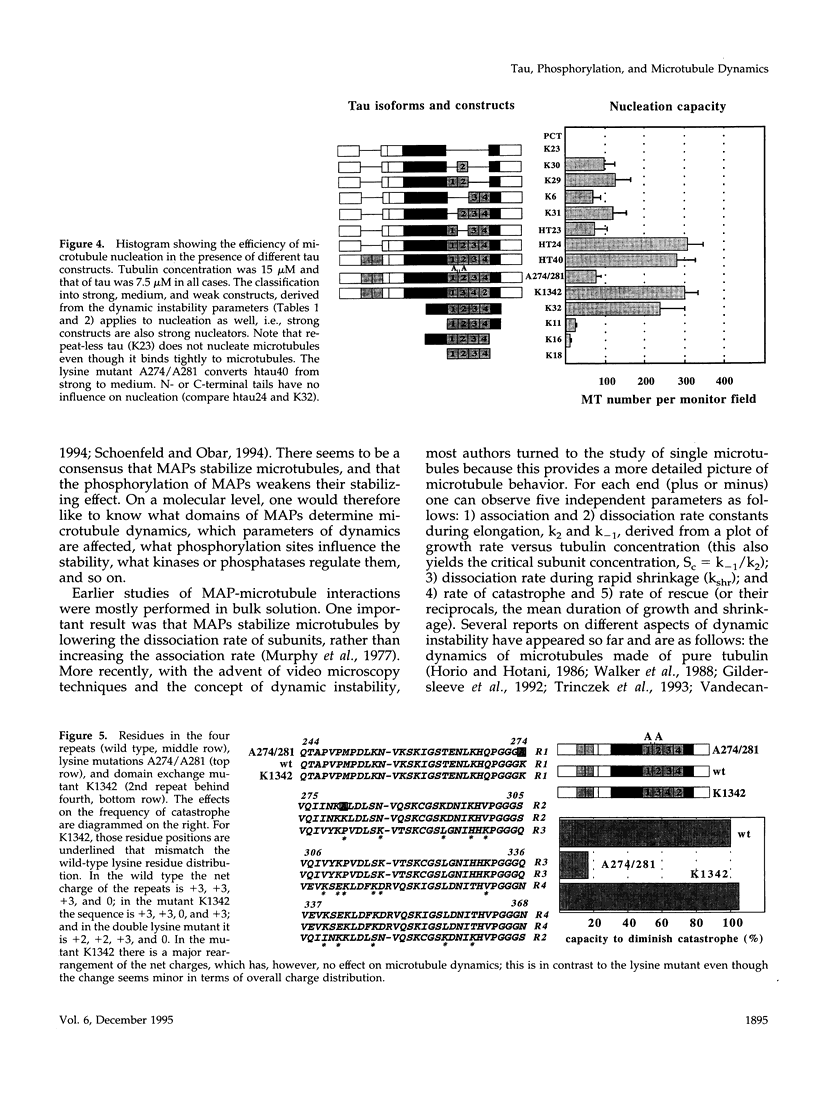
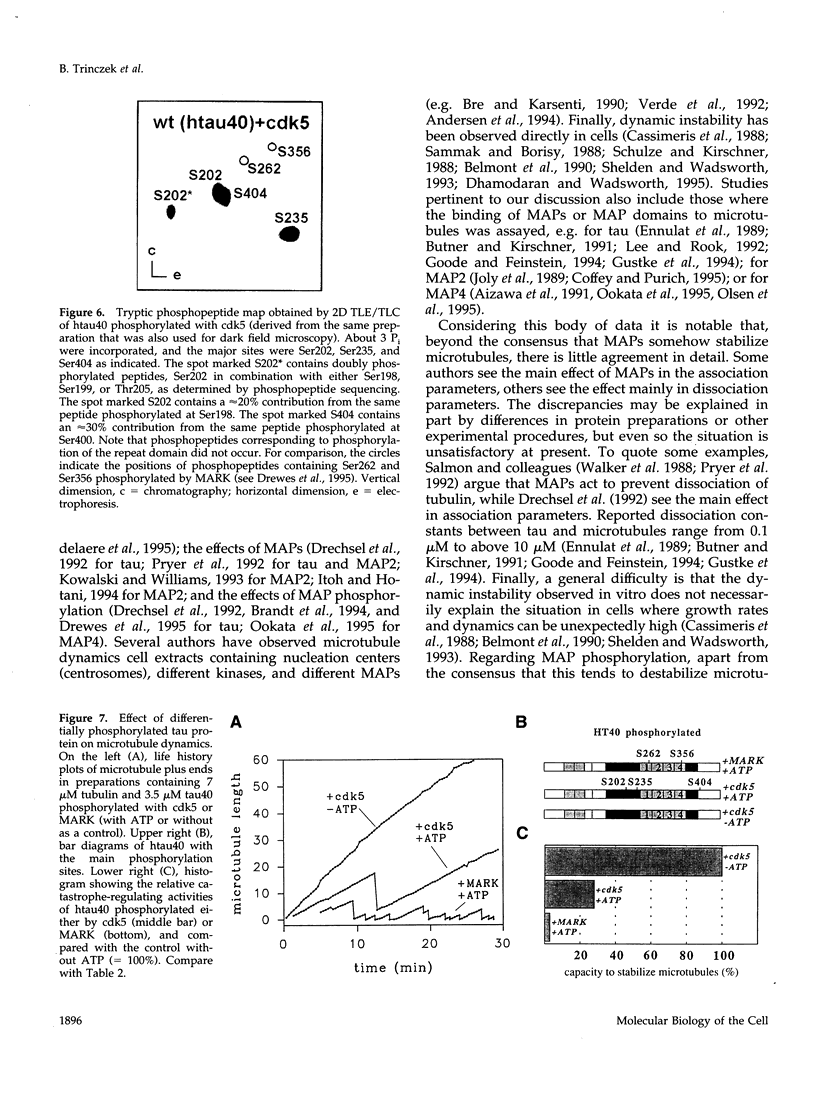
Abstract
The dynamic instability of microtubules is thought to be regulated by MAPs and phosphorylation. Here we describe the effect of the neuronal microtubule-associated protein tau by observing the dynamics of single microtubules by video microscopy. We used recombinant tau isoforms and tau mutants, and we phosphorylated tau by the neuronal kinases MARK (affecting the KXGS motifs within tau's repeat domain) and cdk5 (phosphorylating Ser-Pro motifs in the regions flanking the repeats). The variants of tau can be broadly classified into three categories, depending on their potency to affect microtubule dynamics. "Strong" tau variants have four repeats and both flanking regions. "Medium" variants have one to three repeats and both flanking regions. "Weak" variants lack one or both of the flanking regions, or have no repeats; with such constructs, microtubule dynamics is not significantly different from that of pure tubulin. N- or C-terminal tails of tau have no influence on dynamic instability. The two ends of microtubules (plus and minus) showed different activities but analogous behavior. These results are consistent with the "jaws" model of tau where the flanking regions are considered as targeting domains whereas the addition of repeats makes them catalytically active in terms of microtubule stabilization. The dominant changes in the parameters of dynamic instability induced by tau are those in the dissociation rate and in the catastrophe rate (up to 30-fold). Other rates change only moderately or not at all (association rate increased up to twofold, rates of rescue or rapid shrinkage decreased up to approximately twofold). The order of repeats has little influence on microtubule dynamics (i.e., repeats can be re-arranged or interchanged), arguing in favor of the "distributed weak binding" model proposed by Butner and Kirschner (1991); however, we confirmed the presence of a "hotspot" of binding potential involving Lys274 and Lys281 observed by Goode and Feinstein, 1994. Phosphorylation of Ser-Pro motifs by cdk5 (mainly Ser 202, 235, and 404) in the flanking regions had a moderate effect on microtubule dynamics while phosphorylation at the "Alzheimer"-site Ser262 MARK eliminated tau's interactions with microtubules. In both cases the predominant effects of phosphorylation are on the rates of tubulin dissociation and catastrophe whereas the effects on the rates of association or rescue are comparatively small.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Emori Y., Mori A., Murofushi H., Sakai H., Suzuki K. Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U). J Biol Chem. 1991 May 25;266(15):9841–9846. [PubMed] [Google Scholar]
- Andersen S. S., Buendia B., Domínguez J. E., Sawyer A., Karsenti E. Effect on microtubule dynamics of XMAP230, a microtubule-associated protein present in Xenopus laevis eggs and dividing cells. J Cell Biol. 1994 Dec;127(5):1289–1299. doi: 10.1083/jcb.127.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K., Mandelkow E. M., Biernat J., Piwnica-Worms H., Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993 Dec 28;336(3):417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- Beaudette K. N., Lew J., Wang J. H. Substrate specificity characterization of a cdc2-like protein kinase purified from bovine brain. J Biol Chem. 1993 Oct 5;268(28):20825–20830. [PubMed] [Google Scholar]
- Bell C. W., Fraser C., Sale W. S., Tang W. J., Gibbons I. R. Preparation and purification of dynein. Methods Cell Biol. 1982;24:373–397. doi: 10.1016/s0091-679x(08)60666-4. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Biernat J., Gustke N., Drewes G., Mandelkow E. M., Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993 Jul;11(1):153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Brandt R., Lee G., Teplow D. B., Shalloway D., Abdel-Ghany M. Differential effect of phosphorylation and substrate modulation on tau's ability to promote microtubule growth and nucleation. J Biol Chem. 1994 Apr 22;269(16):11776–11782. [PubMed] [Google Scholar]
- Brugg B., Matus A. Phosphorylation determines the binding of microtubule-associated protein 2 (MAP2) to microtubules in living cells. J Cell Biol. 1991 Aug;114(4):735–743. doi: 10.1083/jcb.114.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bré M. H., Karsenti E. Effects of brain microtubule-associated proteins on microtubule dynamics and the nucleating activity of centrosomes. Cell Motil Cytoskeleton. 1990;15(2):88–98. doi: 10.1002/cm.970150205. [DOI] [PubMed] [Google Scholar]
- Butner K. A., Kirschner M. W. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991 Nov;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., Pryer N. K., Salmon E. D. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin S. J., Bulinski J. C. Microtubule stabilization by assembly-promoting microtubule-associated proteins: a repeat performance. Cell Motil Cytoskeleton. 1992;23(4):236–243. doi: 10.1002/cm.970230403. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Coffey R. L., Purich D. L. Non-cooperative binding of the MAP-2 microtubule-binding region to microtubules. J Biol Chem. 1995 Jan 20;270(3):1035–1040. doi: 10.1074/jbc.270.3.1035. [DOI] [PubMed] [Google Scholar]
- Correas I., Díaz-Nido J., Avila J. Microtubule-associated protein tau is phosphorylated by protein kinase C on its tubulin binding domain. J Biol Chem. 1992 Aug 5;267(22):15721–15728. [PubMed] [Google Scholar]
- Dhamodharan R., Wadsworth P. Modulation of microtubule dynamic instability in vivo by brain microtubule associated proteins. J Cell Sci. 1995 Apr;108(Pt 4):1679–1689. doi: 10.1242/jcs.108.4.1679. [DOI] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G., Lichtenberg-Kraag B., Döring F., Mandelkow E. M., Biernat J., Goris J., Dorée M., Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992 Jun;11(6):2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes G., Trinczek B., Illenberger S., Biernat J., Schmitt-Ulms G., Meyer H. E., Mandelkow E. M., Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995 Mar 31;270(13):7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- Ennulat D. J., Liem R. K., Hashim G. A., Shelanski M. L. Two separate 18-amino acid domains of tau promote the polymerization of tubulin. J Biol Chem. 1989 Apr 5;264(10):5327–5330. [PubMed] [Google Scholar]
- Geisler N., Heimburg T., Schünemann J., Weber K. Peptides from the conserved ends of the rod domain of desmin disassemble intermediate filaments and reveal unexpected structural features: a circular dichroism, Fourier transform infrared, and electron microscopic study. J Struct Biol. 1993 May-Jun;110(3):205–214. doi: 10.1006/jsbi.1993.1023. [DOI] [PubMed] [Google Scholar]
- Gildersleeve R. F., Cross A. R., Cullen K. E., Fagen A. P., Williams R. C., Jr Microtubules grow and shorten at intrinsically variable rates. J Biol Chem. 1992 Apr 25;267(12):7995–8006. [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goode B. L., Feinstein S. C. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol. 1994 Mar;124(5):769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustke N., Trinczek B., Biernat J., Mandelkow E. M., Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994 Aug 16;33(32):9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Morishima-Kawashima M., Takio K., Suzuki M., Titani K., Ihara Y. Protein sequence and mass spectrometric analyses of tau in the Alzheimer's disease brain. J Biol Chem. 1992 Aug 25;267(24):17047–17054. [PubMed] [Google Scholar]
- Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994 Feb;6(1):74–81. doi: 10.1016/0955-0674(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Ishiguro K., Omori A., Sato K., Tomizawa K., Imahori K., Uchida T. A serine/threonine proline kinase activity is included in the tau protein kinase fraction forming a paired helical filament epitope. Neurosci Lett. 1991 Jul 22;128(2):195–198. doi: 10.1016/0304-3940(91)90259-v. [DOI] [PubMed] [Google Scholar]
- Itoh T. J., Hotani H. Microtubule-stabilizing activity of microtubule-associated proteins (MAPs) is due to increase in frequency of rescue in dynamic instability: shortening length decreases with binding of MAPs onto microtubules. Cell Struct Funct. 1994 Oct;19(5):279–290. doi: 10.1247/csf.19.279. [DOI] [PubMed] [Google Scholar]
- Joly J. C., Flynn G., Purich D. L. The microtubule-binding fragment of microtubule-associated protein-2: location of the protease-accessible site and identification of an assembly-promoting peptide. J Cell Biol. 1989 Nov;109(5):2289–2294. doi: 10.1083/jcb.109.5.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y., Chen J., Hirokawa N. Microtubule bundling by tau proteins in vivo: analysis of functional domains. EMBO J. 1992 Nov;11(11):3953–3961. doi: 10.1002/j.1460-2075.1992.tb05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops J., Kosik K. S., Lee G., Pardee J. D., Cohen-Gould L., McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991 Aug;114(4):725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Ishiguro K., Omori A., Takamatsu M., Arioka M., Imahori K., Uchida T. A cdc2-related kinase PSSALRE/cdk5 is homologous with the 30 kDa subunit of tau protein kinase II, a proline-directed protein kinase associated with microtubule. FEBS Lett. 1993 Dec 6;335(2):171–175. doi: 10.1016/0014-5793(93)80723-8. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., McConlogue L. Microtubule-associated protein function: lessons from expression in Spodoptera frugiperda cells. Cell Motil Cytoskeleton. 1994;28(3):195–198. doi: 10.1002/cm.970280302. [DOI] [PubMed] [Google Scholar]
- Kowalski R. J., Williams R. C., Jr Microtubule-associated protein 2 alters the dynamic properties of microtubule assembly and disassembly. J Biol Chem. 1993 May 5;268(13):9847–9855. [PubMed] [Google Scholar]
- Ledesma M. D., Correas I., Avila J., Díaz-Nido J. Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer's disease. FEBS Lett. 1992 Aug 17;308(2):218–224. doi: 10.1016/0014-5793(92)81278-t. [DOI] [PubMed] [Google Scholar]
- Lee G., Rook S. L. Expression of tau protein in non-neuronal cells: microtubule binding and stabilization. J Cell Sci. 1992 Jun;102(Pt 2):227–237. doi: 10.1242/jcs.102.2.227. [DOI] [PubMed] [Google Scholar]
- Lichtenberg-Kraag B., Mandelkow E. M., Biernat J., Steiner B., Schröter C., Gustke N., Meyer H. E., Mandelkow E. Phosphorylation-dependent epitopes of neurofilament antibodies on tau protein and relationship with Alzheimer tau. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5384–5388. doi: 10.1073/pnas.89.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M. M., Fieles A. W., Norris T. E., Dargis P. G., Caputo C. B., Scott C. W., Lee V. M., Goedert M. Human tau isoforms confer distinct morphological and functional properties to stably transfected fibroblasts. Brain Res Mol Brain Res. 1993 Nov;20(3):209–220. doi: 10.1016/0169-328x(93)90043-o. [DOI] [PubMed] [Google Scholar]
- Léger J. G., Brandt R., Lee G. Identification of tau protein regions required for process formation in PC12 cells. J Cell Sci. 1994 Dec;107(Pt 12):3403–3412. doi: 10.1242/jcs.107.12.3403. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Drewes G., Biernat J., Gustke N., Van Lint J., Vandenheede J. R., Mandelkow E. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 1992 Dec 21;314(3):315–321. doi: 10.1016/0014-5793(92)81496-9. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Tau as a marker for Alzheimer's disease. Trends Biochem Sci. 1993 Dec;18(12):480–483. doi: 10.1016/0968-0004(93)90011-b. [DOI] [PubMed] [Google Scholar]
- Meyer H. E., Hoffmann-Posorske E., Heilmeyer L. M., Jr Determination and location of phosphoserine in proteins and peptides by conversion to S-ethylcysteine. Methods Enzymol. 1991;201:169–185. doi: 10.1016/0076-6879(91)01016-u. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Johnson K. A., Borisy G. G. Role of tubulin-associated proteins in microtubule nucleation and elongation. J Mol Biol. 1977 Nov 25;117(1):33–52. doi: 10.1016/0022-2836(77)90021-3. [DOI] [PubMed] [Google Scholar]
- Obar R. A., Dingus J., Bayley H., Vallee R. B. The RII subunit of cAMP-dependent protein kinase binds to a common amino-terminal domain in microtubule-associated proteins 2A, 2B, and 2C. Neuron. 1989 Nov;3(5):639–645. doi: 10.1016/0896-6273(89)90274-2. [DOI] [PubMed] [Google Scholar]
- Olson K. R., McIntosh J. R., Olmsted J. B. Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J Cell Biol. 1995 Aug;130(3):639–650. doi: 10.1083/jcb.130.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Bulinski J. C., Murofushi H., Aizawa H., Itoh T. J., Hotani H., Okumura E., Tachibana K., Kishimoto T. Cyclin B interaction with microtubule-associated protein 4 (MAP4) targets p34cdc2 kinase to microtubules and is a potential regulator of M-phase microtubule dynamics. J Cell Biol. 1995 Mar;128(5):849–862. doi: 10.1083/jcb.128.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel H. K., Lew J., Ali Z., Wang J. H. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J Biol Chem. 1993 Nov 5;268(31):23512–23518. [PubMed] [Google Scholar]
- Preuss U., Döring F., Illenberger S., Mandelkow E. M. Cell cycle-dependent phosphorylation and microtubule binding of tau protein stably transfected into Chinese hamster ovary cells. Mol Biol Cell. 1995 Oct;6(10):1397–1410. doi: 10.1091/mbc.6.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer N. K., Walker R. A., Skeen V. P., Bourns B. D., Soboeiro M. F., Salmon E. D. Brain microtubule-associated proteins modulate microtubule dynamic instability in vitro. Real-time observations using video microscopy. J Cell Sci. 1992 Dec;103(Pt 4):965–976. doi: 10.1242/jcs.103.4.965. [DOI] [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Schoenfeld T. A., Obar R. A. Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system. Int Rev Cytol. 1994;151:67–137. doi: 10.1016/s0074-7696(08)62631-5. [DOI] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. New features of microtubule behaviour observed in vivo. Nature. 1988 Jul 28;334(6180):356–359. doi: 10.1038/334356a0. [DOI] [PubMed] [Google Scholar]
- Schweers O., Mandelkow E. M., Biernat J., Mandelkow E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8463–8467. doi: 10.1073/pnas.92.18.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweers O., Schönbrunn-Hanebeck E., Marx A., Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290–24297. [PubMed] [Google Scholar]
- Scott C. W., Spreen R. C., Herman J. L., Chow F. P., Davison M. D., Young J., Caputo C. B. Phosphorylation of recombinant tau by cAMP-dependent protein kinase. Identification of phosphorylation sites and effect on microtubule assembly. J Biol Chem. 1993 Jan 15;268(2):1166–1173. [PubMed] [Google Scholar]
- Shelden E., Wadsworth P. Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993 Feb;120(4):935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990 Nov;9(11):3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Toso R. J., Jordan M. A., Farrell K. W., Matsumoto B., Wilson L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry. 1993 Feb 9;32(5):1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- Trinczek B., Marx A., Mandelkow E. M., Murphy D. B., Mandelkow E. Dynamics of microtubules from erythrocyte marginal bands. Mol Biol Cell. 1993 Mar;4(3):323–335. doi: 10.1091/mbc.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecandelaere A., Martin S. R., Bayley P. M. Regulation of microtubule dynamic instability by tubulin-GDP. Biochemistry. 1995 Jan 31;34(4):1332–1343. doi: 10.1021/bi00004a028. [DOI] [PubMed] [Google Scholar]
- Verde F., Dogterom M., Stelzer E., Karsenti E., Leibler S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol. 1992 Sep;118(5):1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulliet R., Halloran S. M., Braun R. K., Smith A. J., Lee G. Proline-directed phosphorylation of human Tau protein. J Biol Chem. 1992 Nov 5;267(31):22570–22574. [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Ihara Y. Tau in paired helical filaments is functionally distinct from fetal tau: assembly incompetence of paired helical filament-tau. J Neurochem. 1993 Sep;61(3):1183–1186. doi: 10.1111/j.1471-4159.1993.tb03642.x. [DOI] [PubMed] [Google Scholar]



