Abstract
A concatenation of findings from preclinical and clinical studies support a preeminent role for the corticotropin-releasing factor (CRF) system in mediating the physiological response to external stressors and in the pathophysiology of anxiety and depression. Recently, human genetic studies have provided considerable support to several long-standing hypotheses of mood and anxiety disorders, including the CRF hypothesis. These data, reviewed in this report, are congruent with the hypothesis that this system is of paramount importance in mediating stress-related psychopathology. More specifically variants in the gene encoding the CRF1 receptor interact with adverse environmental factors to predict risk for stress-related psychiatric disorders. In depth characterization of these variants will likely be important in furthering our understanding of the long term consequences of adverse experience.
Keywords: CRF, CRH, receptor, genetic, depression, anxiety
Introduction
Soon after the isolation and chemical characterization of corticotrophin-releasing factor (CRF) from ovine hypothalamus more than 25 years ago, the 41 amino acid containing peptide was unequivocally established as the major physiological regulator of hypothalamic-pituitary-adrenal (HPA) axis activity (1). It’s preeminent role in mediating stress-related behaviors in rodents and primates was quickly demonstrated and hypothesized to be important in the pathogenesis of mood and anxiety disorders (2–8). These early findings were followed by a series of clinical studies and additional laboratory animal experiments that support the hypothesis that alterations in central nervous system (CNS) CRF-containing circuits play an important role in the pathophysiology of anxiety and depression (see (9–11) for review).
Because the CRF hypothesis of depression has previously been comprehensively reviewed by our group and others, this monograph will only briefly summarize the CRF system which contains 4 cloned ligands and 2 receptors, as well as major findings derived from animal and human studies that relate this system to stress, anxiety and depression. Our main focus, hitherto not comprehensively reviewed, is on recent developments in the human molecular genetics of the CRF system, how these findings further support the CRF hypothesis of depression and anxiety, and a discussion of future research directions.
The CRF system
Since the initial isolation of ovine CRF in 1981, 3 related ligands and 2 receptors have been identified (Hauger et al 2003 for review (12)).
As noted above, CRF is a 41 amino-acid neuropeptide, expressed both peripherally (blood vessels, skin, lung, testes, ovaries and placenta) as well as in the CNS -with highest expression in the hypothalamus, amygdala, cerebrocortical areas and the septum (13, 14). Urocortin 1, urocortin 2 (stresscopin-related peptide), and urocortin 3 (stresscopin) are CRF-like neuropeptides (15–18). Human urocortin 1 shares approximately 45% homology to human CRF. In the CNS, urocortin 1 is expressed most strongly in the Edinger-Westphal nucleus and the hypothalamus (19, 20), but overall its expression is more widespread peripherally, including in the pituitary, gastrointestinal tract, testes, cardiac myocytes, thymus, spleen and kidney (13, 20, 21). Urocortin 2 and 3 are also expressed both in the CNS and the periphery. Urocortin 2 is expressed in the magnocellular portion of the hypothalamic paraventricular and arcuate nucleus, the locus coeruleus, brainstem and spinal motor neurons; its expression pattern partially overlaps with that of CRF (17). Urocortin 3 is concentrated in the median preoptic area, the rostral perifornical area, the bed nucleus of the stria terminalis and the medial nucleus of the amygdala (16). Its expression pattern shows little overlap with CRF expression. In the periphery, urocortin 2 has been found in the heart, adrenal gland and peripheral blood cells whereas urocortin 3 has been localized in the gastrointestinal tract, muscle, adrenal gland and skin (13, 22).
The available evidence suggests that these 4 peptide neurotransmitters bind to 2 identified receptors, CRF1 and CRF2 (Hauger et al 2003 for review(12)). CRF and urocortin 1 both bind with high affinity to the CRF1 receptor, whereas urocortin 1, 2 and 3 but not CRF, bind with high affinity to the CRF2 receptor. Both CRF receptors are 7-transmembrane G-protein coupled receptors (GPCRs) and share 70% sequence homology. Both receptors are present in the CNS. CRF1 is expressed in high density in the cerebral cortex, cerebellum, hippocampus, amygdala and pituitary; its peripheral expression is less robust and concentrated to skin, ovaries and testes and the adrenal gland. The CRF2 receptor is also expressed in the CNS, but largely restricted to subcortical areas, including the hypothalamus, amygdala, bed nucleus of the stria terminalis and raphe nuclei, and in peripheral tissues, such as the pituitary, heart, lungs, ovaries, testes and adrenal gland (14, 23–26).
One unique characteristic of this system is that the biological activity of all four peptides is regulated by a CRF-binding protein (CRFBP), which is highly conserved in mammalian as well as non-mammalian species (27–29). It is present in the circulation and the interstitial spaces as a soluble 37 kDa glycoprotein that binds CRF and all urocortins with high affinity, reduces their bioavailability and prevents their binding to CRF receptors (27). CRFBP is highly expressed in a series of tissues, including brain, heart, intestines, lungs and placenta (13, 30, 31). In the CNS, CRFBP is present in all CRF-related pathways and the pituitary. The CRFBP is partially colocalized with CRF, as well as CRF receptor expression, thereby permitting its role in modulation of CRF neurotransmission (30).
The CRF system, stress, depression and anxiety
The CRF system and the stress response
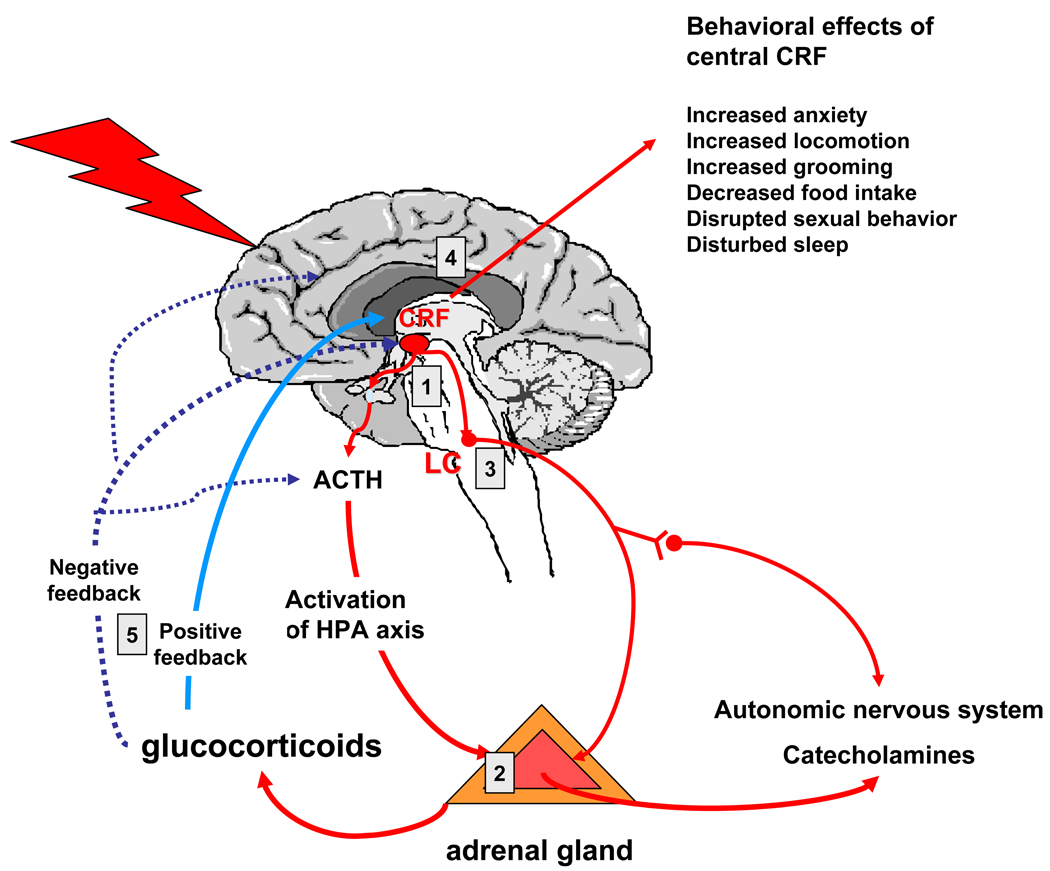
The CRF system plays a multitude of physiological roles that are apparently related to orchestrating the stress response at several different anatomical levels (see figure 1). In addition to its well documented role as a hypothalamic hypophysiotropic factor that stimulates pituitary ACTH synthesis and secretion and thereby controls the activity of the HPA axis (1), CRF neurons also innervate the locus coeruleus, thus activating the other major stress response axis, the CNS noradrenergic and sympathetic nervous systems (32). The CRF system also locally regulates adrenal steroidogenesis and catecholamine synthesis and release from the adrenal gland (33), again, dually influencing the HPA axis and norepinephrine and epinephrine secretion. Furthermore, effects of CRF in limbic brain regions have been associated with increased fear, alertness, decreased appetite and libido, all functions relevant in the fight or flight response and dysregulated in depression and anxiety disorders (9). These effects appear to be mediated mainly by the CRF1 receptor (10, 34, 35). The role of the CRF2 receptor remains more obscure and likely context and brain region dependent. Anxiolytic effects in a brain region-specific and stress level-dependent manner have been described (35–44). Data from CRF2 receptor transgenic animals suggest an inhibitory role on adrenocortical function (38, 45). Overall, it appears that the CRF1 receptor is the principal receptor mediating the stress response, whereas the CRF2 receptor modulates the effects of CRF1 signal transduction (10, 11, 46).
Figure 1. The actions of CRF at different levels of the stress response.
1- CRF nerve terminal in the median eminence release CRF into the hypothalamo-hypophyseal portal system and stimulate ACTH release from the anterior pituitary.
2- CRF directly stimulates cortisol and cathecholamine synthesis from the adrenal gland
3- CRF stimulates noradrenergic neurons in the locus coeruleus
4- CRF mediates a series of behaviors through actions on cortical and limbic brain regions.
5- CRF transcription is negatively regulated by glucocorticoids in the hypothalamus, but positive regulation has been reported in limbic brain regions.
The CRF system and depression
Laboratory animal studies in which brain intracerebroventricular (icv) or brain region-specific microinjections of CRF has been employed have revealed that CRF produces behavioral responses reminiscent of major depression in humans, including increased anxiety, reduced slow wave sleep, psychomotor alterations, anhedonia, decreased appetite and libido (47, 48). These studies are complemented by results obtained in transgenic animals either lacking or over-expressing CRF system ligands or receptors, as well as from studies using selective CRF antagonists. This large database indicates that these behaviors are mainly mediated by CRF1 receptor activation and modulated by CRF2 receptors (10, 49). Conditional CRF1 receptor knock-out mice and CRF overexpressing mice restricted to forebrain areas have further demonstrated that these anxiety- and depression-related phenotypes are specific to activation of the CRF1 receptor in limbic forebrain regions and independent of actions on HPA axis activity (50, 51), though the latter endocrine effects of CRF may indeed contribute to the depressive symptoms. While a negative effect of glucocorticoid receptor activation on CRF expression has been described for the hypothalamus, glucorticoids were shown to increase CRF expression in limbic areas, including the amygdale and the lateral septum (52, 53). In support of the critical role on limbic CRF transmission, recently increased CRF expression in the amygdala induced by use of a lentiviral vector was shown to produce most of the behavioral effects that comprise the depressive syndrome, as well as HPA-axis hyperactivity (54).
In humans, increased CSF CRF concentrations have been repeatedly observed in major depression (8, 55, 56) and suicide victims (57). Moreover, treatment with either electroconvulsive therapy (58) or antidepressants (59–61) reduces CSF CRF concentrations. Notably, persistent elevations of CSF CRF concentration in symptomatically improved depressed patients is associated with early relapse of depression (62).
A series of postmortem studies have provided compelling evidence of increased CRF neurotransmission in major depression and suicide. CRF expression (both mRNA and peptide) is increase in the hypothalamus, cortical areas, pontine nuclei and the locus coeruleus (63–66). This is paralleled by a down regulation of CRF1 but not CRF2, receptors in cortical areas of suicide victims, all pointing to an overactive CRF/CRF1 receptor system in depression (67, 68). In addition, CRFBP has been found to be down-regulated in the amygdala of patients with bipolar disorder (69) and urocortin 1 expression is upregulated in the Edinger-Westphal nuclei of suicide victims (70). The relevance of overactive limbic CRF1 receptor transmission in depression is underlined by the fact that selective CRF1 receptor antagonists exert antidepressant effects at doses that do not influence baseline or stimulated HPA axis activation (71, 72). It is important to note that, although not all studies with CRF1 receptor antagonists are positive in major depression, lack of a CRF1 receptor positron emission tomography (PET) ligand renders choice of dose problematic (73).
The CRF system and early trauma
Overactivity of the CRF/CRF1 receptor system also has been demonstrated to be one of the long term neurobiological sequelae of early life trauma, a major risk factor for the development of affective disorders (74, 75). In fact, both rodents and non-human primates exposed to adverse experiences in early life exhibit evidence of hyperactivity of the CRF system as adults. Bonnet macaques reared under stressful conditions exhibit higher CSF CRF concentrations as adults than monkeys reared under non-stressful condition (76). Similar neuroendocrine results have been obtained in Rhesus monkeys (77). In rodents early life stress is associated with persistent alterations in the CRF system, including increased CRF concentrations, increased CRF mRNA expression, and altered CRF receptor expression and binding in the hypothalamus and limbic brain regions (78–80). This overactivity of the CNS CRF system is paralleled by a hyper-reactive HPA axis response to stress in these animals. These findings in laboratory animals are consistent with findings in humans where early life trauma is a strong predictor of CSF CRF concentrations in adults (81–83) and is associated with an enhanced stress response to standardized psychosocial stressors, such as the Trier Social Stress Test (TSST). In addition in endocrine challenge tests, including the CRF stimulation test and the combined dexamethasone suppression/CRF stimulation test (84–87), patients with early life trauma exhibit evidence of marked HPA-axis hyperactivity.
The CRF system and anxiety disorders
Because of its anxiogenic effects in laboratory animals, the CRF system has also been implicated in the pathophysiology of anxiety disorders (10, 88, 89). Increased CSF CRF concentrations have been reported in PTSD (90–92), but studies in adults with panic disorder and generalized anxiety disorder have failed to demonstrate such abnormalities (93–95).
There is evidence from a series of animal studies that the CRF system is also highly relevant to alcohol dependence (96), particularly during alcohol withdrawal (97), and depression and anxiety disorders exhibit high rates of comorbidity with alcoholism. Indeed several studies have revealed that acute alcohol withdrawal is associated with marked increases in CSF CRF concentrations (98, 99). It is also worth noting that clinically effective benzodiazepine anxiolytics such as alprazolam reduce CRF-ergic activity (100).
In summary, over 3 decades of research have implicated CRF circuits in the pathophysiology of depression and certain anxiety disorders, particularly PTSD, as well as in mediating the long term impact of early trauma. We refer the reader to a series of review articles (10, 11, 46, 101) that comprehensively review the above topics in considerably more detail. This review focuses on the human genetics of the CRF system and how recent advances in this area inform the field on the critical role of the CRF system in depression and anxiety.
Human genetic studies
Susceptibility to depressive or anxiety disorders is now well established to be due to the combined effect of genes and the environment, with heritability estimates for these disorders ranging from about 30% to 40% (102–106). The CRF system, being highly responsive to the environment, has been posited to serve as a key interface between environmental stressors and the development of depression. In fact, differences in the function of the CRF system due to genetic variation may lead to differences in an individual’s response to stressful events and may regulate an individual’s vulnerability to stress-related psychiatric disorders.
Table 1 lists the genes encoding all members of the CRF system with their human chromosomal position.
Table 1.
Genomic position of the genes encoding the ligands and receptors of the CRF system.
| Gene: Symbol |
Gene Product Name | REFSEQ ID ENTREZ Gene ID |
position on chromosome |
Number of exons |
gene size (bases) |
|---|---|---|---|---|---|
| CRH | corticotropin releasing factor (CRF) or hormone (CRH) |
NM_000756, 1392 |
8q13.1 | 2 | 2,080 |
| CRHBP | corticotropin releasing factor/hormone binding protein |
NM_001882, 1393 |
5q13.3 | 7 | 16,619 |
| CRHR1 | corticotropin releasing factor/hormone receptor 1 (CRF1) |
NM_004382, 1394 |
17q21.31 | 13 | 51,525 |
| CRHR2 | corticotropin releasing factor/hormone receptor 2 (CRF2) |
NM_001883, 1395 |
7p15.1 | 12 | 29,278 |
| UCN | urocortin |
NM_003353, 7349 |
2p23.3 | 2 | 866 |
| UCN2 | urocortin 2/ stresscopin-related peptide |
NM_033199, 90226 |
3p21.3 | 2 | 2,049 |
| UCN3 | urocortin 3/ stresscopin |
NM_053049, 114131 |
10p15.1 | 2 | 9,194 |
Linkage studies
Before the sequencing of the human genome (107, 108), studies addressing the genetic basis of affective and anxiety disorders mostly consisted of linkage studies, using large pedigrees of families with multiple affected individuals. Most linkage studies have used short or variable tandem repeats or microsatellite markers which often tag larger regions in the chromosome, so that inference of linkage to a certain candidate gene from these studies usually requires extensive follow-up fine-mapping. Nonetheless, if genetic variation in the CRF system is related to increased vulnerability to depressive and anxiety disorders, one might expect to find regions of linkage for these disorders in the neighborhood of the chromosomal loci encoding these genes.
Although none of the recent meta-analyses of linkage scans for bipolar disorder provide strong support for linkage to regions containing CRF system genes (109, 110), some individual scans in major depressive or anxiety disorders (111, 112) have reported suggestive evidence for linkage in some of these regions. Two meta-analyses of linkage scans in bipolar disorder (109, 110) report linkage to the 8q region distal to the CRH gene. The more recent study actually reports genome-wide significance for this locus when bipolar II patients are included in the analysis (109). However, the signal in these studies (8q23) is distal to the CRH gene, which is located in 8q13.1. Testing of a short tandem repeat marker proximal to the CRH gene did not provide any evidence for linkage with bipolar disorder in a smaller sample of 22 pedigrees (113). In contrast, two studies have reported suggestive linkage (LOD < 2.0) for bipolar disorder in the chromosomal regions 8q13.2 and 8q13.3 including the CRH gene (114, 115), and a recent study has also shown linkage of a region containing 8q13.1 to deficit symptoms in a cohort of families affected with schizophrenia, schizoaffective disorder and bipolar disorder (116). Suggestive evidence for linkage to bipolar disorder has also been reported for 3p21, containing UCN2 (117). Interestingly, strong linkage (LOD 3.88) has been found of the combined phenotype of anxiety disorders and early onset major depression with a large locus on chromosome 3 (3p12.3-q12.3) overlapping with the previous locus (118). UCN3 is located centromeric to a linkage peak for obsessive-compulsive disorders on 10p15.3 (119).
Overall, linkage studies provide some evidence for an involvement of the CRF system in the genetics of depression and anxiety disorders, but the data are not compelling. However, such approaches have significant limitations in the study of the genetics of complex disease because they are usually underpowered to detect the smaller combined effects of multiple genes as well as gene x environment interactions expected in these disorders (120). Lack of evidence from linkage studies can therefore not be extrapolated to an absence of genetic effects of the CRF system in depression and anxiety.
Results from whole-genome association studies
For whole genome association studies, the markers of choice have been single nucleotide polymorphisms (SNPs) because they are common genetic variations (single base exchanges) and their genotyping is amenable to high-throughput methods (121, 122). Over 6.5 million of these variants have been validated in humans so far (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_summary.cgi). In past years, data from the HapMap project, an international consortium to catalogue human genetic variation (HapMap) (123) and other publicly available resources have become available online (http://www.hapmap.org/, http://genome.perlegen.com/, and others) to facilitate the selection of the most informative SNPs to adequately cover the genetic variation in different populations. Most commercially available SNP assays and whole-genome SNP arrays rely on these resources for SNP selection.
Several whole-genome studies have been published for bipolar disorder (124–128) and two for unipolar depression (129, 130) and none show reproducible association of SNPs within CRF system loci in the samples for which association data is readily available online. In fact, data from the GWAS for unipolar depression of the GAIN-study (130) show that within 200 kb 5’ and 3’ of the CRF-system genes, no association with a p-value smaller than 0.01 is observed, except for one SNP about 40 kb proximal of UCN3 (p-value between 0.01 and 0.001). Results from whole-genome studies for anxiety disorders or gene x environment interactions have not been published yet. It is however noteworthy that the arrays used in these studies contained especially few SNPs within the CRH, UCN and UCN2 loci. The Illumina hap550 v3 array for example with over 550,000 SNPs across the whole genome has only 2 SNPs within 20kb surrounding the CRH gene (http://genome.ucsc.edu/). Overall the genetic coverage of the used SNP arrays is not complete and especially weak for rarer variants. Negative results in these studies may thus also be confounded by a lack of coverage of the genetic variation in some of these loci.
Candidate gene approaches
In contrast to the genome wide approaches described above, candidate gene approaches have the advantage of directly testing association of genetic variations within a gene or locus of interest. This focused approach allows to answer specific hypotheses, including gene x gene as well as gene x environment interaction analyses and to home in on the actual functional variant. Candidate gene approaches have investigated a number of different genetic polymorphisms, including putatively functional variable tandem repeats and insertion-deletion. In the absence of a know functional variant, researchers often rely on a selection of marker variants to tag a genetic site making use of information on the linkage disequilibrium structure of the investigated chromosomal region (131).
In the following section we will describe the genetic structure of the genes encoding the various components of the CRF system, as well as evidence for functional polymorphisms within the genes of interest and results from candidate gene association studies.
The CRH locus
While the official name of the peptide is corticotropin releasing factor (CRF), is it also often referred to as corticotrophin releasing hormone (CRH) and CRH is also the official gene symbol for the locus encoding CRF.
Genetic architecture
CRH is a short gene, spanning about 2 kb with 2 exons located on the long arm of chromosome 8. It encodes the 196 amino acid long CRF precursor which is then processed to the 41 amino acid long CRF.
In the HapMap project, 12 SNPs have been genotyped within the CRH locus (+− 1.5 kb), of which most are either non-polymorphic or rare. Shimmin et al (2007) (132) have comprehensively re-sequenced this locus in over 200 individuals of Mexican-, European- and African American descent and have detected 37 SNPs, one insertion-deletion polymorphism and a microsatellite marker in the same region. At the time of publication, only 23% of these variants were listed in dbSNP, the most comprehensive database cataloguing these polymorphisms. This would indicate that current SNP databases and thus commercially available genotyping assays and arrays, vastly under-represent the genetic variation in this locus. Negative association results may therefore also be related to the fact that none of the potentially relevant markers have been genotyped in previous association studies.
Functional polymorphisms
A set of 4 polymorphisms (SNPs) in the 5’ regulatory region of the CRH locus up to 4000 bps upstream of the transcription initiation site have been described and appear to be associated with differential transcription of the gene product using reporter gene assays (133–135). One of these polymorphisms, rs5030876 appears to alter binding of activating transcription factor 6 to the putative promoter region (135, 136), thus providing a potential molecular mechanism for differences in gene transcription. Rosmond et al (2001) tested one of these putatively functional promoter variants (rs503875) for its effects on baseline and dexamethasone-suppressed saliva cortisol measures. This T/G SNP is rare in European populations (8–10% carrying the G allele), but more frequent in Africans (close to 40% carrying at least one G allele) (Hapmap and (133)). In a Swedish sample of 284 men, no effects of the SNP on diurnal saliva cortisol variation or dexamethasone suppression (0.5 mg) were observed. However, its rare allele showed a significant interaction with a functional SNP (ThtIII1) in the glucocorticoid receptor (GR). Only the combination of the rarer CRH promoter variant and the allele of the GR polymorphism previously associated with higher diurnal cortisol secretion was also associated with higher salivary cortisol levels in this study (137). The sample size (N = 12) in the combined genotype group that carried the effect was, however, very small. With the exception of another small study that linked the CRH locus to a congenital isolated ACTH deficiency in one family with two affected and two non-affected siblings (138), we are not aware of any other studies relating genetic variation in the CRH locus to endocrine measures. Recently, a functional variant in the promoter of the CRH gene of Rhesus monkeys has been reported that disrupts a glucocorticoid response element (GRE). This functional variant was associated with lower CSF CRH concentrations as well as more exploratory and bold behavior as infants and adolescents (139).
Association studies
A handful of studies have investigated the association of the CRH locus to anxiety-related phenotypes. Smoller et al (2003, 2005) have investigated the phenotype of behavioral inhibition in children of parents with anxiety disorders and found association with a repeat polymorphisms about 20kb 3’ of the gene (140) and 3 SNPs located just 3’ as well as 5’ of CRH (141, 142). Two of these SNPs were also tested for association with panic disorder in a case-control study in adults, but no significant associations were observed (143). Finally, the synonymous coding SNP rs6159 (amino acid position 96 Gly/Gly), associated with behavioral inhibition (142) as well as two SNPs 5’ of the CRH genes were neither associated with major depression nor with response to antidepressant treatment in a German sample (144). Wasserman et al (2008) also reported negative results for an association of 2 CRH SNPs with suicide attempt (145).
Even though some interesting functional SNPs regulating CRH expression have been identified, so far no definitive conclusions can be reached on the involvement of genetic variation within the CRH locus in anxiety- and depression-related phenotypes due to the limited sample sizes in these studies and the small number of studies overall. However, putatively important gene x environment interactions have not yet been investigated for this gene.
Urocortins and CRHBP
Genetic architecture and functional variants
The genes encoding the preproproteins for the three urocortins are located on different chromosomes and each has 2 exons. The CRHBP locus, encoding CRFBP, is located on chromosome 5 and contains 7 exons. Public databases also indicate two additional exons 3’ of the gene and an alternate isoform skipping exon 7 and containing these 2 3’ exons. We are currently not aware of any validated functional variants in these genes, but putative functional variants such as non-synonymous coding SNPs and other variants potentially influencing transcription or splicing regulation are predicted in silico.
Association studies
To our knowledge, except for negative associations with response to citalopram treatment in major depression (146) no psychiatric genetic association studies have specifically investigated the urocortin gene variants. An initial positive study on the association of CRHBP SNPs with recurrent unipolar depression (147) could not be verified in an enlarged sample of the same cohort and did not replicate in a second independent sample (148). The same group has also reported a lack of association of these variants with bipolar disorder (149). Enoch et al., 2008 report the association of variants within a CRHBP haplotype block with anxiety disorders in Plains Indians and alcohol use disorders in Caucasians. The same haplotypes were also associated with alpha power as measured by resting EEG in these individuals (150). These results may suggest that CRHBP variants could play a role in anxiety and addiction by moderating arousal states. A recent study from our group showed an ethnicity-specific association of a CRHBP SNP with response to citalopram treatment in the STAR*D cohort (146). This study investigated the association of polymorphisms within all CRF system genes discussed in this article and response to antidepressant treatment. The only association withstanding correction for multiple testing was with a SNP located 3’ of the CRHBP gene, rs10473984. In the African American and Hispanic subset of STAR*D patients, the T allele of rs10473984 was associated with poorer treatment outcome. This allele was also associated overall higher plasma ACTH levels, suggesting functional effects of this variant on CRHBP levels and thereby CRF signalling at the pituitary level. Finally, the association with poorer treatment outcome was most pronounced in individuals with anxious depression, supporting the role of this system in anxiety-related behaviors (146).
The CRF receptor genes
In the following, we will present data for the two CRF receptor genes, and begin with the gene encoding the CRF2 receptor (CRHR2), for which fewer studies are available, followed by the more intensely studied CRF1 receptor gene (CRHR1).
The CRHR2 locus
Genetic architecture
The CRHR2 locus contains 15 exons with three different promoter sites and 3 different first exons (151) encoding the CRF2alpha, CRF2 beta and CRF2gamma isoforms (152–154). These three isoforms differ in the initial part of the N-terminal ligand binding domain, but otherwise the receptor structure is the same, starting with the common second exon and they do not appear to differ in their ligand binding profile (152, 153). The CRF2alpha is the predominant isoform in human brain and its expression is responsive to glucocorticoids (155).
Association studies
For variants within the CRHR2 locus, positive associations have been reported. Tochigi et al (156) reported an association of an intronic SNP in CRHR2 with the personality trait openness on the NEO personality inventory in Japanese individuals. DeLuca et al (157) identified a three microsatellite marker haplotype that was associated with the severity of suicidal behavior, but not suicide attempts per se in families with bipolar disorder. Another intronic SNP (not in the same haplotype block as the one reported by Tochigi et al (158) was also associated with response to citalopram treatment in a Spanish cohort. Negative associations of CRHR2 variants with unipolar, bipolar and panic disorder as well as antidepressant treatment response have also been published (143, 146, 159, 160), painting a controversial picture of the association of variants in this gene with depression and anxiety-related phenotypes.
The CRHR1 locus
Genetic architecture
The CRHR1 gene spans 51 kb on the long arm of chromosome 17 (see figure 2). Its 13 exons encode the 415 amino acid CRF1 receptor for which 8 splice variants have been reported (161–163). CRF1alpha is the main isoform, containing all 13 exons. CRF1beta has 29 amino acids inserted into the first intracellular loop (encoded by an additional exon without any homologous sequence in mouse). CRF1c has 40 amino acids deleted from the N-terminus (lacking exon 3) and CRF1d results from a skipping of exon 12 and thus a 14 amino acid deletion from the 7th transmembrane domain. CRF1e lacks exons 3 and 4 - coding for the N-terminus, CRF1f lacks exon 11 and CRF1g lacks exon 10 and part of exons 9 and 11. CRF1h has a cryptic exon inserted between exons 4 and 5. All receptor variants result in either altered/impaired binding affinity or signal transduction. In addition, CRF1h, in which the cryptic exon generates a translation terminator in exon 4, only has a functional CRF binding domain but no transmembrane domains. CRF1e and CRF1h are soluble isoforms and might serve in an analogous function to the CRFBP. In fact in in vitro systems, the presence of CRF1e decreases and CRF1h increases CRF1alpha activation (162). CRF receptors isoform CRF1alpha is expressed in both the central nervous system and the periphery. CRF1e through CRF1h have been discovered in the skin and CRF1beta – 1h in the myometrium (161, 164, 165). The lack of isoform-specific antibodies has made it difficult to test in which tissues the receptor isoform proteins are actually expressed. So far, no report has described how genetic variation might influence this alternative splicing, but polymorphisms altering the ratio of the different isoforms are likely to have a strong impact on CRF1 signaling.
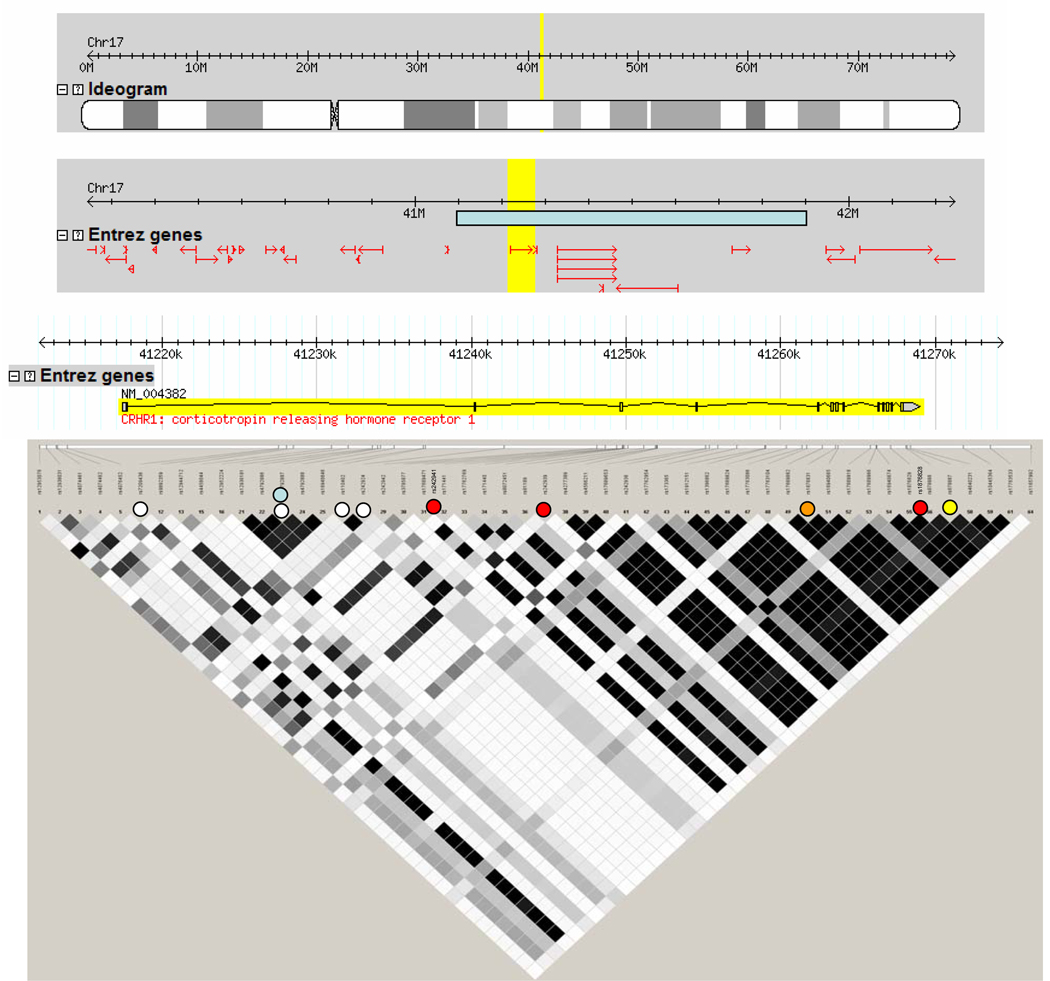
Figure 2. The genetic organization of the CRHR1 gene encoding CRF1.
All representations have been derived from www.hapmap.org. The top panel shows the position of the CRHR1 gene on chromosome 17 (yellow). The second panel shows the 2 mega bases (MB) surrounding the CRHR1 gene (highlighted in yellow) with the position of the neighboring genes (red) and the position of the inversion polymorphism (light blue). The next panel shows the position of the exons and introns of the CRHR1 gene, with the 5’ end of the gene on the left. Below are the SNPs available for this gene in the HapMap project with the linkage disequilibrium (LD) structure for the Caucasian CEU population, as most studies on the genetic of CRF system genes have been performed in Caucasians. LD is represented with the r-squared measure with black squares connecting SNPs in complete LD (r-squared = 1) and white squares connecting SNPs with no LD (s-squared = 0). Shades of grey represent r-squared values between 0 and 1.
White dots denote the position of SNPs interacting with child abuse to predict adult depressive symptoms (177, 179) or cortisol response in the combined dexamethasone/CRF test (180, 181). The light blue dot denotes the SNPs interacting with life events to predict suicide attempts (145). Red dots denote SNPs predicting response to antidepressants in patients with anxious depression (174, 175). The orange dot denotes the SNP interacting with life stress to predict heavy drinking (183, 184) and the yellow dot denotes the SNP associated with panic disorders together with a SNP in AVPR1B (143).
An interesting feature of the chromosomal region containing the CRHR1 locus on chromosome 17q21.31 is the presence of a 900 kb inversion polymorphism (166). This polymorphism is rare in African and Asian populations, but more frequent in populations of European descent. It is associated with a higher number of off-springs in female carriers and thus under positive selection. Due to an increased potential for misalignment during meiosis in heterozygous carriers of this polymorphism, there is an increased risk for deletion and duplications of this locus. Indeed, this inversion polymorphism has been associated with the occurrence of a microdeletion syndrome of this region encompassing the CRHR1 and microtubule-associated protein tau (MAPT) genes (167–171). The clinical features of this syndrome include a marked hypotonia, dysmorphic features and moderate mental retardation. One report describes friendly and amiable behavior in these patients (167). A duplication of this region has been reported in one female patient (172) in whom hirsutism and sleep disturbances were noted besides mental retardation and dysmorphic features. No reports about the effects of this polymorphism on endocrine regulation or a more in-depth psychiatric characterization have been published to our knowledge.
Functional polymorphisms
No data on polymorphisms that change CRHR1 gene function have yet been published. In silico analysis of potentially functional SNPs indicates that several SNPs within this locus might have an impact on gene function (http://fastsnp.ibms.sinica.edu.tw). It is noteworthy that for rs12936511, a synonymous coding SNP in exon 2 (Pro/Pro at amino acid position 20), the presence of the rare allele (minor allele frequency less than 10%) results in the loss of an exonic splice enhancer element and could lead to altered splicing regulation. Two missense mutations in exons 3 and 4 (rs41280114 and rs16940655) result in conservative amino acid exchanges (amino acid position 60 - Val/Ala and amino acid position 96 -His/Arg, respectively) and likely do not have a major impact on protein function.
Association studies
Several association studies with CRHR1 polymorphisms have been published and center around anxiety, depression, response to antidepressant drugs and gene x environment interactions.
Keck et al 2008 (143) have reported an association of CRHR1 polymorphisms with panic disorder. In this study, the authors report nominally significant associations of CRHR1 SNPs in two independent German panic disorder samples. The strongest results were, however, the combined effects of rs878886 in CRHR1 and rs28632197 in AVPR1B, encoding the vasopressin 1B receptor. A two-SNP model showed significant associations with panic disorder in both samples separately and the combined sample of 359 cases and 794 controls. Both SNPs are of potential functional relevance because rs878886 is located in the 3’ untranslated region of the CRHR1 gene and rs28632197 leads to an arginine to histidine amino acid exchange at position 364 of AVPR1B which is located in the intracellular C-terminal domain of the receptor, likely involved in G-protein coupling. These genetic data support the large body of evidence demonstrating interactions of the vasopressin and CRF systems in anxiety (48). Another family-based study failed to find association of four polymorphisms in the CRHR1 locus with panic disorder. However, fewer CRHR1 and no AVPR1B polymorphisms were tested in this study (173).
Four studies in ethnically different samples have investigated associations of CRHR1 SNPs with response to antidepressant treatment in major depression (146, 158, 174, 175). Licinio et al (174) initially reported an association of a 3 SNP haplotype within CRHR1 with response to fluoxetine or desipramine in Mexican-Americans and this has been replicated in a Chinese population (175). Interestingly, both studies reported that the effects of this CRHR1 haplotype were most pronounced in patients with anxious depression. Nominally significant associations with response to citalopram of a SNP within the putative CRHR1 promoter region were also observed in the STAR*D sample (146). Papiol et al (158) found that another intronic SNP (rs110402) was not associated with response to citalopram treatment in 159 Spanish patients, but was correlated with an earlier age at onset for major depression. A significant case-control association of CRHR1 SNPs with major depression was only reported by Liu et al (176).
Another set of studies have examined gene x environment interactions of CRHR1 SNPs and stressful events on different psychiatric phenotypes. Wasserman et al (145) have investigated over 500 family trios of male suicide attempters and found that the rarer T-allele of the intronic CRHR1 SNP rs4792887 is overtransmitted in these families. This overtransmission was restricted to individuals with less stressful life events. Because the vast majority of suicide attempters were also depressed, these data suggest an interaction of CRHR1 polymorphisms with stressful life events on suicide attempts in depression. Our group (177) demonstrated a strong interaction of CRHR1 SNPs with child abuse predicting adult depressive symptoms in an inner city African-American sample of over 400 individuals. We reported protective effects of two 3-SNP CRHR1 haplotypes, whereby individuals with high levels of child abuse and individuals homozygous for the protective haplotypes had significantly less current depressive symptoms than abused individuals carrying other haplotype combinations. In fact the latter groups’ scores on the Beck Depression Inventory were similar to the non-abused group, in which the SNPs had no effects on depressive symptom severity. This result was supported by similar findings in a sample of mostly Caucasian women described in the same report. All three SNPs were intronic and one of the two associated haplotypes contained rs479887 for which interaction with life events and suicide attempts had previously been reported (178). These early trauma x CRHR1 interactions on depression have been replicated and extended in independent studies. Polanczyk et al (2009) report a replication of the interaction on past-year and recurrent major depression using the same 3-SNP haplotype in a female cohort retrospectively assessed for childhood maltreatment (179). The interaction was, however, not observed in the prospective Dunedin cohort and the authors argue that this might be due to differences in the assessment of childhood trauma. Furthermore, two reports corroborate the putative functional role of these same variants using data from an endocrine challenge test, the combined dexamethasone/CRF test. Here the protective alleles are associated with a lower cortisol response in this test, either in interaction with early life events (180) or as a main genetic effect (181). The CRHR1 x environment interaction on depression and HPA-axis regulation are further strengthened by the fact that the effects seem to replicate across different ethnicities. As mentioned above, gene x gene interactions are likely important in the genetics of depression and anxiety. Ressler et al (2009) have reported a significant gene x gene x environment interaction, with these CRHR1 polymorphisms interacting with the serotonin transporter linked polymorphic region (5-HTTLPR) and child abuse to predict adult depression (182).
Blomeyer et al (2007) (183) described the interaction of another CRHR1 tagging polymorphism (rs1876831) and negative life events on adolescent alcohol consumption. In the presence of a large number of negative life events, individuals homozygous for the C allele of this SNP reported about twice as much life time heavy drinking as well as drinks per occasion than carriers of the T allele. This difference was not seen in the presence of a low number of life events. These findings have been extended to young adults, where this tagging SNP and stressful life events interact to predict progression of heavy alcohol use in young adulthood (184). The interaction of genetic variation of CRF system genes and stressful life events on alcohol consumption is further supported by data from rhesus macaques. A functional promoter polymorphisms in the CRH gene interacts with prior stress exposure to predict voluntary alcohol consumption in these animals (185).
In humans, further fine-mapping and re-sequencing will be needed to identify the putative functional variants responsible for these gene x environment interactions and whether the SNPs interacting with life events to predict alcoholism, suicide attempts or depression in fact represent distinct functional loci. Overall, these recent human genetic data strongly support a role for the CRF1 receptor in depression and anxiety, most likely by moderating the effects of early trauma or life events in general.
Conclusions and future directions
Even though the majority of human genetic studies investigating genes encoding the CRF system are limited by small sample sizes or insufficiently dense coverage, the sum of these findings, especially those related to variants within CRHR1, support the concatenation of preclinical and clinical data implicating the CRF system in the pathophysiology of depression and anxiety. Together with the serotonergic and neurotrophic system (111), the CRF system thus represents an example of how human genetic studies can contribute to specific pathophysiologic hypotheses in depression and anxiety.
It has to be noted though that the presented genetic association studies, including data in GWAS are only powered to detect associations with common genetic variants. Rare, functional variants in these genes may, however, also have a relevant impact for these disorders. Next generation sequencing methods will allow to catalogue and compare all sequence variation in these loci and to identify, rare but functionally relevant polymorphisms, including copy number variations.
In addition to expanding genetic association studies of this system in larger samples with sufficiently dense genetic markers as well as re-sequencing, the next generation of CRF-related genetic studies should focus on system genetics, analyzing the impact of the whole system including ligands, receptors as well as other genes upstream and downstream of this signaling pathway. There is already evidence for the association of genetic variants in genes intricately tied to the CRF system with depression and anxiety, such as the glucocorticoid receptor gene (186) and the gene encoding the glucocorticoid receptor co-chaperone FKBP5 (187) for example.
Because of their critical role in stress regulation, it is unlikely that detrimental variants in single genes within the CRF system will be common in the population. More likely, the sum of genetic variations with discrete functional effects in a number of genes within this system will impact stress axis regulation and susceptibility to stress-related psychiatric disorders. Similar to the number of studies now investigating the 5-HTTLPR and the BDNF Val66Met polymorphism (111), gene x gene x environment interactions of CRHR1 variants with this polymorphism have been reported (182). It will now be important to identify and characterize the putative functional variants in CRHR1 and their interaction with the other genes. From the above presented data it appears that loci within this gene moderate the impact of life stress on the development of psychiatric disorders. Stratifying individuals by the protective vs. susceptibility variants of these loci in neuroimaging and endocrine studies for example might yield a better understanding of the long term impact of trauma on neuronal circuitry and hormone systems, and more specifically which compensatory mechanisms are involved in the development of stress-related psychiatric disorders. The characterization of these variants may also be a tool to dissect possible epigenetic impacts of trauma on the CRF system. As described above, early life adversity has been shown to result in persistent effects on CRF neurotransmission and HPA axis regulation (in fact in an animal model of early prenatal stress, methylation of the CRH gene locus correlates with longterm changes in gene expression (188)). Genetic variations within the CRHR1 locus appear to moderate this impact, possibly by enhancing or preventing epigenetic changes within this gene. In fact, early adversity and thus stress have been shown to lead to epigenetic changes, including difference in the methylation status of HPA-axis genes (189). One possibility would be that these putative variants could alter the epigenetic impact of stress, e.g. the methylation of CpG islands by changing a CG sequence. Indeed, a single SNP abolishing one CpG can be associated with global differences in methylation of the whole surrounding CpG island (190).
Despite some controversial results, the initial series of human genetics studies support the importance of this system in mediating stress-related psychopathology. In depth characterization of these variants could represent an important tool in elucidating the long term consequences of early life adverse experience.
Acknowledgements
Support was received by NIH grants MH-42088, MH-69056, MH-58922 and RR-25008. Dr. Binder is supported by a Doris Duke Clinical Scientist Development Award.
Footnotes
Disclosures:
Currently, Dr. Nemeroff serves on the Scientific Advisory Board (SAB) for the American Foundation for Suicide Prevention (AFSP); AstraZeneca; NARSAD, PharmaNeuroboost and CeNeRx. He serves on the Board of Directors of American Foundation for Suicide Prevention (AFSP); George West Mental Health Foundation; NovaDel Pharma, Mt. Cook Pharma, Inc. He owns equity or is stock holder in Corcept; Revaax; NovaDel Pharma; CeNeRx, PharmaNeuroboost, Mt. Cook Pharma. He is inventor on the following patents: Method and devices for transdermal delivery of lithium (US 6,375,990 B1)
Method to estimate serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum (provisional filing April, 2001)
Currently, Dr. Binder receives grant support from NIMH and the Doris Duke charitable foundation.
References
- 1.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 2.Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982 Jul 26;31(4):363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- 3.Kalin NH. Behavioral effects of ovine corticotropin-releasing factor administered to rhesus monkeys. Fed Proc. 1985 Jan;44(1 Pt 2):249–253. [PubMed] [Google Scholar]
- 4.Kalin NH, Shelton SE, Kraemer GW, McKinney WT. Corticotropin-releasing factor administered intraventricularly to rhesus monkeys. Peptides. 1983 Mar-Apr;4(2):217–220. doi: 10.1016/0196-9781(83)90117-1. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Fed Proc. 1985 Jan;44(1 Pt 2):259–263. [PubMed] [Google Scholar]
- 6.Koob GF, Thatcher-Britton K. Stimulant and anxiogenic effects of corticotropin releasing factor. Prog Clin Biol Res. 1985;192:499–506. [PubMed] [Google Scholar]
- 7.Sirinathsinghji DJ, Rees LH, Rivier J, Vale W. Corticotropin-releasing factor is a potent inhibitor of sexual receptivity in the female rat. Nature. 1983 Sep 15–21;305(5931):232–235. doi: 10.1038/305232a0. [DOI] [PubMed] [Google Scholar]
- 8.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984 Dec 14;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 9.Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996 Sep;1(4):336–342. [PubMed] [Google Scholar]
- 10.Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002 Feb;2(1):23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 11.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 12.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003 Mar;55(1):21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 13.Boorse GC, Denver RJ. Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol. 2006 Mar;146(1):9–18. doi: 10.1016/j.ygcen.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, et al. Cloning and characterization of human urocortin. Endocrinology. 1996 Sep;137(9):3896. doi: 10.1210/endo.137.9.8756563. [DOI] [PubMed] [Google Scholar]
- 16.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995 Nov 16;378(6554):287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 19.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999 Dec 20;415(3):285–312. [PubMed] [Google Scholar]
- 20.Wong ML, al-Shekhlee A, Bongiorno PB, Esposito A, Khatri P, Sternberg EM, et al. Localization of urocortin messenger RNA in rat brain and pituitary. Mol Psychiatry. 1996 Sep;1(4):307–312. [PubMed] [Google Scholar]
- 21.Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology. 1999 Dec;140(12):5651–5658. doi: 10.1210/endo.140.12.7223. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN, et al. Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides. 2004 Oct;25(10):1711–1721. doi: 10.1016/j.peptides.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995 Oct;15(10):6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiroi N, Wong ML, Licinio J, Park C, Young M, Gold PW, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001 Sep;6(5):540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 25.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999 Jun 7;408(3):365–377. [PubMed] [Google Scholar]
- 27.Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995 Oct;16(4):362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- 28.Seasholtz AF, Valverde RA, Denver RJ. Corticotropin-releasing hormone-binding protein: biochemistry and function from fishes to mammals. J Endocrinol. 2002 Oct;175(1):89–97. doi: 10.1677/joe.0.1750089. [DOI] [PubMed] [Google Scholar]
- 29.Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991 Jan 31;349(6308):423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- 30.Potter E, Behan DP, Linton EA, Lowry PJ, Sawchenko PE, Vale WW. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitoratos N, Papatheodorou DC, Kalantaridou SN, Mastorakos G. “Reproductive” corticotropin-releasing hormone. Ann N Y Acad Sci. 2006 Dec;1092:310–318. doi: 10.1196/annals.1365.029. [DOI] [PubMed] [Google Scholar]
- 32.Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983 Jul 4;270(2):363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- 33.Tsatsanis C, Dermitzaki E, Venihaki M, Chatzaki E, Minas V, Gravanis A, et al. The corticotropin-releasing factor (CRF) family of peptides as local modulators of adrenal function. Cell Mol Life Sci. 2007 Jul;64(13):1638–1655. doi: 10.1007/s00018-007-6555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999 Jan;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 35.Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997 Jul 23;71(1):15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- 36.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000 Apr;24(4):410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 37.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000 Apr;24(4):403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000 Apr;24(4):415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 39.Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T. Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology (Berl) 2004 Oct;176(1):30–38. doi: 10.1007/s00213-004-1863-1. [DOI] [PubMed] [Google Scholar]
- 40.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004 Jun;28(6):865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 41.Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006 Sep 6;26(36):9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001 Jun 1;902(2):135–142. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- 43.Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003 Aug 8;980(2):206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, et al. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007 Dec;323(3):846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- 45.Preil J, Muller MB, Gesing A, Reul JM, Sillaber I, van Gaalen MM, et al. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology. 2001 Nov;142(11):4946–4955. doi: 10.1210/endo.142.11.8507. [DOI] [PubMed] [Google Scholar]
- 46.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006 Aug;5(4):453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990 May-Aug;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 48.Keck ME. Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids. 2006 Oct;31(3):241–250. doi: 10.1007/s00726-006-0333-y. [DOI] [PubMed] [Google Scholar]
- 49.Keck ME, Holsboer F, Muller MB. Mouse mutants for the study of corticotropin-releasing hormone receptor function: development of novel treatment strategies for mood disorders. Ann N Y Acad Sci. 2004 Jun;1018:445–457. doi: 10.1196/annals.1296.055. [DOI] [PubMed] [Google Scholar]
- 50.Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008 May 13; doi: 10.1038/mp.2008.51. May 13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003 Oct;6(10):1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 52.Kageyama K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J. 2009 Jun;56(3):335–344. doi: 10.1507/endocrj.k09e-075. [DOI] [PubMed] [Google Scholar]
- 53.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998 Apr;23(3):219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 54.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2008 Aug 12; doi: 10.1038/mp.2008.91. August 12, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banki CM, Bissette G, Arato M, O’Connor L, Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987 Jul;144(7):873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- 56.Hartline KM, Owens MJ, Nemeroff CB. Postmortem and cerebrospinal fluid studies of corticotropin-releasing factor in humans. Ann N Y Acad Sci. 1996 Mar 22;780:96–105. doi: 10.1111/j.1749-6632.1996.tb15114.x. [DOI] [PubMed] [Google Scholar]
- 57.Arato M, Banki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989 Feb 1;25(3):355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 58.Nemeroff CB, Bissette G, Akil H, Fink M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta endorphin and somatostatin. Br J Psychiatry. 1991 Jan;158:59–63. doi: 10.1192/bjp.158.1.59. [DOI] [PubMed] [Google Scholar]
- 59.De Bellis MD, Gold PW, Geracioti TD, Jr, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993 Apr;150(4):656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- 60.Veith RC, Lewis N, Langohr JI, Murburg MM, Ashleigh EA, Castillo S, et al. Effect of desipramine on cerebrospinal fluid concentrations of corticotropin-releasing factor in human subjects. Psychiatry Res. 1993 Jan;46(1):1–8. doi: 10.1016/0165-1781(93)90002-x. [DOI] [PubMed] [Google Scholar]
- 61.Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, et al. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8(2):71–79. [PubMed] [Google Scholar]
- 62.Banki CM, Karmacsi L, Bissette G, Nemeroff CB. CSF corticotropin-releasing hormone and somatostatin in major depression: response to antidepressant treatment and relapse. Eur Neuropsychopharmacol. 1992 Jun;2(2):107–113. doi: 10.1016/0924-977x(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 63.Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003 Mar;8(3):324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 64.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006 Apr 1;59(7):594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 65.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994 Oct;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 66.Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003 Jul;28(7):1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 67.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004 Feb 11;24(6):1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988 Jun;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 69.Herringa RJ, Roseboom PH, Kalin NH. Decreased amygdala CRF-binding protein mRNA in post-mortem tissue from male but not female bipolar and schizophrenic subjects. Neuropsychopharmacology. 2006 Aug;31(8):1822–1831. doi: 10.1038/sj.npp.1301038. [DOI] [PubMed] [Google Scholar]
- 70.Kozicz T, Tilburg-Ouwens D, Faludi G, Palkovits M, Roubos E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008 Apr 9;152(4):1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 71.Kunzel HE, Zobel AW, Nickel T, Ackl N, Uhr M, Sonntag A, et al. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003 Nov-Dec;37(6):525–533. doi: 10.1016/s0022-3956(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 72.Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000 May-Jun;34(3):171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 73.Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008 May;165(5):617–620. doi: 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- 74.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003 Aug;160(8):1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 75.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- 76.Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005 Feb 15;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 78.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 79.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993 May;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 80.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005 Dec;30(12):2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 81.Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004 Apr;29(4):777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- 82.Lee R, Geracioti TD, Jr, Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry. 2005 May;162(5):995–997. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- 83.Lee RJ, Gollan J, Kasckow J, Geracioti T, Coccaro EF. CSF corticotropin-releasing factor in personality disorder: relationship with self-reported parental care. Neuropsychopharmacology. 2006 Oct;31(10):2289–2295. doi: 10.1038/sj.npp.1301104. [DOI] [PubMed] [Google Scholar]
- 84.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008 Feb 15;63(4):398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001 Apr;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 86.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000 Aug 2;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 87.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008 Jun 15;63(12):1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008 May 15;148(2):89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- 89.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006 Nov;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997 May;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, et al. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003 Dec 15;54(12):1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 92.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999 Apr;156(4):585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 93.Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord. 1992 May;25(1):39–45. doi: 10.1016/0165-0327(92)90091-j. [DOI] [PubMed] [Google Scholar]
- 94.Fossey MD, Lydiard RB, Ballenger JC, Laraia MT, Bissette G, Nemeroff CB. Cerebrospinal fluid corticotropin-releasing factor concentrations in patients with anxiety disorders and normal comparison subjects. Biol Psychiatry. 1996 Apr 15;39(8):703–707. doi: 10.1016/0006-3223(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 95.Jolkkonen J, Lepola U, Bissette G, Nemeroff C, Riekkinen P. CSF corticotropin-releasing factor is not affected in panic disorder. Biol Psychiatry. 1993 Jan 15;33(2):136–138. doi: 10.1016/0006-3223(93)90315-5. [DOI] [PubMed] [Google Scholar]
- 96.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007 Aug;30(8):399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004 Dec;79(4):671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 98.Adinoff B, Anton R, Linnoila M, Guidotti A, Nemeroff CB, Bissette G. Cerebrospinal fluid concentrations of corticotropin-releasing hormone (CRH) and diazepam-binding inhibitor (DBI) during alcohol withdrawal and abstinence. Neuropsychopharmacology. 1996 Sep;15(3):288–295. doi: 10.1016/0893-133X(95)00212-V. [DOI] [PubMed] [Google Scholar]
- 99.Hawley RJ, Nemeroff CB, Bissette G, Guidotti A, Rawlings R, Linnoila M. Neurochemical correlates of sympathetic activation during severe alcohol withdrawal. Alcohol Clin Exp Res. 1994 Dec;18(6):1312–1316. doi: 10.1111/j.1530-0277.1994.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 100.Skelton KH, Nemeroff CB, Knight DL, Owens MJ. Chronic administration of the triazolobenzodiazepine alprazolam produces opposite effects on corticotropin-releasing factor and urocortin neuronal systems. J Neurosci. 2000 Feb 1;20(3):1240–1248. doi: 10.1523/JNEUROSCI.20-03-01240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004 Nov;311(2):427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 102.Kendler KS. Twin studies of psychiatric illness. Current status and future directions. Arch Gen Psychiatry. 1993 Nov;50(11):905–915. doi: 10.1001/archpsyc.1993.01820230075007. [DOI] [PubMed] [Google Scholar]
- 103.Kendler KS. Genetic epidemiology in psychiatry. Taking both genes and environment seriously. Arch Gen Psychiatry. 1995 Nov;52(11):895–899. doi: 10.1001/archpsyc.1995.03950230009003. [DOI] [PubMed] [Google Scholar]
- 104.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006 Jan;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 105.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001 Oct;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 106.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000 Oct;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 107.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001 Feb 15;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 108.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001 Feb 16;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 109.McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005 Oct;77(4):582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: Bipolar disorder. Am J Hum Genet. 2003 Jul;73(1):49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006 Jul 15;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 112.Smoller JW, Gardner-Schuster E, Covino J. The genetic basis of panic and phobic anxiety disorders. Am J Med Genet C Semin Med Genet. 2008 May 15;148(2):118–126. doi: 10.1002/ajmg.c.30174. [DOI] [PubMed] [Google Scholar]
- 113.Stratakis CA, Sarlis NJ, Berrettini WH, Badner JA, Chrousos GP, Gershon ES, et al. Lack of linkage between the corticotropin-releasing hormone (CRH) gene and bipolar affective disorder. Mol Psychiatry. 1997 Oct-Nov;2(6):483–485. doi: 10.1038/sj.mp.4000268. [DOI] [PubMed] [Google Scholar]
- 114.Liu J, Juo SH, Dewan A, Grunn A, Tong X, Brito M, et al. Evidence for a putative bipolar disorder locus on 2p13–16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21–24, 13q32, 14q21 and 17q11–12. Mol Psychiatry. 2003 Mar;8(3):333–342. doi: 10.1038/sj.mp.4001254. [DOI] [PubMed] [Google Scholar]
- 115.Marcheco-Teruel B, Flint TJ, Wikman FP, Torralbas M, Gonzalez L, Blanco L, et al. A genome-wide linkage search for bipolar disorder susceptibility loci in a large and complex pedigree from the eastern part of Cuba. Am J Med Genet B Neuropsychiatr Genet. 2006 Dec 5;141B(8):833–843. doi: 10.1002/ajmg.b.30314. [DOI] [PubMed] [Google Scholar]
- 116.Fanous AH, Neale MC, Webb BT, Straub RE, O'Neill FA, Walsh D, et al. Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol Psychiatry. 2008 Jul 15;64(2):121–127. doi: 10.1016/j.biopsych.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 117.Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, et al. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):585–590. doi: 10.1073/pnas.011358498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Camp NJ, Lowry MR, Richards RL, Plenk AM, Carter C, Hensel CH, et al. Genome-wide linkage analyses of extended Utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2005 May 5;135B(1):85–93. doi: 10.1002/ajmg.b.30177. [DOI] [PubMed] [Google Scholar]
- 119.Hanna GL, Veenstra-Vanderweele J, Cox NJ, Van Etten M, Fischer DJ, Himle JA, et al. Evidence for a susceptibility locus on chromosome 10p15 in early-onset obsessive-compulsive disorder. Biol Psychiatry. 2007 Oct 15;62(8):856–862. doi: 10.1016/j.biopsych.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996 Sep 13;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 121.Brookes AJ. The essence of SNPs. Gene. 1999 Jul 8;234(2):177–186. doi: 10.1016/s0378-1119(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 122.Ragoussis J. Genotyping technologies for genetic research. Annu Rev Genomics Hum Genet. 2009;10:117–133. doi: 10.1146/annurev-genom-082908-150116. [DOI] [PubMed] [Google Scholar]
- 123.The International HapMap Project. Nature. 2003 Dec 18;426(6968):789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 124.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007 Jun 7;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2007 May 8; doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008 Jun;13(6):558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008 Aug 17; doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J, et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry. 2008 Dec 16; doi: 10.1038/mp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, et al. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Mol Psychiatry. 2008 Dec 23; doi: 10.1038/mp.2008.131. Dec 23, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 130.Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2008 Dec 9; doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007 Oct 18;449(7164):851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shimmin LC, Natarajan S, Ibarguen H, Montasser M, Kim DK, Hanis CL, et al. Corticotropin releasing hormone (CRH) gene variation: comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007 Dec;18(6):434–444. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- 133.Baerwald CG, Mok CC, Fife MS, Tikly M, Lau CS, Wordsworth BP, et al. Distribution of corticotropin-releasing hormone promoter polymorphism in different ethnic groups: evidence for natural selection in human populations. Immunogenetics. 1999 Sep;49(10):894–899. doi: 10.1007/s002510050570. [DOI] [PubMed] [Google Scholar]
- 134.Baerwald CG, Panayi GS, Lanchbury JS. A new XmnI polymorphism in the regulatory region of the corticotropin releasing hormone gene. Hum Genet. 1996 May;97(5):697–698. [PubMed] [Google Scholar]
- 135.Wagner U, Wahle M, Moritz F, Hantzschel H, Baerwald CG. Promoter polymorphisms regulating corticotrophin-releasing hormone transcription in vitro. Horm Metab Res. 2006 Feb;38(2):69–75. doi: 10.1055/s-2006-925115. [DOI] [PubMed] [Google Scholar]
- 136.Wagner U, Wahle M, Malysheva O, Hantzschel H, Baerwald C. Sequence variants of the CRH 5'-flanking region: effects on DNA-protein interactions studied by EMSA in PC12 cells. Ann N Y Acad Sci. 2006 Jun;1069:20–33. doi: 10.1196/annals.1351.003. [DOI] [PubMed] [Google Scholar]
- 137.Rosmond R, Chagnon M, Bouchard C, Bjorntorp P. A polymorphism in the regulatory region of the corticotropin-releasing hormone gene in relation to cortisol secretion, obesity, and gene-gene interaction. Metabolism. 2001 Sep;50(9):1059–1062. doi: 10.1053/meta.2001.25598. [DOI] [PubMed] [Google Scholar]
- 138.Kyllo JH, Collins MM, Vetter KL, Cuttler L, Rosenfield RL, Donohoue PA. Linkage of congenital isolated adrenocorticotropic hormone deficiency to the corticotropin releasing hormone locus using simple sequence repeat polymorphisms. Am J Med Genet. 1996 Mar 29;62(3):262–267. doi: 10.1002/(SICI)1096-8628(19960329)62:3<262::AID-AJMG11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 139.Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, et al. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2008 Aug;65(8):934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gu J, Sadler L, Daiger S, Wells D, Wagner M. Dinucleotide repeat polymorphism at the CRH gene. Hum Mol Genet. 1993 Jan;2(1):85. doi: 10.1093/hmg/2.1.85. [DOI] [PubMed] [Google Scholar]
- 141.Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003 Dec 15;54(12):1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- 142.Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, et al. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol Psychiatry. 2005 Jun 15;57(12):1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 143.Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, Salyakina D, et al. Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008 Apr 2; doi: 10.1002/ajmg.b.30750. Apr. 2nd Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 144.Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004 Dec;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 145.Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2008 Feb;7(1):14–19. doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 146.Binder EB, Owens MJ, Liu W, Deveau TC, AJ R, Trivedi M, et al. Association of polymorphisms in genes regulating the CRF system with antidepressant treatment response in the STAR*D sample. Archives of General Psychiatry. 2009 doi: 10.1001/archgenpsychiatry.2010.18. in press. [DOI] [PubMed] [Google Scholar]
- 147.Claes S, Villafuerte S, Forsgren T, Sluijs S, Del-Favero J, Adolfsson R, et al. The corticotropin-releasing hormone binding protein is associated with major depression in a population from Northern Sweden. Biol Psychiatry. 2003 Nov 1;54(9):867–872. doi: 10.1016/s0006-3223(03)00425-6. [DOI] [PubMed] [Google Scholar]
- 148.Van Den Eede F, Venken T, Del-Favero J, Norrback KF, Souery D, Nilsson LG, et al. Single nucleotide polymorphism analysis of corticotropin-releasing factor-binding protein gene in recurrent major depressive disorder. Psychiatry Res. 2007 Sep 30;153(1):17–25. doi: 10.1016/j.psychres.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 149.Van Den Eede F, Venken T, Van Den Bogaert A, Del-Favero J, Norrback KF, Nilsson LG, et al. Single nucleotide polymorphism analysis of corticotropin-releasing factor-binding protein gene in bipolar disorder. Psychiatr Genet. 2007 Oct;17(5):304–307. doi: 10.1097/YPG.0b013e328133f342. [DOI] [PubMed] [Google Scholar]
- 150.Enoch MA, Shen PH, Ducci F, Yuan Q, Liu J, White KV, et al. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS ONE. 2008;3(10):e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol. 2003 Mar;17(3):395–410. doi: 10.1210/me.2002-0302. [DOI] [PubMed] [Google Scholar]
- 152.Ardati A, Goetschy V, Gottowick J, Henriot S, Valdenaire O, Deuschle U, et al. Human CRF2 alpha and beta splice variants: pharmacological characterization using radioligand binding and a luciferase gene expression assay. Neuropharmacology. 1999 Mar;38(3):441–448. doi: 10.1016/s0028-3908(98)00201-9. [DOI] [PubMed] [Google Scholar]
- 153.Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998 Aug;12(8):1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- 154.Valdenaire O, Giller T, Breu V, Gottowik J, Kilpatrick G. A new functional isoform of the human CRF2 receptor for corticotropin-releasing factor. Biochim Biophys Acta. 1997 May 30;1352(2):129–132. doi: 10.1016/s0167-4781(97)00047-x. [DOI] [PubMed] [Google Scholar]
- 155.Nanda SA, Roseboom PH, Nash GA, Speers JM, Kalin NH. Characterization of the human corticotropin-releasing factor2(a) receptor promoter: regulation by glucocorticoids and the cyclic adenosine 5’-monophosphate pathway. Endocrinology. 2004 Dec;145(12):5605–5615. doi: 10.1210/en.2004-0907. [DOI] [PubMed] [Google Scholar]
- 156.Tochigi M, Kato C, Otowa T, Hibino H, Marui T, Ohtani T, et al. Association between corticotropin-releasing hormone receptor 2 (CRHR2) gene polymorphism and personality traits. Psychiatry Clin Neurosci. 2006 Aug;60(4):524–526. doi: 10.1111/j.1440-1819.2006.01541.x. [DOI] [PubMed] [Google Scholar]
- 157.De Luca V, Tharmalingam S, Kennedy JL. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. Eur Psychiatry. 2007 Jul;22(5):282–287. doi: 10.1016/j.eurpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 158.Papiol S, Arias B, Gasto C, Gutierrez B, Catalan R, Fananas L. Genetic variability at HPA axis in major depression and clinical response to antidepressant treatment. J Affect Disord. 2007 Dec;104(1–3):83–90. doi: 10.1016/j.jad.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 159.Tharmalingam S, King N, De Luca V, Rothe C, Koszycki D, Bradwejn J, et al. Lack of association between the corticotrophin-releasing hormone receptor 2 gene and panic disorder. Psychiatr Genet. 2006 Jun;16(3):93–97. doi: 10.1097/01.ypg.0000218610.45441.c3. [DOI] [PubMed] [Google Scholar]
- 160.Villafuerte SM, Del-Favero J, Adolfsson R, Souery D, Massat I, Mendlewicz J, et al. Gene-based SNP genetic association study of the corticotropin-releasing hormone receptor-2 (CRHR2) in major depression. Am J Med Genet. 2002 Mar 8;114(2):222–226. doi: 10.1002/ajmg.10179. [DOI] [PubMed] [Google Scholar]
- 161.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002 Dec;13(10):436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 162.Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004 Jul;271(13):2821–2830. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ross PC, Kostas CM, Ramabhadran TV. A variant of the human corticotropin-releasing factor (CRF) receptor: cloning, expression and pharmacology. Biochem Biophys Res Commun. 1994 Dec 30;205(3):1836–1842. doi: 10.1006/bbrc.1994.2884. [DOI] [PubMed] [Google Scholar]
- 164.Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors in human and mouse skin: identification of new variants and their differential expression. FASEB J. 2001 Dec;15(14):2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- 165.Jin D, He P, You X, Zhu X, Dai L, He Q, et al. Expression of corticotropin-releasing hormone receptor type 1 and type 2 in human pregnant myometrium. Reprod Sci. 2007 Sep;14(6):568–577. doi: 10.1177/1933719107307821. [DOI] [PubMed] [Google Scholar]
- 166.Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, et al. A common inversion under selection in Europeans. Nat Genet. 2005 Feb;37(2):129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- 167.Koolen DA, Vissers LE, Pfundt R, de Leeuw N, Knight SJ, Regan R, et al. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet. 2006 Sep;38(9):999–1001. doi: 10.1038/ng1853. [DOI] [PubMed] [Google Scholar]
- 168.Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, Hurst JA, et al. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet. 2006 Sep;38(9):1038–1042. doi: 10.1038/ng1862. [DOI] [PubMed] [Google Scholar]
- 169.Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet. 2006 Sep;38(9):1032–1037. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- 170.Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S, et al. Phenotypic Expansion and Further Characterization of the 17q21.31 Microdeletion Syndrome. J Med Genet. 2009 May 15; doi: 10.1136/jmg.2008.065391. [DOI] [PubMed] [Google Scholar]
- 171.Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S, et al. Phenotypic expansion and further characterisation of the 17q21.31 microdeletion syndrome. J Med Genet. 2009 Jul;46(7):480–489. doi: 10.1136/jmg.2008.065391. [DOI] [PubMed] [Google Scholar]
- 172.Kirchhoff M, Bisgaard AM, Duno M, Hansen FJ, Schwartz M. A 17q21.31 microduplication, reciprocal to the newly described 17q21.31 microdeletion, in a girl with severe psychomotor developmental delay and dysmorphic craniofacial features. Eur J Med Genet. 2007 Jul-Aug;50(4):256–263. doi: 10.1016/j.ejmg.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 173.Hodges LM, Weissman MM, Haghighi F, Costa R, Bravo O, Evgrafov O, et al. Association and linkage analysis of candidate genes GRP, GRPR, CRHR1, and TACR1 in panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008 May 1; doi: 10.1002/ajmg.b.30773. May 1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 174.Licinio J, O'Kirwan F, Irizarry K, Merriman B, Thakur S, Jepson R, et al. Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry. 2004 Dec;9(12):1075–1082. doi: 10.1038/sj.mp.4001587. [DOI] [PubMed] [Google Scholar]
- 175.Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007 Mar 6;414(2):155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 176.Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006 Sep 1;404(3):358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 177.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008 Feb;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wasserman D, Sokolowski M, Rozanov V, Wasserman J. The CRHR1 gene: a marker for suicidality in depressed males exposed to low stress. Genes Brain Behav. 2007 Mar 21; doi: 10.1111/j.1601-183X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 179.Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, et al. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009 Sep;66(9):978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009 Oct 1;66(7):681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Heim CM, Bradley B, Mletzko T, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Frontiers in Behavioral Neurosciences. 2009 doi: 10.3389/neuro.08.041.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, et al. Polymorphisms in CRHR1 and the serotonin transporter loci: gene x gene x environment interactions on depressive symptoms American Journal of Medical Genetics, Part B Psychiatric Genetics. 2009 doi: 10.1002/ajmg.b.31052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Blomeyer D, Treutlein J, Esser G, Schmidt MH, Schumann G, Laucht M. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008 Jan 15;63(2):146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 184.Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, et al. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2009 Jul 17;:1–12. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- 185.Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, et al. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Derijk RH, de Kloet ER. Corticosteroid receptor polymorphisms: determinants of vulnerability and resilience. Eur J Pharmacol. 2008 Apr 7;583(2–3):303–311. doi: 10.1016/j.ejphar.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 187.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009 Jun 25; doi: 10.1016/j.psyneuen.2009.05.021. Jun 25 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 188.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008 Sep 3;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009 Sep;1790(9):878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 190.Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, et al. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008 Mar;82(3):696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]