Abstract
Objective
To develop a novel polytrauma model that better recapitulates the immunological response of the severely injured patient by combining long-bone fracture, muscle tissue damage and cecectomy with hemorrhagic shock, resulting in an equivalent Injury Severity Score of greater than 15. We compared this new polytrauma/shock model to historically-used murine trauma-hemorrhage models.
Design
Pre-clinical controlled in vivo laboratory study.
Setting
Laboratory of Inflammation Biology and Surgical Science.
Subjects
6–10 wk old C57BL/6 (B6) mice
Interventions
Mice underwent 90 minutes of shock (MAP 30 mmHg) and resuscitation via femoral artery cannulation followed by either laparotomy (TH), laparotomy with femur fracture (H+FFx), or laparotomy with cecetomy and femur fracture with muscle tissue damage (PT). Mice were euthanized at two hours, one day and three days post injury.
Measurements and Main Results
The spleen, bone marrow, blood, and serum were collected from mice for analysis at the above time points. None of the models were lethal. Mice undergoing PT exhibited a more robust inflammatory response with significant elevations in cytokine/chemokine concentrations when compared to traditional models. PT was the only model to induce neutrophilia (Ly6G+CD11b+ cells) on days 1 and 3 (p<0.05). PT, as compared to TH and H+FFx, induced a loss of circulating CD4+ T cell with simultaneous increased cell activation (CD69+ and CD25+), similar to human trauma. There was a prolonged loss of MHCII expression on monocytes in the PT model (p<0.05). Results were confirmed by genome-wide expression analysis which revealed a greater magnitude and duration of blood leukocyte gene expression changes in the PT model than the TH and sham models.
Conclusions
This novel polytrauma model better replicates the human leukocyte, cytokine, and overall inflammatory response following injury and hemorrhagic shock.
Keywords: hemorrhage, gene expression, femur fracture, inflammation, immune suppression, trauma
Introduction
Trauma with massive injury is the most common cause of death in patients under the age of 45, and the hemorrhagic shock that frequently accompanies trauma increases mortality as well as infectious complications (1–3). Despite advances in trauma care via the advent of aggressive early management and improved intensive care unit (ICU) support, morbidity and mortality, especially from complications remain prohibitively high (3). There appear to be many common immune responses following both trauma and sepsis, and it is thought that immunological dysregulation following trauma contributes to poor outcomes (4–6). There has been a substantial body of research on the immunologic origin of the infectious complications leading to prolonged ICU stays, sepsis, and multiple organ failure (MOF) (7). However, the exact mechanisms behind these phenomena in trauma patients remain unknown, and MOF remains a major source of post-injury morbidity and in-hospital mortality (6, 8).
Currently, our understanding of and capacity to improve the poor outcomes in human trauma patients due to immunological dysregulation has been limited. This is partially due to a lack of adequate translational research with limited animal models. Animal experimentation that recapitulates severe human trauma has been generally unsuccessful due to our inability to utilize a model that allows long term follow-up of severely injured animals, with many studies focusing on early time points less than 24 hours (2, 9, 10). The most commonly used murine trauma-hemorrhage (TH) model, which consists of hemorrhagic shock combined with laparotomy and sometimes femur fracture, is well-established and has been used for the past two decades (9–12). Our experience, as well as data from other studies, suggests that greater injury to the animal may be required to appropriately reflect the inflammatory response that occurs following human trauma beyond the initial phase (4, 5, 13–17). Additionally, since the implementation of standard operating procedures (SOPs), ARDSNET, and computerized systems enforcing evidenced-based protocols (18–20), many patients now survive their initial traumatic insult beyond 24 hours. Subsequently, these patients can develop late MOF or a ‘persistent inflammation-immunosuppression catabolic syndrome’ (PICS), rarely returning to a functional life (7). Thus, we have sought to develop a murine polytrauma model that better recapitulates the human inflammatory response to significant trauma beyond the acute phase of injury and shock. Such a model of murine trauma and hemorrhagic shock, would allow us to better study the dysregulation in the protective immunity that occurs with severe injury, which we believe is in part responsible for a patient’s increased risk of subsequent infections and morbidity and mortality.
In refining the murine trauma and shock model to more accurately reflect the human condition, we sought to develop a model in which the combined insults create the equivalent of an Injury Severity Score (ISS) of greater than 15. This number is typically used as a minimum score for trauma studies of severely injured humans, and it is these patients who have been identified at increased risk for susceptibility to infection, regardless of age (1, 8, 17, 21). Additionally we incorporated multiple compartments of injury into this model, as are seen in severely injured patients. Specifically, in this new polytrauma model (PT) the mouse undergoes hemorrhagic shock, femur fracture with muscle tissue damage, and laparotomy with cecectomy. We then compared this novel ‘polytrauma’ and shock model to previously used models including hemorrhage with laparotomy (TH), and hemorrhage with laparotomy and femur fracture (H+FFx).
Materials and Methods
Mice
Male C57BL/6j (B6) mice aged 6–8 weeks were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The study protocol was approved by the University of Florida IACUC. Mice were acclimated at least one week prior to their use in experiments.
Mouse Injury Models
Groups of mice were anesthetized using inhalational isoflurane and restrained in the supine position. Their femoral arteries were cannulated bilaterally and the mice were awakened from general anesthesia. To simulate shock, the mice were bled to and maintained at a mean blood pressure of 30 mm Hg (± 5 mm Hg) under continuous arterial blood pressure monitoring (BPA; DigiMed) for 90 minutes. Subsequently, the mice were resuscitated with Ringer’s lactate solution at four times the blood volume drawn as previously described (9, 12). The catheters were then removed, the femoral arteries were ligated, and the incisions were closed (22). The mice were again placed under general anesthesia and underwent either: a one centimeter laparotomy to simulate the traditional trauma-hemorrhage model, which has an ISS of zero (TH) (9); a one centimeter laparotomy combined with femur fracture (H+FFx), which has an ISS of nine(10); or polytrauma, comprised of a one centimeter laparotomy with cecectomy combined with medial thigh dissection with femur fracture and muscle tissue damage (PT), which produces an ISS of 18. Specifically, for the femur fracture, careful blunt dissection of the soft tissues to expose the femur was performed and the femur was fractured using two clamps or cut with scissors. The bones were then realigned. In addition, for PT the superior muscle tissue was grasped with a clamp for 30 seconds. The skin was closed in a single layer using 6–0 nylon suture. For the PT model with the addition of cecectomy, the cecum was identified after laparotomy, ligated twice with 3–0 silk suture and resected. The abdominal incision was closed in one layer using surgical staples. Naïve animals underwent no anesthesia or operation while sham mice underwent laparotomy and ligation of their femoral vessels as well as lateral incision and closure of the thigh without hemorrhagic shock. After injury mice were housed in groups and all mice were administered buprenorphrine (0.2 mg/kg BW) prior to arousal from anesthesia and every 12 hours afterward until sacrifice. None of the murine models were lethal and the animals were able to maintain the ability to ambulate and groom, as well as feed and drink. None of the groups of mice lost more than 20% of their body weight. There were no obvious infectious complications.
Sample Collection
The mice were euthanized after two hours and on postoperative days 1 and 3, and their spleens, bone marrow, and blood were collected for analysis. The plasma cytokine and chemokine response was measured by multiplex Luminex™ assay, and specific leukocyte populations were analyzed by flow cytometry in the blood, spleen, and bone marrow for their relative and absolute numbers, phenotype, and activation status as previously described (23, 24). Statistics were performed utilizing one-way ANOVA and Tukey’s multiple comparisons test for post-hoc analysis.
Microarray protocol and gene expression analysis
Genome-wide expression analysis was also performed to compare the severity and duration of the blood leukocyte genomic responses among the three models. Analysis was performed by isolating 100 ng of total RNA from circulating mouse leukocytes isolated by centrifugation and isotonic lysis of contaminating erythrocytes. Nucleic acids were then labeled using the 3′ IVT Express Kit (Santa Clara, CA) and 15 μg of labeled cRNA was hybridized to Mouse Genome 430 2.0 Arrays (Affymetrix, Santa Clara, CA). Arrays were hybridized for 16 hr at 45° C. Following hybridization, arrays were stained and washed using an FS450 Affymetrix fluidics station and Affymetrix FlexFS 450–0004 protocol. Arrays were then scanned in an Affymetrix GeneChip™ scanner 7G Plus.
There were a total of 40 arrays consisting of 14 PT arrays, 14 TH arrays, and 12 sham arrays at two hours, one and three day time points. A log2 transformed expression matrix was calculated from the Affymetrix. cel files using RMA as implemented in the Partek Genomic Suite 6.6, and gene expression patterns were compared among the PT, TH, and sham murine models. The injury responsive genes were identified in a supervised analysis using an F-test with a significance threshold at p<0.001 using the class prediction tool implemented in BRB-ArrayTools version 4.1 Stable Release, developed by Richard Simon & BRB-ArrayToolsDevelopment Team (http://linus.nci.nih.gov/BRB-ArrayTools.html) (24).
Results
Polytrauma induces a significantly greater systemic inflammatory response as compared to traditional TH models
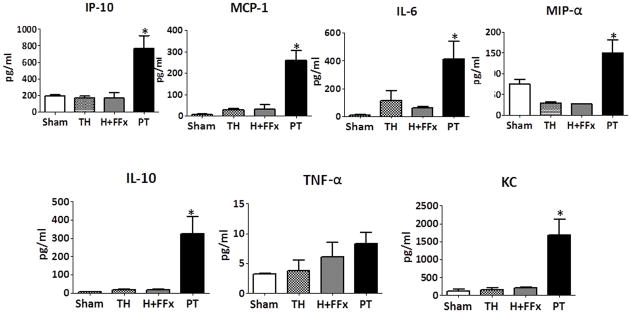
Severe injury in humans is known to cause a significant increase in the concentration of plasma cytokines and chemokines after injury, and these molecules are detectable within 24 hours after injury (5). Examination of plasma cytokines via multiplex Luminex™ assay was performed on day 1 following injury, and demonstrated that the concentrations of plasma cytokines/chemokines (IL-10, IP-10, MIP-1α, KC, IL-6, and MCP-1) were significantly increased in the PT model when compared to both the TH and H+FFx models (Figure 1). There was only a trend for increased TNF-α levels. By day three, the levels of cytokines had significantly decreased in all injury models, and were not statistically different from each other (data not shown).
Figure 1. Inflammatory mediators significantly increased in polytrauma compared to traditional models on Day 1 following injury.
Plasma concentrations of inflammatory mediators (IP-10, MCP-1, IL-6, MIP-α, and KC) as well as the anti-inflammatory mediator IL-10 were increased in the polytrauma (PT) model compared to traditional models on day 1 (* p<0.05 One-way ANOVA, Tukey’s post-hoc test).
Polytrauma produces a sustained leukocytosis that is associated with neutrophilia, as well as a late expansion in myeloid-derived suppressor cells (MDSCs)
Human trauma studies have revealed that there is frequently a leukocytosis with neutrophilia in patients with an ISS of greater than 15 (25, 26). One day after injury, all three models displayed a relative leukocytosis with an associated neutrophilia. However, this neutrophilia was only significant in the PT model when compared to the TH and H+FFx models (p<0.05) (Figure 2A). By day 3, this phenotype of expanded circulating neutrophils persisted only in the PT model, along with the expansion of MDSCs (GR-1+CD11b+) (p=0.05) (Figure 2B). These GR-1+CD11b+ cells were not elevated in either the TH or H+FFx models. Interestingly, the PT model induces a post-injury phenotype that is more similar to the inflammatory response displayed in murine models of polymicrobial sepsis (cecal ligation and puncture) (27).
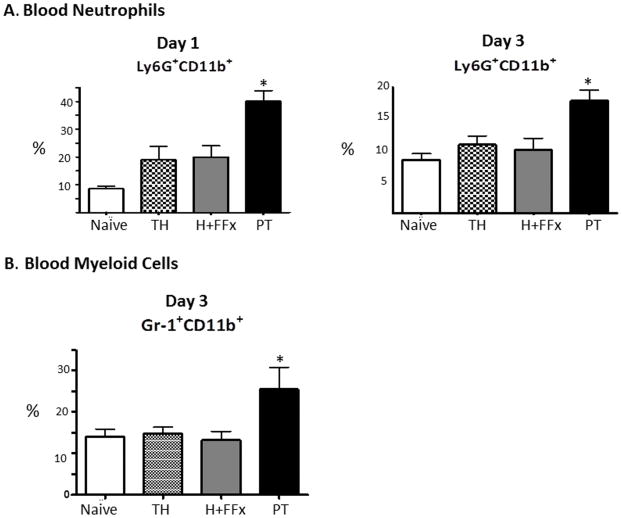
Figure 2. Leukocytosis after trauma associated with neutrophilia.
A. PT was the only model to induce neutrophilia as evidenced by an increased percentage of Ly6G+CD11b+ cells in the blood on days 1 and 3 when compared to the other models (*p<0.0003, p<0.05 One-way ANOVA and Tukey’s post-hoc test, PT versus sham, TH, and H+FFx). B. By day 3, there is also an increase in the circulating GR-1+CD11b+ after PT, which is not displayed in the other models (p=0.05 One-way ANOVA).
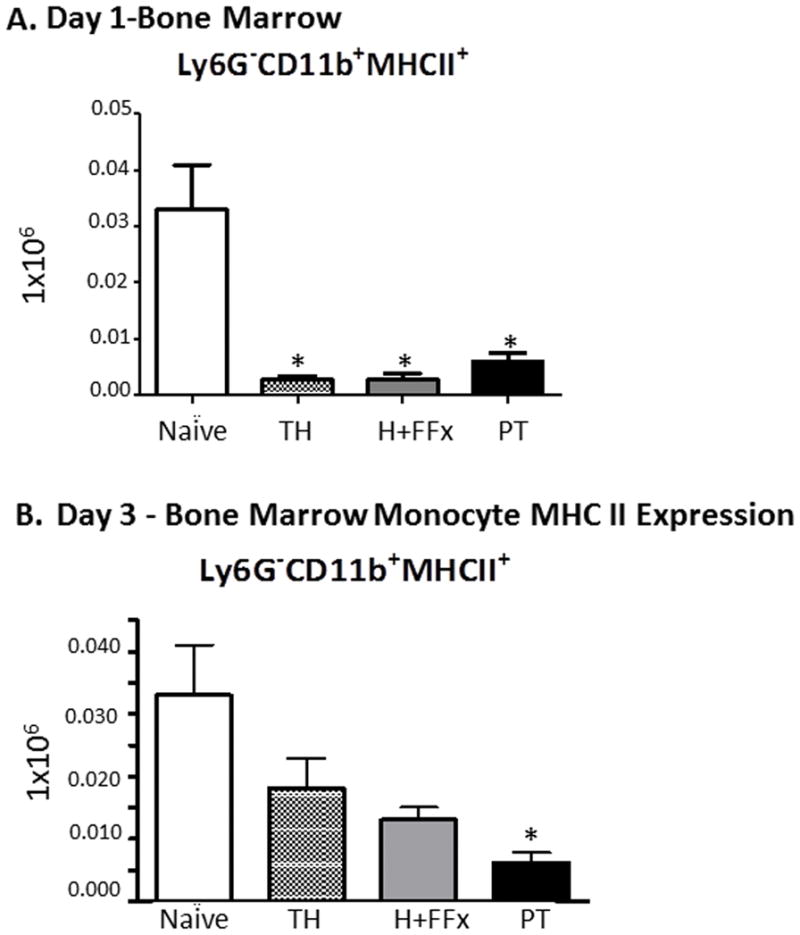
Polytrauma persistently decreases MHCII expression on bone marrow monocytes
All three injury models induce loss of MHC II expression on (MHCII+Ly6G−CD11b+) monocytes in the bone marrow on day 1 following trauma (Figure 3A). This loss of MHCII expression only persists in the PT model, and by day 3, significantly fewer MHCII+Ly6C+CD11b+ cells are present in the bone marrow (Figure 3B), which is consistent with the decreased MHCII expression on monocytes that is seen in trauma patients with poor outcomes (28).
Figure 3. MHC II expression.
A. There were greater numbers of MHCII expressing monocytes on day 1 in the bone marrow in the all three models compared to naïve animals (p=0.0003 One-way ANOVA, p<0.05 Tukey’s post hoc test). B. On day 3, there was persistant decrease in number of MHC II (MHCII+Ly6G−CD11b+) monocytes in the bone marrow (p=0.013 by One-way ANOVA and p<0.05 by Tukey’s post-hoc test, PT versus naïve).
Polytrauma induces an early lymphocyte activation in the spleen and bone marrow with late lymphopenia and increased activation of the remaining circulating lymphocytes
Human studies have also revealed early T helper and cytotoxic T cell lymphocyte activation after trauma (25, 26). Greater lymphocyte activation, including splenic CD8+ cytotoxic T and CD19+ B cells, and bone marrow CD4+ T helper and CD19+ B cells, was observed in on day 1 in the PT model compared to the TH or H+FFx models, as evidenced by the numbers of cells with increased CD69 and/or CD25 expression (Figure 4A). Later, on day 3, a relative CD4+ T helper cell lymphopenia was observed in all the animal models. However, only PT also induced a loss of CD8+ cytotoxic T cells as well as displayed increased activation of the remaining circulating CD4+ T helper lymphocytes in the blood (Figure 4B, p<0.0001). This lymphocyte phenotype demonstrated by PT has also been documented in the human trauma population in the first week after injury (26).
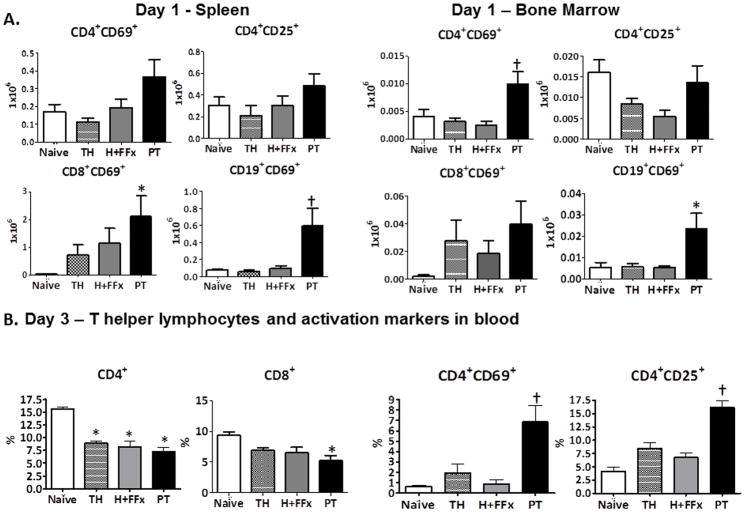
Figure 4. Lymphocyte activation status.
A. On day 1 following injury, the number of activated (CD69+) splenic CD8+ Cytoxic T and CD19+ B cells were increased in the PT model. In addition, only PT increased the number of CD69+ activated CD4+ T helper and CD19+ lymphocytes in the bone marrow. (*p<0.05 One-way ANOVA). Additionally, the PT model was significantly different from TH and H+FFx in the splenic CD19+ B cells and the bone marrow CD69+ activated CD4+ T helper cells († p<0.05 One-way ANOVA, Tukey’s post-hoc). B. On day 3, PT, TH, and H+FFx all induced a loss of CD4+ T helper cells and cytotoxic CD8+ T cells in the blood (* p<0.0001, p=0.0088 One-way ANOVA, Tukey’s Post-hoc). However, only PT also caused a significant increased activation of the remaining circulating CD4+ T helper lymphocytes (CD69+ and CD25+)(†p=0.0015, p<0.0001, p<0.05 One-way ANOVA, Tukey’s post-hoc test).
TH without fracture increases the number splenic monocytes after shock and resuscitation
Human trauma studies have revealed that there is no increase in macrophage numbers in the spleen following human trauma (29). Our analysis of the splenic cells following murine trauma revealed that the Ly6G−CD11b+ splenic and bone marrow population of monocytes consisted of half F4/80+ macrophages (data not shown). We found a significant increase in the number splenic monocytes (including increased macrophages) in the less severe TH model as compared to the PT model and the control population, a response that has not been documented to occur in humans (Figure 5).
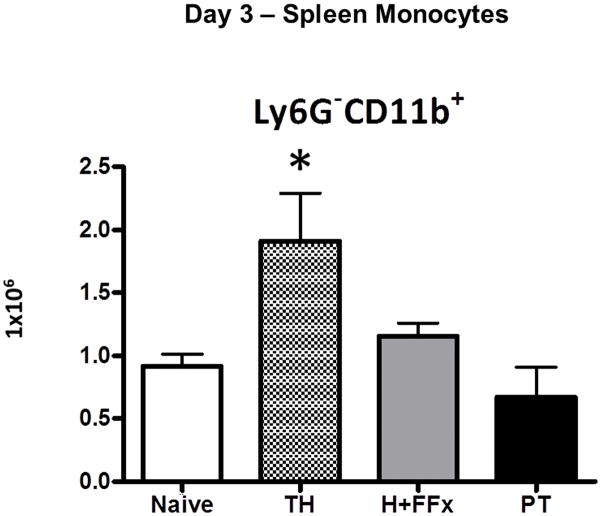
Figure 5. Splenic monocytes after hemorrhagic shock and trauma.
Splenic monocytes (Ly6G− CD11b+) were increased in number in the less severe TH model compared to the PT model and the naive population (* p=0.015 One-way ANOVA, p<0.05 Tukey’s post-hoc test).
Genome-wide expression analysis confirms that the polytrauma model induces a genomic response that varies dramatically from the sham and TH models
Whole blood leukocyte RNA samples from mice in the sham, TH, and PT groups from time points at two hours, one and three days were hybridized to Mouse 430 2.0 microarrays containing 45,101 probe sets representing over 34,000 well-substantiated mouse genes. Forty microarrays were analyzed by ANOVA and revealed that overall there were 21,242 out of the 45,101 probe sets that were significantly different from sham when all three groups at each time point were considered, with a p<0.001. ANOVA performed on each model individually revealed greater than 5,000 probe sets were significant (p<0.001) in both the PT and TH model compared to the sham model in which only 1,732 probe sets were significantly altered over time. Additionally, analysis revealed that gene expression of roughly half (2,933) of the altered probe sets were similar between the PT and TH models, but that 2,373 and 2,605 were unique to the PT and TH models respectively.
When comparing the three models at each individual time point, the magnitude of the fold-changes in gene expression was consistently greater in the PT model (Figure 6). At 2 hours, there were 1,905 probe sets significantly different between the three models at p<0.001 with greater magnitude of change in the PT model compared to the TH model (Figure 6A). Additionally, Figure 6A displays that there were two clusters of genes that were uniquely up-regulated in the PT model that remained unchanged in the TH model (Figure 6A). These clusters included genes involved in NIK, MEK, IKK and NFkB signaling pathways (Table 1). Analysis of individual genes demonstrated that there was significantly greater up-regulation of pro-inflammatory genes in PT as compared to TH. By day 1, the TH model appeared to be returning toward expression patterns similar to the sham-treated animals, whereas the leukocyte gene expression changes persisted in the PT model (Figure 6B). Down-regulation of multiple genes important to protective immunity was more pronounced in PT as compared to TH (Table 1). By day 3, gene expression patterns in the two trauma models remained dissimilar to the sham model. Although the gene expression patterns between the PT and the TH models became more similar, a greater magnitude of gene suppression persisted in the PT model, including some genes associated with adaptive immunity (Figure 6C, Table 1). Evaluation of the canonical pathways most affected with PT and TH demonstrated alterations to many immunological pathways (Table 2). Many of these pathways reflected an overall pattern of increased up and down regulation of genes after PT, especially at the earlier time points (Table 1). Interestingly, findings from the Inflammation and Host Response to Injury Large Scale Collaborative Research Program (Glue Grant) demonstrated the early expression pattern of 63 genes were able to determine whether trauma patients were going to have complicated or uncomplicated outcomes (manuscript in review). Twenty eight of these genes were identified as being altered after PT or TH, with the understanding that there are no direct murine orthologues for the human HLA genes. PT, as compared to TH, induced a further up or down-regulation of 21 of these genes, comparable to that seen in severely traumatized patients with complicated clinical outcomes.
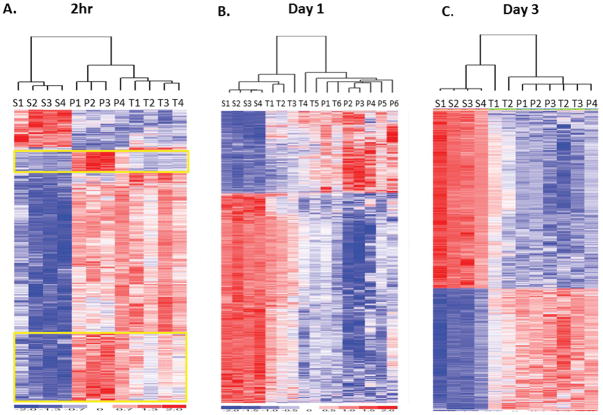
Figure 6. Microarray analysis of PT (P), TH (T), and Sham (S) murine models two hours, one and three days.
A. Two hours after injury an F-test showed 1,905 probe sets significant p<0.001. B. One day after injury an F-test showed 1,925 probe sets significant at p<0.001. C. Three days after trauma, F-test showed 9,802 probe sets significant p<0.001. At two hours the encircled cluster of genes show that there was a greater magnitude of upregulation in the PT model compared to the TH model as evidenced by the coloring on the heatmap. By day 1, the TH model appeared to be returning toward gene changes similar to the sham model, whereas, the PT model gene expression changes persisted, and by three days, the two models remain dissimilar to the sham model, although a greater magnitude of gene suppression persisted in the PT model. S=sham, T= Trauma-Hemorrhage, P=Polytrauma. The number indicates subject number.
Table 1. Differential Gene Expression between PT and TH.
A. The of the top ten differentially expressed genes in the PT and TH models, as compared to sham, at the three times points post shock and injury. B. Five additional selected genes of immunological interest that were differentially expressed in the PT and TH models (as compared to sham) at the three different time points after injury and shock.
| A. Top 10 Differentially Expressed Genes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 Hours | Day 1 | Day 3 | |||||||||
|
|
|
|
|||||||||
| PT | TH | PT | TH | PT | TH | ||||||
|
|
|
|
|||||||||
| Genes | Fold Change | Genes | Fold Change | Genes | Fold Change | Genes | Fold Change | Genes | Fold Change | Genes | Fold Change |
|
|
|
|
|||||||||
| Down-Regulated | Down-Regulated | Down-Regulated | |||||||||
|
|
|
|
|||||||||
| FCER2 | −3.5 | CD55 | −3.2 | MS4A1 | −14 | SS18 | −3.4 | PTGS2 | −15.2 | LY6E | −12.9 |
| HSPH1 | −3.4 | HSPH1* | −3.1 | CD55 | −12.6 | TRIM7 | −3.3 | BACH2 | −11.7 | SBK1 | −12 |
| BCL11A | −3.4 | FCER2 | −3.1 | PTGS2 | −10.1 | RPL41 | −3.2 | LY6E | −11.1 | GNS | −10.2 |
| CD55 | −3.2 | BCL11A | −2.9 | BANK1 | −9.9 | PRPF38B | −3.2 | HIVEP3 | −10.7 | SATB | −10.1 |
| NCR1 | −2.9 | TBX21 | −2.8 | KLRA4 | −9.4 | KLRA4 | −3.1 | SATB1 | −10.4 | BACH2 | −9.6 |
| HPGD | −2.9 | NCR1 | −2.8 | FCER2 | −9.3 | ATR | −3.1 | FCER* | −10.4 | POU2AF1 | −9.1 |
| TARDBP | −2.6 | XCL1 | −2.4 | IVNS1ABP | −9 | RANBP2 | −3 | CCR2 | −10.3 | IL2RG | −9.1 |
| S1PR5 | −2.6 | NRIP2 | −2.2 | P2RY10 | −8.8 | MALAT1 | −3 | III203 | −10.2 | FOXP1 | −8.9 |
| CR2 | −2.6 | EOMES | −2.2 | IGIV1 | −8.8 | HNRPDL | −3 | FILIP1L | −10 | FCER2 | −8.9 |
| XCL1 | −2.5 | CR2 | −2.2 | HLADOB | −8.8 | ZC3H15 | −2.9 | Ifi204 | −9.9 | MLL5 | −8.8 |
|
|
|
|
|||||||||
| Up-Regulated | Up-Regulated | Up-Regulated | |||||||||
|
|
|
|
|||||||||
| CXCL3 | 227.2 | MMP8 | 30 | SAA1 | 35.1 | RHAG | 17.5 | GSTT1 | 46.7 | GSTT1 | 34.7 |
| CXCL1 | 209.7 | ILR2 | 23 | ARG1 | 16.7 | GSTT1 | 15.9 | RHAG | 27.4 | RHAG | 30.8 |
| IRG1 | 103.8 | MT1E | 17.5 | ASNS | 12.8 | TSPAN8 | 10.8 | ASNS | 17.5 | ASNS | 24.1 |
| NGP | 91.7 | ARG2 | 15.3 | NGP | 11.5 | HFE2* | 8 | CTSF | 15.9 | MGST3 | 17.5 |
| CXCL2 | 57.8 | CXCL3 | 14.6 | GSTT1 | 11.3 | EPB42 | 6.8 | MGST3 | 15.3 | CTSF | 15.4 |
| CD14 | 47.6 | CD14 | 14.5 | HFE2 | 9.4 | OLR1 | 6.4 | NQO1 | 12.1 | NQO1 | 13.6 |
| RND1 | 40.1 | PLSCR1 | 13.6 | PROK2 | 8.9 | ASNS* | 6 | UROD | 12.1 | UROD | 12 |
| IL6 | 39.5 | LCN2 | 13.5 | RHAG | 8.4 | RPH3AL | 5.4 | EPB42 | 11.6 | ATG4A | 11.9 |
| MMP8 | 35.7 | HDC | 13.4 | LIPG | 7.3 | PRNP | 4.6 | ATG4A | 11 | EPB42 | 11.2 |
| CAMP | 35.5 | CRISPLD2 | 12.9 | SPP1 | 6.9 | PTGER3 | 4.3 | NIPA1 | 10.7 | HEMGN | 10.9 |
| B. Selected Genes and their Expression | |||
|---|---|---|---|
| Genes | PT | TH | |
| 2 Hours | TNF | 1.9 | −1.4 |
| CXCL10 | 5.8 | −1.1 | |
| NFKB2 | 3.3 | −1.1 | |
| MAP3K8 | 11.8 | 3.2 | |
| IRAK3 | 6.7 | 1.7 | |
| 1 Day | CD55 | −12.6 | −2.5 |
| CCR6 | −3.7 | −1.8 | |
| CD83 | −5.9 | −2.4 | |
| IL1B | −4.4 | 1 | |
| CD163 | 2.4 | −1 | |
| 3 Days | IL16 | −4.3 | −3.5 |
| CCI3 | −6.4 | −4.2 | |
| FCRIA | −4.6 | −3.1 | |
| BCL21 | 3.1 | 2.3 | |
| H2AB1 | 5.1 | 3.8 | |
Table 2.
Top Canonical Pathways Induced by PT and TH.
| 2 Hours |
| IL-10 Signaling |
| Production of NO and ROS in Macrophages |
| IL-6 Signaling |
| NF-κB Signaling |
| iNOS Signaling |
| 1Day |
| DNA Methylation & Transcriptional Repression Signaling |
| RhoGDI Signaling |
| Actin Nucleation by ARP-WASP Complex |
| Signaling by Rho Family GTPases |
| Cdc42 Signaling |
| 3 Days |
| B Cell Receptor Signaling |
| PI3K Signaling in B Lymphocytes |
| Molecular Mechanisms of Cancer |
| T Cell Receptor Signaling |
| RANK Signaling in Osteoclasts |
Discussion
Following severe traumatic injury, human patients enter a state of substantial immune dysregulation consisting of both exaggerated inflammation and immune suppression (13, 31), with significant changes in inflammatory mediators and leukocyte cell populations (5, 6, 26). Murine models of hemorrhagic shock combined with femur fracture and/or laparotomy have been described in the literature for more than a decade (10, 13); however, in many ways they do not appear to fully mimic the leukocyte and cytokine responses that have been demonstrated following severe human trauma after the first day (5, 6, 17, 25, 26). Additionally, these traditional models do not incite severe enough injury to assign an ISS comparable with those used to study human trauma (17). Today, equivalent injuries sustained in human patients that are used in the historical TH murine model would be assigned as ISS score of zero. To address this discrepancy, we sought to develop a novel murine polytrauma model that might better recapitulate the human response to trauma, and compared this model to other previously used TH models. In the PT model, a minimum of two centimeters of cecum/large bowel (a large amount for a mouse) is resected during a laparotomy to mimic an Abbreviated Injury Score (AIS) of 3 awarded to patients with a laparotomy and colon resection without gross contamination in human injury. The same is true for the open femur fracture with anterior muscle damage which is also given AIS of 3. This results in an ISS of 18. Once established, we planned to use this model to study the persistent immune dysregulation commonly seen in the human trauma population.
Most humans who have poor outcomes following trauma have “multi-trauma,” consisting of injury to several systems and compartments. Typically, this may include an intra-abdominal process as well as fractures and hemorrhagic shock with end organ damage, although it could also include an injury to the chest or brain. Our goal was to recapitulate this in a mouse model while being as humane to the animal as possible. Historically, trauma models consisting of long bone fractures and shock have been limited in duration to time points less than 24 hours due to animal welfare restraints (32). Although we understand a superior model would be one in which the animal is not under anesthesia and undergoes a more ‘harsh’ or invasive form of trauma, IACUC restrictions and the necessity for humane treatment of the animals will never allow such a murine model to occur, especially if the animal is to be kept alive for sub-acute and chronic analysis.
In our previous work, we found that a laparotomy itself without further operation (typically our ‘sham’ mouse used for our control groups in polymicrobial sepsis) had some minor effects on the systemic immune system (27). However, when considering the results from others (33, 34), in which minor manipulations of the rodent bowel had significant effects on immune phenotype, we felt that an intra-abdominal process was going to be an essential component of any murine polytrauma model. In addition, an intra-abdominal resection would mimic a typical human severely injured patient. A cecectomy in the mouse is one of the least invasive and painful intra-abdominal resections that could be carried out, essentially being the equivalent of a human appendectomy, although the ‘appendix’ in this case is larger and equivalent in diameter to the colon. Also, lack of a bowel anastomosis allows the animal to immediately resume eating, simplifying post-operative care and distress to the animal, both very important for the model to be both reproducible and practical. Splenectomy or liver resection were not utilized as we wished to preserve the spleen for analysis of immune function and our goal was to incite a sterile inflammatory response, whereas any division of the liver for resection risks inciting inflammation in response to pathogen associated molecular products (PAMPs).
In this report we have demonstrated that a more severe trauma injury model with shock that includes hemorrhage, femur fracture, and laparotomy with cecectomy better mimics the human leukocyte, cytokine, and overall inflammatory response present in severely injured trauma patients than previously published TH and H+FFx models that appear to induce a more transient and less severe injury. When we examined end organ damage in the murine trauma models we found that in both the PT and TH model aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were both elevated at 2 hours and 1 day, and returned to baseline by 3 days (data not shown). The Cr remained at baseline levels in both models. Additionally, both PT and TH models induced similar levels of acute lung injury (ALI) on pathological examination of the lung (data not shown).
In our experience with previous murine models, we were unable to detect significant immunological responses beyond 24 hours, which makes studying sub-acute and chronic leukocyte changes following severe trauma (5, 6, 25, 26) difficult. The polytrauma model incites a robust early immune response with significant increases in chemokine and cytokine concentrations that are more comparable to the robust chemokine and cytokine response seen in humans following severe trauma (5, 24). It is this exaggerated SIRS response that is in part thought to lead to early MOF in severely injured trauma patients (17, 35).
Our previous work in the Inflammation and Host Response to Injury Large Scale Collaborative Research Program (Glue Grant) showed that there is lymphopenia following severe trauma in humans, in addition to an early SIRS response with a prompt induction of the innate immune response with simultaneous immunosuppression (5). Other studies have revealed neutrophilia following severe injury (26). Only the polytrauma model reproduces the early neutrophilia and delayed lymphopenia with activation of circulating leukocytes in the blood, as displayed in human patients (26). In addition, PT increases early myeloid and lymphoid cell activation in the lymphoid tissue and bone marrow, as would be expected from systemic inflammation. We demonstrated that by day 3, the mice enter a state of relative immune suppression evidenced by persistent reductions in myeloid MHCII expression in the bone marrow, which is in accordance with the human blood monocyte responses following severe trauma (28, 36). Additionally, when examining the top five canonical pathways affected by PT on day 3, gene expression of pathways involved in adaptive immunosuppression such as B and T cell receptor signaling were significantly reduced (Table 2). This relative suppression of gene expression involved in adaptive immunity has been assumed to contribute to the increased risk for susceptibility to infection as well as greater morbidity and mortality (7). Data from Hotchkiss, et al. showed that in human patients after trauma, there is no change in macrophage numbers in the spleen, which is contradictory to the findings in the historical TH models as those models exhibit greatly increased numbers of macrophages in the spleen following the traumatic event in the mouse (29). This might be due to the effect of resuscitation without sufficient inflammation. Regardless, this increase in splenic monocytes is not seen in the novel polytrauma model, which is in accordance with the findings in the human condition.
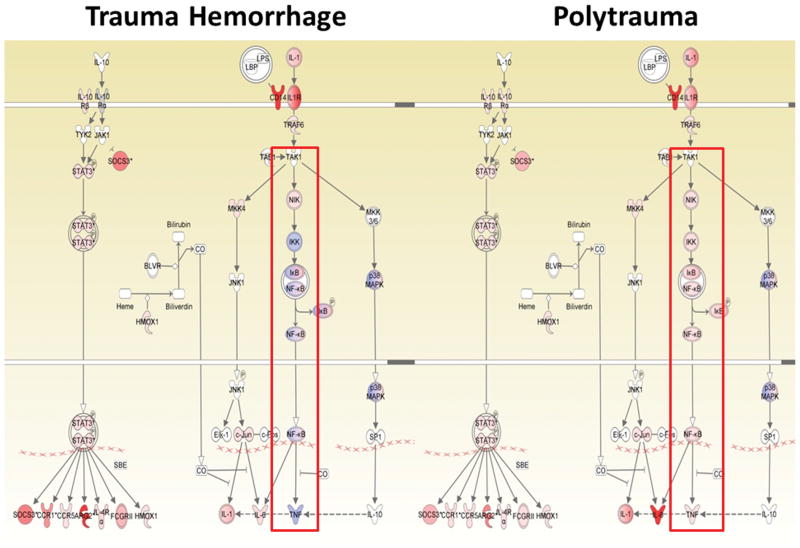
Microarray analysis confirms that the polytrauma model induces a leukocyte genomic response that is markedly different when compared to sham animals and historical murine TH models. Overall there were 21,242 out of the 45,101 probe sets that significantly changed over time when all three time points were considered, which is similar in magnitude to the ‘genomic storm’ seen in humans following severe traumatic injury (30). At all three time points, there are substantial alterations in gene expression between injured animals as compared to sham animals, and the changes are of greater magnitude in the polytrauma model (Table 1). For example, the IL-10 signaling pathway at two hours post injury demonstrates significant differences in gene expression between the two models (Figure 7). Expression of down-stream signaling pathways, including NF-kB are increased in PT, but not in TH. The genomic response between the polytrauma and the historical TH model is remarkably similar to the response seen in severe trauma patients with complicated and uncomplicated clinical outcomes, respectively (37). Trauma patients with complicated clinical outcomes and the polytrauma mouse model share a similar increased magnitude in the elevation of early inflammatory gene expression, as well as increased and prolonged suppression of adaptive immunity gene expression, when compared to the uncomplicated human patients and the TH mouse model (30).
Figure 7. Example of a differentially expressed canonical pathway.
IL-10 signaling pathway at two hours is differentially expressed between the PT and TH murine models.
Conclusions
The polytrauma model appears to be superior to traditional models of murine trauma and hemorrhagic shock in recapitulating the human response to severe injury. The murine immune response to the polytrauma model mimics many of the responses displayed by mice after polymicrobial sepsis, a phenomena not displayed by other murine injury/shock models. Employing this novel polytrauma model will allow us to more appropriately perform translational research in rodents, which, in turn will result in a better understanding of the immune dysregulation that occurs in humans following severe trauma. Although there are many inherent flaws to utilizing animal models in translational research (2), it is the reality of today’s environment that they must be used. Therefore, we must seek to modify existing animal models as new human data become available, in order to have our models most closely mimic the human immune responses. To date, many anti-inflammatory approaches to treat severe trauma and subsequent multiple organ failure have succeeded in mice, but failed in humans, likely due to the redundancy and complex nature of protective immunity (37). A proper understanding of the mammalian response to significant injury and inflammation will hopefully lead to the development of immunomodulatory therapies to help decrease morbidity and mortality in trauma patients.
Acknowledgments
Sources of Support: This work was supported by R01 GM040586-21S1 and R01 GM081923-05, awarded by the National Institute of General Medical Sciences (NIGMS). LFG and AGC were supported by T32 GM008721-12, a training grant in trauma and burns, awarded by the NIGMS.
Footnotes
The authors have not disclosed any potential conflicts of interest
References
- 1.Pfeifer R, Tarkin IS, Rocos B, et al. Patterns of mortality and causes of death in polytrauma patients--has anything changed? Injury. 2009;40(9):907–911. doi: 10.1016/j.injury.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9(1):1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Probst C, Pape HC, Hildebrand F, et al. 30 years of polytrauma care: An analysis of the change in strategies and results of 4849 cases treated at a single institution. Injury. 2009;40(1):77–83. doi: 10.1016/j.injury.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Levy RM, Prince JM, Yang R, et al. Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R970–976. doi: 10.1152/ajpregu.00793.2005. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. The Journal of experimental medicine. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannoudis PV, Hildebrand F, Pape HC. Inflammatory serum markers in patients with multiple trauma. Can they predict outcome? The Journal of bone and joint surgery British volume. 2004;86(3):313–323. doi: 10.1302/0301-620x.86b3.15035. [DOI] [PubMed] [Google Scholar]
- 7.Gentile L, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Peristent inflammation and immunosupression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1–11. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciesla DJ, Moore EE, Johnson JL, et al. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140(5):432–438. doi: 10.1001/archsurg.140.5.432. discussion 438–440. [DOI] [PubMed] [Google Scholar]
- 9.Wang P, Ba ZF, Burkhardt J, et al. Trauma-hemorrhage and resuscitation in the mouse: effects on cardiac output and organ blood flow. Am J Physiol. 1993;264(4 Pt 2):H1166–1173. doi: 10.1152/ajpheart.1993.264.4.H1166. [DOI] [PubMed] [Google Scholar]
- 10.Gill R, Ruan X, Menzel CL, et al. Systemic inflammation and liver injury following hemorrhagic shock and peripheral tissue trauma involve functional TLR9 signaling on bone marrow-derived cells and parenchymal cells. Shock. 2011;35(2):164–170. doi: 10.1097/SHK.0b013e3181eddcab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill R, Ruan X, Menzel CL, et al. Systemic inflammation and liver injury following hemorrhagic shock and peripheral tissue trauma involve functional TLR9 signaling on bone marrow-derived cells and parenchymal cells. Shock. 35(2):164–170. doi: 10.1097/SHK.0b013e3181eddcab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venet F, Chung CS, Huang X, et al. Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol. 2009;183(5):3472–3480. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wichmann MW, Ayala A, Chaudry IH. Severe depression of host immune functions following closed-bone fracture, soft-tissue trauma, and hemorrhagic shock. Crit Care Med. 1998;26(8):1372–1378. doi: 10.1097/00003246-199808000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Matsutani T, Kang SC, Miyashita M, et al. Young and middle-age associated differences in cytokeratin expression after bone fracture, tissue trauma, and hemorrhage. Am J Surg. 2007;193(1):61–68. doi: 10.1016/j.amjsurg.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Maddali S, Stapleton PP, Freeman TA, et al. Neuroendocrine responses mediate macrophage function after trauma. Surgery. 2004;136(5):1038–1046. doi: 10.1016/j.surg.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Kang SC, Matsutani T, Choudhry MA, et al. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine. 2004;26(5):223–230. doi: 10.1016/j.cyto.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 36(2):222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuschieri J, Johnson JL, Sperry J, West MA, Moore EE, Minei JP, Bankey PE, Nathens AB, Cuenca AG, Efron PA the Inflammation and Host Response to Injury. Large Scale Collaborative Research Program Benchmarking Outcomes in the Critically Injured Trauma Patient. Ann Surg 2012. In Press. [Google Scholar]
- 20.McKinley BA, Moore LJ, Sucher JF, et al. Computer protocol facilitates evidence-based care of sepsis in the surgical intensive care unit. The Journal of trauma. 2011;70(5):1153–1166. doi: 10.1097/TA.0b013e31821598e9. discussion 1166–1157. [DOI] [PubMed] [Google Scholar]
- 21.Baker SP, O’Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 22.Knoferl MW, Jarrar D, Angele MK, et al. 17 beta-Estradiol normalizes immune responses in ovariectomized females after trauma-hemorrhage. American journal of physiology Cell physiology. 2001;281(4):C1131–1138. doi: 10.1152/ajpcell.2001.281.4.C1131. [DOI] [PubMed] [Google Scholar]
- 23.Kelly-Scumpia KM, Scumpia PO, Weinstein JS, et al. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 2011;208(8):1673–1682. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederer JA, Brownstein BH, Lopez MC, et al. Comparison of longitudinal leukocyte gene expression after burn injury or trauma-hemorrhage in mice. Physiological genomics. 2008;32(3):299–310. doi: 10.1152/physiolgenomics.00086.2007. [DOI] [PubMed] [Google Scholar]
- 25.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27(4):733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Walsh DS, Siritongtaworn P, Pattanapanyasat K, et al. Lymphocyte activation after nonthermal trauma. Br J Surg. 2000;87(2):223–230. doi: 10.1046/j.1365-2168.2000.01341.x. [DOI] [PubMed] [Google Scholar]
- 27.Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hershman MJ, Cheadle WG, Wellhausen SR, et al. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77(2):204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168(5):2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 30.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahel PF, Smith WR, Moore EE. Role of biological modifiers regulating the immune response after trauma. Injury. 2007;38(12):1409–1422. doi: 10.1016/j.injury.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Menzel CL, Pfeifer R, Darwiche SS, et al. Models of lower extremity damage in mice: time course of organ damage and immune response. The Journal of surgical research. 2011;166(2):e149–156. doi: 10.1016/j.jss.2010.11.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zellweger R, Ayala A, Zhu XL, et al. Effect of surgical trauma on splenocyte and peritoneal macrophage immune function. J Trauma. 1995;39(4):645–650. doi: 10.1097/00005373-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Stephan RN, Saizawa M, Conrad PJ, et al. Depressed antigen presentation function and membrane interleukin-1 activity of peritoneal macrophages after laparotomy. Surgery. 1987;102(2):147–154. [PubMed] [Google Scholar]
- 35.Ertel W, Keel M, Marty D, et al. Significance of systemic inflammation in 1,278 trauma patients. Der Unfallchirurg. 1998;101(7):520–526. doi: 10.1007/s001130050304. [DOI] [PubMed] [Google Scholar]
- 36.MacLean LD, Meakins JL, Taguchi K, et al. Host resistance in sepsis and trauma. Ann Surg. 1975;182(3):207–217. doi: 10.1097/00000658-197509000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock (Augusta, Ga. 2001;16(2):83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]