Summary
Background
Microparticles are submicrometer vesicles that contain RNA and protein derived from their parent cells. Platelet and megakaryocyte microparticles represent 80% of circulating microparticles, and their numbers are elevated in diseases such as cancer and type 2 diabetes. The ability of microparticles to transport protein, lipid and RNA to target cells, as a means of transcellular communication, remains poorly understood. Determining the influence that microparticles have on circulating cells is essential for understanding their role in health and in disease.
Objectives
To develop a novel approach to modify the composition of platelet microparticles, and understand how such changes impact their transcellular communication.
Methods
This novel model utilizes a lentiviral technology to alter the transcription factor peroxisome proliferator-activated receptor-γ (PPARγ) content of megakaryoblastic cell lines and primary megakaryocytes, and also the protein composition of generated platelets and microparticles. The subsequent microparticles were isolated and added to target cells for assessment of uptake and resultant signaling events.
Results
We successfully engineered microparticles to contain green fluorescent protein and elevated levels of PPARγ. We found that these altered microparticles could be internalized by the monocytic cell line THP-1 and primary human microvascular endothelial cells. Importantly, microparticle-delivered PPARγ was shown to increase the expression of fatty acid-binding protein 4 (FABP4), which is a known PPARγ target gene in THP-1 cells.
Conclusion
This proof-of-concept modification of megakaryocyte, platelet and microparticle composition and subsequent change in target cell physiology is an important new tool to address transcellular communication of microparticles.
Keywords: lentivirus, megakaryocyte, microparticle, platelet, PPARγ, thrombopoiesis
Introduction
Circulating microparticles are submicrometer vesicles produced by activated vascular cells such as platelets [1], megakaryocytes [2,3], endothelial cells [4], leukocytes, tumor cells [5], and red blood cells [6]. Estimates of average microparticle counts range from the hundreds of thousands to millions, or 5–50 ng of microparticles per microliter of human blood, with platelet and megakaryocyte microparticles representing 80% of circulating microparticles [7]. Many inflammatory conditions, such as type 2 diabetes [8], cancers [9] and cardiovascular diseases [10], have been reported to involve elevated microparticle numbers. Although the exact mechanism is not yet known, there are many examples of microparticles being internalized, transferring their contents, and eliciting functional changes within target cells [11].
Megakaryocytic cell lines have been widely used to study megakaryopoiesis and the molecular mechanisms of platelet production [12–16]. Circulating human blood platelets can be readily isolated and purified for study of their composition or functional changes in response to treatments. We and others have observed that platelet microparticle composition is dependent on the health of the subject (unpublished from our laboratory), the size class of the microparticle [17], and the type of platelet activation by which they are formed [18]. However, there are no current working models for studying the compositional effects of megakaryocyte-derived or platelet-derived microparticles on cells with which they may interact. Here, we have developed a working platform to modify the composition of platelet microparticles in order to study the function of microparticles. These modified micro-particles are used in ‘transcellular’ (delivery from the microparticle-producing cell to a recipient ‘target’ cell) experiments to better understand how microparticles interact and functionally influence recipient cells.
Dami and Meg-01 cells are two cell lines of megakaryoblastic leukemic origin, and are capable of spontaneously producing platelet-like particles [16,19] and, as we report here, microparticles. The contents of these microparticles can be altered through transduction of the parent megakaryocyte. These altered microparticles were used to investigate the transcellular communication capacities of microparticles on recipient target cells.
Research design and methods
Cell culture
Meg-01 and THP-1 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA), and cultured in RPMI-1640 (Invitrogen, Grand Island, NY, USA) with 5% Hyclone heat-inactivated fetal bovine serum, which has undergone filtration through rated filters of pore size 40–0.04 μm, thus removing microparticles (Thermo Fisher Scientific, Waltham, MA, USA), 10 mM Hepes (Sigma-Aldrich Co., St Louis, MO, USA), 2 mML-glutamine (Invitrogen, Grand Island, NY, USA), 4.5 g L−1 glucose (Invitrogen, Grand Island, NY, USA), and 50 μgmL−1 gentamicin (Invitrogen, Grand Island, NY, USA). Dami cells were a generous gift from P. J. Simpson-Haidaris (University of Rochester), and were cultured according to established conditions [19].
Bone marrow was isolated from C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA), and red blood cells were removed by lysis. The remaining cells were cultured in IMDM (Invitrogen, Grand Island, NY, USA) with 20% BIT 9500 (StemCell Technologies, Vancouver, British Columbia, Canada), 1% penicillin/streptavidin (Invitrogen, Grand Island, NY, USA), 100 ng mL−1 human stem cell factor and 50 ng mL−1 recombinant human thrombopoietin (rhTPO) (R&D Systems, Minneapolis, MN, USA) for 2 days. Half of the cells were subsequently transduced with a green fluorescent protein (GFP)-expressing lentivirus at a transducing unit/cell concentration of 20 for 18 h in OPTIMEM (Invitrogen, Grand Island, NY, USA) in the presence of 6 μgmL−1 polybrene (Sigma-Aldrich). Lentivirus was removed from culture by centrifugation, and cells were incubated in IMDM, 20% BIT 9500, 1% penicillin/streptavidin and 100 ng mL−1 rhTPO for 4 days. Where indicated, platelets were isolated by centrifugation, and activated with phorbol-12-myristate-13-acetate (PMA) (Sigma-Aldrich). Cells were analyzed by flow cytometry using the rat anti-mouse CD41–phycoerythrin antibody (BD Biosciences, San Jose, CA, USA) and GFP fluorescence.
Primary human microvascular endothelial cells (HMVECs) from adult dermis were purchased from Invitrogen (Carlsbad, CA, USA), and cultured as recommended.
Lentiviral production
Lentivirus was made and titered as previously described [20], and the megakaryocytic cell lines used here had a high transduction efficiency, with a transduction unit/cell ratio of 20.
Microscopy
Live culture megakaryocytes were examined by phase-contrast microscopy with an Olympus IX81 inverted microscope. Cytospun cells and adherent fixed cells were analyzed on an Olympus BX51 light microscope (Olympus, Melville, NY, USA). Images were captured and edited with SPOT RT 4.6 (Diagnostic Instruments Inc., New Hyde Park, NY, USA). An FV1000 Olympus Laser Scanning Confocal Microscope (Olympus) was used to capture confocal images, which were subsequently edited with Olympus FV1000 software. Objective lens magnifications are indicated in the figure legends.
Western blotting
Western blotting was performed as previously described [21]. The primary antibodies used for this work were as follows: rabbit anti-human peroxisome proliferator-activated receptor-γ (PPARγ) (Cell Signaling Technology, Boston, MA, USA), mouse anti-Flag (Clonetech Laboratories, Mountain View, CA, USA), mouse anti-human actin, and mouse anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Calbiochem, Darmstadt, Germany).
Flow cytometry
Cells and microparticles were analyzed on the FACSCanto II, LSR II (BD Biosciences) or Accuri C6 flow cytometer (BD Biosciences). Data were analyzed with FLOWJO software (Treestar, Ashland, OR, USA).
Platelet production assay
Megakaryocytes (2 × 105) were plated and cultured with or without the synthetic PPARγ agonist rosiglitazone (10 μM) (Cayman Chemical Company, Ann Arbor, MI, USA). Cells in culture were collected, and directly analyzed at the indicated times by flow cytometry. Platelet-like particles were characterized by size gating, and subgated on GFP-positive events to differentiate intact platelet-like particles from debris or cytometer noise. GFP-positive platelet-like particle counts were normalized to total megakaryocytes to rule out counting errors of initial megakaryocyte plating from the separate transduced cultures.
Microparticle generation from megakaryocyte cultures
In order to generate enough microparticles for subsequent experiments, a minimum of 100 mL of starting megakaryocyte cultures was plated at a density of 4 × 105 cells mL−1, and grown for at least 3 days before beginning microparticle isolation by centrifugation (Table 1). When supernatants were transferred to new tubes, at least 1 mL of medium was Culture-derived microparticles Platelet-derived microparticles left with the undisturbed pellet, to ensure that pelleted cells were not transferred. All steps were performed at room temperature, except where otherwise noted. For estimation of relative microparticle quantity, total lysates of the microparticle pellets were measured with a detergent-compatible protein assay kit (BioRad, Hercules, CA, USA). Beginning with the cell volume and density described above, ~ 300 μg of total protein was measured from each culture-derived microparticle preparation. This amount includes serum proteins that may have accumulated with microparticles during the preparation step. Nevertheless, protein concentration was used to standardize microparticle counts from different starting cultures.
Table 1.
Two processes for the isolation of either culture-derived microparticles or platelet-derived microparticles
| Culture-derived microparticles | Platelet-derived microparticles |
|---|---|
| Megakaryocyte cultures | Megakaryocyte cultures |
| ↓ | ↓ |
| 200 ×g, 15 min | 200 ×g, 15 min |
| ↓ | ↓ |
| Spin supernatant 1200 ×g, 10 min | Spin supernatant 1200 ×g, 10 min |
| ↓ | ↓ |
| Spin supernatant 3000 ×g, 15 min | Resususpend platelet pellet in activation bu3er |
| ↓ | ↓ |
| Spin supernatant 10 000 ×g, 60 min | Activate for 30 min, 37 °C |
| ↓ | ↓ |
| Resuspend microparticle pellet | Spin 3000 ×g 15 min |
| ↓ | ↓ |
| Add to ‘target’cells | Spin supernatant 21 000 ×g, 30 min |
| ↓ | |
| Resuspend microparticle pellet | |
| ↓ | |
| Add to ‘target’cells | |
|
| |
| Megakaryocyte and platelet origin | Platelet origin |
| Higher yield per culture volume | Low yield per culture volume |
In order to generate enough microparticles for subsequent experiments, a minimum of 100 mL of starting megakaryocyte cultures was grown at confluency (4 × 105 cells mL−1) for at least 3 days before beginning isolations by centrifugation. When supernatants were transferred to new tubes, at least 1 mL of medium was left with the undisturbed pellet to ensure that pelleted cells were not transferred. All steps were performed at room temperature, except where otherwise noted.
Microparticle transfer of functional transcription factors
THP-1 cells (50 000 cells per well) were plated in a 96-well culture plate in a 30-μL volume. Culture-derived microparticles were isolated as described in Table 1, resuspended, quantified, and microparticles (20 μg of protein) were added to the THP-1 cells. Half of the wells received the indicated doses of rosiglitazone, and half of the wells received the same doses of rosiglitazone plus the PPARγ antagonist GW9662 (Cayman Chemical Company). After 24 h, cells were lysed in QIAzol Lysis Reagent (Qiagen, Valencia, CA, USA), and RNA was extracted and quantified. cDNA was synthesized with the iScript cDNA Synthesis Kit (BioRad) for subsequent quantitative real-time PCR analysis via iQ SYBR Green Supermix (BioRad). The primers used for PCR were as follows: fatty acid-binding protein 4 (FABP4), 5′-ACAGG AAAGTCAAGAGCACC-3′ and 5′-AACTTCAGTCCAGGTCAACG-3′; and GAPDH, 5′-TGGGTGGAATCATAT TGGAAC-3′and 5′-AGGTGAAGGTCGGAGTCAAC-3′.
Statistics
As indicated in the figure legends, one-way and two-way ANOVA analyses with the Tukey and Bonferroni post tests, respectively, were performed with GraphPad PRISM (Graph-Pad, La Jolla, CA, USA). Error bars depict standard error of the mean.
Results
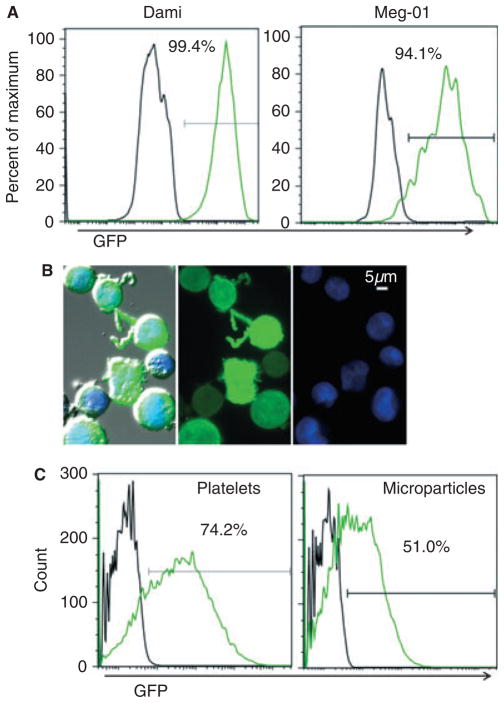
In order to alter the protein composition of microparticles, we first modified the composition of megakaryocytes, the parent cells of platelets and megakaryocytic-derived micro-particles. Transduction with a lentiviral vector allowed permanent insertion of exogenous genes into the host cell DNA. Using this technology in the megakaryoblastic cell lines Meg-01 and Dami, we were able to generate an unlimited source of modified parent cells. A third megakaryoblastic cell line, MO7e, was poorly transduced under the same conditions, and was therefore not used for subsequent microparticle generation (data not shown). We analyzed each transduction through GFP expression by flow cytometry (Fig. 1A), and only used cultures that had a transduction efficiency of > 95% for subsequent experiments. The transduction efficiency in Dami cells was slightly better, and Dami cells proliferated faster, but produced fewer platelet-like particles, than Meg-01 cells (data not shown). Fluorescent imaging demonstrated that GFP was present throughout the megakaryocytes, including extended cytoplasmic processes (Fig. 1B), which have been previously characterized in the Meg-01 cell line and are similar to proplatelet extensions [15]. To determine whether platelet-like particles and microparticles produced by the transduced megakaryocytes contained GFP, we performed flow cytometry analysis of platelet-sized or microparticle-sized events in culture. The flow cytometry gates used to measure the culture-derived platelet-like particles and microparticles are of similar size and granularity to gates used to identify platelets and microparticles from human platelet-rich plasma (Fig. S1). Theserepresentativeresults show that 74% of platelet-like particles and 51% of microparticles contained fluorescent GFP; however, these percentages may be underestimated, as non-platelet or non-microparticle debris could be counted within the GFP-negative populations (Fig. 1C).
Fig. 1.
Green fluorescent protein (GFP) lentiviral transduction of megakaryocytes results in GFP-positive platelets and microparticles. (A) Two megakaryoblastic cell lines were transduced with a GFP-expressing lentiviral vector. Unstained cells were analyzed by flow cytometry 1 week post-transduction, and GFP fluorescence is shown for untransduced (black line) and GFP-transduced (green line) megakaryocytes. (B) Cytospun GFP-transduced Meg-01cells were stained with the nuclear dye 4′,6diamidino-2-phenylindole (DAPI) (blue). From left to right: the triple-merged DIC bright-field image, GFP fluorescence, and DAPI fluorescence; × 40 objective. (C) Culture-derived platelet-like particles and microparticles were pre-gated by size, and analyzed by flow cytometry for GFP fluorescence. Platelet-like particles and microparticles derived from untransduced or GFP-transduced megakaryocyte cultures are depicted with black and green lines, respectively.
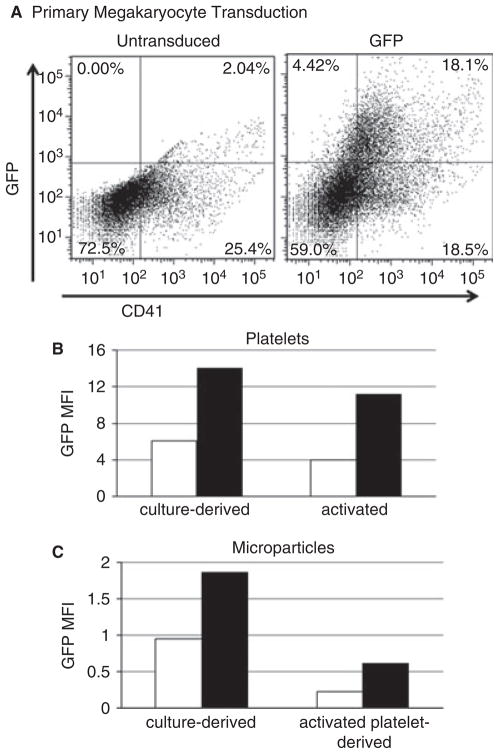
For future applications, this approach of modifying platelet microparticles would be desirable in a primary megakaryocyte system. As a proof of concept, we demonstrated that it was possible to transduce primary mouse bone marrow cells, and used additional cytokines to commit hematopoietic stem cells to the megakaryocytic lineage (represented by CD41+ cells). Megakaryocytes represented a large proportion of the transduced cells (Fig. 2A). We analyzed the culture-derived platelet (unactivated) and microparticle-sized events by flow cytometry, and showed they also contained GFP (Fig. 2B,C). We also showed that activated platelets from transduced cultures could produce GFP-positive platelet microparticles (Fig. 2C). The transduction efficiency of mixed bone marrow cells is lower than in cell lines, and these primary cells are also short-lived (< 2 weeks). Therefore, we chose to continue our investigation of microparticle compositional modification in the Meg-01 and Dami cell line models.
Fig. 2.
Primary mouse megakaryocytes can be transduced, and generate green fluorescent protein (GFP)-positive platelets and microparticles. (A) A mixed bone marrow culture was not transduced (left) or was transduced with a GFP-expressing lentiviral vector (right). Cells were stained with anti-mouse CD41–phycoerythrin (x-axis) and analyzed for GFP fluorescence (y-axis). (B, C) Platelets and microparticles were analyzed directly from culture (left), or platelets were isolated from culture and activated with phorbol-12-myristate-13-acetate (right). Platelets and microparticles were first gated on size and CD41 positivity, and then analyzed for GFP expression. Graphs depict the mean fluorescence intensity (MFI) of GFP from untransduced (white bars) and transduced cultures (black bars). The data shown are from one representative experiment out of three independent experiments that yielded similar results.
Cell cultures of megakaryoblastic cell lines spontaneously produce platelet-like particles and microparticle-sized vesicles [16,19]. We therefore investigated two techniques for isolating microparticles (Table 1). With the use of optimized serial centrifugation, cells and platelet-like particles were removed from culture, and a final spin isolated culture-derived micro-particles. These microparticles could conceivably be derived from megakaryocytes and platelets within the culture. Alternatively, we developed a method that would generate exclusively platelet-derived microparticles, whereby megakaryocytes are first removed by centrifugation, and isolated platelet-like particles are subsequently pelleted and resuspended in an activation buffer to generate fresh platelet-derived microparticles. Activated platelet-like particles were removed before the microparticles were pelleted. Inevitably, microparticles that were attached to cells or platelets within culture were removed in early centrifugation steps, thus lowering the final yield (data not shown). However, this loss of yield is necessary to obtain a unique microparticle pellet. Because of the relatively low yields, most experiments began with 100 mL of megakaryocyte culture supernatant, to produce 200 μL of concentrated microparticles for use in subsequent experiments. We obtained higher quantities of culture-derived microparticles per original culture volume than were obtained with platelet-derived microparticles (data not shown). Therefore, we used this method of culture-derived microparticle isolation for subsequent experiments. We were unable to obtain absolute counts of microparticles with current flow cytometry technology, as the cytometers cannot accurately identify microparticles with a diameter of < 0.5 μm. However, the size of the final micro-particle pellet and the total protein after lysis are reproducible from the same starting megakaryocyte volume and culture density (300 μg; see Research design and methods), and equal volumes and protein concentrations of resuspended microparticles were used from parallel cultures in subsequent transcellular experiments.
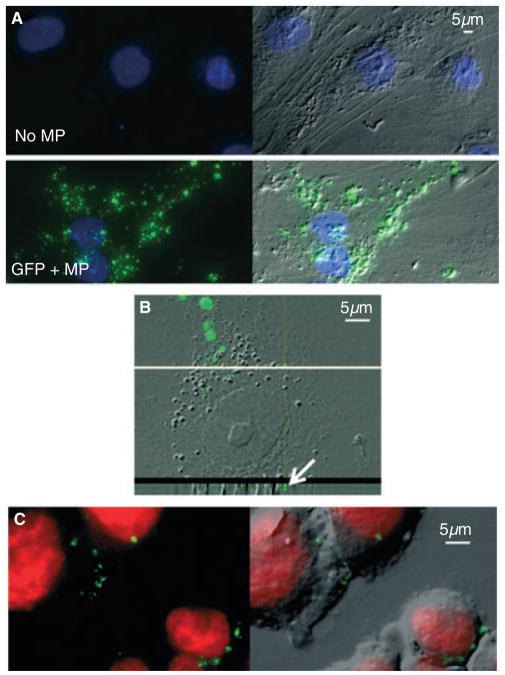
We next wanted to determine whether modified microparticles could interact with target cells and be taken up. We began by using primary HMVECs as target cells, owing to their known interaction with microparticles in vasculature [22]. Unlike HMVECs alone, GFP-positive microparticle-treated HMVECs exhibited GFP fluorescence (Fig. 3A). Both punctate and diffuse areas of GFP fluorescence appeared within the cytoplasm of the target cell. This is a similar pattern of uptake to that seen with fluorescently stained microparticles derived from circulating blood platelets absorbed by adherent cells (Fig. S2). We have also previously observed the absorption of platelet-derived microparticles by suspension THP-1 cells [23]. However, despite thorough washing of labeled microparticles, there was the possibility of free dye integrating into the target cell to falsely display as a microparticle. This caveat supports the advantage of having an internal fluorescent protein (GFP) as a superior marker of the presence of microparticles in our model system. In order to determine whether the GFP-positive microparticles were truly internalized, we examined fluorescence with confocal microscopy. Although the HMVEC cytoplasmic area was spread very thinly, stacked layers showed GFP within the cytoplasm (Fig. 3B). The fluorescent signal appeared to be mostly punctate, but we cannot rule out the existence of a diffuse signal in the cytoplasm, which would not be as bright, and therefore difficult to discern in the thin slice images.
Fig. 3.
Green fluorescent protein (GFP)-positive microparticles are taken up by target cells. (A) Culture-derived microparticles were incubated with preseeded adherent human microvascular endothelial cells (HMVECs) for 6 h. Chamber slides were thoroughly washed before fixation, permeabilization, and 4′,6-diamidino-2-phenylindole (DAPI) staining (blue). Representative examples of HMVECs that were not incubated with microparticles (top) and GFP-positive microparticles (bottom) are displayed as merged GFP (green) and DAPI images (left), and again with the fluorescence overlaying the DIC bright-field image (right); × 40 objective, light microscope. (B) Representative image of confocal microscopy of a GFP-positive microparticle-treated HMVEC. A magnified DIC and GFP merged image of the stacked layers of the cell is shown (top). The horizontal white line shows where the transverse z-stack image is shown below. The white arrow depicts GFP fluorescence within the transverse image of the cytoplasm; × 60 objective, confocal microscope. (C) THP-1 suspension cells were used as other target cells to internalize microparticles. After the cells had been incubated with GFP-positive microparticles for 6 h, they were thoroughly washed before fixation and cytospin centrifugation, followed by Draq5 (red) nuclear staining. Representative images showing GFP and nuclear stain fluorescence (left) are overlaid onto the DIC bright-field image (right); × 40 objective, light microscope. MP, microparticle.
In addition to adherent cells, we were interested in the potential interaction of GFP-positive microparticles with suspension cells, so we tested THP-1 monocytic cells, which are known to interact with microparticles [22,23]. We found that THP-1 cells were able to take up GFP-positive microparticles (Fig. 3C). PMA activation of THP-1 cells causes adherence to the culture dish. These cells were also able to internalize GFP-positive microparticles (data not shown).
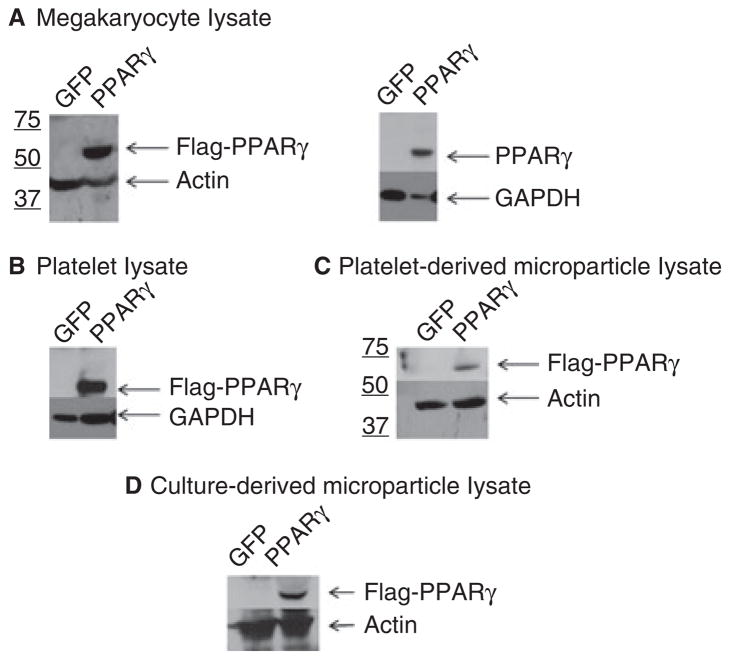
GFP allows microparticles to be easily visualized, but we also wanted to modify the levels of the anti-inflammatory, proadipogenic transcription factor PPARγ in microparticles, to determine whether transcription factors delivered by micro-particles can initiate transcription within a recipient cell. We began by transducing megakaryocyte cell lines as before with the GFP lentivirus or a lentivirus expressing both GFP and Flag-tagged PPARγ. Western blots were performed to assess PPARγ levels in megakaryocytes, platelet-like particles, and microparticle lysates (Fig. 4). All blots showed high levels of the transcription factor as compared with GFP controls, which express very low levels of PPARγ that were not detected in the exposures.
Fig. 4.
Western blot analysis of peroxisome proliferator-activated receptor-γ (PPARγ)-transduced megakaryocyte culture components. Meg-01 megakaryocytes were transduced with either a control lentivirus (green fluorescent protein [GFP]) or a GFP/PPARγ-overexpressing lentivirus (PPARγ). Fractionation of culture components was performed as described in Table 1. Lysates of megakaryocytes (A), platelet-like particles (B), platelet-derived microparticles (C) and culture-derived microparticles (D) were analyzed for protein expression of Flag–PPARγ. Representative western blots were probed with anti-Flag (Flag–PPARγ) or anti-PPARγ (PPARγ) antibodies, as well as actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody as the loading control.
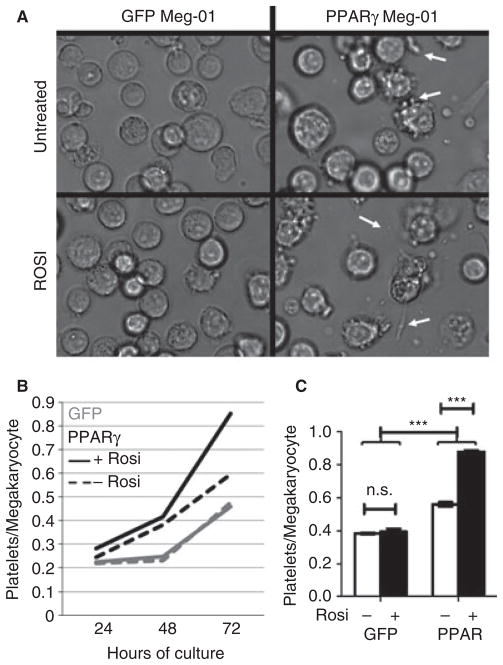
Interestingly, the megakaryocytes that overexpressed PPARγ appeared to divide at a slower rate than GFP-positive control megakaryocytes, causing the percentage of GFP-positive cells in the culture to decrease over time, as the remaining < 5% non-transduced population continued to proliferate normally (data not shown). For this reason, new transductions were performed when the GFP-positive cells in the cultures were reduced to < 85%. Meg-01 cultures had to be freshly transduced more often than Dami cultures. We were interested in identifying other effects of megakaryocytic overexpression of PPARγ, and were particularly interested in platelet-related effects. Morphologically, the PPARγ-transduced megakaryocytes had more cytoplasmic processes and potential platelet-producing morphology in culture (Fig. 5A). With the addition of a synthetic PPARγ-specific agonist, rosiglitazone, the morphology of the GFP-transduced culture remained similar to that of the untreated control, whereas the PPARγ-transduced culture, again, had many visible cytoplasmic processes (Fig. 5A). In order to investigate the observed morphologic changes, we quantified GFP-positive platelet-like particles produced in cultures of untreated or rosiglitazone-treated megakaryocytes via flow cytometry, which is an established method for identifying platelet-like particles produced from megakaryoblastic cell lines [19]. Platelet-like particles accumulated over time in all conditions, but PPARγ-transduced megakaryocytes produced more platelet-like particles than GFP controls (Fig. 5B). In addition, only the PPARγ-transduced megakaryocytes were affected by the rosiglitazone treatment, which resulted in significantly higher numbers of platelet-like particles at 72 h (Fig. 5C). The observed change in morphology correlated with increased platelet-like particles, but further analyses would be needed to determine causality.
Fig 5.
Megakaryocytes that overexpress peroxisome proliferator-activated receptor-γ (PPARγ) respond to rosiglitazone by increasing platelet production. (A) Live culture representative images depict green fluorescent protein (GFP)-transduced (left) or PPARγ-transduced (right) Meg-01 megakaryocytes. Cultures were left untreated (top) or treated with 10 μM rosiglitazone. White arrows highlight cytoplasmic processes; × 40 objective. (B) GFP-transduced (gray) or PPARγ-transduced (black) Dami megakaryocytes were untreated (dashed line) or treated with 10 μM rosiglitazone (solid line) for the indicated times. GFP-positive platelet-like particles were quantified with flow cytometry, and normalized to total megakaryocytes. (C) Normalized platelet-like particle counts from 72-h cultures are plotted and analyzed with a two-way ANOVA.***P < 0.001. NS, not significant; Rosi, rosiglitazone.
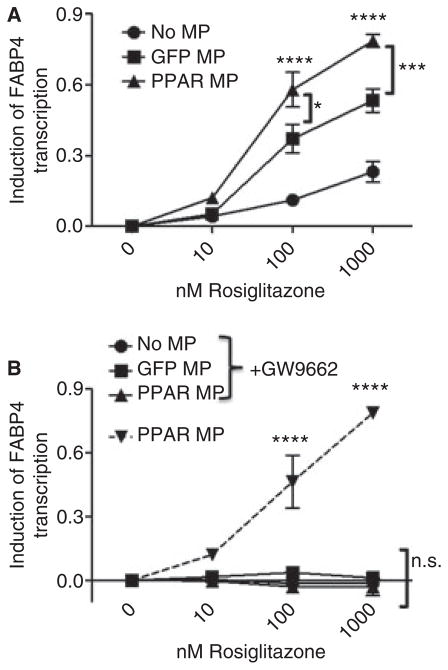
We next wanted to determine whether culture-derived microparticles could transfer the overexpressed PPARγ to target cells, to potentially influence their function. We used the THP-1 monocytic cells as target cells, because of their known interaction with microparticles and their fundamental role in vascular inflammatory conditions, such as atherosclerosis [22]. During the 24-h incubation, we added various doses of rosiglitazone to activate any transferred PPARγ, and additionally blocked that activation by cotreatment with rosiglitazone and 10 μM of the PPARγ-specific antagonist GW9662. To quantify the effects of PPARγ transfer from microparticles and subsequent function after transfer, we measured the mRNA levels of the PPARγ-specific target gene, FABP4,and normalized the starting quantity levels with GAPDH mRNA levels. Next, the values of untreated cells (0 nM rosiglitazone) were subtracted from the normalized FABP4 mRNA values within the same microparticle treatment group, to control for any direct transfer of RNA. Interestingly, the level of FABP4 mRNA was increased in a dose-dependent manner after rosiglitazone treatment in both the GFP-positive and PPARγ-overexpressing microparticle treatment groups (Fig. 6A). However, the PPARγ-overexpressing microparticle-treated cells produced significantly more FABP4 than the GFP-positive microparticle-treated cells at the 100 and 1000 nM rosiglitazone dosages. All increases in FABP4 mRNA were abolished by addition of the antagonist GW9662 (Fig. 6B). Overall, this work supports the hypothesis that PPARγ can successfully transfer from microparticles into target cells, and translocate to the nucleus to modulate gene transcription in recipient cells.
Fig. 6.
Modified microparticles can deliver functional peroxisome proliferator-activated receptor-γ (PPARγ) to target cells. THP-1 cells were cultured for 24 h with no microparticles (No MP) or Meg-01 culture-derived green fluorescent protein (GFP)-positive microparticles (GFP MP) or PPARγ-overexpressing microparticles (PPARγ MP), in the presence of 0, 10, 100 or 1000 nM rosiglitazone alone (A), or with 10 μM GW9662 (B). Quantitative real-time PCR of mRNA was performed, and starting quantities of fatty acid-binding protein 4 (FABP4) were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and then subtracted from the value for the 0 nM concentration of each microparticle treatment group. Values were compared by use of two-way ANOVA and the Bonferroni post test. *P < 0.05, ***P < 0.001, ****P < 0.0001. NS, not significant.
Discussion
We present data to support the proof of concept that lentiviral transduction of megakaryocytes alters the composition of daughter platelets and microparticles. This novel platform technology will be important for future investigations that seek to uncover mechanisms of microparticle uptake on target cells, as well as to uncover the influence of microparticles on cells that internalize them. This is especially important for anucleate cells such as platelets, which cannot be genetically manipulated with standard methods. We thoroughly investigated two platelet-like particle-producing and microparticle-producing megakaryocyte cell line models, Meg-01 and Dami, which were advantageous because of their spontaneous generation of platelet-like particles, allowing them to provide a continuous source of megakaryocyte-derived and platelet-derived micro-particles. We believe that the generation of culture megakaryocyte-derived and platelet-derived microparticles could occur spontaneously from quiescent cells, as previously described [2], or alternatively during activation via pipetting-induced shear stress. The transduction of primary megakaryocytes was also successful, revealing the versatility of our system for the study of megakaryocytes, platelets, and microparticles.
Circulating microparticles in human blood are mostly thought to originate directly from megakaryocytes rather than from activated platelets [3]; however, microparticle shedding from quiescent platelets has not been studied. We speculate that our culture-derived microparticles could originate from megakaryocytes, activated platelets, or quiescent platelets, but the proportions of each are not yet known. Besides identifying their origin, we plan to perform phenotypic and functional comparison tests of culture and blood microparticles. These analyses, followed by further manipulations, may be pertinent in the future to achieve a model that most closely mimics blood microparticles.
As demonstrated by flow cytometry and fluorescence microscopy, the modified microparticles consisted of intact vesicles enclosing native and fluorescently excitable GFP. We have also shown that GFP-positive microparticles can be absorbed by target cells (Fig. 3). Images of target cells show both punctate and diffuse GFP fluorescence. We believe that the punctate areas represent recently internalized intact micro-particles, or, in some cases, groups of microparticles, and the more diffuse green fluorescence could be GFP from internalized microparticles whose membranes have degraded, thus causing them to release their contents into the cytoplasm. However, more mechanistic investigation is needed before these interpretations can be supported. A recent study demonstrated that the uptake of endothelial microparticles is annex-in I/phosphatidylserine receptor-dependent [24]. The same mechanism may be used in the uptake of platelet-derived and megakaryocyte-derived microparticles, as they are also positive for surface phosphatidylserine, but protein interactions may also play a role, and further studies will be necessary to delineate the exact process of uptake and mechanism of content transfer from microparticles into the target cell cytoplasm. In the future, this platform technology could be used to provide the answers to these fundamental questions.
The composition of circulating microparticles from person to person is inevitably different. However, the influence of microparticle composition on maintaining a healthy vasculature, or even in contributing to an inflamed vasculature, remains poorly understood and is under active investigation. The hypothetical routes of communication of microparticles with target cells include surface receptor-driven signal induction and transfer of contents such as protein, RNA and lipids to influence internal signaling pathways [11]. However, little is known about these processes, and these forms of microparticle communication were previously difficult to study. Our new findings demonstrate that the microparticle-delivered transcription factor PPARγ can enter and initiate transcription of the PPARγ target gene, FABP4, within the recipient cell (Fig. 6A). Importantly, between the PPARγ-overexpressing microparticle treatments, FABP4 mRNA increased with rosiglitazone treatment, but not in the presence of the PPARγ antagonist GW9662 (Fig. 6B). This indicates that microparticle-derived PPARγ was delivered to recipient cells and was able to elicit transcription of its target genes, as this increase would only be possible if mRNA were freshly synthesized from target cell genomic DNA. Interestingly, the GFP-positive microparticles were also able to deliver functional PPARγ to the target cells (Fig. 6A). Our laboratory has previously shown transfer of PPARγ from blood platelet-derived microparticles to THP-1 cells [23]. However, the data presented here show, for the first time, that target mRNA transcription is enhanced within the recipient cell when PPARγ is delivered by microparticles. Additionally, these data show that the composition of the microparticle (expression level of PPARγ) impacts the amount of mRNA that can be transcribed.
The breakdown of the microparticle membrane would be crucial for internalized microparticles to release their contents into the cytoplasm to act on machinery within the target cell. This membrane breakdown has not been previously investigated, and will be a crucial component of future investigations of transcellular communication. Overall, this transfer of transcription factors from an altered microparticle to a target cell is of great interest, and will serve as a unique platform with which to alter the levels of other influential transcription factors or proteins found within circulating microparticles.
The frequency of microparticle absorption that occurs in the circulation is not known. Microparticles may specifically be taken up by activated cells, e.g. by cells in inflamed tissue, or may be continuously absorbed by the endothelium or white blood cells. The microparticle composition would then determine the message sent to the target cell. In this manner, microparticles may initiate an inflammatory or, conversely, a resolution response within the target cell, or they may transmit a homeostatic signal to keep target cells in a ‘quiet’ state. In the condition of Scott syndrome, where phosphatidylserine externalization and microparticle shedding are greatly diminished, patients are predisposed to bleeding complications [25]. In this case, the lack of microparticles could also decrease transcellular communication in these patients, which could hinder vascular integrity and maintenance. The overall importance of microparticles is evident in coagulation processes, but the extent of their roles in the vasculature, particularly in transcellular communication, is only beginning to be understood.
A second major finding is that overexpression of PPARγ by megakaryoblastic cells increases platelet-like particle production. Although small amounts of PPARγ are present in the control cells (data not shown), they were not significantly affected by the PPARγ-specific agonist rosiglitazone. In this approach, we used a platelet-counting technique in which GFP-positive platelet-like particles were easily distinguishable from the non-fluorescent background debris detected by flow cytometers in this size range. This novel platelet-counting method may be of interest for future thrombopoietic enhancement investigations. Here, the cells overexpressing PPARγ were not only better platelet producers, but were also able to respond to rosiglitazone through a further increase in platelet-like particle production (Fig. 5). This effect, combined with the observed slower growth rate than that of controls, could result from the ability of PPARγ to cause differentiation in many cancer cell types [20], thus pushing the megakaryocytes through thrombopoiesis and ultimately to death faster than their basal rate. The effect of PPARγ overexpression in primary megakaryocytes was not investigated here; however, on the basis of our cell line data, we predict that these cells would also produce more platelets. These data demonstrate a new potential for future investigations into the role of PPARγ in thrombopoiesis, and introduce the possibility of overexpression and activation of PPARγ as a therapeutic strategy for the treatment of thrombocytopenia.
For the first time, we show here that: (i) microparticle composition can be manipulated; (ii) transfer of microparticle-delivered transcription factors from platelet-derived and megakaryocyte-derived microparticles to target cells is functional, as they translocate into the nucleus, bind genomic DNA, and turn on specific target mRNA; and (iii) modification of transcription factor levels within microparticles is reflected in the amount of target mRNA that is transcribed within the recipient cell (Fig. 6). These exciting conclusions only begin to reveal the capacities of microparticle transcellular communication. Further studies are needed to better understand the kinetics and mechanisms integral to this mode of transcellular signaling. Hereafter, this novel platform technology will provide a useful tool with which to alter internal and/or surface microparticle composition that will aid in investigations of how microparticles influence target cells.
Supplementary Material
Scatterplots of platelets and microparticles from blood and megakaryocyte culture use identical sizing gates.
Membrane-stained platelet microparticles are absorbed by HEK293a cells.
Acknowledgments
We thank T. Bushnell, director of the University of Rochester Medical Center Flow Cytometry Core, for assistance with optimizing microparticle flow cytometry. We also thank L. Callahan, director of the University of Rochester Medical Center Confocal and Conventional Microscopy Core, for her assistance in obtaining images of microparticles within target cells.
Footnotes
Disclosure of conflict of interests
This research was supported in part by T32-HL066988, T90DE021985, HL095467, ES01247, UL1RR024160 and AI091036 (Center for Medical Countermeasures against Radiation Program).
Additional Supporting Information may be found in the online version of this article:
References
- 1.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multive sicularbodies and alpha-granules. Blood. 1999;94:3791–9. [PubMed] [Google Scholar]
- 2.Flaumenhaft R. Formation and fate of platelet microparticles. Blood Cells Mol Dis. 2006;36:182–7. doi: 10.1016/j.bcmd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE. Megakaryocyte derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–21. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 5.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by micro-vesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 6.Tissot JD, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol. 2010;17:571–7. doi: 10.1097/moh.0b013e32833ec217. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and under-appreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 8.Koga H, Sugiyama S, Kugiyama K, Fukushima H, Watanabe K, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of remnant lipoproteins are associated with plasma platelet microparticles in patients with type-2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J. 2006;27:817–23. doi: 10.1093/eurheartj/ehi746. [DOI] [PubMed] [Google Scholar]
- 9.Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36:888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 10.Amabile N, Rautou PE, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin Thromb Hemost. 2010;36:907–16. doi: 10.1055/s-0030-1267044. [DOI] [PubMed] [Google Scholar]
- 11.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 12.Battinelli E, Willoughby SR, Foxall T, Valeri CR, Loscalzo J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc Natl Acad Sci USA. 2001;98:14458–63. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apelseth TO, Hervig T, Wentzel-Larsen T, Petersen K, Reikvam H, Bruserud Ø. A prospective observational study of the effect of platelet transfusions on levels of platelet-derived cytokines, chemokines and interleukins in acute leukaemia patients with severe chemotherapy-induced cytopenia. Eur Cytokine Netw. 2011;22:52–62. doi: 10.1684/ecn.2011.0271. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien JJ, Baglole CJ, Garcia-Bates TM, Blumberg N, Francis CW, Phipps RP. 15-deoxy-Delta12,14 prostaglandin J2-induced heme oxygenase-1 in megakaryocytes regulates thrombopoiesis. J Thromb Haemost. 2009;7:182–9. doi: 10.1111/j.1538-7836.2008.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi K, Satoh M, Kuno H, Yoshida T, Kondo H, Takeuchi M. Platelet-like particle formation in the human megakaryoblastic leukaemia cell lines, MEG-01 and MEG-01s. Br J Haematol. 1998;100:436–44. doi: 10.1046/j.1365-2141.1998.00576.x. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi K, Ogura M, Saito H, Satoh M, Takeuchi M. Production of platelet-like particles by a human megakaryoblastic leukemia cell line (MEG-01) Exp Cell Res. 1991;193:223–6. doi: 10.1016/0014-4827(91)90560-h. [DOI] [PubMed] [Google Scholar]
- 17.Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW. Proteomic and functional characterisation of platelet microparticle size classes. Thromb Haemost. 2009;102:711–18. doi: 10.1160/TH09-04-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shai E, Rosa I, Parguiña AF, Motahedeh S, Varon D, García A. Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. J Proteomics. 2012 doi: 10.1016/j.jprot.2012.02.030. in press. [DOI] [PubMed] [Google Scholar]
- 19.Lev PR, Goette NP, Glembotsky AC, Laguens RP, Meckert PM, Salim JP, Heller PG, Pozner RG, Marta RF, Molinas FC. Production of functional platelet-like particles by the megakaryoblastic DAMI cell line provides a model for platelet biogenesis. Platelets. 2011;22:26–36. doi: 10.3109/09537104.2010.515271. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Bates TM, Bernstein SH, Phipps RP. Peroxisome proliferator-activated receptor gamma overexpression suppresses growth and induces apoptosis in human multiple myeloma cells. Clin Cancer Res. 2008;14:6414–25. doi: 10.1158/1078-0432.CCR-08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahler J, Bernard JJ, Spinelli SL, Blumberg N, Phipps RP. The Feverfew plant-derived compound, parthenolide enhances platelet production and attenuates platelet activation through NF-κB inhibition. Thromb Res. 2011;127:426–34. doi: 10.1016/j.thromres.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158:277–87. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- 23.Ray DM, Spinelli SL, Pollock SJ, Murant TI, O’Brien JJ, Blumberg N, Francis CW, Taubman MB, Phipps RP. Peroxisome proliferator-activated receptor gamma and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, Hussein NA, Kebschull M, Bedorf J, Franklin BS, Latz E, Nickenig G, Werner N. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–35. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 25.Toti F, Satta N, Fressinaud E, Meyer D, Freyssinet JM. Scott syndrome, characterized by impaired transmembrane migration of procoagulant phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87:1409–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplots of platelets and microparticles from blood and megakaryocyte culture use identical sizing gates.
Membrane-stained platelet microparticles are absorbed by HEK293a cells.