Abstract
We examined body mass index (BMI) as a screening tool for gestational diabetes (GDM) and its sensitivity among different racial/ethnic groups. In a retrospective cohort study of 24,324 pregnant women at University of California, San Francisco, BMI was explored as a screening tool for GDM and was stratified by race/ethnicity. Sensitivity and specificity were examined using chi-square test and receiver-operator characteristic curves. BMI of ≥25.0 kg/m2 as a screening threshold identified GDM in >76% of African-Americans, 58% of Latinas, and 46% of Caucasians, but only 25% of Asians (p<0.001). Controlling for confounders and comparing to a BMI of ≤25, African-Americans had the greatest increased risk of GDM (adjusted odds ratio [AOR] 5.1, 95% confidence interval [CI]: 3.0 to 8.5), followed by Caucasians (AOR 3.6, 95% CI: 2.7 to 4.8), Latinas (AOR 2.7, 95% CI: 1.9 to 3.8), and Asians (AOR 2.3, 95% CI: 1.8 to 3.0). BMI’s screening characteristics to predict GDM varied by race/ethnicity. BMI can be used to counsel regarding the risk of developing GDM, but alone it is not a good screening tool.
Keywords: Body mass index, gestational diabetes mellitus, glucose loading test, race/ethnicity, receiver-operator characteristic curve
Gestational diabetes mellitus (GDM) is associated with obstetric complications affecting health outcomes of both the mother and the neonate.1 These complications include macrosomia,2 cesarean delivery,3 shoulder dystocia and birth trauma,4 preeclampsia,5 post-partum maternal development of type 2 diabetes mellitus, 6 as well as increased obesity and type 2 diabetes mellitus in the offspring later in life.7 Furthermore, neonatal metabolic complications may arise in the presence of poorly controlled maternal diabetes in pregnancy; these include hypoglycemia,8 hyperbilirubinemia,9 hypocalcemia, and polycythemia.10
Because treating GDM can lead to a reduction in perinatal complications,11,12 most clinicians in the United States screen some or all of their prenatal patients.13 The current American College of Obstetricians and Gynecologists14 guidelines for GDM recommend screening through history or laboratory testing, including a 50-g, 1-hour oral glucose-loading test (GLT) at 24 to 28 weeks followed by a diagnostic 100-g, 3-hour oral glucose tolerance test (GTT) if screening positive. One area of debate is the cutoff for a positive 50-g GLT. Most institutions use 130 or 140 mg/dL. However, one study demonstrated that different thresholds for various ethnicities may maximize sensitivity and decrease the false-positive rates.15
There remains a debate in the literature regarding whether to screen all pregnant women or only those with risk factors such as Asian, Latina, or Native American race/ethnicity, family history, advanced maternal age, or obesity. Screening based on traditional risk factors (age, body mass index [BMI], prior macrosomia, prior GDM, family history of diabetes) had a low sensitivity and identified <60% of Caucasian women at risk in one study.16 In another study that directly compared the detection rate and false-positive rate of universal versus risk-based screening using a complex screening strategy in which risk scores for GDM were assigned based on age, BMI, and race, the detection rate of GDM remained ~80% for both strategies; there was a minimal reduction in false-positive rate using the complex algorithm of selective screening that may be impractical in clinical settings.17 Although this may suggest minimal benefit of selective screening and possibly explain why at least 94% of current providers in the United States screen all pregnant women,18 the U.S. Preventive Services Task Force indicates that better-quality evidence is needed to support universal screening.19
Obesity, as defined by BMI >29 kg/m2, remains an important and increasing risk factor for GDM.20 Moreover, this association appears to vary by race/ ethnicity. The rate of GDM appears to be twofold higher in obese Latina and Asian women than obese African-American and Caucasian women.1 Despite the high rates of GDM in obese Latina and Asian women, one study reported21 that obese women with a pregestational BMI >29 were most likely to be Latina in origin and least likely to be Asian. Caucasian women fell in between the two groups. Given this background, we sought to investigate BMI as a screening tool for GMD in women of varying race/ethnicity.
MATERIALS AND METHODS
We conducted a retrospective cohort study of 24,325 women with singleton pregnancies who were screened for GDM between 1988 and 2001 at the University of California, San Francisco (UCSF) Medical Center. UCSF is a teaching hospital providing care to a diverse population of patients. The Committee on Human Research at UCSF approved this study. The women studied were stratified by self-reported race/ethnicity into Caucasians, African-Americans, Latinas, and Asians. Prepregnancy weight and height were recorded and utilized to calculate a prepregnancy BMI. Patients with an initial BMI ≥25.0 were identified as overweight and those with a BMI ≥30.0 were identified as obese.
Inclusion criteria consisted of women who had obtained the 50-g GLT, provided information on race/ ethnicity, and had a prepregnancy weight and height recorded at the initial visit. Exclusion criteria included patients with multiple gestations, fetal anomalies, pregestational diabetes, women who did not identify with one of the four racial/ethnic groups mentioned above, and those transferred from outside institutions.
The primary predictor examined was BMI both as a continuous variable and as a series of dichotomized thresholds. Universal screening with a 50-g GLT was the policy at UCSF. Patients with 50-g GLT values of 140 or more were deemed to have positive screening tests and subsequently underwent the diagnostic 3-hour GTT. The primary outcome of interest was the diagnosis of gestational diabetes, which was diagnosed by two abnormal values on a 3-hour GTT by Carpenter-Coustan criteria. Plasma glucose at the UCSF clinical laboratory was measured by the glucose oxidase technique, in which the equipment was calibrated three times a day for quality control during the study period. Secondary outcomes of interest were the diagnosis of GDM in various BMI groups, ranging from normal weight to overweight. Furthermore, the percentage of women by racial/ethnic groups identified with GDM in these BMI subgroups was determined.
STATA software, version 7.0 (Statacorp, College Station, TX) was used to perform analysis of the results. The chi-square test was used to evaluate the statistical differences between the dichotomized variables, and a p value of <0.05 denoted statistical significance. The sensitivity and specificity of BMI as a predictor for GDM in the four racial/ethnic groups was also examined by performing receiver-operator characteristic (ROC) curve analysis. Multivariable analysis was utilized to control for potential confounders including maternal age, parity, insurance status, and marital status.
RESULTS
The 24,325 patients who met study criteria were stratified by race/ethnicity into 10,568 Caucasian, 3275 African-American, 2988 Latina, and 7404 Asian women. The largest percentage of overweight women in the group BMI 26.1 to 29.0 with GDM were Latina (13%), followed by African-American (11%), Caucasian (7%), and then Asian (5%) women. Caucasian and Asian women were more likely to be over the age of 35, nulliparous, and have a higher education level. The presence of government-sponsored (Medicaid) insurance was comparable in all four racial/ethnic groups (Table 1).
Table 1.
Patient Demographics for Each Race/Ethnicity
| Caucasian | African-American | Latina | Asian | |
|---|---|---|---|---|
| n | 10,568 | 3275 | 2988 | 7404 |
| Age >35 (%) | 20 | 8 | 12 | 16 |
| Nulliparous (%) | 56 | 45 | 46 | 52 |
| Education level—some college (%) | 71 | 27 | 27 | 59 |
| Medicaid Insurance (%) | 40 | 44 | 38 | 39 |
| BMI ≤ 19.8 (%) | 22 | 16 | 14 | 34 |
| BMI 19.8–26.0 (%) | 63 | 51 | 59 | 57 |
| BMI 26.1–29.0 (%) | 7 | 11 | 13 | 5 |
| BMI >29.1 (%) | 8 | 22 | 14 | 4 |
All demographics characteristics were significantly different between the racial/ethnic groups with p value <0.05 by chi-square analysis. BMI, body mass index.
A BMI >21.0 identified 91.5% of African-Americans, 90.1% of Latinas, and 79.8% of Caucasians, but only 68.4% of Asians with GDM. At an overweight BMI >25.0, 76.8% of African-American women with GDM were identified as compared with 24.9% of Asian women with GDM (Table 2). When raising the BMI threshold, fewer women with GDM are identified as lower BMI patients with GDM are excluded. Thus, as BMI increases, the sensitivity of BMI to identify GDM in each racial/ethnic group decreases while the specificity increases. When comparing a BMI >21 to a BMI >25, the specificity rose from 41.1 to 81.5% in Caucasians, 27.1 to 62.7% in African-Americans, 27.9 to 69.1% in Latinas, and 53.6 to 88.7% in Asians.
Table 2.
Percent of Women Identified with GDM at Various BMI
| Sensitivity (%) | Specificity (%) | LR (+) | LR (−) | |
|---|---|---|---|---|
| BMI >21* | ||||
| Caucasian | 79.8 | 41.1 | 1.4 | 0.5 |
| African-American | 91.5 | 27.1 | 1.3 | 0.3 |
| Latina | 90.1 | 27.9 | 1.2 | 0.4 |
| Asian | 68.4 | 53.6 | 1.5 | 0.6 |
| BMI >23* | ||||
| Caucasian | 60.1 | 67.3 | 1.8 | 0.6 |
| African-American | 82.9 | 47.9 | 1.6 | 0.3 |
| Latina | 75.2 | 50.4 | 1.5 | 0.5 |
| Asian | 43.5 | 75.7 | 1.8 | 0.7 |
| BMI >25* | ||||
| Caucasian | 46.2 | 81.5 | 2.5 | 0.7 |
| African-American | 76.8 | 62.7 | 2.1 | 0.4 |
| Latina | 58.9 | 69.1 | 1.9 | 0.6 |
| Asian | 24.9 | 88.7 | 2.2 | 0.8 |
| BMI >27* | ||||
| Caucasian | 37.0 | 88.5 | 3.2 | 0.7 |
| African-American | 57.3 | 72.4 | 2.1 | 0.6 |
| Latina | 48.9 | 80.0 | 2.4 | 0.6 |
| Asian | 16.2 | 94.0 | 2.7 | 0.9 |
p<0.01.
BMI, body mass index; GDM, gestational diabetes mellitus; LR (+), positive likelihood ratio; LR (−), negative likelihood ratio.
These findings persisted in the multivariable analysis that controlled for age, parity, insurance status, and marital status. When examining BMI >25.0 as a screening threshold, African-Americans had the greatest increased risk of GDM (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.0 to 8.5) as compared with African-American women with a BMI ≤25.0. This was followed by Caucasians (OR 3.6, 95% CI 2.7 to 4.8), then Latinas (OR 2.7, 95% CI 1.9 to 3.8), and Asians (OR 2.3, 95% CI 1.8 to 3.0; Table 3).
Table 3.
Multivariable Analysis of the Presence of GDM by Prepregnancy BMI
| BMI > 21.0 OR (95% CI) | BMI > 23.0 OR (95% CI) | BMI > 25.0 OR (95% CI) | BMI > 27.0 OR (95% CI) | |
|---|---|---|---|---|
| Caucasian | 2.5 (1.8–3.5) | 3.0 (2.2–4.0) | 3.6 (2.7–4.8) | 4.3 (3.2–5.8) |
| African-American | 3.4 (1.6–7.5) | 4.0 (2.2–7.2) | 5.1 (3.0–8.5) | 3.3 (2.1–5.1) |
| Latina | 3.0 (1.7–5.3) | 2.6 (1.7–3.8) | 2.7 (1.9–3.8) | 3.1 (2.2–4.4) |
| Asian | 2.1 (1.7–2.7) | 2.1 (1.7–2.7) | 2.3 (1.8–3.0) | 2.6 (2.0–3.6) |
Maternal age, parity, insurance status, and marital status were controlled for in the multivariable analysis. BMI, body mass index; CI, confidence interval; GDM, gestational diabetes mellitus; OR, odds ratio.
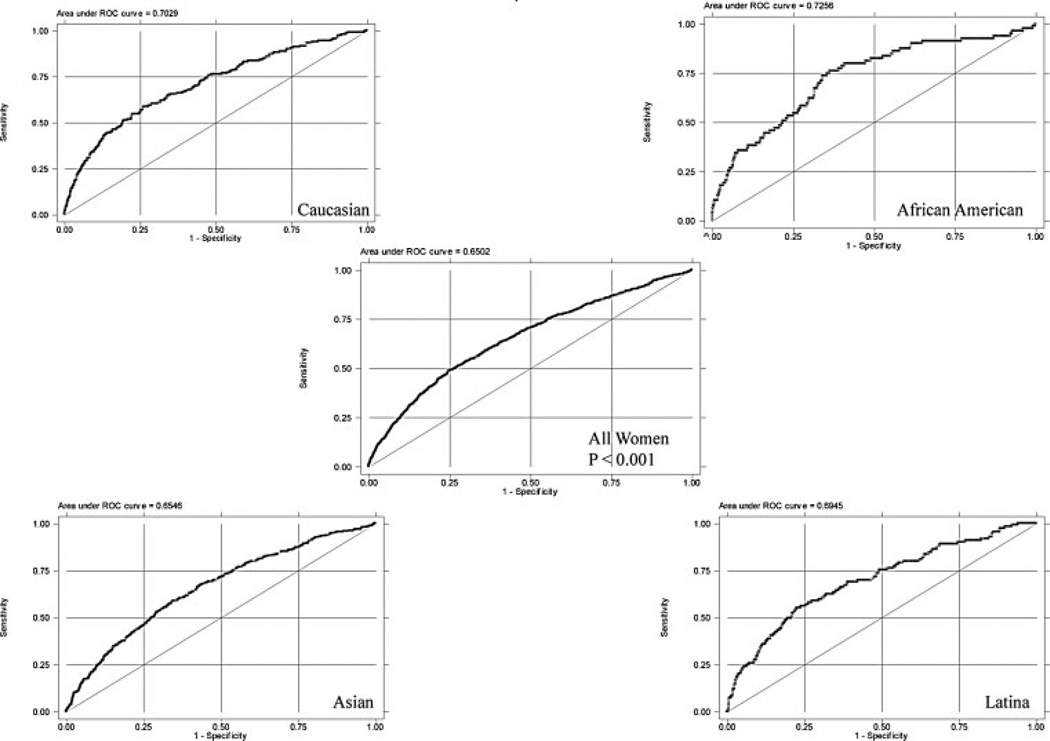
Using the ROC curves to analyze the sensitivity and specificity of BMI as a predictor of GDM in the four race/ethnic groups, the area under the curve was highest for African-Americans at 0.726, followed by Caucasians at 0.703, then Latinas at 0.695, and lowest for Asians at 0.655 (p<0.001; Fig. 1).
Figure 1.
Receiver-operator characteristic (ROC) curves by race/ethnicity. Area under the curve: Caucasian 0.703, African-American 0.726, Latina 0.695, Asian 0.655; p<0.0001 for all racial/ethnic groups.
Finally, when we examined the risk of developing GDM with the particular weight categories—underweight (BMI <20.0), normal weight (BMI 20.0 to 24.9), overweight (BMI 25.0 to 29.9), and obese (BMI >30.0)—Asian American women consistently had the highest rate of GDM within each weight strata (Table 4). In an interesting pattern, Asians had a higher rate of GDM than African-American and Caucasian women who were in the next highest weight group and approximately the same rate of GDM as Latinas in the next highest weight group.
Table 4.
Risk of GDM in Various Weight Strata by Race/Ethnicity
| Underweight (BMI <20.0) |
Normal Weight (BMI 20.0–24.9) |
Overweight (BMI 25.0–29.9) |
Obese (BMI > 30.0) |
|
|---|---|---|---|---|
| Caucasian (%) | 0.93 | 1.45 | 4.17 | 6.81 |
| African-American (%) | 1.15 | 1.19 | 4.71 | 5.48 |
| Latina (%) | 2.39 | 2.93 | 6.81 | 12.93 |
| Asian (%) | 2.72 | 6.09 | 10.22 | 13.78 |
| p value | <0.001 | <0.001 | 0.001 | <0.001 |
BMI, body mass index; GDM, gestational diabetes mellitus.
DISCUSSION
Not surprisingly, we found a greater prevalence of GDM among groups with increasing prepregnancy BMIs. However, the rate of GDM by BMI threshold varied widely by race/ethnicity. Using a BMI cutoff >25.0 as a criterion for screening would only identify ~25% of Asian women who will develop GDM as opposed to ~77% of African-American women, 59% of Latina women, and 46% of Caucasian women. Throughout its range, BMI served as better predictor for GDM in African-American women and was least effective in Asian women, with Caucasian and Latina women between these two groups. Although the ROC curves showed the area under the curve was greatest for African Americans, followed by Caucasians, Latinas, and Asians, the values were all below 0.8. Thus, although BMI was a more effective screening tool in African-American women, the results were not sensitive and specific enough to use BMI ROC curves alone to screen for GDM. However, a prepregnancy BMI threshold, potentially values defining obesity, might be used to identify those women who should be screened at the first visit for preexisting type 2 diabetes mellitus. Given the varying rate of GDM in different race/ethnic groups and the lack of a concrete relationship between GDM and BMI, the mechanism of insulin resistance needs further study. This is especially true in Asian women given the high prevalence of insulin resistance and the weakest correlation to BMI seen in the four race/ethnic groups studied. This finding that Asians had a higher rate of GDM throughout all weight strata is echoed in work in nonpregnant individuals.22 In addition, studying various Asian subgroups as well as other race/ethnicities not discussed in this article should be investigated to target higher-risk populations. Some studies show that certain groups, particularly Asian populations, tend to have more visceral or central fat, which is a known risk factor with insulin resistance and cardiovascular disease.23 Perhaps, measuring waist circumference during the first trimester is a better predictor of the risk of GDM in Asian women than BMI. More studies on stronger markers of GDM, as well, may be of value in the care of our patients.
As type 2 diabetes mellitus is also more prevalent in patients with a prior GDM history, this work reinforces the importance of postpartum counseling. Kousta et al demonstrated that women with GDM had decreased β-cell function and decreased acute insulin response to glucose despite concomitant normal fasting blood glucose levels postpartum.24 During annual visits, primary care physicians and gynecologists, through quality-improvement initiatives, should increase the assessment of the risk of developing type 2 diabetes mellitus in these patients through hemoglobin A1c, homeostatic model assessment, or a 2-hourr GTT.24
Although the current study supports previous studies’ findings regarding varying rates of GDM by race/ethnicity,19 as well as reports new evidence regarding race/ethnicity, weight categories, and GDM, there are several limitations that require discussion. This study focuses on four racial/ethnic groups; although race/ethnicity is difficult to define, all patients were categorized by self-defined/self-reported race/ethnicity, which is considered the current “gold standard.” Because we do not provide care for very many Native American women, we were unable to examine the predictors and outcomes among these women. Additionally, we did not have sufficient numbers to examine further subgroups. It appears that obstetric outcomes vary by Asian subgroups,25 and this is an area that needs exploration with a larger data set.
Another limitation is that the current study is of women receiving care in the Bay Area at UCSF. Population differences may exist, limiting the generalizability among other populations across the country. Conversely, the data do represent women of different backgrounds and socioeconomic status such that we feel our findings can be used to develop a greater understanding of the role of BMI in the occurrence of GDM in different races/ethnicities.
Finally, although we are using race/ethnicity as a proxy for potential biological differences between women, it may be that these differences are better represented by a genetic profile that is able to capsulate one’s a priori risk of GDM or type 2 diabetes mellitus. As such genetic profiles become available studying how BMI modifies GDM and type 2 diabetes mellitus, they may lead to a better understanding of which subgroups may be more or less likely to benefit from GDM screening.
Even though the association between BMI and GDM can still be used to counsel women about their risk of developing GDM, BMI does not appear to be a particularly useful screening tool in the various racial/ethnic groups, particularly Asian women. Furthermore, BMI as a screening tool does not have a high enough sensitivity and specificity to identify a group of women that should or should not receive GDM diagnostic testing. This continues to support the notion for continuing universal screening programs for pregnant women rather than stratifying by BMI.
ACKNOWLEDGMENTS
A.B.C. is supported as a Robert Wood Johnson Physician Faculty Scholar Grant # RWJF-61535 and N.E.S. is supported by the National Institute of Child Health and Human Development, Grant # HD01262 as Women’s Reproductive Health Research Scholars
REFERENCES
- 1.Hollander MH, Paarlberg KM, Huisjes AJ. Gestational diabetes: a review of the current literature and guidelines. Obstet Gynecol Surv. 2007;62:125–136. doi: 10.1097/01.ogx.0000253303.92229.59. [DOI] [PubMed] [Google Scholar]
- 2.Willman SP, Leveno KJ, Guzick DS, Williams ML, Whalley PJ. Glucose threshold for macrosomia in pregnancy complicated by diabetes. Am J Obstet Gynecol. 1986;154:470–475. doi: 10.1016/0002-9378(86)90692-7. [DOI] [PubMed] [Google Scholar]
- 3.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA. 1996;275:1165–1170. [PubMed] [Google Scholar]
- 4.Casey BM, Lucas MJ, Mcintire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869–873. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 5.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158:1148–1153. doi: 10.1093/aje/kwg273. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21:103–113. doi: 10.1046/j.1464-5491.2003.00985.x. [DOI] [PubMed] [Google Scholar]
- 7.Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med. 2009;14:77–84. doi: 10.1016/j.siny.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal RK, Lui K, Gupta JM. Neonatal hypoglycaemia in infants of diabetic mothers. J Paediatr Child Health. 2000;36:354–356. doi: 10.1046/j.1440-1754.2000.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Barnes-Powell LL. Infants of diabetic mothers: the effects of hyperglycemia on the fetus and neonate. Neonatal Netw. 2007;26:283–290. doi: 10.1891/0730-0832.26.5.283. [DOI] [PubMed] [Google Scholar]
- 10.Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20:17–23. doi: 10.1891/0730-0832.20.6.17. [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 12.Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbe SG, Gregory RP, Power ML, Williams SB, Schulkin J. Management of diabetes mellitus by obstetrician-gynecologists. Obstet Gynecol. 2004;103:1229–1234. doi: 10.1097/01.AOG.0000128045.50439.89. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994) Gestational diabetes. Obstet Gynecol. 2001;98:525–538. [PubMed] [Google Scholar]
- 15.Esakoff TF, Cheng YW, Caughey AB. Screening for gestational diabetes: different cut-offs for different ethnicities? Am J Obstet Gynecol. 2005;193(3 Pt 2):1040–1044. doi: 10.1016/j.ajog.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 16.Ogonowski J, Miazgowski T, Homa K, Celewicz Z, Kuczynska M. Low predictive value of traditional risk factors in identifying women at risk for gestational diabetes. Acta Obstet Gynecol Scand. 2007 Jul 2; doi: 10.1080/00016340701505044. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 17.Naylor CD, Sermer M, Chen E, Farine D. Toronto Trihospital Gestational Diabetes Project Investigators. Selective screening for gestational diabetes mellitus. N Engl J Med. 1997;337:1591–1596. doi: 10.1056/NEJM199711273372204. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins-Haug L, Horton JA, Cruess DF, Frigoletto FD. Antepartum screening in the office-based practice: findings from the collaborative Ambulatory Research Network. Obstet Gynecol. 1996;88(4 Pt 1):483–489. doi: 10.1016/0029-7844(96)00231-1. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus: recommendations and rationale. Obstet Gynecol. 2003;101:393–395. doi: 10.1016/s0029-7844(02)03056-9. [DOI] [PubMed] [Google Scholar]
- 20.Ramos GA, Caughey AB. The interrelationship between ethnicity and obesity on obstetric outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 2):1089–1093. doi: 10.1016/j.ajog.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 21.Steinfeld JD, Valentine S, Lerer T, Ingardia CJ, Wax JR, Curry SL. Obesity-related complications of pregnancy vary by race. J Matern Fetal Med. 2000;9:238–241. doi: 10.1002/1520-6661(200007/08)9:4<238::AID-MFM10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Gunton JE, Hitchman R, McElduff A. Effects of ethnicity on glucose tolerance, insulin resistance and beta cell function in 223 women with an abnormal glucose challenge test during pregnancy. Aust N Z J Obstet Gynaecol. 2001;41:182–186. doi: 10.1111/j.1479-828x.2001.tb01205.x. [DOI] [PubMed] [Google Scholar]
- 23.Pi-Sunyer FX. The epidemiology of central fat distribution in relation to disease. Nutr Rev. 2004;62:448. doi: 10.1111/j.1753-4887.2004.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 24.Kousta E, Lawrence NJ, Godsland IF, et al. Early metabolic defects following gestational diabetes in three ethnic groups of anti-GAD antibodies negative women with normal fasting glucose. Hormones (Athens) 2007;6:138–147. doi: 10.14310/horm.2002.111109. [DOI] [PubMed] [Google Scholar]
- 25.Rao AK, Cheng YW, Caughey AB. Perinatal complications among different Asian-American subgroups. Am J Obstet Gynecol. 2006;194:e39–e41. doi: 10.1016/j.ajog.2006.01.027. [DOI] [PubMed] [Google Scholar]