Abstract
Selective delivery of therapeutic molecules to primary and metastatic tumors is optimal for effective cancer therapy. A liposomal nanodelivery complex (scL) for systemic, tumor-targeting delivery of anticancer therapeutics has been developed. scL employs an anti-transferrin receptor (TfR), scFv as the targeting molecule. Loss of p53 suppressor function, through mutations or inactivation of the p53 pathway, is present in most human cancers. Rather than being transiently permissive for tumor initiation, persistence of p53 dysfunction is a continuing requirement for maintaining tumor growth. Herein, we report results of a first-in-man Phase I clinical trial of restoration of the normal human tumor suppressor gene p53 using the scL nanocomplex (SGT-53). Minimal side effects were observed in this trial in patients with advanced solid tumors. Furthermore, the majority of patients demonstrated stable disease. One patient with adenoid cystic carcinoma had his status changed from unresectable to resectable after one treatment cycle. More significantly, we observed an accumulation of the transgene in metastatic tumors, but not in normal skin tissue, in a dose-related manner. These results show not only that systemically delivered SGT-53 is well tolerated and exhibits anticancer activity, but also supply evidence of targeted tumor delivery of SGT-53 to metastatic lesions.
Introduction
The p53 “suppressor gene” is the most frequently annotated gene mutation in human cancer.1,2 Loss of p53 suppressor function, either through mutations or inactivation of the p53 pathway, is present in most if not all human cancers.2,3,4,5,6 Both p53 mutation and pathway dysfunction are associated with poor clinical outcomes,7,8,9,10,11,12 and the presence of the mutation correlates with resistance to chemotherapy and radiation.13 The p53 gene encodes a transcription factor with >540 binding loci thus far identified,14 and plays a dominant role in the induction of cell cycle arrest, DNA repair, apoptosis, and senescence as well as in mediating bystander antitumor effects.6,15,16 In addition, p53 appears to play a role in stem cell, including cancer stem cell, homeostasis in part by regulating the polarity of self-renewing divisions.17,18 Although loss of p53 functionality is known to result in increased genetic instability, impaired growth arrest, and inappropriate cell survival, it was only recently shown that, rather than being merely transiently permissive for tumor initiation, persistence of p53 dysfunction is a continuing requirement for maintaining tumor growth.19,20,21
Several gene therapy clinical trials using adenoviral delivery aimed at restoring functional p53 have been initiated or conducted by us and others,22,23,24,25,26 in which intratumoral administration resulted in both complete local positron emission tomography/computed tomography imaging response,27 and biopsy documented pathological complete responses (in conjunction with radiation therapy).28 Despite these impressive local responses, the widespread clinical application of this documented effective therapeutic gene approach restoring a driver suppressor oncogene has been delayed due to the inability of such intratumoral approaches to treat metastatic disease, as well as to the lack of a systemic delivery vehicle to effectively deliver the gene therapy to both the primary and metastatic sites.
SGT-53 is a therapeutic complex comprised of a cationic liposome encapsulating a plasmid encoding normal human wild-type (wt) p53 DNA. The liposome is decorated with an anti-transferrin receptor (TfR) single-chain antibody fragment (scFv) designed to target cancer cells via the transferrin glycoprotein receptor, which is ubiquitous, highly expressed on tumor cells, and efficiently internalized.29,30,31 This complex has been shown to effectively and specifically deliver the p53 cDNA to tumor cells, both primary and metastatic, via receptor-mediated endocytosis of the cationic liposomal complex.32,33,34,35,36,37,38 More significantly, preclinical studies of the systemic (intravenous) delivery of the SGT-53 nanocomplex have also demonstrated significant antitumor activity, resulting in tumor growth inhibition and long-term regression in a variety of solid tumor preclinical models, when used in combination with chemotherapy and radiation.32,35 Based on the selective tumor-targeting ability and the therapeutic potential shown by the platform technology described above, the prototype of this technology, SGT-53, was advanced into human clinical trials.
Thus, the current Phase I study of systemically delivered SGT-53 in advanced cancer patients was designed to assess safety, to confirm the presence of the transgene in the tumors, and to determine whether there is a dose-dependent level of the transgene in the tumors. Demonstrating that the systemically administered exogenous wt p53 delivered via SGT-53 was indeed present in the tumors would be a significant advance in the field of systemic gene therapy, as this is a result which has been difficult to achieve with other approaches. Human tumors in patients are notoriously difficult to transfect.39 Despite the large number of clinical trials, with the exception of one report by Davis et al.,40 there is very little evidence that the systemically delivered gene therapy payload is actually present in the target tumors.
Results
Patient population
Eleven previously treated patients with refractory cancer (Table 1) and no available standard of care options received 99 infusions of SGT p53 over four sequential dosing cohorts; 0.6, 1.2, 2.4, and 3.6 mg DNA per infusion (Table 2).
Table 1. Patient demographics.
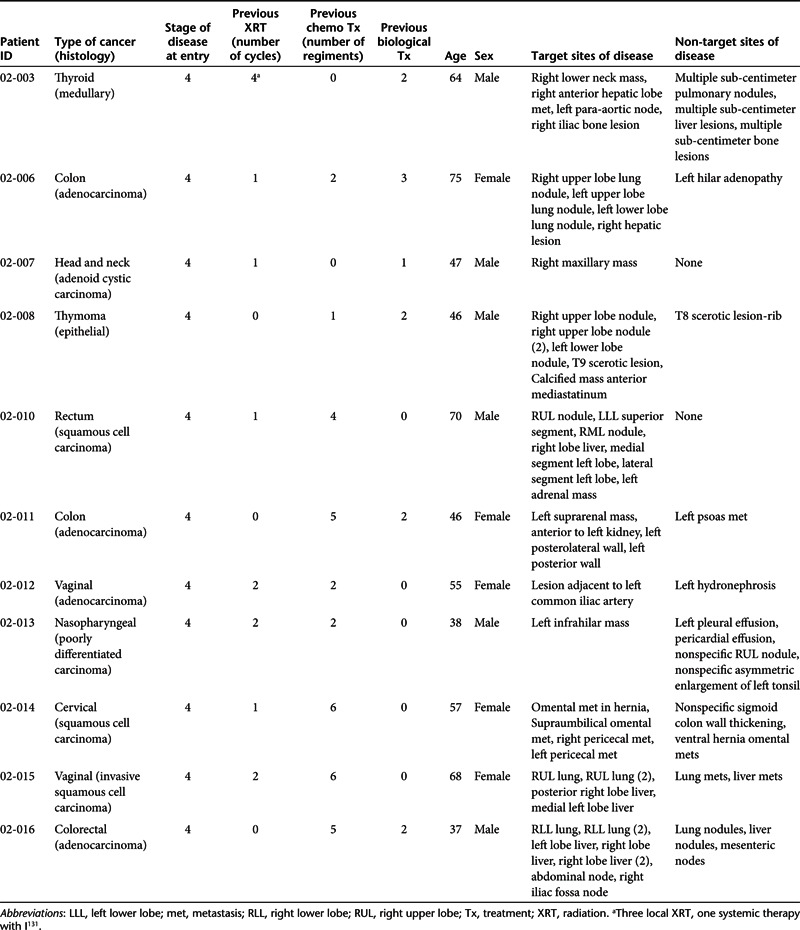
Table 2. Dose summary.
Safety
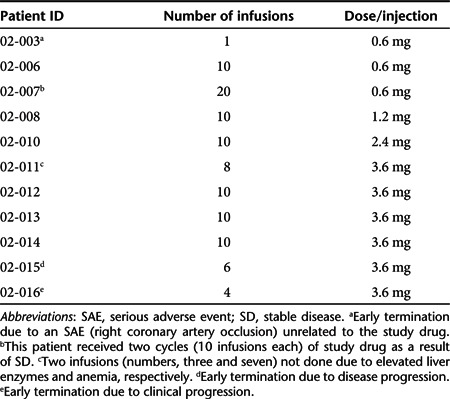
One patient (patient ID 02-007; 0.6 mg DNA per infusion) developed a possibly related grade 3 (fatigue) adverse event. During the course of treatment with SGT-53, this patient, who had head and neck cancer (adenoid cystic carcinoma), experienced severe necrosis of his tumor and underwent debridement of the tumor. The one time grade 3 level of fatigue coincided temporally with this event. Similar to what we observed preclinically in mice, we hypothesize that this massive level of cell death might have been contributory to the fatigue observed.
Two serious adverse events, chest pain and tachycardia were observed in the same patient (patient ID 02-015; 3.6 mg DNA per infusion). These were classified as grade 2 and only possibly related to the study drug. Drug-related grade 1, 2 adverse events occurring in ≥5% of patients are shown in Table 3. These primarily consisted of transient fever starting 6–8 hours after infusion and lasting 12–16 hours (5 of 11; 46%) and transient hypotension over a similar time frame in 7 (7 of 11; 64%). These low-grade toxicities justified our prophylactic regimen involving hydration, tylenol, histamine blockade, and indocin as described in Materials and Methods. No maximum tolerated dose was defined. Insofar, as significant exogenous p53wt levels by PCR were detected in two tumor samples at the 3.6 mg DNA dose level (Figure 1), further dose escalation was discontinued.
Table 3. Relation of grade 1, 2AEs to study drug.
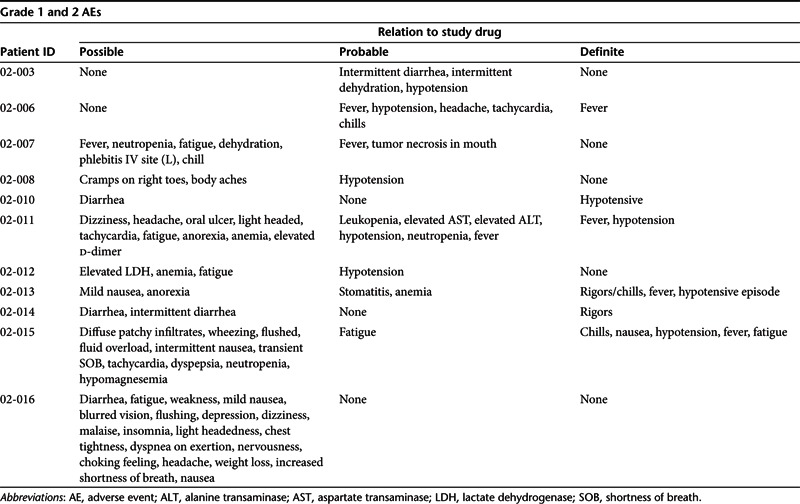
Figure 1.
p53 transgene tumor expression. (a) DNA PCR assessment of the presence of exogenous p53 in three patients' tumors in comparison to negative and positive controls. (b) A separate experiment with just patients 1 and 2 at higher exposure to confirm that exogenous p53 is present in the tumor from the patient who received the 0.6 mg dose. T1: Biopsy was done 100 hours after last treatment (patient 02-007). T2: Biopsy was done 26 hours later (patient 02-011). T3: Biopsy was done 73 hours later (patient 02-014). N: The normal skin sample was taken 71 hours after last dose of SGT-53 (patient 02-014).
Response
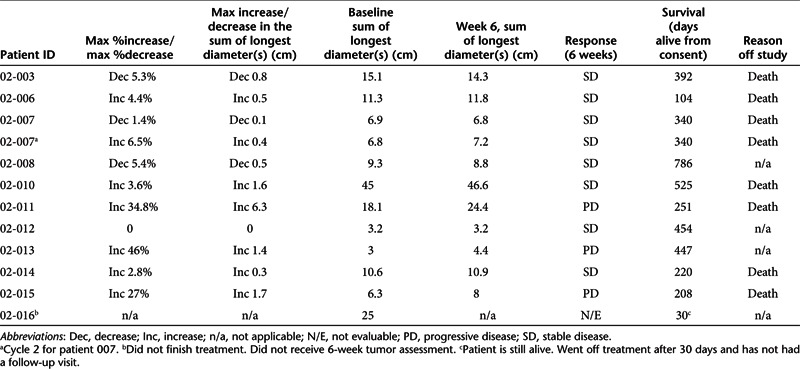
Despite the limitations of a Phase I study vis-à-vis assessment of response, 7 of the 11 patients demonstrated stable disease at week 6 assessment, one was unevaluable and three had progressive disease (Table 4). Median survival was 340 days (Table 4). Moreover, the tumor in patient 02-007 underwent significant necrosis and was reclassified from inoperable to operable. In a second patient (02-010), computed tomography assessment post-treatment showed that the liver metastases all had developed necrotic centers.
Table 4. Response to SGT-53.
Tumor biopsies for assessment of the presence of exogenous p53 were obtained from metastatic lesions in three patients. One patient, 02-007, with adenoid cystic carcinoma sampled at 100 hours post-injection, had received the 0.6 mg dose level, which was the lowest dose administered. Patients 02-011 (adenocarcinoma of the colon), sampled at 26 hours, and 02-014 (squamous cell carcinoma of cervix), sampled at 73 hours, both were treated at the 3.6 mg DNA dose, the highest dose infused. Results from the DNA PCR analysis for the presence of exogenous p53 DNA in the tumors are shown in Figure 1a with positive (pSCMV-p53 plasmid DNA) and negative (normal skin biopsy) controls (patient 02-14, normal tissue sampled at 71 hours). Reverse transcription PCR to determine transgene expression was not applicable for these studies because it would not be able to unequivocally establish that the p53 protein detected was from the exogenous transgene. In contrast, since we could design primers that included part of the plasmid vector backbone, only the exogenous DNA would be amplified. Thus, this DNA PCR approach was used for these studies. These experiments show the clear presence of the exogenous p53 transgene in the metastatic tumor tissue from these three patients, but not in the normal skin sample, suggesting preliminary evidence of tumor localization. Moreover, a DNA dose-dependent presence of the exogenous p53 was also suggested between the tumor biopsies from the patient that received the 0.6 mg dose and those that received the 3.6 mg dose. Figure 1b is from a second experiment to confirm that exogenous p53 is present in the tumor from the patient who received the 0.6 mg dose. It is also significant that all three of the biopsies were obtained from metastatic lesions demonstrating not only the tumor-targeting ability of systemically administered SGT-53, but also its specificity for tumor over the normal skin tissue, consistent with the preclinical studies described here and elsewhere.32,33,35,41,42 Although further evaluation of intratumoral plasmid DNA stability is indicated, previous studies with Tf-lipoplexes in the preclinical studies cited above using human cells showed peak transgene expression at 48–72 hours diminishing by days 7–10.
Discussion
This Phase I study has fulfilled its objectives demonstrating (i) the safety of systemic intravenous infusion of SGT-53 through 3.6 mg DNA per infusion, (ii) significant tumor-specific p53wt transgene presence following systemic delivery, and (iii) an apparent dose–transgene presence relationship. Despite the proven clinical effectiveness of local administration of gene therapeutics, including p53,27,28 clinically applicable systemic delivery allowing for optimized blood clearance, preferential delivery to target tumor tissues including metastases, target tumor cell uptake, and target tumor cell transgene presence/expression, within acceptable safety constraints, has been elusive and continues to be an ongoing challenge.43,44,45 Currently, there has been only one report of systemic targeted delivery of nanoparticles40 in patients with siRNA. However, this report did not examine normal tissue for the presence of the siRNA. Thus, this is the first report to demonstrate tumor specificity as well as uptake in metastatic tumors of a systemically delivered liposomal nanoparticle for gene therapy. The nanocomplex used in this study is a DOTAP:DOPE cationic liposome prepared by a previously described ethanol injection method32 decorated with an anti-TfR single-chain antibody fragment (scFv) targeting the TfR. A symptom-directed prophylactic regimen to attenuate generic lipoplex-associated cytokine-mediated toxicity was devised which allowed safe outpatient administration.
One of the most significant findings to arise from this study is the high level of p53 transgene in the metastatic tumors that was documented at an administered systemic dose of 3.6 mg DNA in two patients without evidence of presence in a concurrent normal skin biopsy. Moreover, evidence of the exogenous SGT-53–delivered p53 was present in the tumor biopsy from the patient who received the lowest DNA dose of 0.6 mg suggesting a dose-dependent uptake in tumors.
For an anticancer therapy to be effective it is crucial that a significant amount of the ID accumulate in the tumor, i.e., an amount sufficient for transgene expression in the therapeutic range in the target organ/tissue with attenuated accumulation in normal tissue so as to decrease potential for toxicity and focus biodistribution. Although, we were not able to determine the percent of the ID present in the tumors of the three patients from whom tumor biopsies were obtained, the exogenous wt p53 DNA, as evidenced by the unique PCR product, was present in the small amounts of metastatic tumor tissues obtained by biopsy from all three patients, even in the individual who had received the lowest dose (0.6 mg p53 DNA) of SGT-53. All three of these patients in this Phase I trial had a heavy tumor burden. Thus, the biopsy tissue obtained represents only a miniscule portion of the complete tumor load. The amount of DNA employed in the PCR assay is equivalent to ~2.5 mg of tumor tissue. Based upon these results and up to a 6.5-fold difference in tumor to normal tissue uptake in vitro (A549 SQ xenograft),46 it is reasonable to hypothesize that a significant percent of the ID may be taken up by the tumors in these patients as a result of the tumor-specific nature of this nanodelivery system.
The effectiveness of p53 restorative therapy has been demonstrated in an elegant series of preclinical models using differing methods of genetic manipulation.19,20,21 However, in all of these the base gene status was p53−/−. At least 75% of the annotated mutations in human cancer are due to missense substitutions of which 80% exert a dominant-negative effect over p53wt.47,48 In at least one Phase III study of the intratumoral injection of a replication-defective adenovirus to deliver p53wt, the presence of a high-mutated level of p53 (as indicated by nuclear staining in ≥20% of tumor cells using a DO-7–based monoclonal antibody), was noted in the subset of patients receiving single modality treatment. This mutated p53 appeared to exhibit a dominant-negative effect inhibiting the functionality of p53wt as evidenced by a lower rate of tumor growth control (20 versus 71%; P = 0.0290) and shorter median survival (2.7 versus 7.2 months; log rank P < 0.0001).24 To the contrary, in an in vitro and in vivo evaluation of p53 restoration via a replication-deficient recombinant adenovirus in 45 human cells lines comprised of wt, mutated, or null p53, there was no evidence that dominant-negative mutations impaired the antiproliferative effects of p53wt in vitro nor did those same mutations impair the p53wt-mediated enhanced survival in vivo.49 A recent study of restorative p53 for spontaneous tumors in a murine model containing the p53R172H gain-of-function dominant-negative mutation showed that not only did restoration produce tumor regression in p53−/− mice, it also blocked tumor growth, albeit without regression, in the p53R172H mice.50 It was also suggested that the response to p53 restoration was tumor type related, inducing apoptosis in lymphomas and senescence in angiosarcomas, similar to the findings reported by Ventura and colleagues.21 Based on their data using the hypomorphicp53neo allele, the authors postulated that the antitumor mechanism of action of p53 is dose-dependent, i.e., high levels of p53 expression inducing apoptosis and low levels inducing senescence.50
Despite the prolongation of survival following p53 restoration in the Eµ-myc lymphoma model, Martins found that the function of the p53 pathway was eventually inactivated followed by lymphoma regrowth and death.19 One of the mechanisms of loss-of-function of the p53 pathway was loss of p19ARF which negated the p53 restoration and in these mice the therapeutic effectiveness of p53 was restored by the addition of a p53-activating stimulus, e.g., radiation or chemotherapy. Likewise, Roth and associates have previously shown that the combination of cisplatin and intratumoral adenoviral p53 transfection produced significant apoptotic cell killing in the H358 non-small cell lung cancer model not seen with either treatment alone.51
As this was a first-in-man safety study of SGT-53, it was administered as a single agent. However, its intended use is in combination with standard radio/chemotherapy treatment modalities. Preclinical studies have demonstrated that this combination treatment yields significant tumor growth inhibition and tumor regression resulting in enhanced survival.16,33,35,36,37,38 The responses observed with the single agent resulting in stable disease, whereas undergoing treatment correlate with what was observed in the preclinical studies when the tumor-targeting liposomal-p53 nanocomplex was administered alone.34,35 Integrating the results of the current study with the data derived from the restoration models, a series of preclinical assessments of in vivo systemic SGT-53 and chemotherapy were devised and implemented resulting in a clinical Phase Ib study of SGT-53 combined with docetaxel, which has been activated and is accruing patients.
Materials and Methods
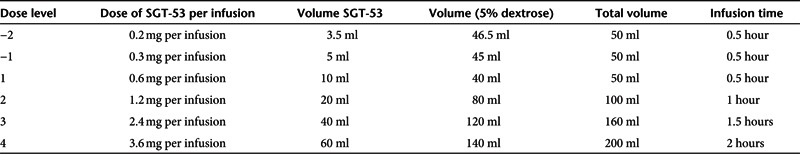
Study design. This study (Trial registration ID: NCT00470613) was an open label, single center, sequential dose-escalating, Phase I study evaluating the safety, pharmacokinetics, and potential activity of SGT-53 in subjects with solid tumors and who had been offered all standard or approved therapies. We utilized an accelerated titration design for inter-participant dose escalation. Study agent administration to each subject was planned in a sequential manner, starting at 0.6 mg DNA per infusion with escalation based on a modified Fibonacci sequence (100% → 50% → 33% → 25% → 20%; 0.6–7.2 mg DNA per infusion). No subject on any dose level would receive the study agent until all preceding subjects completed all first cycle study agent dose administrations plus one additional week after the last study agent dose. Dose escalation did not occur until 2 weeks had elapsed since the last infusion had been administered (Table 5).
Table 5. Dose levels for SGT-53 (based on mg of wt p53).
The study was initiated using single patient dose escalation cohorts, however, if one patient developed grade II drug-related toxic effect, three additional patients would be put on at that dose level. If one of three patients experienced a dose-limiting toxicity (DLT) (see below for defined events) in the expanded cohort 1, cycle 1, then three additional patients would be enrolled at that cohort dose. If ≥2 patients in a cohort experienced a DLT, the study would have been put on hold and dosing parameters would be re-evaluated in consultation with the Drug Safety Monitoring Board (DSMB). If ≤1 of 6 in expanded cohort 1, cycle 1 patients experienced a DLT, a total of three patients would enter cohort 2. If one of three patients in any subsequent cohort experienced a DLT, three additional patients would be enrolled at that cohort dose. If any of the three additional patients experienced a DLT, the previous dose level would be considered the maximum tolerated dose. If the cohort at the previous dose did not already have six patients, three additional patients would be enrolled at the previous dose level. If the highest scheduled dose level was reached in the absence of a DLT, a total of six patients would be enrolled at this dose level. Dose escalation was to be terminated at either (i) the dose resulting in DLT, as detailed below, in which case, following mandated review by the DMSB, the next lower dose level would be expanded by an additional three patients or (ii) at the dose level at which the presence of exogenous normal p53wt DNA was demonstrated in tumor tissue (biologic-determined dose), whichever came first.
Study population. The intended study population included subjects with biopsy-confirmed diagnosis of solid tumors with no available standard treatment options and with biopsy accessible tumor (for assessment of transgene presence) who were registered through Mary Crowley Cancer Research Centers (MCCRC), Dallas, Texas.
Inclusion criteria. Eligible subjects met the following inclusion criteria: biopsy-confirmed diagnosis; had been offered all standard or approved therapies; had solid tumors that could be measured on physical examination or by radiographic imaging studies and were biopsy accessible; were 18 years old or older; had an ECOG (Eastern Cooperative Oncology Group) performance study of 0–2; were able to give protocol-specific informed consent; had recovered from previous therapy; female subjects of childbearing potential must have had a negative pregnancy test within 7 days before initiation of study drug (postmenopausal women must be amenorrheic for at least 12 months to be considered of non-childbearing potential); male and female subjects of reproductive potential must have agreed to use birth control measures (e.g., condoms or birth control pills) to avoid pregnancy throughout the study and for 3 months following discontinuation of the study drug; have adequate organ function characterized by ≤grade 1 scores defined by CTCAE v3.0 and laboratory values within the following criteria: hemoglobin ≥10.0 gm/dl; absolute neutrophil count >1,500/mm3; WBC count >3,000/mm3; platelet count ≥100,000/mm3; prothrombin time/partial thromboplastin time <1.5 times the upper limit of normal; lactate dehydrogenase <3 times the upper limit of normal; total bilirubin ≤ upper limit of normal; aspartate transaminase and alanine transaminase <2.5 × upper limit of normal with alkaline phosphatase ≤2.5 × upper limit of normal; creatinine ≤1.5 mg/dl or creatinine clearance ≥50 ml/minute.
Subjects with the following characteristics were excluded: those with signs and symptoms consistent with an active infection; fever (>38.1 °C); known HIV infection; fasting glucose levels >180 mg/dl; uncontrolled diastolic blood pressure of >90 mm Hg resting at baseline despite medication; an abnormal stress echocardiogram or unfavorable results; uncontrolled congestive heart failure; unstable angina; significant baseline neuropathies (> grade 2 based upon CTCAE v 3.0); requirement for renal dialysis; requirement for systemic steroids within 30 days before study entry; receiving hematopoietic growth factors; receiving anticoagulants other than to maintain patency of venous access lines (≤2 mg warfarin); received an investigational drug within 30 days before study entry; received radiation treatment <4 weeks before study entry; prior exposure to gene vector delivery products; or received treatment with chemotherapeutic agents <4 weeks before study entry.
Study drug administration. Study drug was administered at MCCRC outpatient clinic twice weekly for 5 weeks for a total of 10 infusions. Infusion occurred over 1 hour for dose levels 1 and 2; over 1.5 hours for dose level 3; and over 2 hours for dose level 4. In the event of an infusion reaction, the infusion was discontinued, appropriate medication administered, and the infusion resumed at a slower infusion rate. The infusion was completed within 8 hours of dilution of study agent in dextrose. At least 3 or 4 days separated each infusion. Subjects received intravenous fluids for 2 hours after completion of each fusion.
For the first infusion of SGT-53, subjects were monitored and observed as inpatients for at least 23 hours, and up to 48 hours, from the time of admittance to MCCRC outpatient clinic. Subjects were monitored there for the first 8 hours after completion of the first infusion, during that time pharmacokinetic blood draws were taken at specified intervals. In addition, subjects received intravenous fluids for 2 hours after completion of the first infusion, with continued intravenous access for rapid fluid management if necessary. After 8 hours, subjects were then transferred and admitted to Medical City Hospital for the remainder of the observation period to monitor and manage any reactions to SGT-53. During this time, subjects continued intravenous access with maintenance of the 20-gauge catheter for rapid fluid management if necessary. At the end of the 23–48 hours observation period, subjects were discharged if medically stable.
For subsequent infusions, subjects were infused with SGT-53 in the MCCRC outpatient infusion unit.
All patients received dexamethasone 8 mg intravenously for 1 hour ± 5 minutes before dosing, and a combination of histamine H1 and H2 blockers (e.g., benadryl 25 mg and pepcid 20 mg, both intravenously) 30 ± 15 minutes and indocin 25 mg, orally ~30 ± 5 minutes before receiving SGT-53. All patients also received acetaminophen 650 mg orally just before SGT-53 administration as prophylaxis for pyretic reactions. A final volume of 50–250 ml of SGT-53 in 5% dextrose was infused intravenously via a newly inserted venous access line or following flushing of an intact central venous line. Infusion was completed within 8 hours of dilution of study agent in dextrose. Four hours after completion of each infusion of SGT-53, subjects received acetominophen, 650 mg orally.
Monitoring. Subjects had vital signs monitored every 15 ± 5 minutes during infusion and every 30 ± 5 minutes for 2 hours after each infusion. An electrocardiogram was performed on all subjects at 2 hours after each infusion.
Thereafter, the patients continued to receive intravenous fluids at ≥100 ml/hour during the 24-hour observation period. In addition, the patients continued to receive acetaminophen 650 mg orally alternating with indocin 25 mg orally every 4 hours for the first 24 hours.
Toxicities and DLT. Adverse events were graded according to the CTCAE v3.0 (http://ctep.cancer.gov/forms/). The following grade 2 events defined toxicities that would lead to withholding infusions of SGT-53: Grade 2 total hyperbilirubinemia, aspartate transaminase, alanine transaminase, dyspnea, and disseminated intravascular coagulation if not resolved to grade 1 or less in 2 weeks.
Otherwise, all grade 3 or grade 4 toxicities (as defined by CTCAE v3.0) within the first 5 weeks of study drug administration that had been determined by the principal investigator to be at least possibly related to study drug were considered DLT's.
In addition, subjects who received <4 of 10 of the intended drug infusions, due to experiencing grade 2 events that had not resolved that were determined by the principal investigator to be possibly related to study drug, were considered to have experienced a DLT.
Tumor response. Measurable disease was defined as any lesion with clearly defined borders that could be measured with calipers on physical exam or by imaging, i.e., X-ray, computed tomography, or magnetic resonance imaging. The Response Evaluation Criteria in Solid Tumors (RECIST 1.0) criteria published in 2 February 2000, issue of JNCI (web at http://ctep.cancer.gov/protocolDevelopment/) was utilized to evaluate response.
Levels of exogenous p53wt in tumor via DNA PCR. In three patients, one at 0.6 mg DNA dose level and two at the 3.6 mg DNA dose levels, a biopsy of the most accessible tumor was performed within 24–96 hours after the last administration of SGT-53. At least 20 mg of tumor tissue from a safely accessible site (e.g., liver, abdomen, skin) was obtained. A microscopic examination was performed on the frozen sample to ensure that the sample contained tumor. The tumor tissue was snap frozen and was used for DNA PCR to identify the presence and level of exogenous wt p53 DNA in the tumor. In both cases, the tumor sample was obtained from a metastatic lesion.
In one patient that had received the 3.6 mg DNA dose, a skin biopsy was also performed simultaneously with the tumor biopsy. A 4 mm punch biopsy was obtained from a safely accessible site. The skin was numbed with EMLA cream and the tissue was numbed with lidocaine. The skin biopsy was performed concurrently with the tumor biopsy.
The tissues were pulverized while frozen using a Bessman Tissue Pulverizer and the DNA was isolated using the “High Pure PCR Template Preparation Kit” from Roche (Indianapolis, IN; cat. no. 1796828) and the procedures were supplied by the manufacturer. DNA concentration of each sample was determined by absorbance (260/280 nm).
PCR design. The presence of the exogenous p53 in the tumor and normal skin was detected using DNA PCR employing primers specific for the exogenous p53 gene. To avoid cross-contamination of sample DNA with PCR product, the preparation of the reaction and analysis of the product was performed in separate locations using dedicated reagents and equipment. Only sterile, endotoxin-free tubes, and filter tips were employed. Gloves were worn at all times and changed frequently. A new aliquot of sterile PCR grade water was used for, and disposed off, after each experiment. The pipettors were cleaned with a decontamination agent and ultraviolet-irradiated overnight.
A blank sample containing all reagents except the template was included in the experiments as a negative control. The pSCMV-p53 plasmid DNA, which is used as the payload in SGT-53, at 100 and 500 copies, was used in each set of reactions as a positive control. The amount of template DNA used in each reaction was 0.4 µg. The PCR reactions were performed using the titanium TAQ DNA PCR kit (Clontech, Mountain View, CA; cat. no. 639210). Amplification was performed on an MJ Research thermocycler (MJ Research, Watertown, MA) using the following conditions:
Denaturing:
95 °C, 2 minutes, 0 seconds
Annealing:
For 10 cycles
94 °C, 0 minute, 30 seconds
66 °C, 1 minute, 0 second
72 °C, 2 minutes, 30 seconds
Extension:
For 20 cycles
94 °C, 0 minute, 30 seconds
61 °C, 1 minute, 0 second
72 °C, 2 minutes, 30 seconds
Final extension:
68 °C, 1 minute, 0 second
The PCR product was run on a 1.1% agarose gel, transferred to nylon membrane and hybridized using the 700 bp PCR product obtained from amplification of the positive control.
Schedule of evaluations. The following evaluations and tests were performed as part of pre-treatment screening: written protocol-specific informed consent, medical history, assessment of performance status, and physical examination; also included were: electrocardiogram, chest X-ray, pulmonary function tests, stress echocardiogram, consultation, and evaluation by cardiologist.
Blood samples were taken for: hematology (complete blood count with differential platelet count), chemistries (blood urea nitrogen, Cr, Na+, K+, CO2, Cl, Mg++, Ca++, phosphorus, albumin, alkaline phosphatase, alanine transaminase, aspartate transaminase, total bilirubin, total protein, glucose), prothrombin time/partial thromboplastin time, D-dimer, lactate dehydrogenase, tumor-specific markers (if applicable), and serum pregnancy test in women of childbearing potential; urine analysis; assessment of tumor size by physical measurement or with radiographic imaging, e.g., magnetic resonance imaging, computed tomography scan. Tumor imaging was required within 28 days before study drug infusion.
The following evaluations were performed at visits 2, 4, 6, and 8 (study weeks 1, 2, 3, and 4): symptom-oriented physical examination was done once weekly and symptom-oriented examination was done as needed at intervening study visits; assessment of performance status; review of medical history including concomitant medications and adverse events; on visits 2 and 6 only, before infusion, blood samples (5 ml) were taken for pharmacokinetics analysis of SGT-53.
Acknowledgments
These studies were supported by SynerGene Therapeutics, Inc. and a research grant from SynerGene Therapeutics, Inc. (to K.F.P.). The authors thank Leanne Sleer for her contributions to this study. We also thank Susan W. Mill for manuscript preparation. E.H.C. is an inventor of the described technology for which several patents owned by Georgetown University have been issued. The patents have been licensed to SynerGene Therapeutics Inc. for commercial development. E.H.C. owns equity interests in SynerGene. In addition, E.H.C. serves as a non-paid scientific consultant to SynerGene. The other authors declared no conflict of interest.
References
- Lane DP, Midgley CA, Hupp TR, Lu X, Vojtesek B., and, Picksley SM. On the regulation of the p53 tumour suppressor, and its role in the cellular response to DNA damage. Philos Trans R Soc Lond, B, Biol Sci. 1995;347:83–87. doi: 10.1098/rstb.1995.0013. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Momand J., and, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sørlie T.et al. (1994Database of p53 gene somatic mutations in human tumors and cell lines Nucleic Acids Res 223551–3555. [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ., and, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Gudkov AV., and, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- Cheok CF, Verma CS, Baselga J., and, Lane DP. Translating p53 into the clinic. Nat Rev Clin Oncol. 2011;8:25–37. doi: 10.1038/nrclinonc.2010.174. [DOI] [PubMed] [Google Scholar]
- Isobe T, Hiyama K, Yoshida Y, Fujiwara Y., and, Yamakido M. Prognostic significance of p53 and ras gene abnormalities in lung adenocarcinoma patients with stage I disease after curative resection. Jpn J Cancer Res. 1994;85:1240–1246. doi: 10.1111/j.1349-7006.1994.tb02936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya K, Tsuda H, Hiraide H, Tamaki K, Tamakuma S, Fukutomi T.et al. (1991Nuclear p53 immunoreaction associated with poor prognosis of breast cancer Jpn J Cancer Res 82835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan DC, Davidson AG, Summers CL, Warden HE., and, Doshi HM. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992;52:4828–4831. [PubMed] [Google Scholar]
- Thorlacius S, Börresen AL., and, Eyfjörd JE. Somatic p53 mutations in human breast carcinomas in an Icelandic population: a prognostic factor. Cancer Res. 1993;53:1637–1641. [PubMed] [Google Scholar]
- Thor AD, Moore DH II, Edgerton SM, Kawasaki ES, Reihsaus E, Lynch HT.et al. (1992Accumulation of p53 tumor suppressor gene protein: an independent marker of prognosis in breast cancers J Natl Cancer Inst 84845–855. [DOI] [PubMed] [Google Scholar]
- Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC.et al. (1993Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer J Natl Cancer Inst 85200–206. [DOI] [PubMed] [Google Scholar]
- Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N.et al. (1996The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers J Cancer Res Clin Oncol 122360–365. [DOI] [PubMed] [Google Scholar]
- Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T.et al. (2006A global map of p53 transcription-factor binding sites in the human genome Cell 124207–219. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D., and, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Xu L, Pirollo KF, Chang EH., and, Murray A. Systemic p53 gene therapy in combination with radiation results in human tumor regression. Tumor Targeting. 1999;4:92–104. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B.et al. (2009The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells Cell 1381083–1095. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Cicalese A, Insinga A., and, Pelicci PG. The emerging role of p53 in stem cells. Trends Mol Med. 2012;18:6–12. doi: 10.1016/j.molmed.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Martins CP, Brown-Swigart L., and, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V.et al. (2007Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas Nature 445656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L.et al. (2007Restoration of p53 function leads to tumour regression in vivo Nature 445661–665. [DOI] [PubMed] [Google Scholar]
- Nemunaitis JM., and, Nemunaitis J. Potential of Advexin: a p53 gene-replacement therapy in Li-Fraumeni syndrome. Future Oncol. 2008;4:759–768. doi: 10.2217/14796694.4.6.759. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J., and, Nemunaitis J. Head and neck cancer: response to p53-based therapeutics. Head Neck. 2011;33:131–134. doi: 10.1002/hed.21364. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Clayman G, Agarwala SS, Hrushesky W, Wells JR, Moore C.et al. (2009Biomarkers Predict p53 Gene Therapy Efficacy in Recurrent Squamous Cell Carcinoma of the Head and Neck Clin Cancer Res 157719–7725. [DOI] [PubMed] [Google Scholar]
- Senzer N., and, Nemunaitis J. A review of contusugene ladenovec (Advexin) p53 therapy. Curr Opin Mol Ther. 2009;11:54–61. [PubMed] [Google Scholar]
- Nemunaitis J, Swisher SG, Timmons T, Connors D, Mack M, Doerksen L.et al. (2000Adenovirus-mediated p53 gene transfer in sequence with cisplatin to tumors of patients with non-small-cell lung cancer J Clin Oncol 18609–622. [DOI] [PubMed] [Google Scholar]
- Senzer N, Nemunaitis J, Nemunaitis M, Lamont J, Gore M, Gabra H.et al. (2007p53 therapy in a patient with Li-Fraumeni syndrome Mol Cancer Ther 61478–1482. [DOI] [PubMed] [Google Scholar]
- Swisher SG, Roth JA, Komaki R, Gu J, Lee JJ, Hicks M.et al. (2003Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy Clin Cancer Res 993–101. [PubMed] [Google Scholar]
- Daniels TR, Bernabeu E, Rodríguez JA, Patel S, Kozman M, Chiappetta DA.et al. (2012The transferrin receptor and the targeted delivery of therapeutic agents against cancer Biochim Biophys Acta 1820291–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TR, Delgado T, Helguera G., and, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006;121:159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Delgado T, Rodriguez JA, Helguera G., and, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ.et al. (2002Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes Mol Cancer Ther 1337–346. [PubMed] [Google Scholar]
- Xu L, Pirollo KF, Tang WH, Rait A., and, Chang EH. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther. 1999;10:2941–2952. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM.et al. (2002Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery Hum Gene Ther 13469–481. [DOI] [PubMed] [Google Scholar]
- Xu L, Tang WH, Huang CC, Alexander W, Xiang LM, Pirollo KF.et al. (2001Systemic p53 gene therapy of cancer with immunolipoplexes targeted by anti-transferrin receptor scFv Mol Med 7723–734. [PMC free article] [PubMed] [Google Scholar]
- Yu W, Pirollo KF, Yu B, Rait A, Xiang L, Huang W.et al. (2004Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide Nucleic Acids Res 32e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Pirollo KF., and, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J Control Release. 2001;74:115–128. doi: 10.1016/s0168-3659(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Yu W, Pirollo KF, Rait A, Yu B, Xiang LM, Huang WQ.et al. (2004A sterically stabilized immunolipoplex for systemic administration of a therapeutic gene Gene Ther 111434–1440. [DOI] [PubMed] [Google Scholar]
- Chrastina A, Massey KA., and, Schnitzer JE. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:421–437. doi: 10.1002/wnan.143. [DOI] [PubMed] [Google Scholar]
- Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA.et al. (2010Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles Nature 4641067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R.et al. (2006Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery Hum Gene Ther 17117–124. [DOI] [PubMed] [Google Scholar]
- Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G.et al. (2007Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system Cancer Res 672938–2943. [DOI] [PubMed] [Google Scholar]
- Hallaj-Nezhadi S, Lotfipour F., and, Dass CR. Delivery of nanoparticulate drug delivery systems via the intravenous route for cancer gene therapy. Pharmazie. 2010;65:855–859. [PubMed] [Google Scholar]
- Guo X., and, Huang L. Recent advances in nonviral vectors for gene delivery. Acc Chem Res. 2011;45:971–979. doi: 10.1021/ar200151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahi YS, Bangari DS., and, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011;11:307–320. doi: 10.2174/156652311796150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirollo KF, Rait A, Kim SS, Yang CL, Nixhida M, Han D, Chang EH. Mol Cancer Ther. submitted for publication; 2013. The specificity and efficacy of a tumor targeted system results in the accumulation of a high percent of injected dose in the tumor. [Google Scholar]
- Monti P, Campomenosi P, Ciribilli Y, Iannone R, Inga A, Abbondandolo A.et al. (2002Tumour p53 mutations exhibit promoter selective dominance over wild type p53 Oncogene 211641–1648. [DOI] [PubMed] [Google Scholar]
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P.et al. (2007Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database Hum Mutat 28622–629. [DOI] [PubMed] [Google Scholar]
- Harris MP, Sutjipto S, Wills KN, Hancock W, Cornell D, Johnson DE.et al. (1996Adenovirus-mediated p53 gene transfer inhibits growth of human tumor cells expressing mutant p53 protein Cancer Gene Ther 3121–130. [PubMed] [Google Scholar]
- Wang Y, Suh YA, Fuller MY, Jackson JG, Xiong S, Terzian T.et al. (2011Restoring expression of wild-type p53 suppresses tumor growth but does not cause tumor regression in mice with a p53 missense mutation J Clin Invest 121893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Grimm EA, Mukhopadhyay T, Zhang WW, Owen-Schaub LB., and, Roth JA. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]



