The potential for nonviral gene therapy to treat cancer has been limited by the difficulty of targeting therapeutic genes to tumors. In this issue of Molecular Therapy, Senzer et al.1 show evidence of specific tumor targeting as well as clinical efficacy in a phase I trial of a nonviral vector. This is the first phase I clinical trial to demonstrate tumor specificity and uptake by metastatic tumors of a systemically delivered liposomal nanoparticle. Importantly, the therapeutic nanoparticle elicited minimal side effects and the majority of patients demonstrated stable disease. This phase I trial has shown this delivery system to be a safe, stable, efficient, and tumor-specific drug delivery platform for systemic administration.
The study evaluated systemic delivery of a liposomal nanoparticle vector encoding wild-type p53 (SGT-53), which was engineered with the aim of restoring the expression and proapoptotic function of the latter in tumor tissue (Figure 1). The vector comprised cationic lipids in complex with plasmid DNA and was targeted to tumors using a single-chain antibody specific for the transferrin receptor covalently linked to the lipids by a cysteine–maleimide bond.2 The transferrin receptor is highly expressed on cancer cells, which renders it an attractive targeting moiety.3 Eleven previously treated patients suffering from a wide variety of refractory cancers with no alternative treatment options received SGT-53 intravenously for 5 weeks. The results demonstrated both safety of the therapy with no dose-limiting toxicities and dose-dependent presence of the transgene in tumors. There was also evidence of clinical efficacy, as 7 of the 11 patients had stable disease at 6 weeks after treatment. These results, taken together, illuminate a pathway for the treatment of cancer with gene therapy vectors delivered with targeted nanoparticles.
Figure 1.
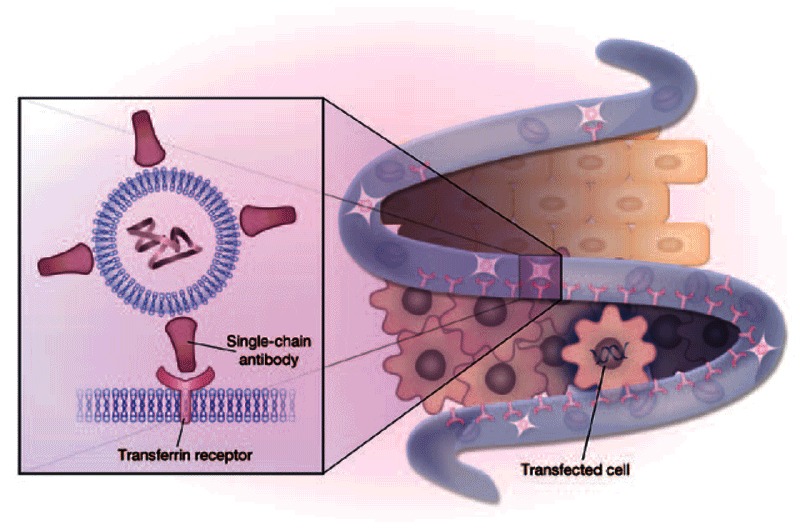
p53 gene delivery with nanoparticles targeted to the transferrin receptor. SGT-53 selectively transfects cancer cells (below) relative to normal cells (above) due to high expression of the transferrin receptor. (Inset): Liposomal nanoparticle vector encoding wild-type p53 targeted with a single-chain antibody specific for the transferrin receptor.
Although cationic lipids have been shown to be effective transfection reagents in vitro, their application in vivo has been hampered by off-target interactions with the reticuloendothelial system and anionic extracellular matrix and proteins.4 They have also been shown to induce lymphopenia and elevated levels of liver enzymes, which can lead to lethal liver damage and mortality.5 In Senzer and colleagues' study, most of the patients had mild reactions (predominantly grade 1–2 transient hypotension and fever); the only grade 3 adverse event was fatigue in one patient that was attributable to extensive tumor necrosis. Because toxicity associated with lipoplexes limits their wide-ranging application, further clinical testing of the SGT-53 formulation for immunogenicity, liver toxicity, and damage in other organs should provide valuable information related to this critical issue.6
Precise selection of the lipid composition and the targeting ligand are important factors for effective nanoparticle delivery systems. Biophysical characterization of similar complexes using transferrin as the targeting molecule showed that although DNA in complex with cationic lipids alone forms large (150- to 400-nm) structures with a very positively charged surface, the addition of transferrin to the complexes leads to a more compact (50- to 90-nm), electrically neutral structure that should prolong its circulation time and ability to target tumors.7 In effect, this provides the charge neutralization and immunoevasive properties of polyethylene glycol without the concomitant decreased transfection efficiency.
Although the delivery mechanism is an important breakthrough in the present study, the nature of the transgene effect is also intriguing. The clinical correlation of p53 status with patient outcome continues to be a hotly debated area of p53 research.8,9 The challenge derives mainly from the inherent complexity of the p53 pathway and the large number of mutations in different tumor types and clinical stages.10 The p53 database includes mutations or variations in the p53 gene (encoding p53) that have been reported in the literature since 1989.11 There have been encouraging findings regarding survival correlations with p53 mutations; for instance, studies have shown that cancers of the breast, head and neck, liver, hematopoietic, and lymphoid systems show an association of p53 mutation with worsened survival, whereas cancers of the pancreas, prostate gland, rectum, and stomach did not.10 Although p53 is an intracellular protein that might be expected to kill only transfected cells, there is evidence that this is not necessarily the case. In a mouse model of breast cancer, therapeutic efficacy was observed even at transfection efficiencies of less than 5%.12 Inhibition of angiogenesis was implicated as a possible explanation for this bystander effect. Controlled phase II and phase III studies stratified for p53 status and that evaluate the metabolic activity of treatment will help elucidate the impact of p53 restoration therapy and identify responding subgroups of patients.
Although these experiments demonstrated plasmid DNA delivery to the tumors, technical difficulties prevented assessment of DNA uptake into the nucleus or expression of vector-derived messenger RNA or protein. Consequently, it is difficult to distinguish whether the therapeutic effect was due to expression of the p53 transgene or whether efficient tumor targeting of high levels of the cationic lipids lead to nonspecific cytotoxicity. Previous preclinical studies offer evidence that the therapeutic effects observed in this study did not arise simply as a result of cytopathic effects of the cationic lipids. For example, similar nanoparticles carrying control plasmids that do not encode p53 did not cause cytotoxicity in vitro, and nanoparticles carrying a plasmid encoding green fluorescent protein (GFP) were injected intravenously into nude mice and induced GFP expression in tumor xenografts but not normal tissue.13 However, confirming this distinction is critical given the important implications regarding the feasibility of using this system in a wider array of applications. For example, treating cancer by delivering therapeutic genes to be highly expressed and secreted for a wide bystander effect (e.g., a prodrug convertase or immunostimulatory cytokine), or treating hemophilia by using a single-chain antibody targeting normal cells and delivering a plasmid encoding clotting factors, would depend on achieving high expression levels of the transgene with minimal cytotoxicity in the transfected cells.
SGT-53 is intended for use in combination with standard radiation treatment or chemotherapy modalities. The loss of p53 function correlates with resistance to standard treatments, and restoration of p53 has resulted in sensitization of tumors to chemotherapy and radiation therapy.14 In vivo studies have shown that combination therapy results in significant tumor regression;15 therefore, clinical studies that evaluate the effect of systemically administered SGT-53 in combination with chemotherapy and/or radiotherapy should be assessed.
The study by Senzer at al. clearly shows that the transferrin receptor is a promising target for gene therapy delivery specifically to cancer and is effective when targeted by a nonviral vector. Wider application of this technology for nonviral gene therapy of diseases other than cancer, for which systemic gene delivery mediated by a virus16 could have undesirable effects, may be possible by targeting a molecule whose role is comparable to that played by the transferrin receptor for delivery to cancer.
References
- Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M.et al. (2013Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors Mol Ther 211096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ.et al. (2002Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes Mol Cancer Ther 1337–346. [PubMed] [Google Scholar]
- Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Satterlee A, Huang L. gene delivery by nonviral vectors: overcoming hurdles. In vivo. Mol Ther. 2012;20:1298–1304. doi: 10.1038/mt.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audouy SL, de Leij LF, Hoekstra D, Molema G. characteristics of cationic liposomes as delivery vectors for gene therapy. In vivo. Pharm Res. 2002;19:1599–1605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- de Kanter R, Monshouwer M, Meijer DK, Groothuis GM. Precision-cut organ slices as a tool to study toxicity and metabolism of xenobiotics with special reference to non-hepatic tissues. Curr Drug Metab. 2002;3:39–59. doi: 10.2174/1389200023338071. [DOI] [PubMed] [Google Scholar]
- Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM.et al. (2002Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery Hum Gene Ther 13469–481. [DOI] [PubMed] [Google Scholar]
- Munro AJ, Lain S, Lane DP. p53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer. 2005;92:434–444. doi: 10.1038/sj.bjc.6602358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff PM. Is there a role for routine p53 testing in colorectal cancer. J Clin Oncol. 2005;23:7395–7396. doi: 10.1200/JCO.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Robles AI, Harris CC.2010Clinical outcomes and correlates of TP53 mutations and cancer Cold Spring Harb Perspect Biol 2a001016 doi: 10.1101/cshperspect.a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier M, Petitjean A, de Caron Fromentel C, Hainaut P, Ayed A, Hupp T.2011TP53 mutations in human cancers: selection versus mutagenesis Ayed A, Hupp T.eds). p53 Landes Bioscience–Springer: Austin, TX 1–18.
- Xu M, Kumar D, Srinivas S, Detolla LJ, Yu SF, Stass SA.et al. (1997Parenteral gene therapy with p53 inhibits human breast tumors in vivo through a bystander mechanism without evidence of toxicity Hum Gene Ther 8177–185. [DOI] [PubMed] [Google Scholar]
- Yu W, Pirollo KF, Yu B, Rait A, Xiang L, Huang W.et al. (2004 Enhanced transfection efficiency of a systemically delivered tumor-targeting immunolipoplex by inclusion of a pH-sensitive histidylated oligolysine peptide Nucleic Acids Res 32e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N.et al. (1996The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers J Cancer Res Clin Oncol 122360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Pirollo KF, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J Control Release. 2001;74:115–128. doi: 10.1016/s0168-3659(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ.et al. (2011Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans Nature 47799–102. [DOI] [PubMed] [Google Scholar]


