Abstract
Prochlorococcus is responsible for a significant part of CO2 fixation in the ocean. Although it was long considered an autotrophic cyanobacterium, the uptake of organic compounds has been reported, assuming they were sources of limited biogenic elements. We have shown in laboratory experiments that Prochlorococcus can take up glucose. However, the mechanisms of glucose uptake and its occurrence in the ocean have not been shown. Here, we report that the gene Pro1404 confers capability for glucose uptake in Prochlorococcus marinus SS120. We used a cyanobacterium unable to take up glucose to engineer strains that express the Pro1404 gene. These recombinant strains were capable of specific glucose uptake over a wide range of glucose concentrations, showing multiphasic transport kinetics. The Ks constant of the high affinity phase was in the nanomolar range, consistent with the average concentration of glucose in the ocean. Furthermore, we were able to observe glucose uptake by Prochlorococcus in the central Atlantic Ocean, where glucose concentrations were 0.5–2.7 nM. Our results suggest that Prochlorococcus are primary producers capable of tuning their metabolism to energetically benefit from environmental conditions, taking up not only organic compounds with key limiting elements in the ocean, but also molecules devoid of such elements, like glucose.
Keywords: high-affinity glucose transport, marine cyanobacteria, multiphasic uptake kinetics
Cyanobacteria is a phylum of the bacterial domain distinguishable by their unique capacity to perform oxygenic photosynthesis. This process, which relies on the existence of two photosystems and a chain of electron transporters, enables these organisms to use light energy and electrons from water to produce reductant molecules and fix atmospheric CO2 to synthesize carbon compounds. Cyanobacteria probably arose on Earth billions of years ago (1), and prolonged evolutionary divergence has made them very diverse in terms of morphology, metabolism, and lifestyle.
A number of cyanobacterial strains can use glucose (2), using it as a source of reduced carbon and energy, although the majority of isolated strains lack this ability. The inability of many species to use carbon sources that are widely used by many prokaryotes is in some cases due to toxic effects (3) or lack of metabolic capabilities, whereas in other cases it is due to the lack of transport systems for the intake of such compounds (4, 5). Unicellular marine cyanobacteria were included in the group of cyanobacteria unable to use glucose (6), long being considered to be photoautotrophic organisms (7). Prochlorococcus is the most abundant marine cyanobacterium (8), being one of the main primary producers on Earth (9, 10), and has hitherto been considered to be photoautotrophic. This fact notwithstanding, different studies have shown that Prochlorococcus strains can take up organic compounds, whose interest was usually considered to come from their containing limited elements, such as nitrogen in amino acids (11), or sulfur in dimethylsulfoniopropionate (12). Initial studies on Prochlorococcus sp. strain PCC 9511 showed no evidence for glucose utilization (13). Nevertheless, we have since shown that Prochlorococcus cultures of different strains do take up glucose (14), showing clear changes in gene expression after glucose addition. The current annotations of Prochlorococcus genomes (15–17) show no genes involved in glucose transport. Nevertheless, we observed that the expression of the gene Pro1404, annotated as melB (a putative melibiose/sodium symporter) in the genomes of Prochlorococcus, showed a clear increase in Prochlorococcus marinus strain SS120, upon glucose addition (14). In this study, our goal was to determine the mechanism of glucose uptake by Prochlorococcus, to investigate the possible involvement of the Pro1404 gene, and to examine whether glucose could be taken up by Prochlorococcus populations in the Atlantic Ocean.
Results and Discussion
To investigate the putative role of the Pro1404 (melB) gene in the uptake of glucose by Prochlorococcus, we chose to express this gene ectopically in a cyanobacterium naturally incapable of glucose transport, Synechococcus elongatus PCC 7942. Two constructions were made where the promoterless Pro1404 gene was placed downstream of kanamycin resistance cassettes, C.K1 or C.K3, respectively. In these constructions, expression of Pro1404 is driven by the promoters of the C.K1 or C.K3 cassettes that show different activity in cyanobacteria, C.K1 (moderate) and C.K3 (strong) (18). Recombinant strains were selected where the C.K1::Pro1404 and C.K3::Pro1404 constructs were introduced by recombination in the S. elongatus chromosome splitting the dispensable asnS locus (coding for asparaginyl-tRNA synthetase).
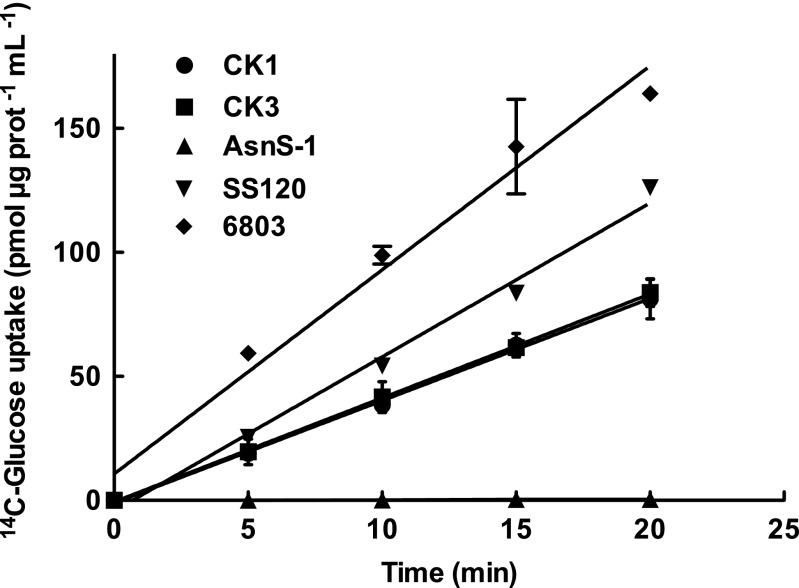
The recombinant strains, named C.K1 and C.K3, were grown and used to study the uptake of 1 µM 14C-glucose. Synechocystis sp. PCC 6803 was used as positive control, whereas an S. elongatus recombinant strain with a cassette insertion in the asnS gene (AsnS-1) was used as a negative control. P. marinus SS120 was also used as reference. The results are shown in Fig. 1: the ectopic expression of Pro1404 in S. elongatus conferred the capability for glucose uptake to the derived recombinant strains C.K1 and C.K3. Therefore, Pro1404 encodes a glucose transporter in P. marinus SS120, and we can infer this gene probably has the same function in the rest of Prochlorococcus and marine Synechococcus strains that have been sequenced and shown to have this gene. Unexpectedly, both recombinant strains exhibited similar glucose transport rates, suggesting that the expression level in the C.K1 strain was sufficient to populate the plasma membrane of the host cell with saturating amounts of the transporter.
Fig. 1.
14C-Glucose uptake in recombinant strains of S. elongatus sp. PCC 7942 expressing the gene Pro1404 from P. marinus SS120. The Pro1404 gene was inserted into asnS of the S. elongatus chromosome as described (Fig. S2), under the control of two constitutive promoters of moderate (C.K1) and high (C.K3) activity in cyanobacteria. A glucose-transporting species, Synechocystis sp. PCC 6803 was used as positive control, whereas the recombinant strain AsnS-1 of S. elongatus was used as negative control. P. marinus sp. SS120 was also used as reference. Data presented are the average of three independent biological replicates. Bars show SD for each sample. SD bars for P. marinus sp. SS120 are smaller than symbols.
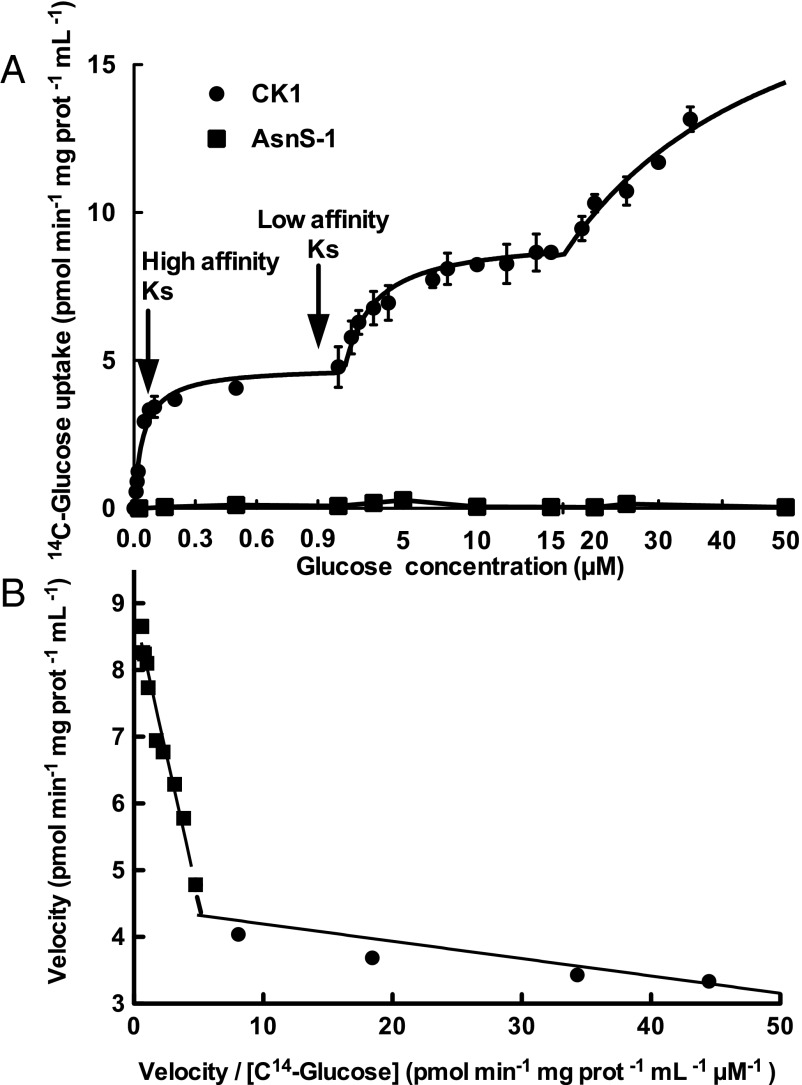
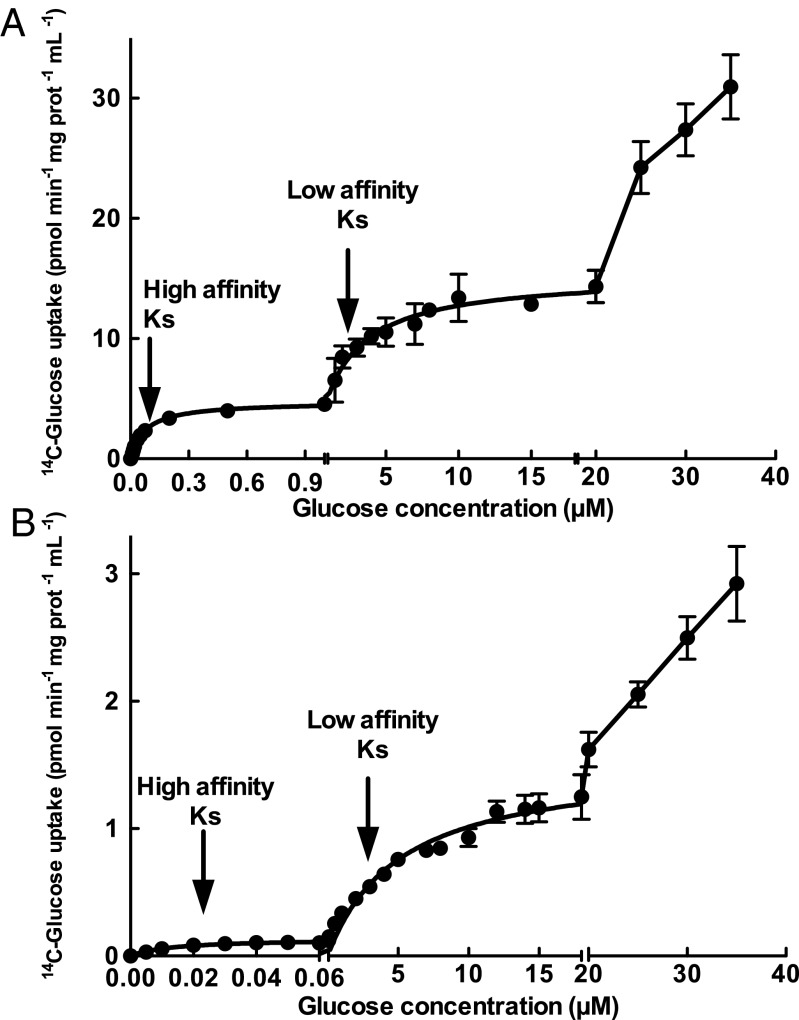
Kinetic properties of glucose uptake were studied in the recombinant S. elongatus C.K1 strain, by testing a range from 25 nM to 50 µM glucose (Fig. 2A). We observed multiphasic kinetic behavior, showing a high affinity Ks constant in the nanomolar range (123.4 nM), in good agreement with the low glucose concentrations estimated in the oligotrophic Atlantic Ocean. Cultures of P. marinus SS120 were assayed for 14C-glucose transport, finding the same high affinity constant (124.6 nM); this value is not significantly different with respect to the Ks obtained in S. elongatus C.K1 (as confirmed by t test, P < 0.05, P value 0.4601); furthermore, glucose transport by Prochlorococcus also shows multiphasic uptake kinetics (see Fig. 4A), thus reinforcing the physiological meaning of the results obtained in the recombinant strains.
Fig. 2.
(A) 14C-Glucose concentration versus its uptake by recombinant S. elongatus C.K1 strain and the control strain AsnS-1. The scale in the x axis is not linear, to allow a clearer representation of the determined glucose uptake values. Data presented are the average of three independent biological replicates. Bars show SD for each sample. (B) Eadie-Hofstee representation of the kinetics of glucose uptake shown in A for the recombinant S. elongatus C.K1 strain.
Fig. 4.
14C-Glucose concentration versus its uptake in cultures of P. marinus sp. SS120 (A) and Synechococcus sp. WH7803 (B). The scale used in the y axis is different to allow a proper representation of results. Data presented are the average of three independent biological replicates. Bars show SD for each sample.
It is worth noting that the high-affinity Ks constant of Pro1404 for glucose is 3,000-fold lower than the one described for the glucose transporters of model freshwater cyanobacteria, with Ks constants in the millimolar range (3, 19, 20).
An interesting aspect of our results is the fact that a single gene product shows multiphasic uptake kinetics, indicating that the same transporter is enabled to behave differently depending on the glucose concentration. Whereas the third phase could not be fully characterized due to the elevated concentrations of glucose required to reach saturation (Fig. 2A), two phases showing Michaelis–Menten kinetics with Ks affinity constants of 123.4 nM and 0.9 µM could be determined. These two phases are reflected in the two lines with clearly different slopes shown in the representation of Eadie-Hofstee (Fig. 2B). Thus, we will refer to Pro1404 as a dual affinity transporter. To our knowledge, only two other transporters, a potassium and a nitrate transporter from plants, have been shown to be dual-affinity transporters with a biphasic kinetics by the use of an ectopic expression approach similar to the one shown here (21–23). In the case of the nitrate transporter, the alternation from the high-affinity to the low-affinity mode and vice-versa is controlled by phosphorylation (24). Whether this transition in Pro1404 is regulated by posttranslational modification remains to be investigated.
Dual affinity transport of solutes has been observed in many organisms, but in most cases this mode of uptake is achieved by the alternate use of distinct high-affinity and low-affinity transporters. The existence of multiple glucose transporters in Prochlorococcus cannot be ruled out by our data. However, we here demonstrate the existence of at least one glucose transporter with biphasic (or multiphasic) kinetic behavior. Glucose concentration in the ocean is generally low, but fleeting glucose enrichments may occur locally by transient fluxes of the sugar (3, 25). Interchanging multiple transporters of varying affinity in response to changes in glucose concentration may take time. By using a single transporter, Prochlorococcus can rapidly adapt the intake of glucose to sudden fluxes, changing from high-affinity, low capacity mode to low-affinity, high-capacity mode and back to the high-affinity, low-capacity mode when the concentration drops and Prochlorococcus needs to scavenge traces of the sugar. Interestingly, similar multiphasic glucose transport has been previously described in marine oligotrophic bacteria (26), with a high affinity Ks constant also in the nanomolar range. However, in these cases, it has not been addressed how many transporters are involved.
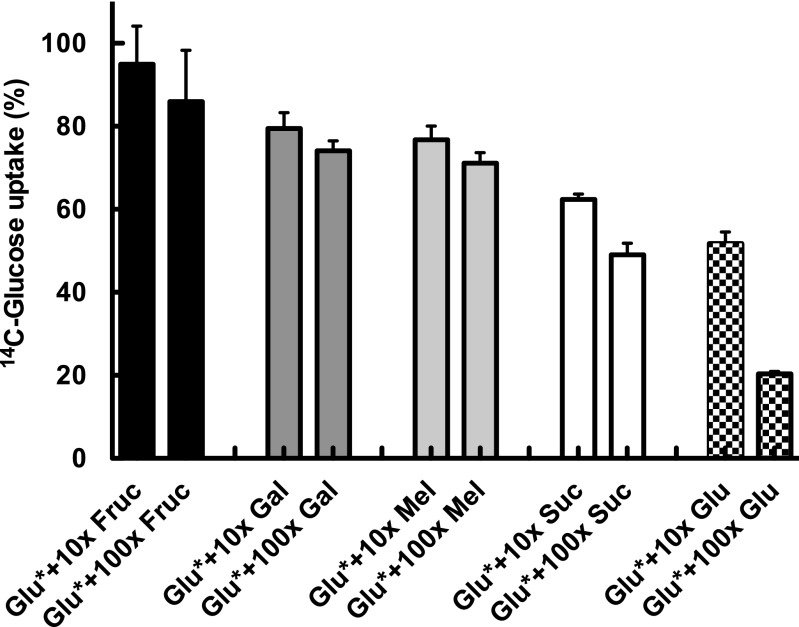
To study the specificity of the Pro1404-encoded transporter, we performed competition experiments where increased concentrations of other sugars were added, in addition to glucose (Fig. 3). 14C-glucose (0.5 µM) was added to all samples, and, additionally, 5 or 50 µM of the competing, unlabeled sugar—fructose, galactose, melibiose, or sucrose—were added. Glucose uptake was determined, assigning 100% to the results obtained with 0.5 µM 14C-glucose alone. The results indicate that, besides being highly efficient, this transporter is more, but not exclusively, specific for glucose. When we compared the decrease in radioactive glucose uptake by addition of cold glucose as control, the observed values (both at 10× and 100× concentrations; Fig. 3) were significantly lower than those observed for all other sugars, according to the Student's t test. Sucrose was the most competitive of the sugars studied, which induced a 50% decrease in glucose uptake when added at 100-fold higher concentration than glucose. Furthermore, our data suggest that, despite its annotation as a melibiose transporter, the main function of this gene product is the uptake of glucose, and its name should be changed to sugar transporter.
Fig. 3.
14C-Glucose uptake in S. elongatus C.K1 in the presence of additional concentrations of different sugars. Fruc, fructose; Gal, galactose; Glu, glucose; Mel, melibiose; Suc, sucrose. Data presented are the average of three independent biological replicates. Bars show SD for each sample.
Genes homologous to Pro1404 exist in the genomes of all marine cyanobacteria. Consequently, we decided to perform comparative experiments addressing the glucose uptake capabilities of P. marinus SS120 and Synechococcus sp. strain WH7803, which belongs to clade V in the marine Synechococcus phylogenetic tree (27), and is representative of one of the open ocean Synechococcus strains inhabiting oligotrophic environments, therefore being potential competitors of Prochlorococcus. Fig. 4 shows glucose uptake by cultures of both strains along the same range of concentrations studied in experiments with recombinant strains. It is worth mentioning that the glucose uptake observed cannot be explained on the basis of the minimal contamination observed in cultures, as described in detail in a previous manuscript (14). As stated above, a multiphasic kinetics was observed in cultures of P. marinus SS120 (Fig. 4A), remarkably similar to that observed in recombinant S. elongatus CK.1 (Fig. 2A), with measurable Ks values of 124.6 nM and 2.37 µM. Both values were not significantly different from those found in the recombinant CK.1, as compared by the t test (P < 0.05). In the case of Synechococcus WH7803, again a multiphasic kinetics was observed, with measurable Ks values of 21.55 nM and 2.86 µM. Interestingly, the high affinity Ks value of Synechococcus WH7803 is roughly sixfold lower than P. marinus SS120 (124.6 nM), but the maximum glucose uptake rate is much higher in Prochlorococcus than in Synechococcus (6.4 vs. 0.16 pmol⋅min−1⋅mg prot−1⋅mL−1, respectively). If we take both parameters into account to calculate the uptake efficiency (maximum uptake rate/Ks) for each strain, the result is that Prochlorococcus is 6.7 times more efficient in glucose uptake than Synechococcus. Although these results have been obtained with laboratory cultures and require further comparative studies in the field, they point to a potentially important difference between the two main genera of marine cyanobacteria living in oligotrophic environments, which might help to explain their clearly different distributions and abundances (27, 28).
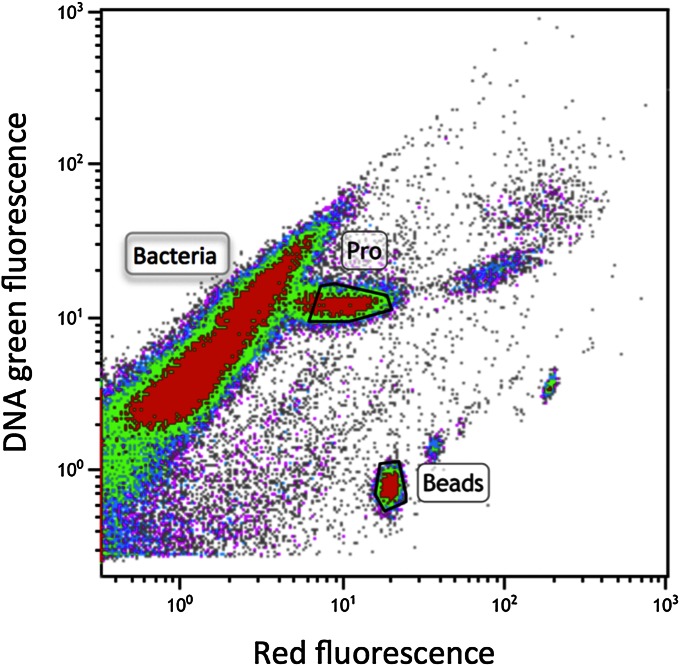
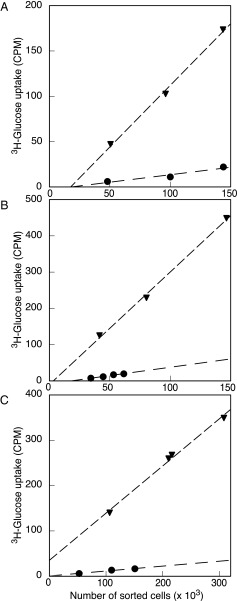
To confirm our laboratory results that Prochlorococcus takes up glucose at low concentrations, we assessed uptake of glucose by Prochlorococcus in the Atlantic Ocean on the Atlantic Meridional Transect 21 (AMT-21) research cruise, September–November 2011 (Fig. S1). Glucose bioavailability, determined using 3H-glucose dilution bioassays (29), was in the nanomolar range (Table 1), with measured values ranging from 0.07 to 2.67 nM, in agreement with previous studies (29). To compare uptake rates of glucose by Prochlorococcus and average bacterioplankton cells, DNA-stained cells were flow sorted after 4 h labeling with 2 nM 3H-glucose. The gated population (Fig. 5) corresponds to Prochlorococcus, showing its distinctive higher red chlorophyll fluorescence, compared with the total bacterioplankton population (11, 30). This type of measurement is technically challenging because of the low specific activity of the glucose tracer and relatively slow uptake of glucose by Prochlorococcus cells. Despite this technical limitation, at three stations, we could determine glucose uptake by Prochlorococcus (Table 1 and Fig. 6). We observed measurable rates of glucose import (Table 1), demonstrating that Prochlorococcus does indeed take up glucose in its natural habitat. Furthermore, we analyzed the relative contribution of populations to total glucose uptake, finding that Prochlorococcus was responsible for 2.6–3.7% of total bacterioplankton glucose uptake observed at those stations (Table 1). On average, the Prochlorococcus population contribution to total bacterioplankton glucose uptake was 3.2 ± 0.56%; Prochlorococcus cellular glucose uptake relative to the average bacterioplankton cell uptake was 13 ± 2% (Fig. 6).
Table 1.
3H-Glucose uptake determined onboard during the AMT-21 cruise, showing the results obtained with sorted Prochlorococcus populations vs. total bacterial uptake
| Geographical coordinates | Station | Depth, m | Total Prochlorococcus uptake, µCi/mL | Total bacterial uptake, µCi/mL | % total uptake by Prochlorococcus | [Glucose], nM |
| 33° 30.11′ N 28° 45.21′ W | 15 | 80 | 4.24 × 10−5 | 128 × 10−5 | 3.3 | 0.50 |
| 10° 45.43′ N 31° 52.40′ W | 35 | 47 | 17.7 × 10−5 | 480 × 10−5 | 3.7 | 0.52 |
| 06° 31.34′ N 29° 07.96′ W | 38 | 55 | 4.18 × 10−5 | 160 × 10−5 | 2.6 | 2.7 |
Fig. 5.
Flow cytometric scatter plot showing a signature of SYBR Green I DNA stained picoplankton from station 35 of cruise AMT-21 (47 m depth). Prochlorococcus cells were identified by their extra red chlorophyll fluorescence, gated (area marked “Pro”) and sorted to determine their 3H-glucose uptake.
Fig. 6.
3H-Glucose uptake vs. number of sorted Prochlorococcus (circles) or total bacterioplankton cells (triangles) at stations 15 (A), 35 (B), and 38 (C). Further details on the results obtained at those stations are shown in Table 1.
The uptake values shown in Fig. 6 are rather low, compared with previous results from our team obtained with Prochlorococcus cultures (Fig. 6 vs. figure 1a in ref. 14 by Gómez-Baena et al.). When we calculated the specific uptake values per cell in natural populations, we obtained 0.642 × 10−10, 1.597 × 10−10, and 0.321 × 10−10 µCi⋅cell−1⋅h−1 for samples obtained at stations 15, 35, and 38 (Table 1), respectively. By contrast, our results with flow sorted cells from cultures of P. marinus strain PCC 9511 were 1.02 × 10−8 µCi⋅cell−1⋅h−1 (14). This fact might be due to the big environmental differences between natural Prochlorococcus populations from the open ocean vs. Prochlorococcus cells growing in the laboratory under optimized conditions of light, nutrients, temperature, etc. Furthermore, it has to be taken into account that glucose uptake of natural populations was determined after incubations with 2 nM glucose (Table 1) whereas glucose uptake in cultures of P. marinus PCC 9511 was determined after incubations with 1 µM glucose (14), which correspond to two different phases of the uptake kinetics, as shown in Figs. 2A and 4A. Finally, it is worth noting that, because glucose uptake was found to be higher in the light (14), laboratory cultures might have higher glucose uptake rates due to an increased light availability, compared with natural samples obtained at 47, 55, and 80 m depth (Table 1).
Pro1404 belongs to the Major Facilitator Superfamily of transporters (31), which couple solute transport to the downgradient flow of an ion. Although the free energy for the transport of a glucose molecule by Pro1404 would need to be calculated, it is predicted to be much lower than the metabolic effort required for the synthesis of a molecule of glucose from CO2 (18 molecules of ATP and 12 of NADPH). Therefore, taking up glucose is energetically advantageous for Prochlorococcus rather than to synthesize it de novo.
Previous works have shown that Prochlorococcus carried out CO2 fixation at a rate of 1.2 fg C⋅cell−1⋅h−1 (32) in the same sampling area used in the present study. According to our estimations, based on the results obtained during this work (Table 1 and Fig. 6), Prochlorococcus was taking up carbon from glucose at rates of 0.16–0.31 fg C⋅cell−1⋅h−1. Therefore, our data suggest that, if carbon from glucose is incorporated to metabolism by Prochlorococcus, its ratio with respect to that incorporated from CO2 would be roughly 1/6. Although this is an initial estimation that needs confirmation in larger field studies, it indicates that the importance of glucose uptake cannot be considered as negligible.
The ecological meaning of the results described above is very significant. We provide conclusive evidence that Prochlorococcus does take up glucose in the open Atlantic Ocean. Laboratory experiments demonstrate that the gene Pro1404 is coding for a high affinity glucose importer in Prochlorococcus. This means that, besides being possibly the most abundant autotrophic organism on Earth, Prochlorococcus can use organic molecules devoid of other essential elements (such as nitrogen or sulfur) except carbon. Moreover, initial estimates indicate that the ratio of carbon obtained from glucose is noteworthy and might constitute a significant part of total carbon incorporated by Prochlorococcus cells when glucose is available in the environment. This estimate is consistent with the bioenergetic advantage of glucose uptake vs. glucose synthesis de novo, summarized above in this paper. Although glucose is usually detected at very low concentrations in the open ocean, there might be events of sudden increase in its availability [such as excretions from other organisms, or detrital particles (26)], which might be exploited by Prochlorococcus to save energy for other metabolic uses.
Different studies suggest that the metabolism of Prochlorococcus is optimized to be extremely efficient in growing autotrophically at very oligotrophic environments. In our view, Prochlorococcus is essentially a phototroph but is also capable of taking up glucose when available as a way to save energy for other purposes.
If we take into account the abundance and wide distribution of Prochlorococcus, it seems reasonable to expect it to be an important player in the global carbon cycle for an unexpected reason: in addition to its important and widely recognized contribution to primary production, one should consider its role in the consumption of sugars. The implications of this conclusion are far reaching because they could significantly alter the ecological role of these cyanobacteria as primary producers capable of tuning their metabolism to energetically benefit from environmental conditions.
Materials and Methods
Cyanobacterial Strains and Growth Conditions.
P. marinus strain SS120 (low-irradiance adapted) was routinely cultured in polycarbonate Nalgene flasks (10 L) using PCR-S11 medium as described (13). The sea water used as basis for this medium was kindly provided by the Instituto Español de Oceanografía (Madrid). Synechococcus sp. strain WH7803 was grown using artificial seawater medium, as described (33). Cells were grown in a culture room set at 24 °C under continuous blue irradiances (4 µE⋅m−2⋅s−1). The marine cyanobacterial cultures were clonal but not axenic although the contamination level was extremely low (< 3%), as assessed by flow cytometry.
Synechococcus sp. strain PCC 7942 was grown axenically at 30 °C in the light (75 µE⋅m−2⋅s−1) in BG11 medium (34). Cultures of S. elongatus and derived strains were tested for axenicity in two ways, by microscopic observation and by plating samples of each culture on BG11 plates and on LB plates supplemented with 1% glucose that were cultured for at least 1 wk at 30 °C. The data presented in the manuscript correspond only to experiments where all strains involved were axenic. Liquid cultures were bubbled with a mixture of CO2 and air (1%, vol/vol). For recombinant strains, kanamycin was used at 7 µg⋅ml−1 final concentration. For plates, the medium was solidified with 1%, separately autoclaved agar (Difco).
Construction of Recombinant Synechococcus sp. PCC 7942 Strains.
The Pro1404 gene from P. marinus SS120 (currently annotated as melB) was amplified by PCR using genomic DNA as a template and primers MELB-1F and MELB-1R:
MELB-1F: 5′ GGAGGTCCATGGTTTCCTATGGAT 3′; and
MELB-1R: 5′ TGCCAAGCTTTTTAAGCCAACGG 3′.
The amplified fragment was cloned between the NcoI and HindIII sites of the pTrc99A plasmid vector (35) generating plasmid pcMM1. This molecule was used as a template for subsequent PCR amplification with phosphorylated primers PCMM1-1F and PCMM1-1R to generate a PCR fragment, including the melB gene and the downstream rrnB transcriptional terminator from pTrc99A, which was ligated with pCA12 plasmid (36) linearized by PCR with divergent primers 7942_ASNS_3F and 7942_ASNS_3R. The resulting plasmid, pcMM2, contained the melB gene from Prochlorococcus with a downstream rrnB terminator interrupting the Synechococcus sp. PCC 7942 asnS ORF, which has been shown to be dispensable for this organism (36). Kanamycin-resistance cassettes C.K1 and C.K3 (37) were cloned in the same orientation as the melB gene in the HpaI site upstream of it in pcMM2, generating plasmids pcMM3 and pcMM4, respectively. The construction of plasmids is outlined in Fig. S2.
Plasmids pcMM3 and pcMM4, which do not replicate in cyanobacteria, were introduced by transformation in S. elongatus sp. PCC 7942. Kanamycin-resistant transformants were selected, and the genomic structure of the asnS locus was analyzed. Those transformants that had integrated the constructions described above by double recombination in the asnS locus were selected for further analysis (Fig. S2).
Determination of Glucose Uptake by Cultured Cyanobacterial Strains.
[U-14C]glucose (281 mCi/mmol; American Radiolabeled Chemicals, Inc) was added to 0.7–4.5 mL of culture sample to reach a final concentration of 0.01 µM to 20 µM, depending on the experiment. Mixtures of radiolabeled and unlabeled glucose were added to achieve the desired concentration; furthermore, unlabeled sugars were used in the competition experiments. Aliquots were taken at the indicated times, filtered through 0.22-µm (for Prochlorococcus sp. SS120 and Synechococcus sp. WH7803) and 0.45-µm (for Synechocystis sp. PCC 6803, S. elongatus sp. PCC 7942, and its recombinant strains) Millipore filters, washed with 25 mM Tris⋅HCl pH 7.5 and then placed into scintillation vials. In studies on cultured marine cyanobacterial strains, the aliquots were taken at 3, 6, and 9 min (for Prochlorococcus sp. strain SS120) and at 2, 5, and 10 min (for Synechococcus sp. strain WH7803), due to the different saturation times observed for the glucose uptake kinetics of each strain. Scintillation was started by the addition of 5 mL of Ready Protein Mixture (Beckman Coulter) and measured in an LS6000IC Scintillation Counter (Beckman Coulter).
Determination of Protein Concentration.
Protein concentration was determined using the Bio-Rad Protein Assay kit, based on the method described by Bradford (38).
Dilution Bioassay to Estimate Glucose Concentration in the Atlantic Ocean.
A series of dilution bioassays for the determination of glucose concentration, following the method described by Wright and Hobbie (39), were carried out at different sampling points, along the Atlantic Meridional Transect 21 cruise (AMT-21), from September 29 to November 14, 2011 (Fig. S1). At each of 38 stations, surface seawater was collected in Niskin bottles and decanted into HCl-washed vacuum flasks. Samples were analyzed within 1 h of sampling.
d-[5,6 3H]glucose (specific activity 60 Ci/mmol; Hartmann Analytic) was added to 1.6 mL of seawater samples, in a final concentration range from 0.25 to 1.25 nM. The samples were incubated in sterile 2-mL capped polypropylene microcentrifuge tubes at in situ temperature and fixed with 1% paraformaldehyde (PFA) at 30, 50, 70, and 90 min. Following fixation, samples were filtered onto 0.2-µm polycarbonate filters (Nuclepore; Whatman) and washed twice with 4 mL of deionized water. The radioactivity retained on filters was measured as cpm, following the addition of 4 mL of scintillation mixture, in a liquid scintillation counter (Tri-Carb 3100; Perkin-Elmer). Glucose turnover times were plotted against the concentrations of added glucose; in this representation, the regression lines intercepts to the x axis are the ambient glucose concentration at each sampling point. Further details of the bioassay method have been described by Zubkov et al. (29).
Determination of Glucose Uptake by Natural Prochlorococcus Populations Using Flow Cytometry.
To determine cell abundance, 1 mL of untreated seawater was fixed with 1% PFA for 1 h, then stained with SYBR Green I nucleic acid stain (Sigma) in the presence of 30 mM potassium citrate (38) for 1 h. Samples were analyzed by flow cytometry (FACSCalibur; Beckton Dickinson Biosciences) at a flow rate of 15–60 mL⋅min−1, depending on the cell concentration, for 1 min. Multifluorescent 0.5-µm beads (Fluoresbrite Microparticles; Polysciences) were used to check particle concentration and fluorescence.
To measure glucose uptake by natural Prochlorococcus populations, 1.6–8 mL of seawater was incubated with 2 nM d-[5,6 3H]glucose for 4 h. Samples were fixed with 1% PFA, and DNA was stained as described above. Prochlorococcus cells were sorted by flow cytometry, from a plot of red chlorophyll fluorescence vs. green DNA fluorescence (Fig. 5) using CellQuest software (BD Biosciences). The total bacterioplankton population was sorted from a plot of side scatter against green DNA fluorescence, to compare the relative uptake of Prochlorococcus with the total community (Fig. 6, Table 1). Sorted cells were filtered onto 0.2-µm polycarbonate filters (Nuclepore; Whatman) and washed twice with 4 mL of deionized water. The radioactivity retained on filters was measured as cpm, following the addition of 20 mL of scintillation mixture, using a liquid scintillation counter (Tri-Carb 3100; Perkin-Elmer).
Supplementary Material
Acknowledgments
We thank Prof. W. R. Hess (University of Freiburg) for providing cultures of Synechococcus WH7803, Mr. R. Holland (National Oceanography Centre, Southampton, United Kingdom) for help with flow cytometry analyses, and Prof. E. Flores (Instituto de Bioquímica Vegetal y Fotosíntesis) for insightful discussions. We acknowledge kind support from Dr. G. Tarran (principal scientist of the cruise D371-AMT21), as well as the crew of the R.R.S. Discovery, and thank Prof. S. García Navarro (Universidad de Córdoba) for kindly providing access to radioactivity facilities. We also thank the Roscoff Culture Collection (Station Biologique) for providing Prochlorococcus strains (ASSEMBLE project, Grant Agreement 227799, from the “Capacities” program, Seventh Framework Programme, European Union). This work was supported by Grants BFU-2009-08008/BMC and BFU2010-19544 (Spanish Ministerio de Educación y Ciencia cofunded by the European Union European Social Fund) and by Universidad de Córdoba (Programa Propio de Investigación). M.d.C.M.-M. received a grant from Projects BFU-2009-08008/BMC and P07-CVI-3055 (Proyectos de Excelencia). This study is a contribution to the international Integrated Marine Biogeochemistry and Ecosystem Research project and was supported by the UK Natural Environment Research Council National Capability funding to Plymouth Marine Laboratory and the National Oceanography Centre, Southampton. This is contribution number 232 of the Atlantic Meridional Transect (AMT) programme.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8323.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221775110/-/DCSupplemental.
References
- 1.Knoll A. Cyanobacteria and Earth history. In: Herrero A, Flores E, editors. The Cyanobacteria: Molecular Biology, Genomics, and Evolution. Norfolk, UK: Caister Academic; 2008. [Google Scholar]
- 2.Pelroy RA, Rippka R, Stanier RY. Metabolism of glucose by unicellular blue-green algae. Arch Mikrobiol. 1972;87(4):303–322. doi: 10.1007/BF00409131. [DOI] [PubMed] [Google Scholar]
- 3.Flores E, Schmetterer G. Interaction of fructose with the glucose permease of the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 1986;166(2):693–696. doi: 10.1128/jb.166.2.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang CC, Jeanjean R, Joset F. Obligate phototrophy in cyanobacteria: More than a lack of sugar transport. FEMS Microbiol Lett. 1998;161(2):285–292. doi: 10.1111/j.1574-6968.1998.tb12959.x. [DOI] [PubMed] [Google Scholar]
- 5.Ungerer JL, Pratte BS, Thiel T. Regulation of fructose transport and its effect on fructose toxicity in Anabaena spp. J Bacteriol. 2008;190(24):8115–8125. doi: 10.1128/JB.00886-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waterbury JB, Watson SW, Valois FW, Franks DG. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can Bull Fish Aquat Sci. 1986;214:71–120. [Google Scholar]
- 7.Béjà O, Suzuki M. Photoheterotrophic marine prokaryotes. In: Kirchman DL, editor. Microbial Ecology of the Oceans. 2nd Ed. Hoboken, NJ: John Wiley & Sons; 2008. pp. 131–157. [Google Scholar]
- 8.Chisholm S, et al. A novel free living prochlorophyte abundant in the oceanic euphotic zone. Nature. 1988;334:340–343. [Google Scholar]
- 9.Partensky F, Hess WR, Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev. 1999;63(1):106–127. doi: 10.1128/mmbr.63.1.106-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman ML, Chisholm SW. Code and context: Prochlorococcus as a model for cross-scale biology. Trends Microbiol. 2007;15(9):398–407. doi: 10.1016/j.tim.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Zubkov MV, Fuchs BM, Tarran GA, Burkill PH, Amann R. High rate of uptake of organic nitrogen compounds by Prochlorococcus cyanobacteria as a key to their dominance in oligotrophic oceanic waters. Appl Environ Microbiol. 2003;69(2):1299–1304. doi: 10.1128/AEM.69.2.1299-1304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vila-Costa M, et al. Dimethylsulfoniopropionate uptake by marine phytoplankton. Science. 2006;314(5799):652–654. doi: 10.1126/science.1131043. [DOI] [PubMed] [Google Scholar]
- 13.Rippka R, et al. Prochlorococcus marinus Chisholm et al. 1992 subsp. pastoris subsp. nov. strain PCC 9511, the first axenic chlorophyll a2/b2-containing cyanobacterium (Oxyphotobacteria) Int J Syst Evol Microbiol. 2000;50(Pt 5):1833–1847. doi: 10.1099/00207713-50-5-1833. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Baena G, et al. Glucose uptake and its effect on gene expression in prochlorococcus. PLoS ONE. 2008;3(10):e3416. doi: 10.1371/journal.pone.0003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocap G, et al. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature. 2003;424(6952):1042–1047. doi: 10.1038/nature01947. [DOI] [PubMed] [Google Scholar]
- 16.Dufresne A, et al. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc Natl Acad Sci USA. 2003;100(17):10020–10025. doi: 10.1073/pnas.1733211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettler GC, et al. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet. 2007;3(12):e231. doi: 10.1371/journal.pgen.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paz-Yepes J, Flores E, Herrero A. Expression and mutational analysis of the glnB genomic region in the heterocyst-forming Cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 2009;191(7):2353–2361. doi: 10.1128/JB.01381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joset F, Buchou C, Zhang C, Jeanjean R. Physiological and genetic analysis of the glucose-fructose permeation system in two Synechocystis species. Arch Microbiol. 1988;149(5):417–421. [Google Scholar]
- 20.Raboy B, Padan E. Active transport of glucose and alpha-methylglucoside in the cyanobacterium Plectonema boryanum. J Biol Chem. 1978;253(9):3287–3291. [PubMed] [Google Scholar]
- 21.Liu KH, Huang CY, Tsay YF. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell. 1999;11(5):865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morère-Le Paven MC, et al. Characterization of a dual-affinity nitrate transporter MtNRT1.3 in the model legume Medicago truncatula. J Exp Bot. 2011;62(15):5595–5605. doi: 10.1093/jxb/err243. [DOI] [PubMed] [Google Scholar]
- 23.Fu HH, Luan S. AtKuP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10(1):63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu KH, Tsay YF. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003;22(5):1005–1013. doi: 10.1093/emboj/cdg118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stocker R. Marine microbes see a sea of gradients. Science. 2012;338(6107):628–633. doi: 10.1126/science.1208929. [DOI] [PubMed] [Google Scholar]
- 26.Nissen H, Nissen P, Azam F. Multiphasic uptake of D-glucose by an oligotrophic marine bacterium. Mar Ecol Prog Ser. 1984;16(1-2):155–160. [Google Scholar]
- 27.Scanlan DJ, et al. Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev. 2009;73(2):249–299. doi: 10.1128/MMBR.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partensky F, Blanchot J, Vaulot D. Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: A review. In: Charpy L, Larkum A, editors. Marine Cyanobacteria (Bulletin de l'Institut Océanographique, Monaco, Special Paper 19) Monaco: Institut Océanographique; 1999. pp. 457–476. [Google Scholar]
- 29.Zubkov M, Tarran G, Mary I, Fuchs B. Differential microbial uptake of dissolved amino acids and amino sugars in surface waters of the Atlantic Ocean. J Plankton Res. 2008;30:211–220. [Google Scholar]
- 30.Zubkov M, Tarran G. Amino acid uptake of Prochlorococcus spp. in surface waters across the South Atlantic Subtropical Front. Aquat Microb Ecol. 2005;40(3):241–249. [Google Scholar]
- 31.Law CJ, Maloney PC, Wang DN. Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol. 2008;62:289–305. doi: 10.1146/annurev.micro.61.080706.093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jardillier L, Zubkov MV, Pearman J, Scanlan DJ. Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J. 2010;4(9):1180–1192. doi: 10.1038/ismej.2010.36. [DOI] [PubMed] [Google Scholar]
- 33.Waterbury J, Willey J. Isolation and growth of marine planktonic cyanobacteria. Methods Enzymol. 1988;167:100–105. [Google Scholar]
- 34.Rippka R, Deruelles J, Waterbury J, Herdman M, Stanier R. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 35.Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 36.Luque I, Riera-Alberola ML, Andújar A, Ochoa de Alda JA. Intraphylum diversity and complex evolution of cyanobacterial aminoacyl-tRNA synthetases. Mol Biol Evol. 2008;25(11):2369–2389. doi: 10.1093/molbev/msn197. [DOI] [PubMed] [Google Scholar]
- 37.Elhai J, Wolk CP. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 38.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 39.Wright RT, Hobbie JE. Use of glucose and acetate by bacteria in aquatic ecosystems. Ecology. 1966;47:447–464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.