Abstract
Calcium-binding protein 1 (CaBP1) is a neuron-specific member of the calmodulin superfamily that regulates several Ca2+ channels, including inositol 1,4,5-trisphosphate receptors (InsP3Rs). CaBP1 alone does not affect InsP3R activity, but it inhibits InsP3-evoked Ca2+ release by slowing the rate of InsP3R opening. The inhibition is enhanced by Ca2+ binding to both the InsP3R and CaBP1. CaBP1 binds via its C lobe to the cytosolic N-terminal region (NT; residues 1–604) of InsP3R1. NMR paramagnetic relaxation enhancement analysis demonstrates that a cluster of hydrophobic residues (V101, L104, and V162) within the C lobe of CaBP1 that are exposed after Ca2+ binding interact with a complementary cluster of hydrophobic residues (L302, I364, and L393) in the β-domain of the InsP3-binding core. These residues are essential for CaBP1 binding to the NT and for inhibition of InsP3R activity by CaBP1. Docking analyses and paramagnetic relaxation enhancement structural restraints suggest that CaBP1 forms an extended tetrameric turret attached by the tetrameric NT to the cytosolic vestibule of the InsP3R pore. InsP3 activates InsP3Rs by initiating conformational changes that lead to disruption of an intersubunit interaction between a “hot-spot” loop in the suppressor domain (residues 1–223) and the InsP3-binding core β-domain. Targeted cross-linking of residues that contribute to this interface show that InsP3 attenuates cross-linking, whereas CaBP1 promotes it. We conclude that CaBP1 inhibits InsP3R activity by restricting the intersubunit movements that initiate gating.
Keywords: EF hand, intracellular Ca2+ channel, ion channel, ryanodine receptor
Dynamic increases in cytosolic free Ca2+ concentration ([Ca2+]c) regulate many cellular events, including acute and long-term changes in neuronal activity (1–3). Release of Ca2+ from intracellular stores is controlled by intracellular Ca2+ channels (4), the most common of which are inositol 1,4,5-trisphosphate receptors (InsP3Rs) (5, 6). Dual regulation of InsP3Rs by InsP3 and Ca2+ facilitates regenerative Ca2+ release (6), generating Ca2+ signals of remarkable versatility and spatiotemporal complexity (1, 2, 7). The sites through which Ca2+ biphasically regulates InsP3Rs are unresolved (5, 8, 9). There is, however, evidence, that proteins with EF-hand Ca2+-binding motifs can regulate gating of InsP3Rs. These include calmodulin (CaM) (10–12), calmyrin (CIB1) (13), and neuronal Ca2+ sensor (NCS) proteins (2). The latter comprise a branch of the CaM superfamily that includes NCS-1 (14) and Ca2+-binding protein 1 (CaBP1) (15, 16).
CaBP1–5 proteins (2, 17) have four EF hands that form pairs within the N lobe (EF1 and 2) and C lobe (EF3 and 4). The two lobes are structurally independent (18) and connected by a flexible linker (18). Whereas all four EF hands bind Ca2+ in CaM, EF2 in CaBP1 does not bind Ca2+, and EF1 has reduced selectivity for Ca2+ over Mg2+. EF3 and EF4 in the C lobe of CaBP1 exhibit canonical Ca2+-induced conformational changes (18, 19). Many splice variants and isoforms of CaBPs are expressed in different neurons (20–22), and their targets include a variety of ion channels (2). CaBP1, for example, regulates voltage-gated P/Q-type (23) and L-type Ca2+ channels (24) and a transient receptor potential channel, TRPC5 (25). Furthermore, the prevailing view that InsP3Rs open only after binding InsP3 was challenged by evidence that CaBP1 (15) and related proteins (13) might, in their Ca2+-bound forms, gate InsP3Rs. The suggestion that Ca2+, via CaBP1, might directly gate InsP3Rs proved to be contentious, but it spawned further evidence that CaBP1 regulates InsP3Rs (14, 16, 20).
InsP3Rs are large tetrameric channels (5, 6). Their activation is initiated within the N-terminal domain (NT; residues 1–604) by binding of InsP3 to the InsP3-binding core (IBC; residues 224–604) of each subunit (26). This process leads, via rearrangement of the suppressor domain (SD; residues 1–223) (27), to opening of an intrinsic pore (28, 29). Despite extensive studies of CaBP1 (2) and of the many proteins that modulate InsP3Rs (5), little is known about the structural basis of these protein interactions with InsP3Rs or of CaBP1 with any ion channel. Here we combine NMR, mutagenesis, cross-linking, and functional analyses to define, at the atomic level, the interactions between CaBP1 and InsP3Rs.
Results
CaBP1 Inhibits InsP3-Evoked Ca2+ Release.
CaBP1 is found only in neurons (2), and they predominantly express InsP3R1. We therefore used permeabilized DT40 cells lacking endogenous InsP3Rs, but stably expressing rat InsP3R1, to assess the effects of CaBP1 on Ca2+ release from intracellular stores. The two splice variants of CaBP1 expressed in brain (17) regulate InsP3Rs and Ca2+ channels similarly (16). Throughout this study, we used the short variant (Table S1) because its solubility makes it more amenable to NMR analysis. Across a range of [Ca2+]c, CaBP1 alone had no effect on the Ca2+ content of the intracellular stores of DT40–InsP3R1 cells (Fig. S1 A–D). The lack of effect of Ca2+–CaBP1 on InsP3R1 was confirmed by nuclear patch-clamp analyses of single InsP3R1 (Fig. S1E). These results are inconsistent with the notion that Ca2+–CaBP1 stimulates Ca2+ release via InsP3R (15), a suggestion that has also been challenged by others, who argue that CaBP1 inhibits InsP3-evoked Ca2+ release (16, 20).
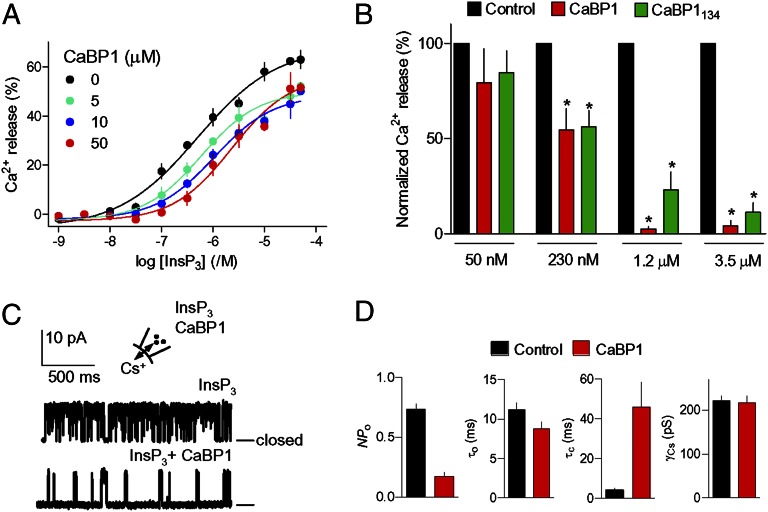
CaBP1 caused a concentration-dependent decrease in the sensitivity of InsP3-evoked Ca2+ release (Fig. 1A and Table S2) without affecting 3H–InsP3 binding (Fig. S1 G–I). Inhibition of InsP3-evoked Ca2+ release by CaBP1 was increased at higher [Ca2+]c, but was evident even at the [Ca2+]c of a resting cell, although not at lower [Ca2+]c (Fig. 1B and Table S2). In single-channel analyses recorded under optimal conditions for InsP3R activation (30), CaBP1 (10 µM) massively reduced channel activity (NPo) without affecting unitary conductance (γCs) or mean channel open time (τo) (Fig. 1 C and D). Analysis of records that included only a single functional InsP3R established that an increase in mean channel closed time (τc) from 4.2 ± 0.9 ms to 46 ± 13 ms accounted for the 5.3-fold reduction in NPo in the presence of CaBP1 (Fig. S1F). The lack of effect on τo and γCs indicates that CaBP1 inhibits gating rather than blocking the InsP3R pore.
Fig. 1.
Inhibition of InsP3R1 by CaBP1. (A) CaBP1 inhibits InsP3-evoked Ca2+ release. Permeabilized DT40–IP3R1 cells in CLM with a [Ca2+]c of 1.2 μM were incubated with CaBP1 (10 min) before adding InsP3. Results (means ± SEM; n = 3, with duplicate determinations in each) show the concentration-dependent release of Ca2+ by InsP3. (B) Effects of CaBP1 and CaBP1134 (50 μM) on InsP3-evoked Ca2+ release at the indicated [Ca2+]c show that CaBP1 is not the only Ca2+ sensor. For each [Ca2+]c, results (percentage of control, means ± SEM; n = 3–4, with duplicate determinations in each) show the Ca2+ release evoked by the concentration of InsP3 that evoked half-maximal Ca2+ release (EC50) under control conditions. *P < 0.05 relative to control. (C) Typical patch-clamp recordings from single InsP3R1 in medium with [Ca2+]c of 1.5 μM stimulated with InsP3 (10 μM) alone or with CaBP1 (10 μM). Bars show the closed state. The holding potential was +40 mV. (D) Summary data (mean ± SEM; n given in Fig. S1F) show NPo, mean channel open (τo) and closed (τc) times and unitary conductance (γCs).
Inhibition of InsP3R by CaBP1 Is Enhanced by Ca2+ Binding to both CaBP1 and InsP3R.
Cytosolic Ca2+ enhances the inhibition of InsP3R by CaBP1 (Fig. 1B and Table S2). We mutated residues within each of the three functional EF hands of CaBP1 to prevent Ca2+ binding (CaBP1134) (Fig. S2F). CaBP1134 was as effective as CaBP1 in causing Ca2+-dependent inhibition of InsP3-evoked Ca2+ release at the typical [Ca2+]c of a resting cell (230 nM), but less effective than CaBP1 at higher [Ca2+]c (Fig. 1B and Fig. S2 A–D). When only the last pair of EF hands was mutated (CaBP134), the results were similar to those obtained with CaBP1134 (Fig. S2E). Although CaBP1134 does not bind Ca2+ (Fig. S2F), its ability to inhibit InsP3-evoked Ca2+ release was enhanced by increasing [Ca2+]c. However, at the highest [Ca2+]c, the inhibition by mutant CaBP1 was less than with native CaBP1 (Fig. 1B and Fig. S2). This result suggests that the last pair of EF hands in CaBP1 contributes to the enhanced inhibition of InsP3R at elevated [Ca2+]c. We conclude that CaBP1 inhibits InsP3-evoked Ca2+ release at resting [Ca2+]c. At the [Ca2+]c of stimulated cells, the inhibition is potentiated by Ca2+ binding to both InsP3R and the last pair of EF hands in CaBP1. This complex regulation of CaBP1–InsP3R interactions by cytosolic Ca2+ may have contributed to conflicting reports of their Ca2+ dependence (13, 15, 16, 20) and requirement for functional EF hands (15, 16).
Local Hydrophobic Interactions Between InsP3R and the C Lobe of CaBP1.
Ca2+–CaBP1 binds via its C lobe to the NT, in both its apo and InsP3-bound forms, with a 1:1 stoichiometry and an equilibrium dissociation constant (KD) of ∼3 μM (18). In the absence of Ca2+, CaBP1 binds with 10-fold lower affinity (18), consistent with our functional analyses (Fig. 1B). We used NMR-based approaches, including chemical shift perturbation and paramagnetic relaxation enhancement (PRE) (31), to examine the structure of the NT–CaBP1 complex. Our PRE experiments measure distances between side-chain methyl groups in CaBP1 that are <10 Å away from nitroxide spin labels attached to specific Cys residues in the NT. The starting point was the NT in which all Cys residues were replaced by Ala (NTCL) (Table S1). Extensive structural and functional studies confirmed that NTCL mimics the behavior of wild-type NT (29). Isothermal titration calorimetry demonstrated that Ca2+–CaBP1 binds to NTCL (KD = 16 μM, pKD = 4.8 ± 0.1) (Fig. S3A), although with lower affinity than native NT (KD = 3 μM, pKD = 5.5 ± 0.1) (18). This small difference in Gibbs free energy of binding (ΔΔG° = 0.9 kcal/mole) suggests that CaBP1 has a similar structural interaction with NT and NTCL, consistent with the similar NMR spectra of Ca2+–CaBP1 bound to NTCL (Fig. S4A, red) and wild-type NT (Fig. S4A, blue). We then introduced single Cys residues into strategic sites on the surface of NTCL and used them in PRE experiments. The NMR spectra of CaBP1 bound to wild-type and mutant NTs are similar, confirming that each NT mutant is folded and bound similarly to CaBP1.
Binding of NTCL to 15N-labeled CaBP1 caused nearly all backbone amide resonances to broaden beyond detection in 15N–1H heteronuclear single quantum coherence spectra, preventing use of backbone amide resonances in the PRE analysis. Only side-chain methyl NMR resonances of CaBP1 were detected with enough sensitivity to be analyzed using PRE. 13C-labeled CaBP1 binding to unlabeled NTCL was monitored by using 1H–13C methyl transverse relaxation-optimized spectroscopy (TROSY) NMR (Fig. S4A). Binding of NTCL had large effects on the NMR resonances assigned to CaBP1 residues in the C lobe, whereas residues in the N lobe were unaffected. This finding is consistent with the NT binding via the C lobe of CaBP1 (18). The 13C-labeled methyl resonances of V101, L104, and V162 in CaBP1 became severely broadened after the addition of NTCL, suggesting that these residues directly contact NTCL. Mutation of each of these residues to Ser massively reduced the affinity of CaBP1 for NTCL (Fig. S3 B and C). In addition, exposed residues in CaBP1 (I124, L131, L132, and L150) have methyl resonances that show perturbations in chemical shifts, indicating a change in their magnetic environment upon binding of the NT. By monitoring the NMR spectral changes of 13C-methyl–labeled CaBP1 complexed with NTCL in the presence (paramagnetic) and absence (diamagnetic) of attached spin label, the proximity of NTCL and CaBP1 was defined. A methyl TROSY spectrum of 13C-labeled CaBP1 bound to NTCL with a single Cys insertion, NTCL(E20C), was similar to that of CaBP1 bound to NTCL (Fig. S4 A and B), indicating that NTCL(E20C) is structurally intact. Attachment of a nitroxide spin label to NTCL(E20C) caused a marked decrease in NMR peak intensity for some CaBP1 residues (I124, L131, L132, V148, and L150), whereas others (L99, V136, L141, L145, and V156) were less affected (Fig. S4B). The ratios of peak intensities in the presence and absence of spin label (IParamagnetic/IDiamagnetic) were taken as a measure of the distance between the methyl groups and spin label. The PRE ratios are listed for all single Cys insertions in Fig. S4C.
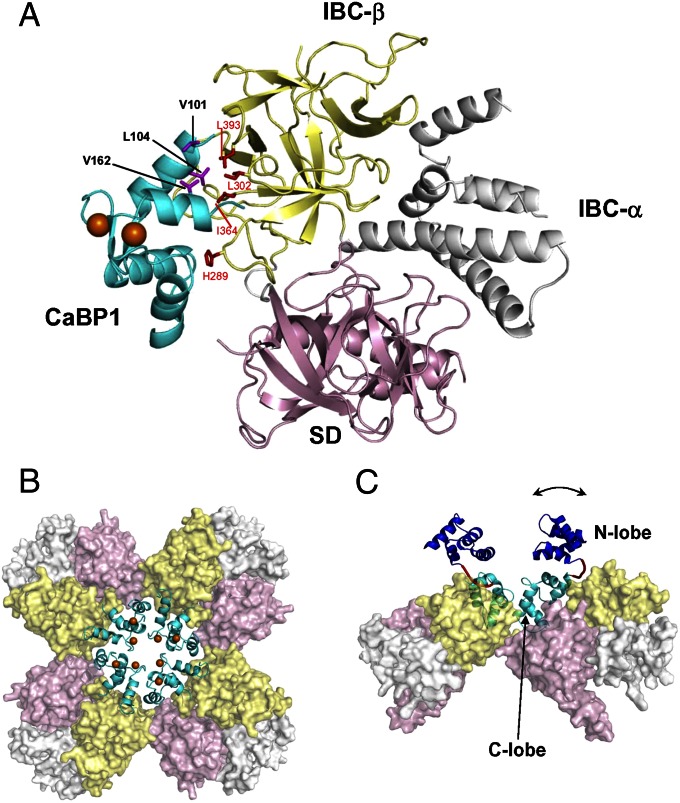
We used the PRE restraints and chemical shift perturbation data within HADDOCK (32) to dock the NMR structure of Ca2+-bound C lobe of CaBP1 (18) onto the crystal structure of the NT (29) (Fig. 2A). Within the complex, the Ca2+-bound C lobe of CaBP1 is in the familiar open conformation typical of Ca2+-bound EF hands in CaM (33). Exposed hydrophobic residues in Ca2+–CaBP1 (V101, L104, and V162) interact with clustered hydrophobic residues (L302, I364, and L393) in the IBC-β domain of InsP3R1 (Fig. 2A). These residues are conserved in InsP3Rs but not in ryanodine receptors, consistent with evidence that CaBP1 binds to all three InsP3R subtypes (15, 16) but not to ryanodine receptors (16). Exposed CaBP1 residues in EF4 (I144, M164, and M165) made contacts with H289 in the IBC-β domain, and side-chain atoms of R167 (CaBP1) were within 5 Å of conserved residues (N47 and N48) in the SD. These interactions align with evidence that CaBP1 binding to InsP3R is mediated by the NT (15) and with both the IBC and SD being required for high-affinity binding (16, 18). The key hydrophobic residues in CaBP1 (V101, L104, and V162) are less exposed in the closed conformation of Ca2+-free CaBP1, consistent with the reduced affinity of CaBP1 for NT in the absence of Ca2+ (18). The importance of the hydrophobic residues within the IBC was confirmed by mutagenesis. A triple mutant of NTCL (L302S/I364S/L393S) bound InsP3, but not CaBP1 (Fig. S5 A–C), confirming that InsP3 and CaBP1 bind to distinct sites (18). Interaction of the NT with Ca2+–CaBP1 via localized clusters of hydrophobic residues probably explains why mutation of single residues in CaBP1 (V101S, L104S, and V162S) massively attenuates its binding to the NT (Fig. S3 B and C).
Fig. 2.
Structure of CaBP1 bound to InsP3R. (A) The C lobe of Ca2+-bound CaBP1 [cyan; Protein Data Bank (PDB) ID code 2K7D] bound to NT (PDB ID code 3UJ4) in a 1:1 complex. Key residues at the binding interface are highlighted in magenta (CaBP1) and red (InsP3R). NT subdomains are colored pink (SD), yellow (IBC-β), and gray (IBC-α). (B) Model of tetrameric NT (pink, yellow, and gray) generated by superimposing the NT crystal structure (29) onto a cryo-EM structure of InsP3R1 (34). NMR structural restraints were used to define contacts between each NT and C lobe of CaBP1 (cyan, with Ca2+ in orange). (C) Side view of tetrameric NT bound to full-length CaBP1 in a 4:4 complex. The N lobe of CaBP1 (blue) is connected to the C lobe (cyan) by a flexible linker (red) that allows the N lobe to adopt multiple orientations (indicated by the arrow).
CaBP1 Forms a Ring Around the Cytosolic Entrance of the InsP3R.
Native InsP3R is a tetramer with a central ion-conducting pore (34). Docking crystal structures of the NT (29) onto a cryo-EM structure of full-length tetrameric InsP3R1 (34) suggests the arrangement shown in Fig. 2B, which is similar to that proposed for ryanodine receptors (35). Overlaying the structure of the CaBP1/NT complex onto the tetrameric NT generates a structure in which four molecules of CaBP1 associate, via the clustered hydrophobic residues in their C lobes, with the three clustered hydrophobic residues in each of the four IBC-β domains. The latter contribute to the lining of the central cytosolic vestibule, and the four molecules of CaBP1 form a ring-like structure around it. The position of the N lobe of CaBP1 within the tetrameric complex could not be defined because NMR signals assigned to it were unaffected by the NT. Previous studies showed that CaBP1 and its isolated C lobe bind to the NT with very similar affinity (18), consistent with an absence of contacts between the NT and N lobe. The location of the N lobe within the complex was estimated by first generating an ensemble of full-length CaBP1 structures in which the two lobes are connected by a flexible linker and are free to adopt many different relative orientations during simulated annealing. This ensemble of full-length CaBP1 structures was then docked into the NT structure (Fig. S4E). The CaBP1 C-lobe interaction with the NT is well defined in the ensemble of docked structures (cyan in Fig. S4E with rmsd = 0.5 Å), whereas the relative location of the N lobe in the complex is highly variable (Fig. S4E, blue). Each structure from the ensemble was then overlaid and docked into the tetrameric NT structure. The lowest energy model (Fig. 2C) placed the N lobe above the C lobe and projecting into the cytosol away from any contact with the NT. This elongated organization of the two lobes of CaBP1 differs from their compact, hexameric arrangement in the CaBP1 crystal structure (19). Solution NMR has shown that, as with CaM (36) and troponin C (37), the CaBP1 N and C lobes fold independently and do not interact structurally (18). This finding is consistent with a lack of contact between the lobes of CaBP1 when complexed with InsP3R (Fig. 2C).
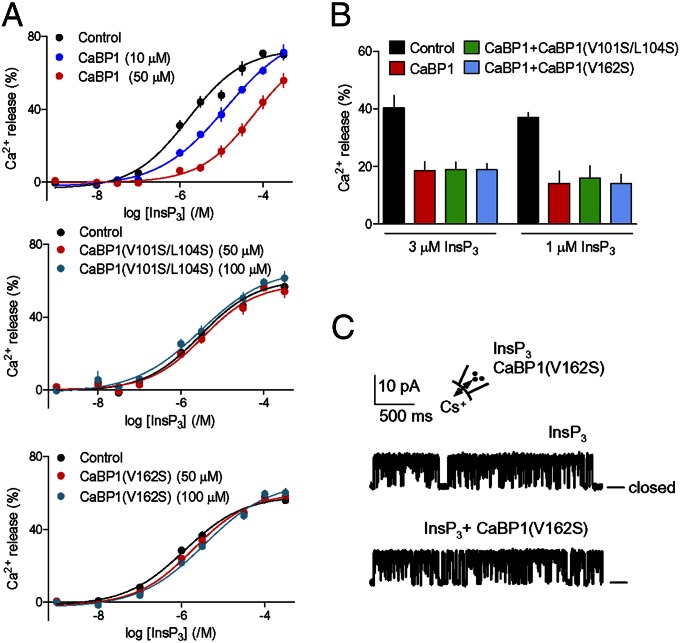
Functional analyses confirmed the importance of the critical residues within CaBP1. CaBP1 with mutations to the key hydrophobic residues [CaBP1(V101S/L104S) or CaBP1(V162S)] (Fig. 2A) had no effect on InsP3-evoked Ca2+ release at any [Ca2+]c examined, even when the CaBP1 concentrations were increased to 100 µM (Fig. 3A, Fig. S5D, and Table S3). Furthermore, the mutant CaBP1s did not affect inhibition of InsP3-evoked Ca2+ release by CaBP1 (Fig. 3B and Fig. S5E), confirming that they do not compete with CaBP1 for binding to InsP3Rs. Furthermore, in patch-clamp analyses of nuclear InsP3R, responses to InsP3 were unaffected by CaBP1(V162S) (Fig. 3C and Fig. S1F). These functional analyses support the proposed structure of CaBP1 bound to InsP3R (Fig. 2).
Fig. 3.
Hydrophobic residues in CaBP1 are essential for inhibition of InsP3R. (A) Inhibition of InsP3-evoked Ca2+ release by CaBP1 is abolished after mutation of its key hydrophobic residues. Permeabilized DT40–InsP3R1 cells in CLM with [Ca2+]c of 3.5 μM were incubated with the indicated concentrations of CaBP1, CaBP1(V162S), or CaBP1(V101S/L104S) (10 min) before adding InsP3. Results show the concentration-dependent release of Ca2+ by InsP3. (B) Ca2+ release evoked by 1 or 3 μM InsP3 alone, with CaBP1 (50 μM), or with CaBP1 (50 μM) and mutant CaBP1 (100 μM). Results (A and B) are means ± SEM; n = 4, with duplicate determinations in each. Similar results performed in CLM with 1.2 μM [Ca2+]c are shown in Fig. S5 D and E. Summary results are in Table S3. (C) Typical patch-clamp recordings from single InsP3R1 in medium with [Ca2+]c of 1.5 μM stimulated with InsP3 (10 μM) alone or in combination with CaBP1(V162S) (10 μM) shows that the mutant CaBP1 is inactive. Bars show the closed state. The holding potential was +40 mV. Summary results are shown in Fig. S1F. Fig. 2A shows the positions of mutated residues.
CaBP1 Stabilizes Interactions Between the NTs of Tetramic InsP3R.
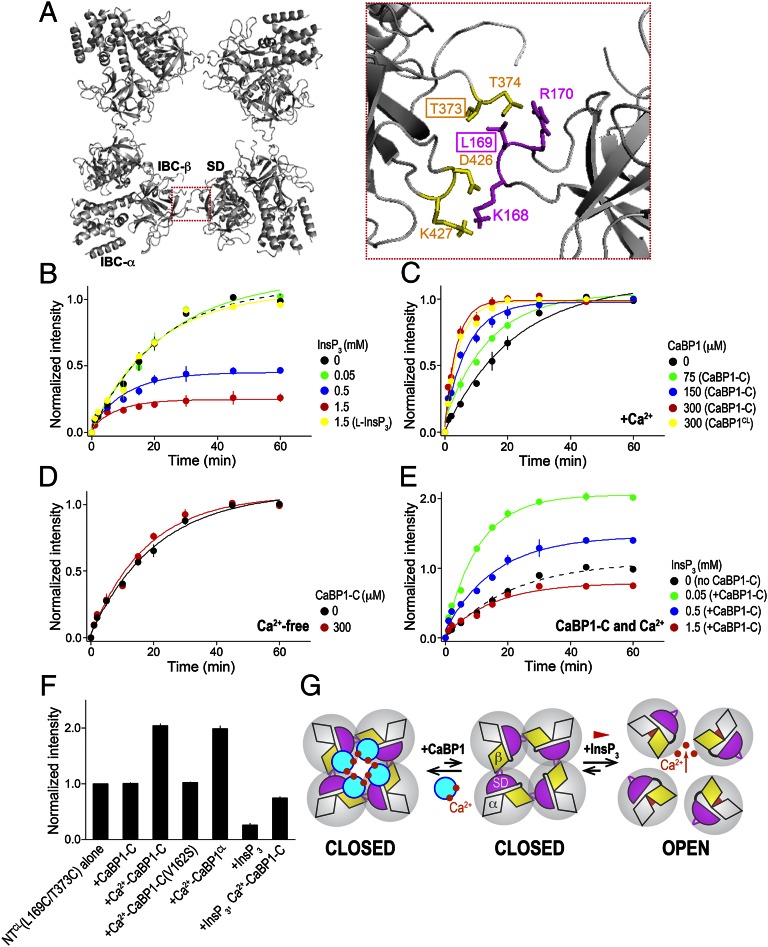
Activation of InsP3R is proposed to begin with InsP3-stimulated rearrangement of an intersubunit interface between the SD and IBC-β domain. This movement then disrupts interaction of the “hot-spot” (HS) loop (residues 165–180) of the SD with the IBC-β domain of an adjacent subunit leading to channel gating (29) (Fig. 4A). This model predicts key interactions at the intersubunit interfaces, notably between residues within the SD (K168, L169, and R170) and IBC (T373, T374, D426, K427). We tested this prediction by inserting pairs of Cys residues into NTCL and assessing their proximity by oxidative cross-linking with copper–phenanthroline (CuP). Three double Cys-substituted mutants (L169C/T373C, L169C/T374C, and K168C/K427C) were engineered as candidates for cross-linking in light of the modeled intersubunit interface (Fig. 4A). NTCL(L169C/T373C) provided the most convincing evidence of a concentration- and time-dependent formation of tetramers in the presence of CuP (Fig. S6 A and B). NTCL or NTCL with a single Cys insertion (L169C or T373C) or a pair of Cys that are not expected to be in proximity (A61C/A553C) did not produce tetramers (Fig. S6C). These results confirm the proximity of L169C/T373C to the tetrameric NT interface and demonstrate the utility of CuP cross-linking for analyses of intersubunit interactions between NTs. We used NTCL(L169C/T373C) for subsequent analyses and confirmed that its InsP3-binding affinity was similar to that of NTCL (Fig. S6D).
Fig. 4.
Opposing effects of CaBP1 and InsP3 on interactions between NTs. (A) Top view of the tetrameric structure of NTs, and close-up view of the boxed area of the intersubunit interface between the HS loop of the SD (magenta) and IBC-β (yellow) (29). The two residues that were replaced by Cys for CuP cross-linking analyses are boxed. (B) InsP3 weakens the interactions between NT subunits and thereby the rate of CuP-mediated cross-linking of tetrameric NT. NTCL(L169C/T373C) (75 μM) in medium containing 5 mM CaCl2 was incubated with CuP (100 μM) alone or with the indicated concentrations of InsP3 (the naturally occurring d-isomer) or biologically inactive l-InsP3. Results are expressed as fractions of the average intensity of the tetramer band detected at 60 min in the control incubation (no InsP3). The results with NTCL(L169C/T373C) alone (black) are shown for comparison. (C and D) Similar cross-linking experiments show that CaBP1 has the opposite effect to InsP3. Effects on tetramer formation of the indicated concentrations of the C lobe of CaBP1 (CaBP-C) and of the Cys-less form of full-length CaBP1 (CaBP1CL) in medium containing 5 mM CaCl2 (C) or in Ca2+-free medium (D). (E) The C-terminal of CaBP1 substantially blocks the destabilization of NT subunit interactions by InsP3. Effects of the indicated concentrations of InsP3 with CaBP1 C lobe (300 μM) in medium containing 5 mM CaCl2. Results are expressed as fractions of the average intensity of the tetramer band detected at 60 min in the control incubation (no InsP3 or CaBP1). The results with NTCL(L169C/T373C) alone (black) are shown for comparison. (F) Summary data show amounts of cross-linked NTCL(L169C/T373C) tetramer detected at 60 min relative to NTCL(L169C/T373C) alone at 60 min. Results in B–F show means ± standard deviation from three independent experiments. InsP3 denotes d-InsP3 unless indicated otherwise. The data from which these analyses derive are shown in Fig. S6, and the rate constants and normalized band intensities in Table S4. (G) Interactions between adjacent NTs mediated by IBC-β (yellow) and the HS loop of the SD (magenta) hold the tetrameric InsP3R in a closed state. InsP3 binding closes the clam-like IBC, disrupting these intersubunit interactions, and allowing the channel to open. The cytosolic vestibule of the InsP3R with four CaBP1s (cyan) bound is probably at least 5 Å across and unlikely to impede the flow of ions. Instead, we suggest that CaBP1 clamps the intersubunit interactions and thereby inhibits channel opening.
InsP3R activation proceeds via disruption of intersubunit interactions between NT domains (29). Our structure shows CaBP1 forming a tetrameric cap tethered to the NTs of the tetrameric InsP3R (Fig. 2B). This structure suggests that CaBP1 may lock InsP3Rs in a closed state by restricting the usual InsP3-evoked disruption of intersubunit interactions. We used CuP cross-linking and NTCL (L169C/T373C) to assess this possibility. As predicted by our model, InsP3 caused a concentration-dependent inhibition of CuP-mediated formation of cross-linked tetrameric NTCL(L169C/T373C) (Fig. 4B, Fig. S6E, and Table S4). The biologically inactive isomer of InsP3 (l-InsP3) had no effect on cross-linking (Fig. 4B). In contrast, the C lobe of CaBP1, which lacks endogenous Cys, caused a concentration-dependent increase in the rate and extent of formation of cross-linked tetrameric NTCL(L169C/T373C) (Fig. 4C, Fig. S6F, and Table S4). Similar results were obtained with a Cys-less form of full-length CaBP1 (CaBP1CL) (Fig. 4C, Fig. S6F, and Table S4). These results demonstrate that the C lobe of CaBP1 is largely responsible for the observed effects on NT cross-linking. The CaBP1 C lobe in the absence of Ca2+ had no effect on NT tetramer cross-linking (Fig. 4D, Fig. S6G, and Table S4), and neither did CaBP1(V162S), which did not bind the NT (Fig. S6H and Table S4). CaBP1 also partially blocked the inhibition of cross-linking by InsP3 (Fig. 4E, Fig. S6I, and Table S4). These results (Fig. 4F and Table S4) support our suggestion that InsP3 activates InsP3R by disrupting an intersubunit interface between the SD and IBC-β. We suggest that, in the presence of Ca2+, CaBP1 forms a tetrameric cap on the InsP3R that restricts these intersubunit movements and thereby stabilizes a closed state of the channel (Fig. 4G).
Discussion
Gating of InsP3Rs is regulated by InsP3 binding, but modulated by many additional signals, notably Ca2+ and a variety of proteins (5), including such Ca2+-regulated proteins as CaM (12), CIB1 (13), and CaBP1 (2). These proteins are either highly (CaM) or exclusively (CaBP1) expressed in neurons, where they have been proposed to attenuate basal InsP3R activity (12), provide the Ca2+ sensor for inhibitory feedback of InsP3Rs (5, 11), modulate InsP3R activity (5), or, for CaBP1 and CIB1, allow Ca2+ directly to gate InsP3Rs (5, 13). The latter suggested that, within neurons, InsP3Rs, like ryanodine receptors, might mediate regenerative Ca2+ signals without the need for coincident production of InsP3. Our results demonstrate that CaBP1 does not directly activate InsP3R1, the predominant InsP3R subtype in neurons (Fig. S1 A–D). CaBP1 does, however, massively reduce InsP3-activated InsP3R activity by stabilizing a closed state of the channel (Fig. 1 C and D), an effect that is enhanced by Ca2+ binding to both CaBP1 and the InsP3R (or a protein tightly associated with InsP3R). These dual effects of Ca2+ may allow cooperative inhibition of InsP3Rs by increases in [Ca2+]c. However, even at resting [Ca2+]c, there is detectable inhibition of InsP3Rs by CaBP1 (Fig. 1B and Table S3), suggesting that CaBP1 may also contribute to setting the basal sensitivity of neuronal InsP3Rs.
We identified hydrophobic residues within the C lobe of CaBP1 that become more exposed when CaBP1 binds Ca2+ (V101, L104, and V162) and showed by both NMR and functional analyses that they make essential contacts with hydrophobic residues in the IBC-β domain (L302, I364, and L393) (Fig. 2A). Additional minor contacts between the CaBP1 C lobe and residues within the SD contribute further to high-affinity binding of CaBP1 (18). The hydrophobic interactions between CaBP1 and the IBC are essential for CaBP1 binding and inhibition of InsP3R (Fig. 3 and Figs. S1F and S5). Docking the NT–CaBP1 complex into the structure of a full-length InsP3R reveals an arrangement in which tetrameric CaBP1 is anchored by its hydrophobic contacts to the underlying NT domains. CaBP1 thereby forms a ring-like structure around the cytosolic vestibule that leads to the InsP3R pore (Fig. 2B). This arrangement has the Ca2+-binding sites of CaBP1 lining the route through which Ca2+ passes via the InsP3R to the cytosol (Fig. 2 B and C).
The InsP3-induced conformational change that initiates InsP3R activation involves rearrangement of an interface between the SD and IBC-β domain. This intrasubunit rearrangement then disrupts an interaction between subunits mediated by the HS loop of the SD (29). This loop includes a residue (Y167 in InsP3R1) that is important for gating of InsP3Rs (38) and ryanodine receptors (39). Our cross-linking analyses support this scheme because residues that contribute to the intersubunit interface are less readily cross-linked in the presence of InsP3 (Fig. 4B and Fig. S6E). Ca2+–CaBP1 has the opposite effect: It increases cross-linking (Fig. 4) and inhibits InsP3R gating by stabilizing a closed state of the channel (Fig. 1). We suggest that CaBP1 counteracts the InsP3-induced conformational change by “clamping” the underlying InsP3R subunits and restricting their relative motion. We speculate that CaBP1 held loosely to neuronal InsP3R at resting [Ca2+]c tightens its grip as Ca2+ passing through an open InsP3R binds to CaBP1 to cause rapid feedback inhibition (Fig. 4G).
Materials and Methods
Expression and Purification of CaBP1, NT, and Their Mutants.
The short form of CaBP1 was used throughout. CaBP1 and its mutants were expressed and purified from Escherichia coli strain BL21(DE3) as described (40). NT (residues 1-604) and NTCL (in which native Cys are replaced by Ala) from rat InsP3R1 were expressed and purified as described (29, 41). Individual Cys residues were introduced into NTCL using the QuikChange site-directed mutagenesis kit. Sequences of all plasmids were confirmed. Table S1 lists the proteins used.
NMR Spectroscopy.
Samples were prepared by dissolving perdeuterated and uniformly 15N/13C-labeled CaBP1 (0.2 mM) containing protonated methyl groups (for Val, Leu, and Ile) (42) in 0.3 mL of 95% [2H]H2O containing 10 mM [2H11]Tris, pH 7.4, 0.1 mM KCl, and either 5 mM EDTA or 5 mM CaCl2 in the presence of 0.2 mM NTCL or NTCL with single Cys substitutions (E20C, A61C, R170C, H289C, N300C, A394C, and K424C). Methyl TROSY experiments on 13C-labeled CaBP1 bound to unlabeled NTCL were performed as described (43). NMR-PRE experiments were performed on samples that contained isotopically labeled CaBP1 bound to NTCL with a single Cys insertion with or without an attached nitroxide spin label. Spin labeling was performed as described (31). All NMR experiments were performed at 30 °C on a Bruker Avance 800 MHz spectrometer equipped with triple-resonance cryoprobe and z axis gradient. NMR assignments were described (18).
Molecular Docking.
Atomic coordinates for the NT (PDB ID code 3UG4) and CaBP1 C lobe (PDB ID code 2K7D) were used to generate the docked structure in Fig. 2A. For docking of full-length CaBP1 to NT (Fig. 2C), an ensemble of structures of full-length CaBP1 (with a flexible linker between the two lobes) was generated by a simulated annealing protocol within CYANA using distance restraints derived for the CaBP1 C lobe and N lobe (PDB ID code 2K7B). All docking calculations were performed by using the HADDOCK Guru interface (http://haddock.science.uu.nl/services/HADDOCK/haddock.php) (44). Mutagenesis data and chemical-shift perturbation data were used as inputs to define active and passive residues to generate ambiguous restraints (44). The PRE ratios (Fig. S4C) were converted into unambiguous restraints and docking calculations were performed as described (45). The final docked structure in Fig. 2A is the average of 148 calculated structures that converged within a single cluster (red dots in Fig. S4D).
InsP3-Evoked Ca2+ Release and 3H-InsP3 Binding.
DT40 cells stably expressing only rat InsP3R1 (DT40–InsP3R1 cells) were loaded with a low-affinity luminal Ca2+ indicator, permeabilized in cytosol-like medium (CLM), and the Ca2+ content of the endoplasmic reticulum was continuously monitored during additions of ATP (to allow Ca2+ uptake), CaBP1, and InsP3 as described (46). Ca2+ release evoked by InsP3 is expressed as a percentage of the ATP-dependent Ca2+ uptake. 3H-InsP3 binding to rat cerebellar membranes or purified NT was performed in CLM at 4 °C as described (28). Results were fitted to Hill equations.
Patch-Clamp Recording.
Currents were recorded from patches excised from the outer nuclear envelope of DT40–InsP3R1 cells by using symmetrical cesium methanesulfonate (140 mM) as charge carrier. The composition of recording solutions and methods of analysis were otherwise as described (47).
Cross-Linking of Cys Residues.
For CuP cross-linking, a mixture of 50 mM CuSO4 and 65 mM 1,10-phenanthroline (Sigma) was freshly prepared. Concentrations in the text refer to final CuP concentration (≥50 μM). NTCL or its mutants (75 μM) were incubated on ice with CuP in medium containing 360 mM NaCl, 20 mM Tris⋅HCl, pH 8.4, 2.5% (vol/vol) glycerol, and 0.2 mM Tris(2-carboxyethyl)phosphine (TCEP). Ca2+-free buffer was prepared by using Chelex 100 resin (Bio-Rad Laboratories). NTCL(L169C/T373C) and CaBP1 C lobe were used after dialysis in Ca2+-free buffer. Reactions were quenched by addition of 10 mM N-ethylmaleimide and 10 mM EDTA (final concentrations). Samples were mixed with 4× nonreducing SDS loading buffer, heated at 55 °C for 15 min, and subjected to SDS/PAGE by using NuPAGE 3–8% Tris⋅acetate gels (Invitrogen). After Coomassie Brilliant Blue staining, band intensities of tetrameric NT were quantified by densitometry using ImageJ. For analyses of time courses, the intensity of the tetramer band at each time for the experimental condition was expressed relative to the intensity of the tetramer band at 60 min for control condition (no InsP3 or CaBP1). These normalized intensities were fitted with a single exponential time course by using IGOR Pro-6 (WaveMetrics).
Supplementary Material
Acknowledgments
We thank Dr. Jerry Dallas for help with NMR experiments. This work was supported by National Institutes of Health Grants EY012347 and NS045909 (to J.B.A.) and RR11973 (to the University of California at Davis NMR Facility); Canadian Institutes of Health Research (CIHR) and the Canada Foundation for Innovation grants (to M.I.); Wellcome Trust Grant 085295; Biotechnology and Biological Sciences Research Council Grant BB/H009736/1 (to C.W.T.). A.M.R. is a Fellow of Queens’ College. T.R. was a Fellow of Pembroke College.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220847110/-/DCSupplemental.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Haynes LP, McCue HV, Burgoyne RD. Evolution and functional diversity of the Calcium Binding Proteins (CaBPs) Front Mol Neurosci. 2012;5:e9. doi: 10.3389/fnmol.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbara JG. IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: Functional implications for synaptic plasticity. Biochim Biophys Acta. 2002;1600(1-2):12–18. doi: 10.1016/s1570-9639(02)00439-9. [DOI] [PubMed] [Google Scholar]
- 4.Taylor CW, Dale P. Intracellular Ca2+ channels—a growing community. Mol Cell Endocrinol. 2012;353(1-2):21–28. doi: 10.1016/j.mce.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor CW, Tovey SC. IP3 receptors: Toward understanding their activation. Cold Spring Harb Perspect Biol. 2010;2(12):a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konieczny V, Keebler MV, Taylor CW. Spatial organization of intracellular Ca2+ signals. Semin Cell Dev Biol. 2012;23(2):172–180. doi: 10.1016/j.semcdb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Miyakawa T, et al. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J. 2001;20(7):1674–1680. doi: 10.1093/emboj/20.7.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor CW, Laude AJ. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+ Cell Calcium. 2002;32(5-6):321–334. doi: 10.1016/s0143416002001859. [DOI] [PubMed] [Google Scholar]
- 10.Adkins CE, et al. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1, -2 and -3 inositol trisphosphate receptors. Biochem J. 2000;345(Pt 2):357–363. [PMC free article] [PubMed] [Google Scholar]
- 11.Michikawa T, et al. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23(4):799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Morris SA, Adkins CE, O’Beirne G, Taylor CW. Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulin: Redistribution of calmodulin as a possible means of regulating Ca2+ mobilization. Proc Natl Acad Sci USA. 1997;94(21):11627–11632. doi: 10.1073/pnas.94.21.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White C, Yang J, Monteiro MJ, Foskett JK. CIB1, a ubiquitously expressed Ca2+-binding protein ligand of the InsP3 receptor Ca2+ release channel. J Biol Chem. 2006;281(30):20825–20833. doi: 10.1074/jbc.M602175200. [DOI] [PubMed] [Google Scholar]
- 14.Schlecker C, et al. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest. 2006;116(6):1668–1674. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, et al. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci USA. 2002;99(11):7711–7716. doi: 10.1073/pnas.102006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasri NN, et al. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004;23(2):312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeseleer F, et al. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem. 2000;275(2):1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, et al. Structural insights into Ca2+-dependent regulation of inositol 1,4,5-trisphosphate receptors by CaBP1. J Biol Chem. 2009;284(4):2472–2481. doi: 10.1074/jbc.M806513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Findeisen F, Minor DL., Jr Structural basis for the differential effects of CaBP1 and calmodulin on CaV1.2 calcium-dependent inactivation. Structure. 2010;18(12):1617–1631. doi: 10.1016/j.str.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes LP, Tepikin AV, Burgoyne RD. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J Biol Chem. 2004;279(1):547–555. doi: 10.1074/jbc.M309617200. [DOI] [PubMed] [Google Scholar]
- 21.Menger N, Seidenbecher CI, Gundelfinger ED, Kreutz MR. The cytoskeleton-associated neuronal calcium-binding protein caldendrin is expressed in a subset of amacrine, bipolar and ganglion cells of the rat retina. Cell Tissue Res. 1999;298(1):21–32. doi: 10.1007/s004419900060. [DOI] [PubMed] [Google Scholar]
- 22.Seidenbecher CI, Reissner C, Kreutz MR. Caldendrins in the inner retina. Adv Exp Med Biol. 2002;514:451–463. doi: 10.1007/978-1-4615-0121-3_27. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, et al. Differential modulation of Cav2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci. 2002;5(3):210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou H, Yu K, McCoy KL, Lee A. Molecular mechanism for divergent regulation of Cav1.2 Ca2+ channels by calmodulin and Ca2+-binding protein-1. J Biol Chem. 2005;280(33):29612–29619. doi: 10.1074/jbc.M504167200. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita-Kawada M, et al. Inhibition of TRPC5 channels by Ca2+-binding protein 1 in Xenopus oocytes. Pflugers Arch. 2005;450(5):345–354. doi: 10.1007/s00424-005-1419-1. [DOI] [PubMed] [Google Scholar]
- 26.Bosanac I, et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420(6916):696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- 27.Bosanac I, et al. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell. 2005;17(2):193–203. doi: 10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 28.Rossi AM, et al. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol. 2009;5(9):631–639. doi: 10.1038/nchembio.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo MD, et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483(7387):108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman T, Taylor CW. Nuclear patch-clamp recording from inositol 1,4,5-trisphosphate receptors. Methods Cell Biol. 2010;99:199–224. doi: 10.1016/B978-0-12-374841-6.00008-6. [DOI] [PubMed] [Google Scholar]
- 31.Clore GM, Tang C, Iwahara J. Elucidating transient macromolecular interactions using paramagnetic relaxation enhancement. Curr Opin Struct Biol. 2007;17(5):603–616. doi: 10.1016/j.sbi.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominguez C, Boelens R, Bonvin AM. HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125(7):1731–1737. doi: 10.1021/ja026939x. [DOI] [PubMed] [Google Scholar]
- 33.Ikura M. Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci. 1996;21(1):14–17. [PubMed] [Google Scholar]
- 34.Ludtke SJ, et al. Flexible architecture of IP3R1 by cryo-EM. Structure. 2011;19(8):1192–1199. doi: 10.1016/j.str.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468(7323):585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Tanaka T, Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat Struct Biol. 1995;2(9):758–767. doi: 10.1038/nsb0995-758. [DOI] [PubMed] [Google Scholar]
- 37.Gagné SM, Tsuda S, Li MX, Smillie LB, Sykes BD. Structures of the troponin C regulatory domains in the apo and calcium-saturated states. Nat Struct Biol. 1995;2(9):784–789. doi: 10.1038/nsb0995-784. [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki H, Chan J, Ikura M, Michikawa T, Mikoshiba K. Tyr-167/Trp-168 in type 1/3 inositol 1,4,5-trisphosphate receptor mediates functional coupling between ligand binding and channel opening. J Biol Chem. 2010;285(46):36081–36091. doi: 10.1074/jbc.M110.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amador FJ, et al. Crystal structure of type I ryanodine receptor amino-terminal beta-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc Natl Acad Sci USA. 2009;106(27):11040–11044. doi: 10.1073/pnas.0905186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingard JN, et al. Structural analysis of Mg2+ and Ca2+ binding to CaBP1, a neuron-specific regulator of calcium channels. J Biol Chem. 2005;280(45):37461–37470. doi: 10.1074/jbc.M508541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan J, et al. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J Mol Biol. 2007;373(5):1269–1280. doi: 10.1016/j.jmb.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 42.Tugarinov V, Kay LE. An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR. 2004;28(2):165–172. doi: 10.1023/B:JNMR.0000013824.93994.1f. [DOI] [PubMed] [Google Scholar]
- 43.Tugarinov V, Sprangers R, Kay LE. Line narrowing in methyl-TROSY using zero-quantum 1H-13C NMR spectroscopy. J Am Chem Soc. 2004;126(15):4921–4925. doi: 10.1021/ja039732s. [DOI] [PubMed] [Google Scholar]
- 44.de Vries SJ, van Dijk M, Bonvin AM. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc. 2010;5(5):883–897. doi: 10.1038/nprot.2010.32. [DOI] [PubMed] [Google Scholar]
- 45.Battiste JL, Wagner G. Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry. 2000;39(18):5355–5365. doi: 10.1021/bi000060h. [DOI] [PubMed] [Google Scholar]
- 46.Tovey SC, Sun Y, Taylor CW. Rapid functional assays of intracellular Ca2+ channels. Nat Protoc. 2006;1(1):259–263. doi: 10.1038/nprot.2006.40. [DOI] [PubMed] [Google Scholar]
- 47.Taufiq-Ur-Rahman, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458(7238):655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.