Abstract
Tumor heterogeneity of high-grade glioma (HGG) is recognized by four clinically relevant subtypes based on core gene signatures. However, molecular signaling in glioma stem cells (GSCs) in individual HGG subtypes is poorly characterized. Here we identified and characterized two mutually exclusive GSC subtypes with distinct dysregulated signaling pathways. Analysis of mRNA profiles distinguished proneural (PN) from mesenchymal (Mes) GSCs and revealed a pronounced correlation with the corresponding PN or Mes HGGs. Mes GSCs displayed more aggressive phenotypes in vitro and as intracranial xenografts in mice. Further, Mes GSCs were markedly resistant to radiation compared with PN GSCs. The glycolytic pathway, comprising aldehyde dehydrogenase (ALDH) family genes and in particular ALDH1A3, were enriched in Mes GSCs. Glycolytic activity and ALDH activity were significantly elevated in Mes GSCs but not in PN GSCs. Expression of ALDH1A3 was also increased in clinical HGG compared with low-grade glioma or normal brain tissue. Moreover, inhibition of ALDH1A3 attenuated the growth of Mes but not PN GSCs. Last, radiation treatment of PN GSCs up-regulated Mes-associated markers and down-regulated PN-associated markers, whereas inhibition of ALDH1A3 attenuated an irradiation-induced gain of Mes identity in PN GSCs. Taken together, our data suggest that two subtypes of GSCs, harboring distinct metabolic signaling pathways, represent intertumoral glioma heterogeneity and highlight previously unidentified roles of ALDH1A3-associated signaling that promotes aberrant proliferation of Mes HGGs and GSCs. Inhibition of ALDH1A3-mediated pathways therefore might provide a promising therapeutic approach for a subset of HGGs with the Mes signature.
Keywords: cancer stem cell, epithelial-to-mesenchymal transition, glioblastoma multiforme, glioblastoma, proneural-to-mesenchymal transition
A hallmark of malignant high-grade gliomas (HGGs), including anaplastic glioma and glioblastoma multiforme (GBM), is their intrinsic resistance to current therapies that leads to extremely poor clinical outcomes (1). Even patients with well-demarcated tumors in noneloquent areas that allow maximal gross total removal at surgery and respond well to initial combined therapies inevitably develop subsequent tumor recurrence with minimal survival (2). Therefore, there is an urgent need to better understand the underlying mechanisms of such malignancy, thereby providing an opportunity to develop novel therapies and approaches to treat patients with aggressive HGGs.
It is established that multiple genetic and metabolic pathways create intricate networks to facilitate cross-talk between oncogenic and oncometabolic pathways that contribute to tumor progression and therapy resistance of human cancers, including HGGs (3). Recent genomewide transcriptome analyses suggest that HGGs can be divided into four clinically relevant subtypes: proneural (PN), neural, classic, and mesenchymal (Mes) HGGs (4, 5). Distinct signals are activated in these individual HGG subtypes that may account for the observed differential responses to therapy. Therefore, therapeutic strategies for HGGs should be designed based on tumor subtype instead of applying them to all patients with HGGs (6).
HGG tumors are composed of heterogeneous tumor cell populations that include tumor cells with stem cell properties termed glioma initiating/propagating cells or glioma stem cells (GSCs) (7). The unique properties of GSCs are considered to contribute to the therapeutic resistance of HGG (8, 9). Thus, understanding and targeting tumor-propagating GSCs could be beneficial in developing effective strategies that overcome therapeutic resistance of HGG. Given the distinct gene sets and signaling pathways that are differentially expressed in each subtype of HGG (4, 5), GSCs in each subtype may also harbor distinct and dysregulated pathways that render their unique phenotypes in tumor growth, progression, and resistance to therapy.
In this study, we tested a hypothesis that HGG subtypes also contain distinct GSC subtypes that could be differentiated by transcriptome array analyses, and we then determined individual expression and phenotypic signatures in two mutually exclusive GSC subtypes.
Results
GSC Cultures Derived from HGG Surgical Specimens Displayed Two Distinct Stem Cell–Related Phenotypes In Vitro.
We collected 40 patient specimens of HGGs from surgeries for patients with HGGs and established 19 HGG-derived tumor cultures in defined serum-free medium that enriches tumorigenic self-renewing and multipotent GSCs as previously described (Table S1) (10). Under these culture conditions, we observed two phenotypically different GSC cultures with distinct morphologies. One set of GSC cultures (n = 10) displayed round neurosphere-like floating aggregates (cluster 1), and the other set (n = 9) formed irregular-shaped floating aggregates with some adherent cells on the bottom of the culture dish (cluster 2; Fig. S1A). Immunocytochemistry with several stem cell–associated markers demonstrated that GSCs in cluster 1 were highly positive for Sox2 (markers for PN HGGs) and capable of differentiating into GFAP-positive glial cells and TuJ1-positive neuronal cells, whereas GSCs in cluster 2 were positive for CD44 but negative for Sox2 (Fig. S1B). Additionally, both types of GSCs retain their multipotency properties (Fig. S1 C and D). However, no single factor that correlates with a statistically significant difference distinguishes the clinical characteristics of patients with the HGGs from which these two groups of GSCs were derived.
Distinct Expression Profiles of mRNA Distinguish Mes from PN GSC Subtypes.
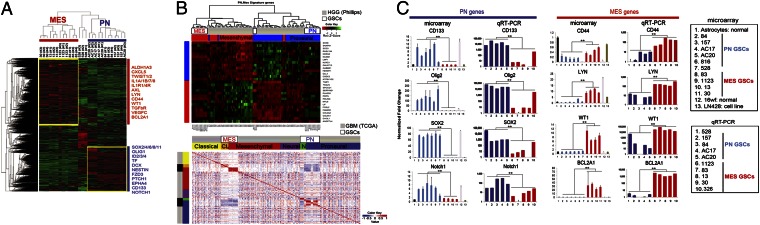
Recently, two reports proposed the existence of two GSC subtypes as determined by microarray based gene expression profiles (11, 12). To investigate whether our GSC samples display two distinct expression profiles in a similar manner, we performed transcriptome microarray analysis of 27 GSC samples (triplicate samples from nine patient-derived GSC cultures), as well as cells comprising human astrocytes, fetal neural progenitors, and five glioma cell lines. For expression signature analysis, we performed a differential expression analysis of GSCs (PN vs. Mes) and found 5,796 genes to be differentially expressed (false discovery rate < 0.05; Fig. S2A). As shown in the heat map of differentially expressed genes in Fig. 1A, one group showed high expression of what are considered PN-associated genes and the other with Mes-associated genes. Our samples cluster accordingly with the PN and Mes HGG subtypes from the Philips dataset, as shown in the hierarchical cluster analysis (Fig. 1B, Upper) (4). Furthermore, 3,376 genes (FDR < 0.05) were found differentially expressed between the PN and Mes samples in the TCGA dataset (Fig. 1B, Lower). A total of 1,986 differentially expressed genes were present in both analyses (GSCs and TCGA datasets). Interestingly, fewer genes were found statistically significantly and differentially expressed in the TCGA dataset than in our dataset, a fact that might be explained by the higher heterogeneity of TCGA tumor samples (Fig. S2B). To validate these microarray results, we performed quantitative real-time–PCR (qRT-PCR) analysis of some of the most highly differentially expressed genes (lowest P value). The data shown in Fig. 1C highlight the consistent results of expressions between the microarray and qRT-PCR analysis with the PN-associated genes (CD133, Olig2, Sox2, and Notch1) and the Mes-associated genes (CD44, Lyn, WT1, and BCL2A1) in PN and Mes GSCs (4, 5), respectively. The expression of these representative PN and Mes genes displayed similar, if not identical, expression patterns in the matched original tumors and their derived tumor spheres (Fig. S3A). We also found that within the PN or Mes groups, the GSC samples were better correlated with each other than the TCGA samples, likely due to the higher heterogeneity among the TCGA tumors (Fig. 1B). As for isocitrate dehydrogenase 1 (IDH1) mutation status, all four PN and two Mes GSCs we sequenced were IDH1 WT (Fig. S3B).
Fig. 1.
Microarray analysis of two distinctive GSC samples. (A) Hierarchical biclustering of genes differentially expressed between PN and Mes cell lines. (B) Heat map with pairwise Pearson correlation for the Phillips HGG dataset and TCGA GBM dataset with the microarray samples. Stronger correlation is observed among microarray samples of the same type compared among and with TCGA samples of the same subtype. (C) qRT-PCR and microarray of two GSC subtypes (**P < 0.01). Data are representative of three independent experiments with similar results.
Mes GSCs Show More Aggressive Phenotypes than PN GSCs In Vitro and In Vivo.
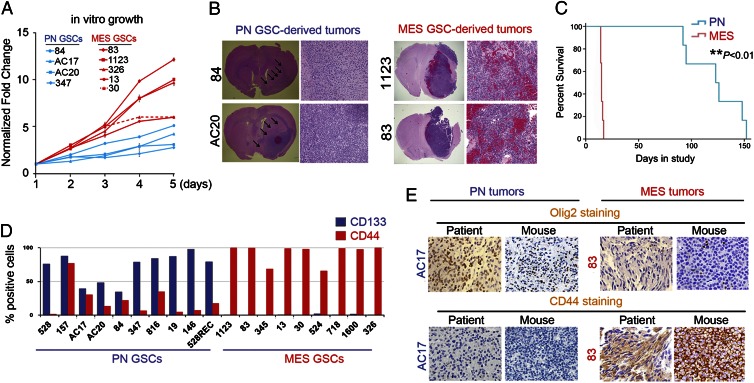
Next, we investigated whether PN and Mes GSCs display any differences in cellular and tumorigenic behaviors. As shown in Fig. 2A, under identical growth conditions, Mes GSCs showed higher potential for in vitro cell growth than PN GSCs (Fig. 2A; Fig. S4). We then implanted individual PN GSCs (84, AC17, and AC20) or Mes GSCs (83, 326, and 1123) into the brains of immunocompromised mice. As shown in Fig. 2 B and C, mice that received Mes GSCs succumbed to intracranial GBM-like tumors within 18 d, with a median survival of 15 d after implantation. In contrast, mice that received PN GSCs developed brain tumors at a much slower rate, with a median survival of 123 d. Mes GSC–derived brain tumors contained multiple large hemorrhagic lesions and abundant tumor vessels, as well as tumor-associated necrosis, whereas PN GSC–derived tumors showed minimal or no increase of angiogenesis or necrosis compared with the adjacent normal brain (Fig. 2B, arrows).
Fig. 2.
Phenotypic differences between PN and Mes GSCs. (A) In vitro growth curves of PN GSC samples (n = 4) and Mes GSC samples (n = 5). (B) Representative H&E staining of various mouse brain sections with tumors established by two PN and two Mes GSCs. (C) Kaplan-Meier survival curves of mice bearing PN GSC– and Mes GSC–derived tumors (**P < 0.01). (D) FACS analyses of cell surface expression of CD133 and CD44 in cultured PN (n = 10) and Mes (n = 9) GSCs. (E) Representative images of IHC with the original patient tumors and PN or Mes GSCs–derived mouse intracranial tumors. PN GSCs are Olig2high; CD44-/low, whereas Mes GSCs are Olig2low; CD44high. Data in A–E are representative of three independent experiments with similar results.
We then assessed the expression of CD133 and CD44, two cell surface proteins that often associate with cancer stem cells, in these GSCs by FACS analysis (13, 14). As shown in Fig. 2D, most PN GSCs were positive for CD133, whereas Mes GSCs presented with only minimal to undetectable CD133-expressing cells. Conversely, the majority of Mes GSCs were positive for CD44, whereas PN GSCs had minimal to modest levels of CD44 expression. We next tested whether these data recapitulate the expression of the original clinical HGG tumors from which these GSCs were derived and whether these expression patterns are maintained in the intracranial xenografts. As a PN-associated marker for immunohistochemistry (IHC), we used Olig2 because IHC staining for CD133 did not reliably correlate with our FACS results of dissociated tumor cells. Most of the original HGG samples that gave rise to PN GSCs expressed the PN marker Olig2 at high levels but were negative or only faintly stained for the Mes marker CD44 (Fig. 2E; Fig. S5). In contrast, those HGG specimens that gave rise to Mes GSCs exhibited high levels of expression of CD44 but minimal levels of Olig2. Interestingly, most of the GSC-derived intracranial xenograft tumors maintained similar patterns of immunoreactivity to Olig2 and CD44 (six of eight samples). Taken together, these results suggested two distinct GSC subtypes established from clinical HGG samples retained, at least to a major extent, the expression signatures of the original HGG tumors and recapitulated the major phenotypes of the original clinical HGGs in their xenograft brain tumors.
Aldehyde Dehydrogenase 1A3 Activity Is Markedly Elevated in Mes GSCs Compared with Mes Non-GSCs and PN GSCs.
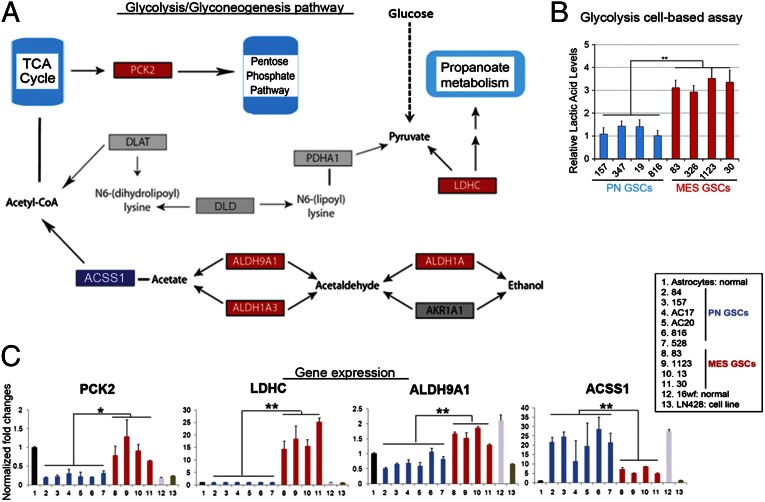
Based on the transcriptome array analyses, we found that a total of 5,796 genes are differentially expressed between our PN and Mes GSCs (Fig. S2A). Pathway enrichment analysis [Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways] for all differentially expressed genes identified the pathway of glycolysis and gluconeogenesis containing aldehyde dehydrogenase (ALDH) genes to be the most significantly enriched in Mes GSCs (P = 0.000315; Fig. 3A). Furthermore, glycolytic activity was significantly elevated in Mes GSCs (n = 4) compared with PN GSCs (n = 4; Fig. 3B). In particular, four key enzymes in this pathway, PCK2, LDHC, ALDH1A3, and ALDH9A1, were significantly up-regulated in Mes GSCs, whereas ACSS1 is expressed at higher levels in PN GSCs (Fig. 3C).
Fig. 3.
Glycolysis pathway as the most differentially activated pathway in Mes GSCs. (A) Glycolytic pathway containing aldehyde dehydrogenase genes (KEGG ID: hsa00010) was significantly enriched in Mes GSCs (P = 0.0001315). Genes in red were differentially expressed between Mes and PN tumors. (B) Elevated glycolytic activity in Mes GSCs (n = 4) compared with PN GSCs (n = 4; **P < 0.01). (C) Difference of expression of individual genes in the glycolytic pathway in PN and Mes GSC samples, normal astrocytes, and neural progenitors (16wf), and the glioma cell line LN486 (LN) (*P < 0.05, **P < 0.01).
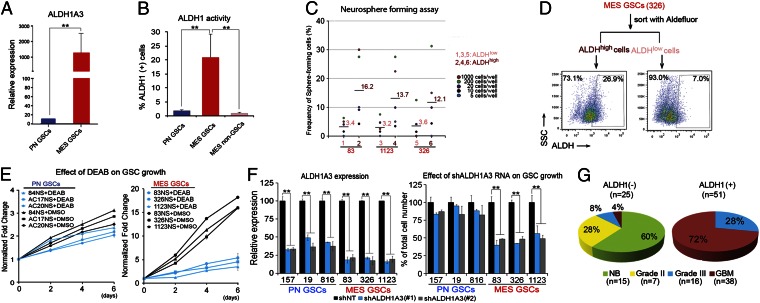
Of note in the transcriptome analyses, ALDH1A3, an isoenzyme of ALDH1 in the glycolysis and gluconeogenesis pathway, showed an ∼150-fold increase in mRNA levels in Mes GSCs compared with that in PN GSCs (Fig. 4A). Thus, we examined the expression of all 19 members of the ALDH family in these GSCs and found that only ALDH1A3 was up-regulated in Mes GSCs compared with PN GSCs (Fig. S6A). Next, we investigated ALDH1 activity of these GSCs using FACS analysis after staining with Aldefluor (Fig. 4B). Aldefluor is a nontoxic substrate that diffuses into living cells and then is broken down to the fluorescent molecule Bodipy-aminoacetate by ALDH1. Thus, the amount of intracellular accumulated fluorescent reaction product is directly proportional to intracellular ALDH1 activity (15). As shown in Fig. 4B and Fig. S6B, ALDH1 activity-high (ALDH1-high) cells are only found in Mes GSCs (n = 3) but not in PN GSCs (n = 3) or non-GSCs derived from Mes GSCs (n = 3). We then assessed whether ALDH1 activity in Mes GSCs correlates with the stemness of these GSCs (e.g., in vitro clonality and bipotent capacity) by a clonal sphere formation assay. After separation of Mes GSCs into ALDH1-high cells and ALDH1-low cells by FACS, ALDH1 activity positively correlated with the in vitro clonogenic potential of ALDH1-high GSCs (Fig. 4C). Then we performed bipotency tests of ALDH1-high and ALDH1-low cells and determined whether FACS-sorted Mes ALDH1-high and ALDH1-low cells give rise to both ALDH1-high and ALDH1-low cells 7 d following FACS separation (Fig. 4D; Fig. S6C). Although the majority of ALDH1-low cells remained as ALDH1-low cells (92.1–95.5%), ∼16.2–28.3% of the sorted ALDH1-high cells retained the ALDH1-high phenotype, similar to the proportions within the unsorted Mes GSCs. These results suggest that ALDH1 activity corresponds to bipotency of Mes GSCs in vitro.
Fig. 4.
ALDH1A3 is a functional Mes GSC marker. (A) qRT-PCR analysis of ALDH1A3 expression in PN and Mes GSCs (**P < 0.01). (B) FACS analysis using Aldeflour. ALDH activities in PN GSCs (n = 3), Mes GSCs (n = 3), and non-GSCs (n = 3) derived from Mes GSCs (**P < 0.01). (C) Frequency of sphere-forming cells between ALDH1high and ALDH1low Mes GSCs. FACS-sorted based on ALDH expression Mes GSCs were used in the assays (**P < 0.01). (D) FACS reanalysis: ALDH activity after 1-wk postcell sorting of Mes 326 ALDHhigh cells. ALDHhigh Mes GSC spheres generated both ALDHhigh and ALDHlow cells, whereas the majority of ALDHlow sphere cells retain as ALDHlow cells. (E) Effect of an ALDH inhibitor DEAB on cell growth of PN (n = 3) and Mes (n = 3) GSCs. DEAB abrogates the in vitro growth of Mes GSCs but has a marginal effect on PN GSCs. (F) Effect of shALDH1A3 knockdown on growth and ALDH1A3 gene expression of both PN and Mes GSCs. The growth of Mes GSCs is significantly reduced by shRNA-mediated depletion of ALDH1A3 compared with PN GSCs. RNA interference with 2 shALDH1A3 constructs significantly reduced ALDH1A3 expression levels in PN and Mes GSCs (n = 3 each, **P < 0.01). (G) Pie chart indicating the number of samples that were analyzed in different WHO tumor grades of clinical glioma samples or normal brain tissues that are ALDH(+) or (−). Data in A–F are representative of three independent experiments with similar results.
Next, we tested whether the ALDH1 activity is required for growth of Mes GSCs in vitro. When various GSCs were incubated with the ALDH1 inhibitor diethylaminobenzaldehyde (DEAB) (15), Mes GSCs, but not PN GSCs, showed marked decreases in in vitro growth (Fig. 4E; Fig. S7A). We then used two shRNA constructs to deplete ALDH1A3 in three PN GSCs and three Mes GSCs. As shown in Fig. 4F and Fig. S7B, knockdown of ALDH1A3 by shRNA constructs markedly inhibited the growth of Mes GSCs yet had minimal impact on the growth of PN GSCs. Decreased expression of ALDH1A3 by ALDH1A3 knockdown was confirmed in both PN and Mes GSCs (Fig. 4F; Fig. S7C). Collectively, ALDH1A3 appears to be required for the in vitro growth of Mes GSCs but not PN GSCs. To further demonstrate clinical relevance of ALDH1A3 expression, we performed IHC staining for ALDH1A3 expression in a collection of 76 clinical glioma samples containing WHO grade II–IV tumors, as well as adjacent normal brain tissues (Fig. 4G; Fig. S7D). In sharp contrast to the observed negative expression of ALDH1A3 in 15 normal brain tissues and 7 low-grade glioma tissues, expression of ALDH1A3 in HGG tissue specimens was markedly increased (n = 51; Fig. 4G). Taken together, these results demonstrate that ALDH1A3 is highly expressed in clinical HGG tumor specimens and suggest that ALDH1A3 could be a unique functional biomarker for Mes GSCs.
Mes GSCs Are More Resistant to Radiation Treatment than PN GSCs In Vitro.
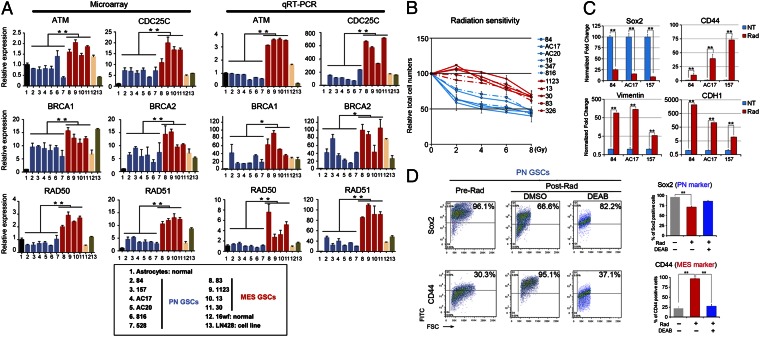
A recent study demonstrated that the mesenchymal phenotype is a hallmark of tumor aggressiveness in brain tumors (16). Thus far, our in vitro and in vivo data suggest that Mes GSCs display a more aggressive tumor phenotype than PN GSCs (Fig. 2). Therefore, we postulated that Mes GSCs could be more resistant than PN GSCs to radiation treatment, the current first-line therapy for HGGs. Thus, we first examined the expression profiles of several genes involved in the repair of DNA double-strand breaks, comparing the PN and Mes GSCs by transcriptome microarray and qRT-PCR. Expression of most, if not all, of these DNA damage signaling and DNA repair genes was significantly higher in Mes GSCs than PN GSCs (Fig. 5A). Furthermore, a striking difference in radiosensitivity was observed between PN and Mes GSCs (n = 11; Fig. 5B; Fig. S8A). As expected, Mes GSCs were significantly resistant to radiation treatment compared with PN GSCs.
Fig. 5.
Prominent radioresistance of Mes GSCs and radiation induces transformation of PN GSCs into Mes GSCs. (A) DNA microarray analyses and qRT-PCR validation of DNA damage-repair gene expression in GSCs. Various DNA damage-repair genes are expressed at higher levels in Mes GSCs than that in PN GSCs (*P < 0.05, **P < 0.01). Box: various GSC cells depicted in bar graphs. (B) Effect of radiation treatment on in vitro growth of PN and Mes GSCs (11 samples in total) at indicate doses. (C) Representative PN and Mes gene expressions in PN GSC samples (n = 3) with and without radiation treatment (5 Gy, tested at day 5; **P < 0.01). (D) FACS analyses for Sox2 (PN marker) and CD44 (Mes marker) in PN GSCs (84, AC17, and AC20) pre- and postradiation (5 Gy) at day 5. DEAB (100 μM), a selective inhibitor of ALDH1, partially blocked the changes of expression of the markers (**P < 0.01). Bar graphs: the average of levels of marker expression among 3 PN GSC neurospheres. Data in A–D are representative of three independent experiments with similar results.
Radiation Treatment Induces Mes-Associated Marker Expression in PN GSCs.
The transition of epithelial tumors to a Mes phenotype plays a critical role in advancing aggressiveness, tumor cell motility, and metastasis in various types of human cancers (17). Given that Mes GSCs appear to be more radioresistant, we predicted that radiation treatment may induce a shift of the GSC phenotype from PN to Mes [PN-to-Mes transformation (PMT)]. To test this hypothesis, we first performed qRT-PCR and found that Sox2, a PN marker, was decreased, whereas CD44, Vimentin, and CDH1, all of which are mesenchymal markers, increased by radiation treatment of PN GSCs (n = 3; Fig. 5C). Then we examined the expression of the Mes marker CD44 and the PN marker Sox2 in three PN and three Mes GSCs before and after radiation treatment in vitro (Fig. 5D; Fig. S8B). Although the Mes GSCs (n = 3) did not show any detectable difference in the expression of these markers when comparing expression before and after radiation (Fig. S8 B and C), all three PN GSCs (n = 3) showed a marked increase in the expression of CD44 and an appreciable decrease in expression of Sox2 after radiation treatment, suggesting radiation may induce expression of the Mes signature in PN GSCs, leading to PMT in these HGG-derived PN GSCs (Fig. 5D; Fig. S8D). Because ALDH1A3 is aberrantly up-regulated in Mes GSCs compared with PN GSCs (Fig. 4), we examined whether ALDH1 is required for the putative PMT in these GSCs. Treatment with the ALDH1 inhibitor DEAB before radiation for PN GSCs (n = 3) blocked the shift of expression of Sox2 and CD44 in the treated PN GSCs (Fig. 5D; Fig. S8D), suggesting that inhibition of ALDH1 attenuates radiation-induced transformation of PN GSCs into Mes GSCs. Taken together, these data suggest that radiation treatment of PN GSCs induces expression of Mes-associated markers and inhibition of the operational Mes GSC marker ALDH1A3 in irradiated PN GSCs attenuates their transformation into Mes GSCs.
Discussion
In our analysis of well-characterized clinical HGG-derived GSCs, we report the following findings. First, we are able to classify two distinct tumor-derived GSC subtypes in HGGs (PN GSCs and Mes GSCs) by genomewide transcriptome microarray analysis, as well as in vitro and in vivo tumor growth assays. Second, genes involved in glycolysis and gluconeogenesis pathways including ALDH family genes, in particular ALDH1A3, are significantly up-regulated in Mes GSCs compared with PN GSCs. Third, activities of ALDH1, the enzyme that catalyzes the conversion of acetate from acetaldehyde, is markedly increased in Mes GSCs but not PN GSCs. Fourth, inhibition of ALDH1 by a pharmacological inhibitor or shRNA knockdown of ALDH1A3 attenuates PMT and in vitro growth of Mes GSCs. Fifth, ALDH1A3 is highly expressed in clinical HGGs but not in low-grade glioma or normal brain samples. Sixth, Mes GSCs display a significantly higher radioresistance, with markedly elevated levels of expression of genes associated with DNA repair. Last, irradiation induces transformation of PN GSCs into a Mes-like GSC phenotype (PMT) that is highly resistant to radiation treatment, and inhibition of ALDH1 reverses the radiation-resistant phenotype of Mes GSCs. Taken together, our data suggest that subtypes of GSCs in clinical HGG tumor tissues are identifiable by their in vitro and in vivo behaviors, as well as their global mRNA expression profiles. Up-regulation of ALDH1A3 and DNA repair genes not only distinguishes Mes GSCs from PN GSCs but also contributes to the irradiation-induced PMT.
One novelty of this study is the significance related to our identification of elevated expression of ALDH1A3 in Mes GSCs. First, ALDH1A3 can be a potentially useful biomarker for Mes GSCs. The ALDH gene superfamily is composed of 19 isoenzymes, and their expression appears to be cancer type dependent (18). We found that most Mes GSCs do not express the commonly used GSC marker CD133. Instead, of all 19 members of the ALDH gene family, the expression level of ALDH1A3 is increased up to 150-fold in Mes compared with PN GSCs. Additionally, ALDH1 has been recently recognized as a surrogate marker for cancer stem cells (CSCs), and knockdown of ALDH1 inhibited CSC growth and sensitized CSCs to chemotherapies in various types of human cancers including breast (19), colon (20), and pancreatic (18) cancers. Consistent with these reports, we demonstrate that ALDH1A3 could be a biomarker for Mes GSCs in HGG. Second, increased ALDH1 activity in Mes GSCs is associated with stem cell properties. This observation is clinically relevant because the increased ALDH1 expression correlates with malignancy of glioma in patients. Third, up-regulated ALDH1A3 could also be a potential biomarker to indirectly monitor lipid metabolism in gliomas. Activated cell metabolism (e.g., glycolysis) in cancer cells play critical roles in rendering malignancy and poor responses to therapies of malignant gliomas (21). In the clinic, 18F-fludeoxyglucose (FDG) PET is routinely used to evaluate increased glucose consumption and glycolysis in tumors. We found that ALDH1A9 and ALDH1A3, the enzymes that catalyze conversion of acetaldehyde into acetate, are markedly increased in Mes GSCs. Detection of malignant HGG by 11C-acetate PET based on increased activity of these two enzymes has been reported recently (22). Thus, a potential clinical application of 11C-acetate PET would be useful for evaluation of subtypes of human HGGs.
We also demonstrate a potential role for ALDH1A3 in radiation-induced PMT. Previous studies described that in some, if not all, GBMs, tumor recurrence after failure of standard therapies is accompanied with a phenotypic shift from PN to Mes tumors (4). Activated MET activity in Mes HGGs was shown to induce a program reminiscent of the epithelial-to-mesenchymal transition and enhanced Mes features (23). In this study, we revealed that such a PMT also occurs in GSCs. We found that PN GSCs are more sensitive to radiation than Mes GSCs, and radiation treatment of PN GSCs down-regulated PN markers and up-regulated Mes markers, suggesting radiation-induced PMT. In contrast, radiosensitivity of individual GSCs did not show any statistically significant correlation with tumor grade, despite the distinct genetic background of WHO grade III gliomas compared with grade IV tumors (e.g., 1p/19q LOH in malignant oligodendrogliomas). Our observation of more PN GSCs derived from grade III tumors including oligodendrogliomas and more Mes GSCs derived from grade IV GBMs demonstrates the significance of a Mes signature, specifically in GSCs. The molecular insight of this unique phenomenon warrants further investigation.
Moreover, our results do suggest several open questions that will require further discussion. First, during this study, we noticed that some of the grade III tumor samples (e.g., 1123) gave rise to Mes GSCs, and some of the recurrent GBM (e.g., 347, 816, and 528Rec) after failure of whole brain radiation gave rise to PN GSCs. This result may reflect the existence of both PN and Mes GSC subtypes within individual tumors. Second, the clinical significance of PN and Mes signatures in HGGs needs further in-depth investigation. Although contribution of a Mes signature in glioma (and other cancers) for aggressiveness and poorer prognosis of affected patients is also well recognized, a recent study by Sturm et al. (24) reported that a small subset of PN GBM (after removing IDH1 mutant samples) has a worse prognosis. It appears still debatable which subtype of gliomas has a better or worse prognosis. Third, although we observed a clear increase of Mes-associated genes and proteins in irradiated PN GSCs, further investigation is needed to determine whether radiation treatment causes a complete and irreversible transformation from the PN to Mes phenotype. In fact, both the Phillips study and the Verhaak study identified all three (or four) subtypes in newly diagnosed and recurrent tumors (4, 5). In addition, our data do not rule out the possibility that radiating mixed populations in PN GSCs could potentially allow for a small subset of radiation-resistant Mes-like cells to preferentially survive and emerge as the dominant population under our experimental condition (radiation). In fact, preirradiated PN GSCs contain a subset of CD44-expressing/Sox2-negative cells (Fig. 5D), suggesting that our hypothesis could be at least a potential possibility.
In conclusion, in this study, we identified two mutually exclusive GSCs in HGGs (PN and Mes) with striking phenotypic and genetic differences including aberrantly high expression of ALDH1A3 in Mes GSCs. We also showed that irradiation induces a change of expression of the PN and Mes representative markers indicating a transformation of PN to Mes GSCs and ALDH1A3 is required for this transformation. Collectively, our data provide a set of evidence suggestive of a unique signaling mechanism underlying the transformation of PN GSCs to Mes-like cells and maintenance of stemness of Mes GSCs. Future characterization of the ALDH1-mediated pathways could potentially elucidate novel molecular mechanisms of GSC maintenance and/or propagation and eventually lead to the development of novel and effective molecularly targeted therapies for HGGs.
Materials and Methods
Experimental methods are detailed in SI Materials and Methods. Methods include generation of HGG tumor-derived neurospheres, reagents and antibodies, immunostaining, gene expression profiling and genetic analyses, qRT-PCR and FACS, in vitro cultures, xenotransplantation, glycolysis cell-based assay, IDH1 mutation detection, and statistical analyses. A complete list of cohort demographics is provided in Table S1.
Supplementary Material
Acknowledgments
We thank Drs. H. Kornblum (University of California at Los Angeles) and K. Palanichamy (Ohio State University) for sharing their tumor samples for this study. This work was supported in part by the start-up fund from the Ohio State University; the Department of Neurological Surgery; American Cancer Society Grant MRSG-08-108-01; National Institutes of Health (NIH) Grants CA135013 (to I.N.), LM009657 (to P.V.B.), UL1RR024153 (Reis, Clinical and Translational Science Institute University of Pittsburgh), CA130966 and CA158911 (to S.-Y.C. and B.H.), CA148629, GM087798, NS037704, ES019498, and GM099213 (to R.W.S.), and P30 CA047904 for the University of Pittsburgh Cancer Institute Core Facility (the Lentiviral and the Cancer Biomarkers Facility, to R.W.S.); a Brain Cancer Research award from the James S. McDonnell Foundation (to B.H.); a Zell Scholar award from the Zell Family Foundation; funds from Northwestern Brain Tumor Institute and Department of Neurology Northwestern University (to S.-Y.C.); and the Basic Science Research Program through National Research Foundation of Korea (NRF) Grant 2011-0024089 (to S.-H.K.). P.M. was supported by the China Scholarship Council.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221478110/-/DCSupplemental.
References
- 1.Dunn GP, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26(8):756–784. doi: 10.1101/gad.187922.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson C, et al. Glioblastoma multiforme: A review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18(8):1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 3.Haar CP, et al. Drug resistance in glioblastoma: A mini review. Neurochem Res. 2012;37(6):1192–1200. doi: 10.1007/s11064-011-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips HS, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Verhaak RG, et al. Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai A, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Joo KM, et al. MET signaling regulates glioblastoma stem cells. Cancer Res. 2012;72(15):3828–3838. doi: 10.1158/0008-5472.CAN-11-3760. [DOI] [PubMed] [Google Scholar]
- 9.Guvenc H, et al. Impairment of glioma stem cell survival and growth by a novel inhibitor for Survivin-Ran protein complex. Clin Cancer Res. 2013;19(3):631–642. doi: 10.1158/1078-0432.CCR-12-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazaki T, et al. Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res. 2012;18(5):1268–1280. doi: 10.1158/1078-0432.CCR-11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lottaz C, et al. Transcriptional profiles of CD133+ and CD133- glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res. 2010;70(5):2030–2040. doi: 10.1158/0008-5472.CAN-09-1707. [DOI] [PubMed] [Google Scholar]
- 12.Günther HS, et al. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27(20):2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 13.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 14.Nakano I, Chiocca EA. Finding drugs against CD133+ glioma subpopulations. J Neurosurg. 2011;114(3):648–, discussion 648–650. doi: 10.3171/2009.11.JNS091668. [DOI] [PubMed] [Google Scholar]
- 15.Huang EH, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69(8):3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carro MS, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463(7279):318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarti R, et al. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat Cell Biol. 2012;14(11):1212–1222. doi: 10.1038/ncb2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10(9):1378–1384. doi: 10.4161/cc.10.9.15486. [DOI] [PubMed] [Google Scholar]
- 19.Marcato P, et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells. 2011;29(1):32–45. doi: 10.1002/stem.563. [DOI] [PubMed] [Google Scholar]
- 20.Carpentino JE, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69(20):8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf A, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchida T, Takeuchi H, Okazawa H, Tsujikawa T, Fujibayashi Y. Grading of brain glioma with 1-11C-acetate PET: Comparison with 18F-FDG PET. Nucl Med Biol. 2008;35(2):171–176. doi: 10.1016/j.nucmedbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Lu KV, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22(1):21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturm D, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.